Abstract
Background
Analyses of cancer patterns by detailed racial/ethnic groups in the Northeastern US are outdated.
Methods
Using 2008–2014 death data from the populous and diverse New York state (NYS), mortality rates and regression-derived ratios with corresponding 95% confidence intervals (CI) were computed to compare Hispanic, non-Hispanic white (NHW), non-Hispanic black (NHB), Asian populations; and specific Hispanic and NHB subgroups: Puerto Rican, Dominican, South American, Central American, US-born black, and Caribbean-born black. Special analyses on liver cancer mortality, given the higher prevalence of hepatitis C infection among the 1945–1965 birth cohort, were performed.
Results
244,238 cancer deaths were analyzed. Mortality rates were highest for US-born blacks and lowest for South Americans and Asians. Minority groups had higher mortality from liver and stomach cancer than NHWs; Hispanics and NHBs also had higher mortality from cervical and prostate cancers. Excess liver cancer mortality among Puerto Rican and US-born black men was observed, particularly for the 1945–1965 birth cohort, with mortality rate ratios of 4.27 (95%CI 3.82–4.78) and 3.81 (95%CI 3.45–4.20), respectively.
Conclusions
US-born blacks and Puerto Ricans, who share a common disadvantaged socioeconomic profile, bear a disproportionate burden for many cancers, including liver cancer among baby boomers. The relatively favorable cancer profile for Caribbean-born blacks contrasts with their US-born black counterparts, implying that race per se is not an inevitable determinant of higher mortality among NHBs.
Impact
Disaggregation by detailed Hispanic and Black subgroups in US cancer studies enlightens our understanding of the epidemiology of cancer and is fundamental for cancer prevention and control efforts.
Keywords: New York, Cancer, Hispanics, Blacks, Liver Cancer
Introduction
Few states reflect the United States’ racial/ethnic diversity as well as New York State (NYS); its 2016 population of 20 million was over 45% minority: 19% Hispanic, 18% Black, and 10% Asian/Pacific Islander (API) (1). Yet, no comprehensive analysis to date has leveraged this diversity to critically examine on a population level the heterogeneity by race, ethnicity, and birthplace in site-specific cancer mortality patterns, including distinguishing between Afro-Caribbean and US-born black populations as well as detailing the patterns for diverse Hispanic subgroups.
Cancer is the leading cause of death for Hispanics and Asian/Pacific Islanders in the United States (US); nonetheless, their cancer burden is less than that of non-Hispanic whites (NHWs) and especially non-Hispanic blacks (NHBs), who bear the most disproportionate share of the national cancer burden (2). Hispanic, Black and Asian minorities, projected to increase in both absolute number as well as proportion (3), are heterogeneous, with varying socioeconomic circumstances, nativity and/or immigration experiences, and cultural values and practices. Aggregating these distinct groups in cancer research masks considerable diversity in cancer incidence (4,5,6) and mortality (7,8,9) among subgroups. Moreover, it limits the ability to detect and address determinants of differences in cancer incidence, survival, and mortality, and to discern the extent to which biological, cultural, or socioeconomic factors explain revealed cancer disparities.
While comprehensive data on Asian/Pacific Islander subgroups is available from the SEER cancer surveillance program, that is not the case for non-Mexican Hispanic subgroups and Afro-Caribbeans, who primarily reside in two non-SEER states, New York and Florida. Moreover, unbiased cancer incidence and survival studies for these groups have been impeded by the incompleteness of birthplace and racial/ethnic subgroup information in cancer surveillance systems in general, as well as the incompleteness of follow-up in some registries (10). However, unlike available federal mortality data, some states make specific birthplace information available in their mortality data upon request. Because death certificate information on birthplace, has been found to be highly accurate for minority populations (11), this allows for the more complete and accurate identification of minority subgroups in cancer data. NYS, with 11.3 million NHWs, 3.5 million Hispanics, 3 million NHBs and 1.6 million APIs, is ideal for studying race/ethnicity-specific cancer patterns for populations living within the same geography (12).
To fully capture the diversity of cancer patterns, this study aims to compare cancer mortality between Hispanic, Black and Asian decedents, as well as racial/ethnic subgroups, referencing NHWs. In addition, given the rise in liver cancer mortality (13), a cancer that disproportionately impacts minority populations (14), we analyze this cancer site in greater detail. NYS’s unique diversity includes a large Caribbean-born black subgroup and large Puerto Rican (PR) and Dominican subgroups. In anticipation of meeting the cancer prevention and control needs of these burgeoning minority populations, public health professionals, clinicians, and policymakers alike will require this accurate characterization of patterns within very diverse racial and ethnic groups.
Materials and Methods
Cancer mortality data for 2008–2014 for NYS residents were obtained from the New York State Department of Health. All-sites-combined cancer as well as the most common cancer-specific causes of death were analyzed. Cancer sites were coded according to the ICD-10; cancers of unknown primary (CUP) included C79 and C80 as causes of death. Female breast cancer rates were presented in aggregate as well as divided into two age groups, younger than age 50 and 50 or older, to approximate pre-menopausal and post-menopausal breast cancers.
Major racial/ethnic groups analyzed were non-Hispanic whites, non-Hispanic blacks (including single race and in combination with one other race, i.e. NHB and NHW were coded as black), Hispanics, and Asian/Pacific Islanders, (hereafter referred to as Asians due to proportionally very few (2%) Pacific Islanders in population and only 180 deaths.) A small number of decedents of Native American/Alaskan Native origin (n=341) and those with unknown or more than 2 races (n=1003) were excluded from analyses.
To minimize misclassification, all codes for race and ethnicity were examined, including text fields and birthplace of decedents, to obtain four clearly delineated Hispanic subgroups: Puerto Rican, Dominican, Central American (CA), and South American (SA). Decedents from Spanish-speaking countries in Central America, as well as those coded Hispanic from Belize, were aggregated into the CA group; likewise, SA was designated for all decedents from Spanish-speaking countries in South America, including any from Guyana, French Guiana, and Suriname who were identified as Hispanic. Included in the All Hispanics category but not reported as standalone subgroups were Cubans and those born in Spain due to relatively few cancer deaths in NYS, as well as Mexicans, for whom the extremely young population structure and scant number of cancer deaths prohibited a standalone group. Only 2% of all Hispanic (US-born) decedents (n=466), were of unknown Hispanic subgroup after careful data assembly. For mortality rate calculations, these were proportionally assigned to subgroups based on age, sex, and cancer site combinations, using methodology described elsewhere (4).
Analyzed subgroups among NHB populations were based on race codes and birthplace and included Caribbean-born blacks and US-born blacks. Caribbean-born black decedents were residents of NYS born in the following Caribbean island nations/territories: Anguilla, Antigua and Barbuda, Aruba, Bahamas, Barbados, Cayman Islands, Dominica, Grenada, Guadeloupe, Haiti, Jamaica, Martinique, Montserrat, Saint Kitts and Nevis, Saint Barthelemy, Saint Lucia, Saint Martin, Saint Vincent and the Grenadines, the Netherlands Antilles, the British Virgin Islands, Trinidad and Tobago, Turks and Caicos, and West Indies not-otherwise-specified. NHBs born in other countries (e.g. Guyana, Nigeria, etc.) were included in the All NHB category but not analyzed as standalone groups.
Population denominators for NYS, presented in Table 1, were obtained from the US Census Bureau, using 2008 to 2014 single-year American Community Survey data (15).
Table 1.
Study Population Characteristics. New York. 2008–2014.
| Population Dataa | Cancer Decedents Data (2008–2014) | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Total Populationb | Foreign-bornc | Cancer Deaths | Foreign-Bornc | Black Raced | From New York City | Top country of birth | |
|
|
|||||||
| Non-Hispanic white | 11,328,035 | 10% | 182,696 | 13% | 0% | 22% | USA, 87% |
| All Non-Hispanic black | 2,968,524 | 27% | 33,499 | 26% | 100% | 69% | USA, 74% |
| US-born black | 2,154,932 | 0% | 24,725 | 0% | 65% | USA,100% | |
| Caribbean-born black | 575,260 | 100% | 6,346 | 100% | 81% | Jamaica, 40% | |
| Other foreign-born black | 238,332 | 100% | 2,428 | 100% | 85% | Guyana, 30% | |
| Asiane | 1,600,999 | 68% | 8,516 | 96% | 0% | 80% | China, 50% |
| All Hispanic | 3,505,408 | 50% | 19,527 | 85% | 5% | 79% | Puerto Rico, 38% |
| Puerto Rican | 1,122,741 | 28% | 9,774 | 79% | 2% | 80% | Puerto Rico, 78% |
| Central American | 405,819 | 64% | 1,626 | 97% | 26% | 70% | Panama, 24% |
| South American | 608,785 | 68% | 2,746 | 98% | 1% | 74% | Ecuador, 29% |
| Dominican | 775,273 | 61% | 3,585 | 97% | 8% | 90% | Dominican Republic, 96% |
| Other Hispanicsf | 592,790 | 52% | 1,796 | 69% | 4% | 68% | Cuba, 34% |
Single-year American Community Surveys, 2008–2014
Annualized
Outside 50 US states
Includes single race as well as black in combination with one other race
Includes 2% Pacific Islanders
Includes Cubans, Mexicans and Spaniards
Cancer mortality rates stratified by sex for the seven-year period of 2008–2014 were calculated per 100,000 persons, annualized and age-standardized to the 2000 US Standard Population using eighteen age group bands, all 5-year except the last, which was 85 and older. Gamma intervals modification was used to calculate corresponding 95% confidence intervals (CIs) (16). To directly compare rates for all analyzed populations, we computed age-adjusted site-specific mortality rate ratios (MRRs) using negative binomial regression, which compounds age-specific ratios between populations of remarkably different age structures more effectively than the US Standard population weights (17). Models included decedents ages 35 and over, except prostate, which included ages 45 and older.
