Abstract
The architectural features of branching morphogenesis demonstrate exquisite reproducibility among various organs and species despite the unique functionality and biochemical differences of their microenvironment. The regulatory networks that drive branching morphogenesis employ cell-generated and passive mechanical forces, which integrate extracellular signals from the microenvironment into morphogenetic movements. Cell-generated forces function locally to remodel the extracellular matrix (ECM) and control interactions among neighboring cells. Passive mechanical forces are the product of in situ mechanical instabilities that trigger out-of-plane buckling and clefting deformations of adjacent tissues. Many of the molecular and physical signals that underlie buckling and clefting morphogenesis remain unclear and require new experimental strategies to be uncovered. Here, we highlight soft material systems that have been engineered to display programmable buckles and creases. Using synthetic materials to model physicochemical and spatiotemporal features of buckling and clefting morphogenesis might facilitate our understanding of the physical mechanisms that drive branching morphogenesis across different organs and species.
Keywords: Programmable mechanical instabilities, branching morphogenesis, buckling, wrinkling, clefting, creases, passive forces, morphodynamics
Graphical Abstract
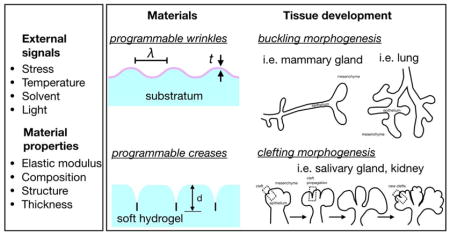
Introduction
The innate property of embryonic cells to proliferate, polarize, differentiate and collectively develop into populations of distinct shapes defines the process of tissue morphogenesis. Despite the unique physiological functionality of different organs, their architectures frequently display striking similarities even among different species. The lung, kidney, salivary and mammary glands are some of the organs that are comprised of a branching network, which results from the morphogenesis of epithelial cells arranged in tubular topologies [1]. However, whether the development of the branched morphologies of these organs results from similar physical mechanisms remains unclear. For instance, in contrast with the lung or prostate, the mammary glands undertake major steps of their development during the postnatal period, affected by the geometric features of the tissue and the concentration of biochemical factors [2,3]. Exploring the cellular and physical principles that direct branching morphogenesis in different organs requires the generation of new experimental strategies that can efficiently unravel the underlying mechanisms for a wide range of tissues and their surrounding microenvironments.
Engineering biomimetic cell culture systems to recapitulate biochemical and biomechanical signals found in developing tissues can greatly facilitate our understanding of epithelial morphogenesis. Biomaterials have been used extensively for similar purposes in tissue engineering, and they have been shown to replicate several features of the natural microenvironment [4]. Following this paradigm, earlier studies of branching morphogenesis combined hydrogel protein scaffolds with dissected embryonic organs [5]. The transplanted organoids reproduced critical features of branching morphogenesis, stimulated by the presentation of soluble growth factors. When particles were embedded in the surrounding hydrogel to establish a spatial gradient of growth factors, the embryonic airway epithelium proliferates and extends towards sites of higher fibroblast growth factor (FGF) concentration [6]. This culture model was thought to replicate the patterned expression of FGF10 by the adjacent mesenchyme and its effects on epithelial proliferation and branching during the earliest stages of lung development [6,7]. Nonetheless, when FGF10 was uniformly added in suspension, the lung epithelium maintained its ability to form branches [7]. Hence, branching morphogenesis is the result of coordinated biophysical and biochemical signals that together control tissue development.
One possible avenue for understanding these complex morphogenetic mechanisms is the design of advanced culture systems that integrate active and passive forces previously found to act during branching morphogenesis [8]. Active forces are generated by actomyosin contractility and transduced through the cytoskeleton and across transmembrane cell receptors that bind selectively to ECM proteins or to neighboring cells. These active forces probe the surrounding mechanical and biochemical microenvironment via cell-ECM and cell-cell interactions, thus influencing the morphological and functional characteristics of the cells and the forming tissue. In this context, biomimetic systems with tunable stiffness [9], viscosity [10], and viscoelastic characteristics [11] were previously engineered to reveal how the mechanical signals of the microenvironment affect cytoplasmic organization and cell functionality through cell-mediated mechanical perturbations. Hybrid biomaterials that permit the controlled presentation of adhesive motifs were formulated to unveil the spatial requirements for ligand-mediated mechanotransduction [12]. Similarly, three-dimensional (3D) hydrogels were engineered to investigate how the interplay between cell-ECM and cell-cell interactions modulates the effects of mechanical properties on cell differentiation [13].
However, branching morphogenesis is also influenced by passive mechanical forces generated by mechanical instabilities in the microenvironment of the developing tissue [14,15]. The effect of these mechanical instabilities on tissue architecture depends on the mechanical properties of the tissues and the mode of branching. During buckling morphogenesis, mechanical instabilities define the spatial patterns associated with branch initiation, while active forces reinforce the acquired tissue morphology and drive its further development. In contrast, the physical characteristics that drive clefting morphogenesis are more ambiguous than those responsible for buckling; new modeling approaches are required to elucidate the underlying mechanisms. Although certain mechanical instabilities can easily be modeled using synthetic materials, there has only been limited effort to engineer cell culture systems with programmable mechanical instabilities. Here, we provide a brief overview of the mechanical instabilities associated with branching morphogenesis and highlight recent progress on the design of synthetic materials with similar mechanical features. We envision that new approaches to engineer biomimetic systems with tunable mechanical instabilities will enable the modeling of a wider variety of physical mechanisms of branching morphogenesis.
Buckling morphogenesis
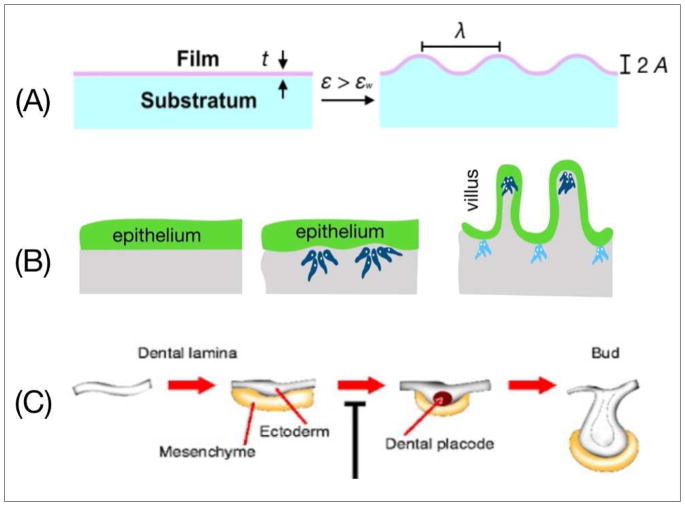
Several recent studies have highlighted the role of physical mechanisms on the regulation of buckling morphogenesis [16]. Varner et al. [17] (**) showed that the initial buckling of mesenchyme-free mouse airway epithelium acts as a local physical pattern to direct branch extension [18]. In this example, the characteristic wavelength of buckling depends on the growth rate of the epithelium and is independent of the stiffness of the surrounding gel. These findings suggest that the physical characteristics of the participating cellular and ECM components act together with biochemical cues to drive branching morphogenesis. An analogous case of buckling deformation in synthetic materials can be sufficiently modeled by a bilayer film that is comprised of a rigid upper layer and a softer foundation. Compressing the bilayer film above a critical strain (εw) triggers out-of-plane deformation (Fig. 1A). This type of mechanical instability is described by a characteristic wavelength (λ) and amplitude (A), the values of which depend on the relative Young’s modulus, thickness, and Poisson ratio of the two layers.
Fig. 1.