Lastly, for liver cancer, common among all minorities, we studied age and cohort-specific patterns, with a focus on the 1945–1965 birth cohort, which is subject to the Centers for Disease Control and Prevention (CDC) recommendation of one-time HCV testing due to comparatively high HCV prevalence (18). We studied age-specific rates as well as age-adjusted rates, and computed liver cancer MRRs stratified by the 1945–1965 birth cohort (possible ages 43–69 at death during 2008–2014) and all those born outside the cohort, here also called the “normal-risk” cohort, before 1945 or after 1965 (possible ages 40–48 and 64+ at death).
SAS 9.3 was used for data analyses. The University of Nevada, Las Vegas Institutional Review Board determined this research to be Exempt per Common Rule 45 CFR 46.101(a).
Results
Between 2008–2014, cancer was the cause of death for 245,582 NYS residents; 244,238 were included in this analysis (Table 1). Among males and females, lung was the leading cause of cancer death for all populations except Caribbean-born blacks and Central Americans, for whom lung cancer was second to prostate cancer in males and breast cancer in females. Mortality from liver cancer was second for Asian males, while prostate cancer was second for all other male groups. Among NHW, Asian, and US-born black females, breast cancer rates were second, but much lower than lung cancer, while for the Hispanic subgroups other than CAs, breast cancer and lung cancer were similar as the top two leading causes of death for women. Colorectal cancer was the third leading cause of death for most populations (Table 2 and Table 3).
Table 2.
Annual Age-Adjusteda Mortality Rates per 100,000 for Selected Cancers by Race/Ethnicity, New York. 2008–2014. Male.
| Non-Hispanic white | Asian/Pacific Islander |
Non-Hispanic
black Allf |
Selected Black Subgroups | Hispanic Allf | Selected Hispanic Subgroups | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| US-born black | Caribbean-born black | Puerto Rican | Dominican | Central American | South American | |||||||||||||||
| n=91,500 | n=4,709 | n=15,850 | n=11,801 | n=2,871 | n=9,899 | n=5,177 | n=1,644 | n=744 | n=1,322 | |||||||||||
|
| ||||||||||||||||||||
| Rate | 95%CI | Rate | 95%CI | Rate | 95%CI | Rate | 95%CI | Rate | 95%CI | Rate | 95%CI | Rate | 95%CI | Rate | 95%CI | Rate | 95%CI | Rate | 95%CI | |
| Oral Cavity & Pharynx | 3.2 | (3.0–3.3) | 3.2 | (2.6–3.8) | 4.3 | (3.8–4.8) | 5.8 | (5.1–6.6) | 1.4 | (0.9–2.3) | 2.9 | (2.5–3.4) | 4.9 | (4.1–5.8) | 1.8 | (1.1–2.6) | 1.5 | (0.7–2.8) | 0.9 | (0.4–1.6) |
| Esophagus | 7.8 | (7.6–8.1) | 3.0 | (2.5–3.6) | 6.1 | (5.5–6.7) | 7.7 | (6.9–8.6) | 3.5 | (2.7–4.8) | 4.6 | (4.1–5.1) | 7.2 | (6.2–8.3) | 4.0 | (2.9–5.3) | 2.7 | (1.4–4.6) | 1.8 | (1.1–2.8) |
| Stomach | 4.2 | (4.0–4.4) | 9.5 | (8.5–10.6) | 9.2 | (8.5–10.0) | 9.5 | (8.5–10.5) | 8.3 | (7.0–9.9) | 7.2 | (6.6–8.0) | 7.8 | (6.7–9.0) | 5.2 | (4.0–6.6) | 11.5 | (8.7–14.8) | 7.1 | (5.7–8.8) |
| Colorectum | 16.9 | (16.5–17.3) | 12.8 | (11.6–14.1) | 22.7 | (21.5–23.9) | 26.7 | (25.1–28.3) | 14.5 | (12.9–16.5) | 15.6 | (14.6–16.6) | 21.7 | (19.9–23.6) | 11.2 | (9.4–13.3) | 13.0 | (10.0–16.6) | 11.7 | (9.8–13.9) |
| Liverb | 7.2 | (6.9–7.4) | 13.1 | (11.9–14.3) | 13.1 | (12.3–13.9) | 17.7 | (16.6–19.0) | 5.1 | (4.2–6.5) | 12.7 | (11.9–13.6) | 21.3 | (19.7–23.1) | 7.8 | (6.3–9.5) | 8.5 | (6.0–11.5) | 6.6 | (5.2–8.2) |
| 1945–1965 Birth Cohortc | 2.7 | (2.5–2.8) | 4.6 | (4.0–5.3) | 6.8 | (6.2–7.4) | 10.0 | (9.1–11.0) | 1.7 | (1.2–2.7) | 5.8 | (5.3–6.4) | 11.5 | (10.3–12.8) | 2.7 | (1.9–3.7) | 2.5 | (1.4–4.3) | 2.0 | (1.4–2.9) |
| Outside Birth Cohort | 4.5 | (4.3–4.7) | 8.4 | (7.4–9.7) | 6.3 | (5.6–7.1) | 7.7 | (6.7–8.9) | 3.4 | (2.6–4.9) | 6.9 | (6.1–7.9) | 9.8 | (8.3–11.8) | 5.1 | (3.8–7.3) | 5.9 | (3.6–10.0) | 4.6 | (3.4–6.9) |
| Pancreas | 13.7 | (13.4–14.0) | 8.1 | (7.2–9.1) | 13.3 | (12.4–14.2) | 15.8 | (14.6–17.0) | 8.2 | (6.9–9.8) | 9.1 | (8.4–9.9) | 10.4 | (9.2–11.7) | 8.9 | (7.3–10.8) | 8.8 | (6.3–12.0) | 7.8 | (6.2–9.6) |
| Lungd | 53.1 | (52.5–53.8) | 32.5 | (30.6–34.5) | 53.8 | (52.0–55.6) | 73.3 | (70.7–76.0) | 22.0 | (20.0–24.4) | 29.2 | (27.9–30.6) | 40.0 | (37.5–42.5) | 24.1 | (21.3–27.2) | 21.5 | (17.2–26.4) | 16.7 | (14.2–19.3) |
| Prostate | 17.6 | (17.2–18.0) | 7.7 | (6.7–8.8) | 43.4 | (41.7–45.2) | 45.3 | (43.1–47.6) | 36.8 | (33.9–40.0) | 19.3 | (18.1–20.6) | 21.6 | (19.6–23.7) | 20.0 | (17.3–23.0) | 29.5 | (24.0–35.7) | 13.2 | (10.9–15.9) |
| Kidney | 5.3 | (5.1–5.5) | 2.4 | (1.9–2.9) | 4.1 | (3.7–4.7) | 5.0 | (4.3–5.7) | 2.2 | (1.6–3.3) | 2.8 | (2.4–3.2) | 3.7 | (3.0–4.5) | 1.3 | (0.7–2.1) | 2.8 | (1.5–4.6) | 2.8 | (1.9–4.0) |
| Bladder | 9.1 | (8.9–9.4) | 2.9 | (2.3–3.5) | 5.5 | (4.9–6.2) | 6.6 | (5.8–7.5) | 3.8 | (2.9–5.2) | 4.3 | (3.8–4.9) | 5.3 | (4.4–6.4) | 3.9 | (2.8–5.3) | 2.5 | (1.1–4.7) | 3.0 | (2.0–4.4) |
| Brain | 5.4 | (5.2–5.6) | 2.1 | (1.7–2.6) | 2.7 | (2.3–3.1) | 3.1 | (2.6–3.6) | 2.0 | (1.5–3.1) | 2.7 | (2.4–3.2) | 3.1 | (2.5–3.8) | 2.6 | (1.8–3.6) | 2.4 | (1.3–4.0) | 2.5 | (1.8–3.5) |
| CUP | 10.1 | (9.9–10.4) | 4.8 | (4.1–5.6) | 10.5 | (9.7–11.3) | 13.1 | (12.0–14.2) | 5.6 | (4.6–7.0) | 7.1 | (6.5–7.8) | 9.6 | (8.4–10.9) | 5.8 | (4.5–7.4) | 4.8 | (2.9–7.1) | 5.0 | (3.7–6.6) |
| NHL | 7.8 | (7.5–8.0) | 4.3 | (3.6–5.0) | 5.8 | (5.2–6.4) | 5.9 | (5.2–6.7) | 5.4 | (4.4–6.8) | 6.2 | (5.6–6.9) | 6.8 | (5.8–7.8) | 6.0 | (4.7–7.6) | 6.1 | (4.1–8.6) | 6.5 | (5.0–8.2) |
| Myeloma | 3.6 | (3.4–3.8) | 1.4 | (1.0–1.9) | 6.5 | (5.9–7.2) | 6.6 | (5.8–7.4) | 6.5 | (5.5–8.0) | 3.4 | (3.0–4.0) | 3.4 | (2.7–4.2) | 3.9 | (2.8–5.2) | 3.6 | (2.0–5.7) | 3.8 | (2.7–5.2) |
| Leukemia | 9.5 | (9.2–9.8) | 4.1 | (3.5–4.8) | 7.0 | (6.4–7.7) | 7.5 | (6.6–8.3) | 6.1 | (5.0–7.5) | 5.9 | (5.3–6.5) | 5.5 | (4.7–6.5) | 6.2 | (4.8–7.7) | 5.7 | (3.7–8.3) | 6.8 | (5.3–8.6) |
| All-Sites-Combinede | 193.3 | (192.0–194.6) | 120.4 | (116.7–124.2) | 224.3 | (220.6–228.0) | 269.5 | (264.5–274.6) | 140.7 | (135.3–146.4) | 146.3 | (143.2–149.4) | 190.7 | (185.2–196.2) | 122.8 | (116.4–129.5) | 134.0 | (123.1–145.5) | 106.9 | (100.6–113.4) |
2000 US Standard Population
Includes intrahepatic bile duct
High HCV prevalence birth cohort; age-adjusted rates for liver cohorts originate from different age groups and thus should only be compared across populations, not within.