(A) Buckling morphogenesis of a stratified film due to implementation of a critical strain. The wavelength and the amplitude of the out-of-plane deformations depend on the mechanical properties and the thickness of the film. (B) Gradual deformation of an initially flat epithelial layer in the mouse intestine. The intestinal epithelium secretes growth factors that locally cluster mesenchymal cells. Reciprocal epithelial and mesenchymal signaling causes villi to emerge followed by shortening of the height of the epithelial layer [20]. (C) Early stages of tooth morphogenesis. Induction of odontogenic mesenchyme by growth factors secreted by the epithelium. Subsequent folding and bud formation of the epithelium under mesenchyme-assisted spatial confinement [23].
During buckling morphogenesis, the critical strain (εw) is accrued across the epithelium under the influence of an asymmetry in growth or the presence of a dynamic mechanical mismatch between the epithelium and its adjacent mesenchyme [16]. For example, in the chicken midgut the higher growth rate and compression ratio of the epithelium compared to those of the mesenchyme can induce the sequential formation of longitudinal ridges and zigzag patterns that precede villus formation [19]. Similar to the model of the bilayer film, the buckling characteristics delineating this phenomenon are the spatial periodicity and amplitude of the folds that form. In the mouse intestine, villus morphogenesis begins with an initially flat epithelium (Fig. 1B) [20]. Epithelial cells secrete soluble signals that induce proliferation and migration of the adjacent mesenchyme and its differentiation into smooth muscle. Through this process, the smooth muscle constrains the growing epithelial layer, giving rise to cell shortening and basal deformation that enables the formation of an out-of-plane villus morphology [21,22]. During tooth morphogenesis (Fig. 1C), concentration gradients of several growth factors (i.e. FGF and bone morphogenetic protein) locally alter the density of mesenchymal cells [23] and define regions of highly proliferative epithelial cells. This asymmetry in growth causes inward folding of the epithelium and formation of the tooth bud.
Given that the process results from a mechanical instability, it is not surprising that epithelial buckling and wrinkling have occasionally been enabled independent of adjacent mesenchymal cells. When distal lung epithelium is denuded of its adjacent mesenchyme and is embedded in a hydrogel comprised of basement membrane proteins, the tissue maintains its ability to buckle [17], consistent with similar findings in kidney [24] and salivary gland [5]. These examples highlight the ubiquity of buckling morphogenesis throughout the development of different organs and despite the operation of different signaling pathways. Nonetheless, it remains unclear how buckling morphogenesis maintains highly precise architectural features without information from a genetic blueprint. This structural complexity is increased by the presence of multiple physical (e.g. stiffness, geometry, thickness) and chemical (e.g. growth factors, ECM proteins) heterogeneities across different organs and species. Engineered cell culture systems that allow control over these features can be potentially used to investigate how different physical properties of the microenvironment orchestrate buckling morphogenesis.
Materials with programmable instabilities for modeling buckling morphogenesis
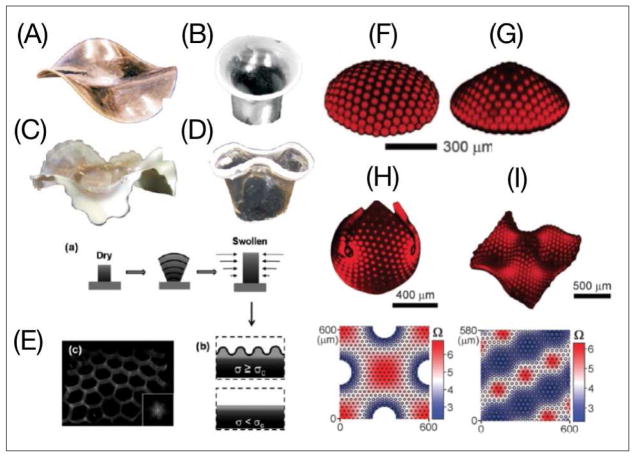
Soft materials have increasingly paved their way into our lives through a vast number of products such as sensors, drug delivery agents, and surgical implants. With control over their chemistry and mechanical properties, soft materials can be used to generate synthetic microenvironments with biologically relevant shape-forming characteristics. Following this concept, synthetic materials prescribed with programmable shape transformations can benefit future efforts in modeling tissue morphogenesis. In previous studies, engineered materials acquired a wrinkled/buckled morphology through a range of stimulatory cues (e.g. thermal, mechanical, or solvent-based) [25]. In the pioneering work of Klein et al. [26], poly(N-isopropylacrylamide) (PNIPAM) gels were created with a one-dimensional (1D) radial gradient of monomer density on top of a Hele-Shaw cell (Fig. 2A–D). Above a critical temperature, the gel transforms into 3D buckles whose shape depends on the concentration gradient of the monomer. Similarly, Guvendiren et al. [27] designed poly(2-hydroxyethyl methacrylate) (PHEMA) films crosslinked with ethylene glycol dimethacrylate to induce a vertical 1D crosslinking gradient and depth-wise variation of the elastic modulus. Solvent-induced swelling of these films while they are attached on a solid substratum generates anisotropic compressive stresses that lead to tunable wrinkling morphology (Fig. 2E).
Fig. 2.
(A–D) Large–scale buckling and wrinkling instabilities in response to different radial gradients of monomer concentration and crosslinking density [26]. (E) Wrinkling induced by the accumulation of critical stress in response to a perpendicular gradient of crosslinking density [27]. Creation of 2D axisymmetrically patterned polymer sheets tuned buckling into a (F) spherical cap and (G) cone geometry in response to heterogeneous swelling. (H–I) 2D non-axisymmetric patterning induces 3D buckling and wrinkling following the prescribed metrics [28].
These initial studies motivated the incorporation of two-dimensional (2D) instructive patterns in swelling gels to enable control over 3D shape deformations. Kim et al. [28] employed halftone gel lithography to obtain programmable buckling. Using lithographic patterning on PNIPAM films through a multiple-mask approach, they formulated gels with well-defined crosslinked regions to function as 2D instructive patterns. With control over the distribution, size, and degree of crosslinking in the patterned regions, this strategy provides a unique method to engineer programmable instabilities. Axisymmetric patterning enables control over the geometrical configuration of the buckles (Fig. 2F–G), while non-axisymmetric patterning generates 3D shapes of arbitrary complexity (Fig. 2H–I). More recently, gray-scale lithography was used to prescribe patterning of even higher spatial resolution without the use of masks [29] (**). Alternative strategies created light-activated programmable instabilities by incorporating gold nanoparticles in the corresponding gels [30]. In this case, near-infrared (IR) radiation of the gel induces 3D buckles due to nanoparticle-generated heat ablation. Controlling the deformation kinetics in materials with prescribed shape characteristics is another advantage towards modeling branching morphogenesis. A recent study showed that the kinetics of buckling formation can simply be tuned by changing the concentration of encapsulated nanotubes or by varying the power of the radiating light [31]. Using photodegradable hydrogels, Kapyla et al. [32] designed cell culture systems that deform in response to exposure to UV light (**). Overall, materials with programmable buckling and wrinkling instabilities provide a unique platform to engineer different mechanical states that replicate the initiation of buckling morphogenesis by passive forces.