Includes bronchus
All sites combined includes those listed as well as those not listed here.
“All” includes those who fall in this race/ethnicity category but not specifically studied here.
Abbreviations: CI Confidence Interval; CUP: Cancers of Unknown Primary; NHL: Non-Hodgkin’s Lymphoma
Table 3.
Annual Age-Adjusteda Mortality Rates per 100,000 for Selected Cancers by Race/Ethnicity, New York. 2008–2014. Female.
| Non-Hispanic white | Asian/Pacific Islander |
Non-Hispanic black Allg |
Selected Black Subgroups | Hispanic Allg | Selected Hispanic Subgroups | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| US-born black | Caribbean-born black | Puerto Rican | Dominican | Central American | South American | |||||||||||||||
| n=91,196 | n=3,807 | n=17,649 | n=12,924 | 3,475 | n=9,628 | n=4,597 | n=1,941 | n=882 | n=1,424 | |||||||||||
| Rate | 95%CI | Rate | 95%CI | Rate | 95%CI | Rate | 95%CI | Rate | 95%CI | Rate | 95%CI | Rate | 95%CI | Rate | 95%CI | Rate | 95%CI | Rate | 95%CI | |
| Oral Cavity & Pharynx | 1.1 | (1.0–1.2) | 1.2 | (0.9–1.5) | 1.5 | (1.3–1.7) | 1.9 | (1.6–2.3) | 0.8 | (0.5–1.5) | 0.9 | (0.7–1.1) | 1.1 | (0.8–1.5) | 0.8 | (0.5–1.3) | 0.7 | (0.3–1.6) | 0.7 | (0.4–1.2) |
| Esophagus | 1.7 | (1.6–1.8) | 0.6 | (0.4–0.9) | 1.9 | (1.6–2.2) | 2.6 | (2.2–3.0) | 0.8 | (0.5–1.5) | 1.1 | (0.9–1.3) | 1.6 | (1.2–2.0) | 0.8 | (0.5–1.3) | 1.0 | (0.4–1.9) | 0.8 | (0.4–1.3) |
| Stomach | 2.1 | (2.0–2.2) | 4.0 | (3.5–4.7) | 4.6 | (4.2–5.0) | 4.5 | (4.0–5.0) | 4.5 | (3.8–5.5) | 3.9 | (3.5–4.3) | 3.8 | (3.2–4.4) | 2.8 | (2.2–3.6) | 6.7 | (5.0–8.6) | 4.5 | (3.6–5.6) |
| Colorectum | 12.3 | (12.0–12.6) | 8.0 | (7.2–8.8) | 15.2 | (14.4–15.9) | 17.3 | (16.3–18.2) | 11.1 | (10.0–12.5) | 10.0 | (9.3–10.6) | 13.3 | (12.1–14.5) | 8.4 | (7.2–9.7) | 9.4 | (7.4–11.8) | 6.5 | (5.4–7.8) |
| Liverb | 2.9 | (2.8–3.1) | 4.9 | (4.3–5.6) | 4.0 | (3.6–4.4) | 4.5 | (4.0–5.0) | 3.0 | (2.4–3.9) | 5.2 | (4.7–5.7) | 6.2 | (5.5–7.1) | 5.5 | (4.5–6.6) | 4.7 | (3.3–6.4) | 4.4 | (3.4–5.4) |
| 1945–1965 Birth Cohortc | 0.7 | (0.7–0.8) | 1.0 | (0.8–1.4) | 1.6 | (1.4–1.9) | 2.2 | (1.8–2.7) | 0.6 | (0.3–1.4) | 1.6 | (1.3–1.8) | 2.2 | (1.8–2.8) | 1.2 | (0.8–1.9) | 1.1 | (0.5–2.3) | 1.1 | (0.7–1.8) |
| Outside Birth Cohort | 2.2 | (2.1–2.3) | 3.9 | (3.3–4.7) | 2.4 | (2.1–2.7) | 2.3 | (1.9–2.8) | 2.3 | (1.8–3.4) | 3.6 | (3.2–4.2) | 4.0 | (3.3–5.0) | 4.2 | (3.3–5.6) | 3.7 | (2.4–7.9) | 3.3 | (2.3–4.6) |
| Pancreas | 10.2 | (9.9–10.4) | 6.5 | (5.7–7.2) | 11.3 | (10.6–11.9) | 13.2 | (12.4–14.1) | 7.0 | (6.1–8.1) | 7.1 | (6.5–7.6) | 8.4 | (7.5–9.3) | 5.4 | (4.5–6.5) | 7.3 | (5.6–9.4) | 6.7 | (5.5–8.0) |
| Lungd | 39.4 | (38.9–39.9) | 15.6 | (14.5–16.8) | 29.3 | (28.3–30.3) | 41.6 | (40.1–43.1) | 8.3 | (7.4–9.5) | 14.4 | (13.7–15.2) | 20.6 | (19.2–22.1) | 11.0 | (9.6–12.5) | 9.3 | (7.3–11.6) | 9.4 | (8.0–11.0) |
| Female Breast | 21.0 | (20.7–21.4) | 9.1 | (8.3–9.9) | 27.9 | (27.0–28.9) | 31.2 | (30.0–32.6) | 20.7 | (19.2–22.5) | 14.7 | (13.9–15.4) | 18.1 | (16.8–19.5) | 13.3 | (11.9–14.9) | 15.1 | (12.6–17.8) | 11.2 | (9.7–12.8) |
| Premenopausale | 3.0 | (2.8–3.1) | 1.8 | (1.5–2.2) | 5.7 | (5.2–6.2) | 6.0 | (5.4–6.7) | 5.0 | (4.2–6.1) | 2.4 | (2.1–2.7) | 2.9 | (2.4–3.5) | 2.8 | (2.2–3.5) | 1.6 | (1.0–2.5) | 2.2 | (1.6–2.9) |
| Postmenopausal | 18.0 | (17.8–18.4) | 7.2 | (6.5–8.0) | 22.3 | (21.4–23.1) | 25.2 | (24.1–26.4) | 15.7 | (14.5–17.2) | 12.3 | (11.6–13.0) | 15.2 | (14.0–16.5) | 10.6 | (9.3–12.0) | 13.5 | (11.1–16.1) | 9.0 | (7.7–10.5) |
| Cervix | 1.8 | (1.7–1.9) | 1.6 | (1.2–1.9) | 4.4 | (4.0–4.8) | 4.8 | (4.3–5.4) | 3.7 | (3.1–4.6) | 2.9 | (2.6–3.2) | 3.9 | (3.3–4.5) | 2.8 | (2.2–3.6) | 2.5 | (1.7–3.7) | 2.2 | (1.6–2.9) |
| Endometrium | 4.9 | (4.7–5.1) | 2.1 | (1.7–2.5) | 10.2 | (9.7–10.8) | 10.8 | (10.0–11.5) | 8.5 | (7.6–9.7) | 4.1 | (3.8–4.6) | 4.8 | (4.1–5.5) | 3.8 | (3.0–4.7) | 7.6 | (5.8–9.7) | 2.5 | (1.8–3.3) |
| Ovary | 8.4 | (8.2–8.6) | 4.5 | (3.9–5.1) | 6.6 | (6.1–7.1) | 7.0 | (6.4–7.6) | 5.2 | (4.5–6.2) | 4.8 | (4.4–5.3) | 5.6 | (4.8–6.4) | 4.1 | (3.3–5.0) | 6.4 | (4.9–8.3) | 4.0 | (3.1–5.0) |
| Kidney | 2.2 | (2.1–2.3) | 1.0 | (0.8–1.4) | 1.7 | (1.4–1.9) | 2.1 | (1.8–2.5) | 0.7 | (0.4–1.4) | 1.1 | (0.9–1.3) | 1.4 | (1.0–1.8) | 0.6 | (0.3–1.0) | 1.4 | (0.7–2.4) | 1.0 | (0.6–1.6) |
| Bladder | 2.6 | (2.5–2.7) | 1.2 | (0.9–1.5) | 2.2 | (1.9–2.5) | 2.6 | (2.3–3.0) | 1.4 | (1.0–2.2) | 1.5 | (1.3–1.8) | 2.1 | (1.7–2.6) | 0.9 | (0.6–1.4) | 1.5 | (0.7–2.6) | 0.8 | (0.4–1.4) |
| Brain | 3.7 | (3.5–3.8) | 1.5 | (1.2–1.9) | 1.8 | (1.5–2.0) | 1.7 | (1.4–2.0) | 1.9 | (1.4–2.7) | 2.0 | (1.8–2.3) | 2.4 | (1.9–2.9) | 2.1 | (1.6–2.8) | 1.3 | (0.7–2.1) | 1.9 | (1.3–2.6) |
| CUP | 7.4 | (7.2–7.6) | 3.2 | (2.7–3.8) | 7.3 | (6.8–7.8) | 8.7 | (8.0–9.4) | 4.9 | (4.2–5.9) | 4.6 | (4.2–5.1) | 5.2 | (4.5–5.9) | 4.4 | (3.6–5.4) | 5.1 | (3.7–6.8) | 4.1 | (3.2–5.2) |
| NHL | 4.8 | (4.6–5.0) | 2.5 | (2.1–3.0) | 3.5 | (3.1–3.8) | 3.5 | (3.1–4.0) | 3.0 | (2.5–3.9) | 3.5 | (3.1–3.9) | 4.1 | (3.5–4.8) | 3.2 | (2.5–4.0) | 3.3 | (2.2–4.7) | 3.4 | (2.6–4.4) |
| Myeloma | 2.3 | (2.2–2.5) | 0.9 | (0.6–1.2) | 4.3 | (4.0–4.7) | 4.6 | (4.1–5.2) | 3.9 | (3.2–4.8) | 2.2 | (1.9–2.6) | 2.4 | (1.9–3.0) | 3.3 | (2.6–4.2) | 1.8 | (1.0–2.9) | 1.5 | (1.0–2.2) |
| Leukemia | 5.1 | (5.0–5.3) | 2.5 | (2.0–2.9) | 3.6 | (3.3–4.0) | 3.8 | (3.3–4.2) | 2.7 | (2.2–3.6) | 3.9 | (3.5–4.3) | 4.3 | (3.7–5.0) | 3.6 | (2.9–4.5) | 3.9 | (2.7–5.3) | 3.2 | (2.4–4.1) |
| All-Sites-Combinedf | 145.4 | (144.4–146.3) | 76.5 | (74.0–79.1) | 151.7 | (149.5–154.0) | 178.2 | (175.1–181.3) | 99.9 | (96.5–103.5) | 96.2 | (94.3–98.2) | 119.7 | (116.2–123.2) | 83.5 | (79.7–87.4) | 98.3 | (91.7–105.2) | 75.8 | (71.8–80.0) |
2000 US Standard Population
Includes intrahepatic bile duct
High HCV prevalence birth cohort; age-adjusted rates for liver cohorts originate from different age groups and thus should only be compared across populations, not within.