Clefting morphogenesis
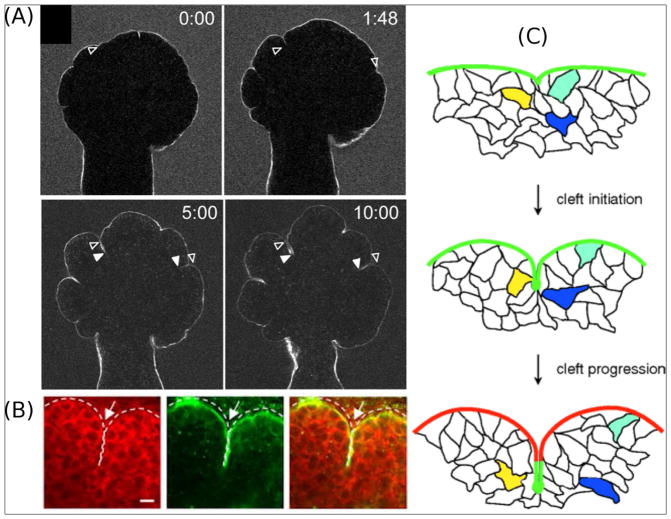
Buckling morphogenesis is not the only mechanical process that can drive the formation of branches. In the salivary and mammary glands, branching morphogenesis occurs through cleft formation wherein a parent branch ruptures in half to form two daughter branches [33]. While in the mammary gland the molecular and physical characteristics of clefting morphogenesis are poorly defined, in the salivary gland the mesenchymal ECM plays an instructive role [18]. This matrix-driven morphogenesis involves highly motile epithelial cells (Fig. 3A and C) [34,35] that along with their adjacent mesenchymal cells remodel the ECM and rearrange cell-cell and cell-ECM interactions near the sites of cleft nucleation (Fig. 3B) [36–38] to begin to form a branching network. In this process, the epithelial buds turn into reservoirs of ECM proteins that modulate cell adhesion and contractile activity through the actomyosin network. The kinetics of stress fiber formation seems to approximate the kinetics of cleft propagation [39], which becomes reversible in the case of low ECM levels [40]. Although their functional role is poorly defined, mesenchymal cells are located close to the tip of the cleft, suggesting that these cells might secrete chemical factors or facilitate ECM remodeling [40–42].
Fig. 3.
(A) Dynamics of clefting morphogenesis in the mouse salivary gland. The bright line corresponds to fluorescently tagged fibronectin (FN), which follows and fills the cleft opening [34]. (B) Cytoplasmic vinculin (red) accumulates in cells at the cleft region of the salivary gland and overlaps with localized collagen type IV in the basement membrane [36]. (C) Schematic of matrix-driven branching. FN is initially deposited at the site of cleft formation and it remains subject to remodeling as the cleft deepens. Colored cells demonstrate that cleft formation is a dynamic process during which cell migration and time-dependent ECM presentation control branching morphogenesis [34].
Investigating the role of the mesenchyme in epithelial bifurcation during lung morphogenesis, Kim et al. [43] showed that new clefts form at sites of smooth muscle differentiation. In turn, smooth muscle cells define spatial constraints that compress the growing epithelial tissue and trigger bifurcation of the epithelium. Smooth muscle differentiation has also been hypothesized to instruct clefting morphogenesis through other physical mechanisms including ECM remodeling [44] and diffusion-barrier contributions [45], which coordinately mediate the interplay of cell-ECM and cell-cell forces.
The increased epithelial dynamics and the critical role of ECM remodeling during clefting morphogenesis are indicative of a softer, more compliant tissue that is distinct from the mechanically constrained epithelial layer involved in buckling morphogenesis. Using computational modeling, Bi et al. [46] showed that in confluent 2D tissues a liquid-like state is rendered when cell-cell forces exceed the constraints of cortical tension. This spatial intermingling of epithelial and mesenchymal cells in a compliant microenvironment can be more sufficiently modeled by soft, single-layer hydrogels. Correspondingly, creases, which are the mechanical instabilities in similarly soft materials, are better candidates than buckles for modeling the physical features of cleft formation and propagation.