Includes bronchus
Cutoff of age 50 used to approximate pre- and post-menopausal status; Age-adjusted breast cohorts originate from different age groups and thus should only be compared across populations, not within.
All sites combined includes those listed as well as those not listed here.
“All” includes those who fall in this race/ethnicity category but not specifically studied here.
Abbreviations: CI Confidence Interval; CUP: Cancers of Unknown Primary; NHL: Non-Hodgkin’s Lymphoma
South American Hispanics of both sexes in NYS had the lowest all-cancers-combined mortality, 106.9 per 100,000 (95%CI:100.6–113.4) for males and 75.8 (95%CI: 71.8–80.0) for females, closely followed by Asians and Dominicans. Slightly higher rates were seen for CA and Caribbean-born black groups, but not nearly as high as Puerto Rican or NHWs. US-born blacks were the highest of all groups, with mortality rates per 100,000 for all-cancers-combined of 269.5 (95%CI: 264.5–274.6) for males and 178.2 (95%CI:175.1–181.3) for females. Compared to Caribbean-born blacks, US-born black populations showed considerably higher mortality rates: nearly five times higher for lung, three times higher for male liver, and twice as high for colorectal (in males only) and pancreas, kidney, and bladder cancers. Among Hispanics, the Puerto Rican group had the highest all-cancers-combined rates, not significantly different than NHWs males, with 190.7 per 100,000 (95%CI:185.2–196.2) among males and 119.7 per 100,000 (95%CI:116.2–123.2) among females (Table 2 and Table 3).
Patterns varied greatly by cancer site. Compared to NHW men, risk of mortality from lung cancer was 56% and 41% lower among Caribbean-born black and Asian men, respectively, and 20% lower among Puerto Rican men (Table 4). In women, the risk differentials were even greater: 79%, 63%, and 45% lower lung cancer mortality among Caribbean-born black, Asian, and PR women, respectively. Yet, US-born black men and women had 49% and 15% higher risk of lung cancer mortality, respectively, than NHWs in NYS. Conversely, Puerto Rican, US-born black and Caribbean-born black populations had significantly higher risk of death from stomach, prostate, pre-menopausal breast, and cervical cancer compared to NHWs, for whom risk of death from bladder, kidney, leukemia and ovarian cancers was greater than all other analyzed populations. Notably, both US-born black as well as Caribbean-born black populations had significantly higher mortality from prostate, myeloma (both sexes), pre-menopausal breast, and endometrial cancers.
Table 4.
Mortality Rate Ratiosa for Selected Cancers, New York. 2008–2014
| NHW | Aggregated Racial/Ethnic Groups | Selected Racial/Ethnic Subgroups | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| Non-Hispanic black | Asian | Hispanic | Puerto Rican | US-born black | Caribbean-born black | ||||||||
| MRR | 95% CI | MRR | 95% CI | MRR | 95% CI | MRR | 95% CI | MRR | 95% CI | MRR | 95% CI | ||
|
|
|||||||||||||
| MALES | |||||||||||||
| Oral | 1 | 1.42 | (1.26–1.59) | 0.97 | (0.82–1.16) | 0.93 | (0.81–1.07) | 1.48 | (1.09–2.02) | 1.93 | (1.70–2.19) | 0.48 | (0.34–0.67) |
| Esophagus | 1 | 0.80 | (0.72–0.88) | 0.37 | (0.31–0.44) | 0.58 | (0.52–0.65) | 0.87 | (0.69–1.09) | 1.03 | (0.92–1.14) | 0.42 | (0.34–0.53) |
| Stomach | 1 | 2.20 | (2.01–2.41) | 2.26 | (2.03–2.53) | 1.80 | (1.63–1.99) | 1.85 | (1.56–2.18) | 2.30 | (2.07–2.57) | 1.92 | (1.64–2.25) |
| Colorectum | 1 | 1.41 | (1.32–1.51) | 0.72 | (0.65–0.79) | 0.94 | (0.87–1.01) | 1.32 | (1.09–1.59) | 1.68 | (1.52–1.86) | 0.90 | (0.79–1.03) |
| Liverb | 1 | 2.01 | (1.59–2.54) | 2.20 | (1.71–2.81) | 1.72 | (1.36–2.16) | 2.95 | (1.89–4.63) | 2.48 | (2.02–3.05) | 0.73 | (0.55–0.96) |
| −1945–1965 Birth Cohortc | 1 | 2.54 | (2.31–2.79) | 1.67 | (1.46–1.90) | 2.12 | (1.92–2.34) | 4.27 | (3.82–4.78) | 3.81 | (3.45–4.20) | 0.63 | (0.47–0.83) |
| −Outside Birth Cohort | 1 | 1.51 | (1.17–1.95) | 2.03 | (1.55–2.66) | 1.51 | (1.19–1.93) | 2.22 | (1.92–2.56) | 1.70 | (1.49–1.94) | 0.72 | (0.55–0.95) |
| Pancreas | 1 | 1.01 | (0.94–1.08) | 0.55 | (0.49–0.62) | 0.68 | (0.63–0.74) | 0.82 | (0.62–1.09) | 1.23 | (1.09–1.39) | 0.57 | (0.47–0.68) |
| Lungd | 1 | 1.12 | (0.99–1.25) | 0.59 | (0.52–0.67) | 0.55 | (0.49–0.62) | 0.80 | (0.71–0.89) | 1.49 | (1.35–1.65) | 0.44 | (0.39–0.50) |
| Prostatee | 1 | 2.74 | (2.51–3.00) | 0.43 | (0.37–0.50) | 1.15 | (1.04–1.27) | 1.29 | (1.13–1.48) | 3.00 | (2.67–3.37) | 2.23 | (1.97–2.54) |
| Kidney | 1 | 0.78 | (0.69–0.89) | 0.44 | (0.36–0.54) | 0.52 | (0.45–0.61) | 0.71 | (0.58–0.86) | 0.96 | (0.83–1.10) | 0.39 | (0.29–0.53) |
| Bladder | 1 | 0.62 | (0.55–0.69) | 0.30 | (0.24–0.36) | 0.47 | (0.41–0.53) | 0.61 | (0.51–0.72) | 0.75 | (0.67–0.86) | 0.38 | (0.30–0.49) |
| NHL | 1 | 0.90 | (0.75–1.09) | 0.56 | (0.45–0.70) | 0.90 | (0.75–1.09) | 1.01 | (0.78–1.31) | 0.93 | (0.73–1.19) | 0.78 | (0.58–1.03) |
| Myeloma | 1 | 2.06 | (1.73–2.44) | 0.43 | (0.32–0.58) | 1.02 | (0.85–1.24) | 1.03 | (0.79–1.36) | 2.10 | (1.70–2.59) | 2.04 | (1.61–2.59) |
| Leukemia | 1 | 0.82 | (0.73–0.93) | 0.43 | (0.36–0.52) | 0.61 | (0.54–0.70) | 0.59 | (0.47–0.75) | 0.93 | (0.77–1.11) | 0.69 | (0.54–0.87) |
| All-Sites-Combinedf | 1 | 1.21 | (1.18–1.24) | 0.61 | (0.59–0.63) | 0.77 | (0.75–0.79) | 1.05 | (0.96–1.16) | 1.49 | (1.36–1.63) | 0.72 | (0.66–0.80) |
|
| |||||||||||||
| FEMALES | |||||||||||||
| Stomach | 1 | 2.15 | (1.94–2.39) | 1.95 | (1.68–2.27) | 1.85 | (1.65–2.08) | 1.85 | (1.56–2.20) | 2.15 | (1.90–2.42) | 2.09 | (1.76–2.48) |
| Colorectum | 1 | 1.26 | (1.19–1.34) | 0.65 | (0.58–0.72) | 0.81 | (0.76–0.88) | 1.14 | (1.01–1.29) | 1.51 | (1.36–1.67) | 0.93 | (0.82–1.07) |
| Liverb | 1 | 1.48 | (1.26–1.74) | 1.68 | (1.39–2.02) | 1.82 | (1.55–2.12) | 2.22 | (1.74–2.82) | 1.67 | (1.32–2.10) | 0.96 | (0.72–1.28) |
| −1945–1965 Birth Cohortc | 1 | 2.18 | (1.83–2.60) | 1.38 | (1.05–1.81) | 2.10 | (1.75–2.52) | 2.97 | (2.28–3.87) | 2.94 | (2.34–3.69) | 0.75 | (0.48–1.17) |
| −Outside Birth Cohort | 1 | 1.09 | (0.95–1.26) | 1.78 | (1.50–2.12) | 1.64 | (1.44–1.86) | 1.80 | (1.50–2.17) | 1.05 | (0.89–1.24) | 1.08 | (0.84–1.38) |
| Pancreas | 1 | 1.10 | (1.04–1.17) | 0.61 | (0.54–0.68) | 0.69 | (0.64–0.75) | 0.86 | (0.74–0.99) | 1.36 | (1.22–1.53) | 0.67 | (0.58–0.79) |
| Lungd | 1 | 0.80 | (0.71–0.90) | 0.40 | (0.35–0.46) | 0.37 | (0.33–0.42) | 0.55 | (0.48–0.63) | 1.15 | (1.02–1.29) | 0.21 | (0.18–0.25) |
| Breast | 1 | 1.33 | (1.27–1.39) | 0.45 | (0.41–0.49) | 0.69 | (0.65–0.74) | 0.86 | (0.79–0.93) | 1.49 | (1.41–1.57) | 0.97 | (0.89–1.05) |
| −Premenopausalf | 1 | 1.88 | (1.69–2.09) | 0.63 | (0.52–0.78) | 0.79 | (0.69–0.91) | 0.97 | (0.79–1.20) | 2.06 | (1.82–2.32) | 1.47 | (1.22–1.77) |
| −Postmenopausal | 1 | 1.24 | (1.18–1.30) | 0.42 | (0.38–0.46) | 0.68 | (0.64–0.72) | 0.84 | (0.77–0.92) | 1.40 | (1.32–1.48) | 0.90 | (0.82–0.98) |
| Cervix | 1 | 2.46 | (2.21–2.75) | 0.83 | (0.66–1.05) | 1.60 | (1.40–1.82) | 2.10 | (1.76–2.51) | 2.66 | (2.35–3.02) | 2.01 | (1.67–2.41) |
| Endometrium | 1 | 2.01 | (1.80–2.24) | 0.42 | (0.34–0.52) | 0.84 | (0.74–0.96) | 0.97 | (0.83–1.13) | 2.15 | (1.97–2.36) | 1.69 | (1.49–1.92) |
| Ovary | 1 | 0.78 | (0.73–0.84) | 0.56 | (0.49–0.63) | 0.57 | (0.52–0.62) | 0.65 | (0.56–0.74) | 0.83 | (0.76–0.91) | 0.62 | (0.54–0.72) |
| Kidney | 1 | 0.75 | (0.65–0.87) | 0.47 | (0.35–0.62) | 0.50 | (0.41–0.60) | 0.61 | (0.46–0.81) | 0.97 | (0.82–1.15) | 0.30 | (0.20–0.46) |
| Bladder | 1 | 0.86 | (0.75–0.98) | 0.45 | (0.34–0.59) | 0.57 | (0.48–0.68) | 0.80 | (0.64–1.00) | 1.01 | (0.87–1.17) | 0.56 | (0.42–0.75) |
| NHL | 1 | 0.99 | (0.77–1.28) | 0.55 | (0.41–0.74) | 0.82 | (0.64–1.06) | 1.03 | (0.74–1.44) | 0.96 | (0.69–1.32) | 0.84 | (0.59–1.19) |
| Myeloma | 1 | 1.84 | (1.67–2.04) | 0.38 | (0.28–0.52) | 0.96 | (0.83–1.10) | 1.05 | (0.85–1.29) | 1.96 | (1.74–2.21) | 1.64 | (1.38–1.96) |
| Leukemia | 1 | 0.68 | (0.61–0.76) | 0.46 | (0.38–0.56) | 0.70 | (0.63–0.79) | 0.80 | (0.68–0.95) | 0.71 | (0.62–0.81) | 0.52 | (0.42–0.64) |
| All-Sites-Combinedf | 1 | 1.14 | (1.05–1.23) | 0.54 | (0.50–0.59) | 0.67 | (0.62–0.73) | 0.86 | (0.78–0.95) | 1.33 | (1.22–1.46) | 0.74 | (0.67–0.81) |
MRRs derived from negative binomial regression including ages 35+ for all cancers, prostate 45+;
Includes intrahepatic bile duct
High HCV prevalence birth cohort (1945–1965)
Includes bronchus
includes ages 45+
cutoff of age 50 used to approximate pre- and post-menopausal status
All sites combined includes those listed as well as those not listed here
Abbreviations: CI: Confidence Interval; CUP: Cancers of Unknown Primary; HCV: Hepatitis C virus; NHL: Non-Hodgkin’s Lymphoma; NHW: non-Hispanic white
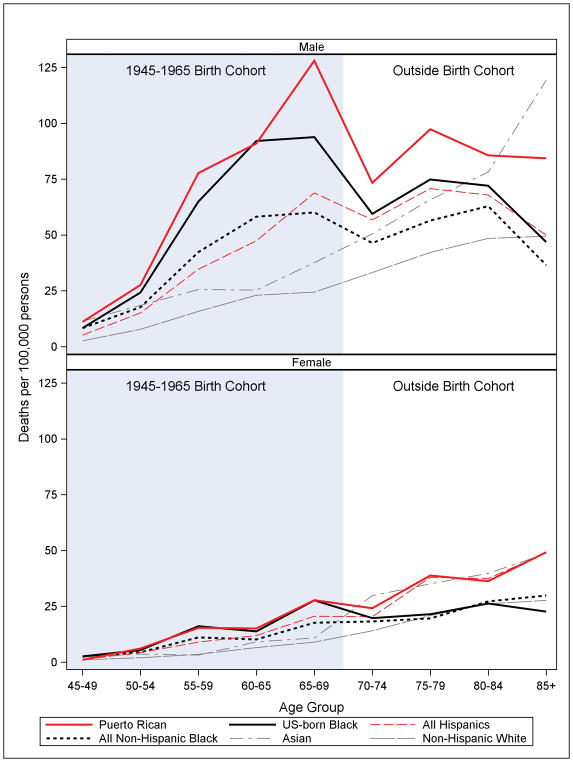
Liver cancer mortality risk was between 1.5 – 2 times higher than NHWs for each of the aggregated minority groups: NHBs, Asians, and Hispanics. However, for Puerto Rican and US-born black males born in the 1945–1965 cohort, risk of death from liver cancer was high: MRR: 4.52; 95%CI (4.05–5.04) and MRR: 3.71; 95%CI (3.37–4.08), respectively. Likewise, Puerto Rican and US-born black women from the 1945–1965 cohort also had significantly higher liver cancer mortality than NHWs: MRR: 3.05; 95%CI (2.45–3.82) and MRR: 2.90; 95%CI (2.38–3.53), respectively (Table 4). Age-group specific rates shown in Figure 1 for the 1945–1965 cohort (beginning from age group 45–49 through age group 65–69) and for the “normal-risk” cohort (beginning from age group 65–69) visually depict a very discernible “hump and dip” pattern for Puerto Rican males and US-born black males and females for the 1945–1965 birth cohort, reflecting their excess mortality.
Figure 1. Increased mortality for liver cancer among Puerto Rican men and US-born Black men and women in the 1945–1965 birth cohort.
Age-specific rates by racial/ethnic group. (Age-specific rates per 100,000 for the age group 65–69 born outside the birth cohort were as follows: Males NHW 26.9, NHB 50.9, Asian 41.5, Hispanic 51.5, USB Black 67.5, PR 76.8; Females NHW 9.4, NHB 10.5, Asian 12.6, Hispanic 21.0, USB Blacks 10.8, PR 33.8) New York State, 2008–2014.
Discussion
Presented here is the first comprehensive analysis of recent cancer mortality patterns in the populous state of New York by detailed racial and ethnic subgroup. Novelties include the intra-racial comparison of US-born blacks to Caribbean-born blacks in NYS, unique Hispanic patterns driven by large Puerto Rican and Dominican populations, and a presentation of East Coast cancer patterns for Asians, more commonly studied on the West Coast, especially California (7).
Overall, a clear contrast is evident between majority immigrant populations in NYS - including Asians, Dominicans, Central Americans, South Americans, and Caribbean-born blacks - and the populations native to the United States and Puerto Rico; the former showed significantly lower overall cancer mortality. Reasons for the advantage of foreign-born populations are complex and undoubtedly have specificities by population group. However, differences on a population basis in three modifiable determinants of many cancers can likely explain much of this advantage: a lower historical prevalence of smoking (19,20) and obesity (21) among immigrants, as well as reproductive patterns among immigrant women that reduce the risk of breast cancer (22).