Materials with programmable instabilities for modeling clefting morphogenesis
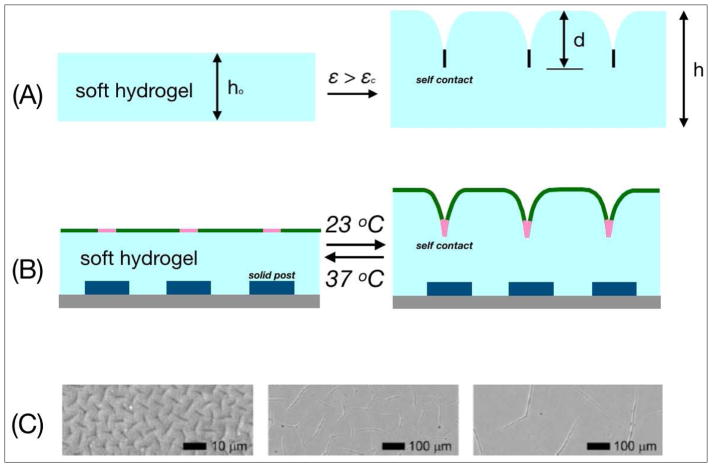
In contrast to the formation of wrinkles in elastic bilayer films, the mechanical perturbation of soft hydrogels or elastomers induces morphological deformations of their surfaces with sharp self-contacting regions (Fig. 4A) [25]. These instabilities are called creases and their onset strain (εc) is relatively smaller than that which induces buckling/wrinkling instability [47]. To address the singular shapes of creases, non-linear perturbation analysis on a skin layer with finite bending stiffness under a critical strain εc revealed the discontinuous transition from a flat surface to creases of accountable depth [48]. The geometric features of creases approximate those of epithelial clefts (Fig. 3), while their nucleation in soft elastomers is favored by compositional heterogeneities and the presentation of physical defects or solid barriers that are engineered inside their structure [49]. Similarly, clefting morphogenesis is enabled by ECM patterning at the sites of nucleation, although the mechanical role of ECM patterning in this event remains poorly explored. Reversibility is another property common to crease and cleft propagation. The fraction of nucleated clefts that accumulates an inadequate amount of ECM and incomplete remodeling is more vulnerable to retraction. On the other hand, crease reversibility in synthetic materials is coordinated by the functional interplay between the material surface and its elastic energy [49].
Fig. 4.
(A) Creasing instability induced by a critical strain εc on a soft elastomer or hydrogel with thickness ho. In the out-of-plane deformed state, the surface of the elastomer contains self-contacting features. (B) Composite polymer film for formation of thermoreversible creases [50]. The blue boxes are solid-like spots for controlled crease formation. The pink segments refer to regions of selective chemical modification on the self-contacting regions. (C) Control of crease size by adjustment of film thickness. Crease size increases with gel thickness (3, 40, 160 μm respectively) [52].
Thus, materials engineered with programmable creases can serve as physical models of clefting morphogenesis. Creases have been induced in soft hydrogels using different stimuli including temperature, pH, and light gradients [25]. By patterning solid posts at the supporting substratum (Fig. 4B: blue boxes), Kim et al. [50] generated reversible crease instabilities that are temperature-responsive. Furthermore, when the creases were modified with biological motifs at the self-contacting regions (Fig. 4B: pink-colored regions), they were able to induce ligand-specific cell adhesion. Conversely, Chen et al. [51] photo-labeled non-contacting regions of the creases with adhesive motifs, forming stretchable channels that can be used to study the dynamics of receptor-mediated cell adhesion by harnessing the kinetic characteristics of crease reversibility.
Changing the physical and chemical characteristics of synthetic materials can provide control over the geometry of crease instabilities. Trujillo et al. [52] engineered hydrogels of controllable thickness to create creases with similar structure but different size (Fig. 4C). In this case, the critical strain (εc) for crease formation was independent of gel stiffness. Recently, Takahashi et al. [53] created free-swelling gels that sequentially form rectangular patterns of creases and bulk bending. Altogether, materials with programmable crease instabilities have the potential to model combinatorial signals of clefting morphogenesis, and thus to help elucidate the role of different factors such as ECM composition, cellular presentation, and geometrical constraints.