Non-Hispanic black populations
Of all analyzed populations and subgroups, US-born blacks stand out as most afflicted by cancer death in NYS. Cancer disparities for Black populations in the US have been extensively documented and are usually attributed to higher prevalence of risk factors, especially those associated with lower socioeconomic status (SES) (23,34), as well as racial disparities in health care access and quality (25). Consistent with a recent study in Florida (8), US-born black populations in NYS had considerably higher mortality rates than Caribbean-born blacks for nearly all cancers. For cancers typical of the “Western” lifestyle, associated with obesity and smoking (lung, colorectal, post-menopausal breast, pancreas), US-born blacks had higher rates than the NHW referent population; in contrast, Caribbean-born blacks had significantly lower rates (except for colorectal). However, for some cancers, specifically myeloma, prostate, endometrial and pre-menopausal breast, both US-born and Caribbean-born blacks sustained significantly higher mortality than any of the other analyzed populations, suggesting a possible racial vulnerability, genetic or other, as seen in the Florida study (8). In NYS, the racial component of these cancers is supported further by the uniquely elevated rates for prostate and endometrial cancers among the Hispanic subgroup with the largest proportion (26%) of reported black race on death certificates (See Table 1) in NYS - Central Americans. Overall, the findings here among disaggregated Black populations reinforce the notion that race per se is not synonymous with worse cancer patterns, which, as shown here, specifically afflict US-born blacks more so than their Caribbean-born counterparts. The known unique historical context of discrimination for Black populations combined with current socioeconomic disadvantage may result in increased prevalence of cancer risk factors by pathways not yet entirely understood, requiring clarification specifically for those US-born. Other striking mortality disparities that were observed uniquely for US-born blacks, in relation to other races, included stomach, cervical, liver and colorectal cancers, as well as oral cancer among males.
Hispanics
Contrary to the narrative for black populations, and despite similar socioeconomic profiles (26,27), Hispanics have generally been shown to have lower mortality risk from cancer than NHWs (28). However, our study found all-cancer-combined mortality for Puerto Rican men to be similar to the majority NHW population, albeit with considerable variation by specific cancer sites. Compared to NHWs in NYS, Puerto Ricans showed higher rates of infection-related cancers (stomach, liver, cervix), as well as prostate and colorectal cancers. Moreover, in comparison to their Dominican, Central American, and South American counterparts, Puerto Ricans distinguished themselves with the highest mortality for almost every cancer, at least partially explained by their higher prevalence of major risk factors for cancer, including obesity, smoking (21,29,30) and excessive alcohol use (31,32) compared to other Hispanic subgroups. Additionally, 21% of Puerto Ricans in the current study were born in the continental US, which typically translates into earlier adoption of a “Western” lifestyle and greater acculturation, with the cascading effect of increasing prevalence of modifiable cancer risk factors sustained for a longer period of time, indelibly impacting health patterns in later life (21,33,34). Conversely, Central Americans, South Americans, and Dominicans likely benefit from healthier diets and more active lifestyles when growing up in their countries of origin and continue to maintain lower rates of obesity (29,30), alcohol drinking (32) and tobacco use (35) upon migration.
Asians
Understandably, most studies examining the cancer experience of Asian populations in the US are based in California (7), home to 32% of all Asian Americans (12). Nonetheless, the state of NY has the second largest Asian population, representing 10% of all Asian Americans (12). Largely mimicking their low incidence rates for most cancers (5), Asians had some of the lowest mortality rates of all populations in the current study. As in California (7,36), Asians in NYS showed high rates for only two cancers analyzed - liver and stomach. Liver cancer rates for Asians are largely driven by high prevalence of chronic infection with hepatitis B (14,37) due to immigration from countries with later implementation of mass hepatitis B (HBV) vaccination campaigns (38). Additionally, it is likely that higher prevalence of chronic infection with Helicobacter pylori in Asian countries (39,40) largely explains the high stomach cancer rates. While the leading causes of cancer death for Asians (excepting liver) are lung, colorectal, prostate and breast, similar to all populations, their burden for these lifestyle-related cancers are relatively low, at least partially reflecting their lower prevalence of obesity (41,42) and smoking compared to NHWs (42,43). Notably, lung cancer mortality for Asian men and women in NYS was higher than the other majority foreign-born subgroups, consistent with reports showing higher smoking prevalence among Asians in NY than those subgroups (29,42).
Liver Cancer
Considered in aggregate, all major US minority populations suffer from higher liver cancer incidence and mortality (13,14), which are closely related for this poor prognosis cancer (44), than NHWs. Hepatocellular cancer (HCC) is by far the most common liver cancer histology (45). Prevalence of the major risk factors for HCC, chronic infection with hepatitis B (HBV) and hepatitis C virus (HCV) (37,46), obesity (41), diabetes (47), and heavy alcohol consumption (48), are quite unevenly distributed between racial/ethnic groups, by age group and by sex (49,50). Chronic HCV infection has been at the core of liver cancer increases in the US in the last decade (14). While hepatitis infection data is not directly collected for new liver cancer cases in the US, a population-based study from New York City (NYC) (37) and studies from several liver transplantation centers have documented HCV prevalence in HCC cases at approximately 50% (51).
In the current study, all minority populations have high liver cancer mortality rates compared to NHWs; yet, we aptly demonstrate how aggregation of heterogenous populations obscures important evidence. Specifically, the liver cancer rates are relatively subdued in the aggregate NHB and Hispanic groups, clearly tempered by the inclusion of foreign-born subgroups with lower liver cancer mortality, specifically Caribbean-born blacks (the lowest of all groups analyzed), Dominicans, Central and South Americans.
However, when Puerto Rican and US-born black subgroups are considered separately, particularly in the context of the 1945–1965 birth cohort with higher prevalence of chronic HCV infection (18), striking patterns emerge. The “hump and dip” pattern seen in Figure 1 for the PR and US-born black populations portrays an obvious excess mortality in the “hump” representing the 1945–1965 cohort. The “dip” shows age-specific rates that decrease with age, which is not only counterintuitive for liver cancer, but also discordant with the age-specific rate pattern for NHWs and Asians. This pattern is further confirmed by rate ratios showing distinct and substantial cohort differences in mortality for Puerto Rican men, 4.3 times higher than NHWs in the 1945–1965 cohort, but only 2.2 times higher in the “normal-risk” cohort, with a similar pattern for US-born black men and women, although less clear for PR women. This unusual evidence points to an independent causal factor affecting specific age groups in certain US populations. While both cohorts carry the impact of all risk factors combined (HCV, HBV, obesity, diabetes, alcohol abuse, etc.), the striking differential in mortality rates within the 1945–1965 cohort correlates with known higher levels of chronic HCV infection (46,52), for which the dominant risk factors in the US are past intravenous drug use, particularly during the decades when needle-sharing was most common (1960s–1980s) (53), as well as contaminated blood transfusions before 1992 (18). Thus, while HCV may be impacting all populations at some level, we observe that the excess liver cancer mortality likely associated with chronic HCV infection impacts two specific populations most extremely: Puerto Rican and US-born black subgroups. The results for Puerto Rican males, in particular, are consonant with results for US-born Hispanic populations (majority Mexican) in Texas and California (54,55), other immigrant populations (56); results are also consistent with the high prevalence of chronic HCV infection documented for US-born Hispanics and Puerto Ricans (46) and high rates of incarceration, linked to HCV transmission (57,58). Unfortunately, neither Hispanics in aggregate nor Puerto Ricans specifically are recognized as priority populations in the National Viral Hepatitis Action Plan (59), which is especially concerning since effective HCV antiviral treatment that reduces liver cancer risk is now available (59).
Conversely, different risk factors clearly play a more distinctive role in liver cancer etiology for other population groups. For Asians, higher HBV prevalence likely drives their high liver cancer rates (37), especially given the low prevalence of obesity (41), diabetes (except South Asians) (47), and heavy drinking (48). For the majority NHW population in NYS, a more balanced distribution of viral and non-viral risk factors by age group likely drives rates. Generally, liver cancer mortality patterns seem less clear for women. Thus, future research must clarify the risk profile driving the excess liver cancer in minority women, especially foreign-born, whose liver cancer rates consistently surpass NHW women, especially among the “normal-risk” cohort as shown in Figure 1. Increased awareness of the liver cancer patterns revealed here is critical for clinicians making decisions with their patients about viral hepatitis testing, as well as for public health program planners.
Strengths and Limitations
Our state-level population-based study circumvents biases arising from disparate baseline risks across different geographies. While a few studies have reported disaggregated cancer mortality rates for Hispanic subgroups (6,9,54), and NHB populations (8), none to date have included them altogether with Asians for the broadest possible portrayal of cancer mortality patterns among distinctive American racial/ethnic populations in the same state. NYS was well-suited for this undertaking. Additionally, our study benefitted from very high completeness of the relevant information that allowed for reliable classification of decedents into Hispanic and NHB subgroups (more than 97% complete for all race, ethnicity and birthplace variables).
The standard limitations of descriptive epidemiology apply to the current cross-sectional study based solely on death data. Cancer mortality reflects primarily incidence, but also survival from a cancer diagnosis. Thus, while our results are consistent with previous studies on cancer incidence for US minority populations (4,28), it is also possible that racial/ethnic differences in health care access and quality, both extensively documented (60), may have resulted in worse cancer survival, especially for NHBs, thus impacting the mortality burden. However, neither survival time information nor individual-level indicators of access to quality health care are available from death data. Also lacking is specific risk factor and comorbidity profiles for each decedent, as well as information on individual-level socioeconomic factors, year of immigration, language dominance, or other acculturation measures for immigrants. Theoretically, our mortality numbers could be affected by the Salmon Bias, whereby immigrants return to their home countries of origin to die, although this effect has been shown to be small (61). Asian, Central American and South American rates are themselves aggregates of diverse populations, whose cancer determinants may also differ greatly; sparse numbers prevented a more detailed accounting of these populations. Lastly, only 22% of NHWs come from NYC; yet, for all minority groups that proportion exceeds 65%. Since adjusted cancer rates are higher in NYS than in NYC (62), our differences, as expressed by MMRs with NHWs as references, are slightly underestimated.