Conclusions
Branching morphogenesis underlies the development of an enormous variety of tissues across biological species. Some general tissue characteristics that can determine the mode of branching morphogenesis include the presence of adjacent tissue layers (e.g., epithelium and mesenchyme) with distinct growth rates and compression ratios or the presentation of different types of gradients (e.g., ECM composition, cell differentiation) on the epithelial layer, which can potentially induce buckling and clefting morphogenesis, respectively. Moreover, the role of ubiquitous cell-cell and cell-ECM interactions is not conserved among different organs and modes of branching morphogenesis [54], despite the fact that their contributions affect tissue topology at different stages of morphogenesis [55].
However, it remains unclear how the physical and chemical mechanisms that are involved in this process preserve such reproducibility independent of a genetic blueprint. One promising solution towards understanding what determines the morphogenetic movements is the design of cell culture systems with controllable shape-changing characteristics. We anticipate that one strategy to formulate similar systems will employ biomaterials with time-dependent mechanical properties and signal-induced mechanical instabilities. Materials that exhibit these general characteristics can establish a platform to recapitulate the conditions that govern buckling and clefting morphogenesis. Sculpting the shape of artificial cell culture microenvironments will enable the investigation of a wide range of physical factors that are hypothesized to induce stereotyped patterns and folds during tissue development, such as the geometry of unfolded tissues, the arrangement of folds and patterns, and any mechanical mismatches. It is expected that similar findings will further explain the physical signals that result from the relative rates of epithelial growth [17] and smooth muscle differentiation [43] in buckling and clefting morphogenesis, respectively. Overall, soft materials with programmable instabilities show the potential to facilitate interdisciplinary studies on branching morphogenesis, benefitting future understanding of organ development and regeneration.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health (HL110335, HL118532, HL120142, CA187692, CA214292), the David & Lucile Packard Foundation, the Alfred P. Sloan Foundation, the Camille & Henry Dreyfus Foundation, the Burroughs Wellcome Fund, and a Faculty Scholars Award from the Howard Hughes Medical Institute
Abbreviations
- 1D
one-dimensional
- 2D
two-dimensional
- 3D
three-dimensional
- BMP
bone morphogenetic protein
- ECM
extracellular matrix
- FGF
fibroblast growth factor
- IR
infrared
Footnotes
Disclosure Statement
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lubkin SR. Branched organs: Mechanics of morphogenesis by multiple mechanisms. Multiscale Modeling of Developmental Systems. 2008;81:249. doi: 10.1016/S0070-2153(07)81008-8. [DOI] [PubMed] [Google Scholar]
- 2.Nelson CM, Vanduijn MM, Inman JL, Fletcher DA, Bissell MJ. Tissue geometry determines sites of mammary branching morphogenesis in organotypic cultures. Science. 2006;314:298–300. doi: 10.1126/science.1131000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sternlicht MD, Kouros-Mehr H, Lu P, Werb Z. Hormonal and local control of mammary branching morphogenesis. Differentiation. 2006;74:365–381. doi: 10.1111/j.1432-0436.2006.00105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Magin CM, Alge DL, Anseth KS. Bio-inspired 3D microenvironments: a new dimension in tissue engineering. Biomed Mater. 2016;11:022001. doi: 10.1088/1748-6041/11/2/022001. [DOI] [PubMed] [Google Scholar]
- 5.Qiao J, Sakurai H, Nigam SK. Branching morphogenesis independent of mesenchymal-epithelial contact in the developing kidney. Proc Natl Acad Sci U S A. 1999;96:7330–7335. doi: 10.1073/pnas.96.13.7330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weaver M, Dunn NR, Hogan BLM. Bmp4 and Fgf10 play opposing roles during lung bud morphogenesis. Development. 2000;127:2695–2704. doi: 10.1242/dev.127.12.2695. [DOI] [PubMed] [Google Scholar]