Conclusions
Considerable heterogeneity in cancer mortality is observed between different racial/ethnic populations in NYS. At the extremes are US-born blacks on one side and Asian and South American populations on the other: the former has the highest cancer mortality burden; the latter have very low rates relative to other analyzed groups. Generally, Caribbean-born blacks and the Hispanic populations that are majority immigrant - Dominicans, Central and South Americans - have relatively low mortality. However, overall cancer mortality rates for Puerto Rican men match that of NHWs, a novel finding. Subgroup analyses can facilitate the identification not only of high-risk groups, but also low-risk populations who may have protective factors that can be preserved upon immigration or even replicated in other populations.
For liver cancer, the burden is high for all minority groups. However, the excess mortality among Puerto Ricans and US-born blacks, especially in men, is substantial, the most extreme excess of any cancer, especially in the 1945–1965 birth cohort. By considering NHBs and Hispanics in aggregate, the unique HCV- related burden for these specific groups has previously been masked. Awareness of the severity of this problem is critical to clinicians making decisions with their patients about screenings as well as public health program planners.
In the broader context, disparities for some cancers that are commonly associated with foreign-born populations from developing countries, especially infection-related cancers, are increasingly characteristic of US-born minority populations. These cancers, along with colorectal and oral cancer (in males), consistent with evidence seen for US-born Latinos in California (54,55,63), are disproportionately burdening these groups. This commonality among US-born minorities speaks to an undercurrent of entrenched socioeconomic disparities that determine the risk factors for these cancers, including infection with HPV, HCV, H. pylori, obesity, diabetes, and alcohol abuse. Contextualizing cancer prevention and control efforts for the burgeoning minority populations in the US will require addressing the social determinants of health.
Acknowledgments
Funding: Paulo S. Pinheiro was partially funded by the National Institute of General Medical Sciences (8 P20 GM103440-11).
The authors would like to acknowledge Maria Schymura, Director of the New York State Cancer Registry, NYS Department of Health, for her comments to the manuscript draft.
Footnotes
Conflict of Interest Disclosure Statement: The authors declare no potential conflicts of interest.
Disclaimers:
Any conclusions are the authors’ own and do not necessarily reflect the opinion of the data source, the New York State Department of Health, Bureau of Vital Statistics.
References
- 1.US Census Bureau. [Accessed November 2017];Quick Facts New York. Available at: https://www.census.gov/quickfacts/NY.
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA: a cancer journal for clinicians. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 3.Colby SL, Ortman JM. Projections of the Size and Composition of the US Population: 2014 to 2060. Washington, DC: US Department of Commerce, Economics and Statistics Administration, US Census Bureau; Mar, 2015. Report No. P25–1143. [Google Scholar]
- 4.Pinheiro PS, Sherman RL, Trapido EJ, et al. Cancer incidence in first generation U.S. Hispanics: Cubans, Mexicans, Puerto Ricans, and new Latinos. Cancer Epidemiol Biomarkers Prev. 2009;18(8):2162–9. doi: 10.1158/1055-9965.EPI-09-0329. [DOI] [PubMed] [Google Scholar]
- 5.Jin H, Pinheiro PS, Xu J, Amei A. Cancer incidence among Asian American populations in the United States, 2009–2011. International Journal of Cancer. 2016;138(9):2136–45. doi: 10.1002/ijc.29958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howe HL, Lake A, Schymura MJ, Edwards BK. Indirect method to estimate specific Hispanic group cancer rates. Cancer Causes & Control. 2009;20(7):1215–26. doi: 10.1007/s10552-009-9398-8. [DOI] [PubMed] [Google Scholar]
- 7.McCracken M, Olsen M, Chen MS, Jemal A, Thun M, Cokkinides V, et al. Cancer incidence, mortality, and associated risk factors among Asian Americans of Chinese, Filipino, Vietnamese, Korean, and Japanese ethnicities. CA: a cancer journal for clinicians. 2007;57(4):190–205. doi: 10.3322/canjclin.57.4.190. [DOI] [PubMed] [Google Scholar]
- 8.Pinheiro PS, Callahan KE, Ragin CR, Hage RW, Hylton T, Kobetz EN. Black heterogeneity in cancer mortality: US-Blacks, Haitians, and Jamaicans. Cancer Control. 2016;23(4):347–358. doi: 10.1177/107327481602300406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pinheiro PS, Callahan KE, Siegel RL, Jin H, Morris CR, Trapido EJ, et al. Cancer Mortality in Hispanic Ethnic Groups. Cancer Epidemiol Biomarkers Prev. 2017 Mar;26(3):376–82. doi: 10.1158/1055-9965.EPI-16-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinheiro PS, Morris CR, Liu L, Bungum TJ, Altekruse SF. The impact of follow-up type and missed deaths on population-based cancer survival studies for Hispanics and Asians. J Natl Cancer Inst Monographs. 2014;2014(49):210–7. doi: 10.1093/jncimonographs/lgu016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomez SL, Glaser SL. Quality of birthplace information obtained from death certificates for Hispanics, Asians, and Pacific Islanders. Ethn Dis. 2004;14(2):292–5. [PubMed] [Google Scholar]
- 12.U.S. Census Bureau, Population Division. Annual Estimates of the Resident Population by Sex, Race, and Hispanic Origin for the United States, States, and Counties. Table PEPST6H, 2011 Both Sexes, Non-Hispanic Race. Released June 2017. Available from: https://factfinder.census.gov.
- 13.Jemal A, Ward EM, Johnson CJ, Cronin KA, Ma J, Ryerson AB, et al. Annual report to the nation on the status of cancer, 1975–2014, featuring survival. JNCI: Journal of the National Cancer Institute. 2017;109(9) doi: 10.1093/jnci/djx030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ryerson AB, Eheman CR, Altekruse SF, et al. Annual Report to the Nation on the Status of Cancer, 1975–2012, featuring the increasing incidence of liver cancer. Cancer. 2016;122(9):1312–37. doi: 10.1002/cncr.29936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruggles S, Genadek K, Goeken R, Grover J, Sobek M. American Community Survey 2008–2014 1-Year Estimates. Minneapolis: University of Minnesota; [Accessed September 2017]. Integrated Public Use Microdata Series: Version 6.0 [Machine-readable database] [Google Scholar]
- 16.Tiwari RC, Clegg LX, Zou Z. Efficient interval estimation for age-adjusted cancer rates. Stat Methods Med Res. 2006 Dec;15(6):547–69. doi: 10.1177/0962280206070621. [DOI] [PubMed] [Google Scholar]
- 17.Gardner W, Mulvey EP, Shaw EC. Regression analyses of counts and rates: Poisson, overdispersed Poisson, and negative binomial models. Psychol Bull. 1995;118(3):392. doi: 10.1037/0033-2909.118.3.392. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention (CDC) MMWR: Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945–1965. United States: US Department of Health and Human Services; 2012. Report No.: 61(No. RR-4) [Google Scholar]
- 19.Blue L, Fenelon A. Explaining low mortality among US immigrants relative to native-born Americans: the role of smoking. Int J Epidemiol. 2011 Jun;40(3):786–93. doi: 10.1093/ije/dyr011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ng M, Freeman MK, Fleming TD, et al. Smoking prevalence and cigarette consumption in 187 countries, 1980–2012. JAMA. 2014;311(2):183–92. doi: 10.1001/jama.2013.284692. [DOI] [PubMed] [Google Scholar]
- 21.Bates LM, Acevedo-Garcia D, Alegría M, Krieger N. Immigration and generational trends in body mass index and obesity in the United States: results of the National Latino and Asian American Survey, 2002–2003. Am J Public Health. 2008;98(1):70–7. doi: 10.2105/AJPH.2006.102814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ewertz M, Duffy SW, Adami H, Kvåle G, Lund E, Meirik O, et al. Age at first birth, parity and risk of breast cancer: A meta-analysis of 8 studies from the nordic countries. International journal of cancer. 1990;46(4):597–603. doi: 10.1002/ijc.2910460408. [DOI] [PubMed] [Google Scholar]
- 23.Singh GK, Jemal A. Socioeconomic and Racial/Ethnic Disparities in Cancer Mortality, Incidence, and Survival in the United States, 1950–2014: Over Six Decades of Changing Patterns and Widening Inequalities. Journal of environmental and public health. 2017;2017 doi: 10.1155/2017/2819372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Keefe EB, Meltzer JP, Bethea TN. Health disparities and cancer: racial disparities in cancer mortality in the United States, 2000–2010. Front Public Health. 2015 Apr 15;3:51. doi: 10.3389/fpubh.2015.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bach PB, Schrag D, Brawley OW, Galaznik A, Yakren S, Begg CB. Survival of blacks and whites after a cancer diagnosis. JAMA. 2002;287(16):2106–13. doi: 10.1001/jama.287.16.2106. [DOI] [PubMed] [Google Scholar]
- 26.Musu-Gillette L, Robinson J, McFarland J, Kewal-Ramani A, Zhang A, Wilkinson-Flicker S. Status and trends in the education of racial and ethnic groups 2016 (NCES 2016–007) U.S. Department of Education, National Center for Education Statistics; Washington, DC: 2016. [Google Scholar]
- 27.Lopez MH, Cohn D. Hispanic poverty rate highest in new supplemental census measure. Pew Research Center; Washington, DC: Nov 8, 2011. Available from: http://www.pewhispanic.org/2011/11/08/hispanic-poverty-rate-highest-in-new-supplemental-census-measure/ [Google Scholar]
- 28.Siegel RL, Fedewa SA, Miller KD, et al. Cancer statistics for Hispanics/Latinos, 2015. CA Cancer J Clin. 2015;65(6):457–80. doi: 10.3322/caac.21314. [DOI] [PubMed] [Google Scholar]
- 29.Greer S, Naidoo M, Hinterland K, Archer A, Lundy De La Cruz N, Crossa A, Gould LH. Health of Latinos in NYC. 2017:1–32. [Google Scholar]
- 30.Daviglus ML, Talavera GA, Avilés-Santa ML, Allison M, Cai J, Criqui MH, et al. Prevalence of major cardiovascular risk factors and cardiovascular diseases among Hispanic/Latino individuals of diverse backgrounds in the United States. JAMA. 2012;308(17):1775–84. doi: 10.1001/jama.2012.14517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Welte JW, Barnes GM. Alcohol and other drug use among Hispanics in New York State. Alcoholism: Clinical and Experimental Research. 1995;19(4):1061–6. doi: 10.1111/j.1530-0277.1995.tb00989.x. [DOI] [PubMed] [Google Scholar]
- 32.Caetano R, Ramisetty-Mikler S, Rodriguez LA. The Hispanic Americans Baseline Alcohol Survey (HABLAS): rates and predictors of alcohol abuse and dependence across Hispanic national groups. J Stud Alcohol Drugs. 2008 May;69(3):441–8. doi: 10.15288/jsad.2008.69.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lara M, Gamboa C, Kahramanian MI, Morales LS, Hayes Bautista DE. Acculturation and Latino health in the United States: a review of the literature and its sociopolitical context. Annu Rev Public Health. 2005;26:367–97. doi: 10.1146/annurev.publhealth.26.021304.144615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abraido-Lanza AF, Chao MT, Florez KR. Do healthy behaviors decline with greater acculturation?: Implications for the Latino mortality paradox. Soc Sci Med. 2005;61(6):1243–55. doi: 10.1016/j.socscimed.2005.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaplan RC, Bangdiwala SI, Barnhart JM, Castañeda SF, Gellman MD, Lee DJ, et al. Smoking among US Hispanic/Latino adults: the Hispanic community health study/study of Latinos. Am J Prev Med. 2014;46(5):496–506. doi: 10.1016/j.amepre.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang ET, Yang J, Alfaro-Velcamp T, So SK, Glaser SL, Gomez SL. Disparities in liver cancer incidence by nativity, acculturation, and socioeconomic status in California Hispanics and Asians. Cancer Epidemiol Biomarkers Prev. 2010 Dec;19(12):3106–18. doi: 10.1158/1055-9965.EPI-10-0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moore MS, Ivanina E, Bornschlegel K, Qiao B, Schymura MJ, Laraque F. Hepatocellular Carcinoma and Viral Hepatitis in New York City. Clinical Infectious Diseases. 2016;63(12):1577–1583. doi: 10.1093/cid/ciw605. [DOI] [PubMed] [Google Scholar]
- 38.Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11(2):97–107. doi: 10.1046/j.1365-2893.2003.00487.x. [DOI] [PubMed] [Google Scholar]
- 39.Plummer M, Franceschi S, Vignat J, Forman D, de Martel C. Global burden of gastric cancer attributable to Helicobacter pylori. International Journal of Cancer. 2015;136(2):487–90. doi: 10.1002/ijc.28999. [DOI] [PubMed] [Google Scholar]
- 40.World Gastroenterology Organization. Global Guidelines: Helicobacter pylori in developing countries. 2010 Available at: http://www.worldgastroenterology.org/assets/downloads/en/pdf/guidelines/11_helicobacter_pylori_developing_countries_en.pdf.
- 41.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311(8):806–14. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Public Health Information Group, Office of Minority Health and Health Disparities Prevention, Center for Community Health, New York State Department of Health. New York State Minority Health Surveillance Report. New York, NY: 2012. Available at: https://www.health.ny.gov/statistics/community/minority/docs/surveillance_report_2012.pdf. [Google Scholar]
- 43.U.S. Department of Health and Human Services. Tobacco use among US racial/ethnic minority groups-African Americans, American Indians and Alaska Natives, Asian Americans and Pacific Islanders, and Hispanics: A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 1998. [Google Scholar]
- 44.Momin BR, Pinheiro PS, Carreira H, Chunyu L, Weir HK. Liver cancer survival in the United States by race and stage (2001–2009): findings from the CONCORD -2 study Cancer. 2017 doi: 10.1002/cncr.30820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Altekruse SF, Devesa SS, Dickie LA, McGlynn KA, Kleiner DE. Histological classification of liver and intrahepatic bile duct cancers in SEER registries. J Registry Manag. 2011 Winter;38(4):201–5. [PMC free article] [PubMed] [Google Scholar]
- 46.Kuniholm MH, Jung M, Everhart JE, et al. Prevalence of hepatitis C virus infection in US Hispanic/Latino adults: results from the NHANES 2007–2010 and HCHS/SOL studies. J Infect Dis. 2014;209(10):1585–90. doi: 10.1093/infdis/jit672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Setiawan VW, Hernandez BY, Lu SC, Stram DO, Wilkens LR, Le Marchand L, et al. Diabetes and racial/ethnic differences in hepatocellular carcinoma risk: the multiethnic cohort. J Natl Cancer Inst. 2014 Oct 18;106(12) doi: 10.1093/jnci/dju326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.US Department of Health and Human Services, Office of the Surgeon General. Facing Addiction in America: The Surgeon General’s Report on Alcohol, Drugs, and Health. Vol. 2016. Washington (DC): 2016. Nov, [PubMed] [Google Scholar]
- 49.Makarova-Rusher OV, Altekruse SF, McNeel TS, Ulahannan S, Duffy AG, Graubard BI, et al. Population attributable fractions of risk factors for hepatocellular carcinoma in the United States. Cancer. 2016;122(11):1757–65. doi: 10.1002/cncr.29971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.El–Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132(7):2557–76. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 51.Di Bisceglie AM, Lyra AC, Schwartz M, Reddy RK, Martin P, Gores G, et al. Hepatitis C–related hepatocellular carcinoma in the United States: influence of ethnic status. Am J Gastroenterol. 2003;98(9):2060–3. doi: 10.1111/j.1572-0241.2003.t01-1-07641.x. [DOI] [PubMed] [Google Scholar]
- 52.Denniston MM, Jiles RB, Drobeniuc J, Klevens RM, Ward JW, McQuillan GM, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160(5):293–300. doi: 10.7326/M13-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Armstrong GL, Alter MJ, McQuillan GM, Margolis HS. The past incidence of hepatitis C virus infection: implications for the future burden of chronic liver disease in the United States. Hepatology. 2000;31(3):777–82. doi: 10.1002/hep.510310332. [DOI] [PubMed] [Google Scholar]
- 54.Pinheiro PS, Callahan KE, Gomez SL, Marcos-Gragera R, Cobb TR, Roca-Barcelo A, et al. High cancer mortality for US-born Latinos: Evidence from California and Texas. BMC Cancer. 2017;17(1):478. doi: 10.1186/s12885-017-3469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pinheiro PS, Callahan KE, Stern MC, de Vries E. Migration from Mexico to the United States: A High−Speed Cancer Transition. Int J Cancer. 2018;142(3):477–488. doi: 10.1002/ijc.31068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Vries E, Arroyave IZ, Pinheiro PS. The effect of migration on cancer mortality among Colombians: a natural experiment. J Immigrant Minority Health. 2018 In Press. [Google Scholar]
- 57.Sakala L. Breaking down mass incarceration in the 2010 census: State-by-state incarceration rates by race/ethnicity. Prison Policy Initiative. 2014 May [Google Scholar]
- 58.Larney S, Kopinski H, Beckwith CG, Zaller ND, Jarlais DD, Hagan H, Rich JD, Bergh BJ, Degenhardt L. Incidence and prevalence of hepatitis C in prisons and other closed settings: results of a systematic review and meta−analysis. Hepatology. 2013 Oct 1;58(4):1215–24. doi: 10.1002/hep.26387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.US Dept. of Health and Human Services. National Viral Hepatitis Action Plan 2017–2020. 2017 Jan; Available from: https://www.hhs.gov/hepatitis/action-plan/national-viral-hepatitis-action-plan-overview/index.html.
- 60.Smedley BD, Stith AY, Nelson AR, editors. Institute of Medicine (US) Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care. Unequal treatment: Confronting racial and ethnic disparities in health care. Washington DC: National Academies Press; 2003. Available from: https://www.ncbi.nlm.nih.gov/books/NBK220358/ [DOI] [PubMed] [Google Scholar]
- 61.Abraido-Lanza AF, Dohrenwend BP, Ng-Mak DS, Turner JB. The Latino mortality paradox: a test of the “salmon bias” and healthy migrant hypotheses. Am J Public Health. 1999 Oct;89(10):1543–8. doi: 10.2105/ajph.89.10.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.New York State Department of Health, & New York State Cancer Registry. Cancer Incidence and Mortality by Gender and Hispanic Ethnicity, 2010–2014. Retrieved from https://www.health.ny.gov/statistics/cancer/registry/table5.htm.
- 63.Martinsen RP, Morris CR, Pinheiro PS, Parikh-Patel A, Kizer KW. Colorectal Cancer Trends in California and the Need for Greater Screening of Hispanic Men. Am J Prev Med. 2016;51(6):e155–63. doi: 10.1016/j.amepre.2016.05.019. [DOI] [PubMed] [Google Scholar]