- 7.Bellusci S, Grindley J, Emoto H, Itoh N, Hogan BLM. Fibroblast Growth Factor 10(FGF10) and branching morphogenesis in the embryonic mouse lung. Development. 1997;124:4867–4878. doi: 10.1242/dev.124.23.4867. [DOI] [PubMed] [Google Scholar]
- 8.Heisenberg CP, Bellaiche Y. Forces in tissue morphogenesis and patterning. Cell. 2013;153:948–962. doi: 10.1016/j.cell.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 9.Discher DE, Sweeney L, Sen S, Engler A. Matrix elasticity directs stem cell lineage specification. Biophysical Journal. 2007:32a–32a. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 10.Kourouklis AP, Lerum RV, Bermudez H. Cell adhesion mechanisms on laterally mobile polymer films. Biomaterials. 2014;35:4827–4834. doi: 10.1016/j.biomaterials.2014.02.052. [DOI] [PubMed] [Google Scholar]
- 11.Chaudhuri O, Gu L, Klumpers D, Darnell M, Bencherif SA, Weaver JC, Huebsch N, Lee HP, Lippens E, Duda GN, et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nature Materials. 2016;15:326. doi: 10.1038/nmat4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang J, Grater SV, Corbellini F, Rinck S, Bock E, Kemkemer R, Kessler H, Ding J, Spatz JP. Impact of order and disorder in RGD nanopatterns on cell adhesion. Nano Lett. 2009;9:1111–1116. doi: 10.1021/nl803548b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cosgrove BD, Mui KL, Driscoll TP, Caliari SR, Mehta KD, Assoian RK, Burdick JA, Mauck RL. N-cadherin adhesive interactions modulate matrix mechanosensing and fate commitment of mesenchymal stem cells. Nature Materials. 2016;15:1297–1306. doi: 10.1038/nmat4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirashima T. Pattern formation of an epithelial tubule by mechanical instability during epididymal development. Cell Rep. 2014;9:866–873. doi: 10.1016/j.celrep.2014.09.041. [DOI] [PubMed] [Google Scholar]
- 15.Taber LA. Morphomechanics: transforming tubes into organs. Curr Opin Genet Dev. 2014;27:7–13. doi: 10.1016/j.gde.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nelson CM. On Buckling Morphogenesis. Journal of Biomechanical Engineering-Transactions of the Asme. 2016:138. doi: 10.1115/1.4032128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •17.Varner VD, Gleghorn JP, Miller E, Radisky DC, Nelson CM. Mechanically patterning the embryonic airway epithelium. Proc Natl Acad Sci U S A. 2015;112:9230–9235. doi: 10.1073/pnas.1504102112. This work demonstrates that purely physical mechanisms can support epithelial patterning in response to asymmetric growth. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varner VD, Nelson CM. Cellular and physical mechanisms of branching morphogenesis. Development. 2014;141:2750–2759. doi: 10.1242/dev.104794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shyer AE, Tallinen T, Nerurkar NL, Wei Z, Gil ES, Kaplan DL, Tabin CJ, Mahadevan L. Villification: how the gut gets its villi. Science. 2013;342:212–218. doi: 10.1126/science.1238842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chin AM, Hill DR, Aurora M, Spence JR. Morphogenesis and maturation of the embryonic and postnatal intestine. Semin Cell Dev Biol. 2017;66:81–93. doi: 10.1016/j.semcdb.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walton KD, Kolterud A, Czerwinski MJ, Bell MJ, Prakash A, Kushwaha J, Grosse AS, Schnell S, Gumucio DL. Hedgehog-responsive mesenchymal clusters direct patterning and emergence of intestinal villi. Proc Natl Acad Sci U S A. 2012;109:15817–15822. doi: 10.1073/pnas.1205669109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grosse AS, Pressprich MF, Curley LB, Hamilton KL, Margolis B, Hildebrand JD, Gumucio DL. Cell dynamics in fetal intestinal epithelium: implications for intestinal growth and morphogenesis. Development. 2011;138:4423–4432. doi: 10.1242/dev.065789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thesleff I. Epithelial-mesenchymal signalling regulating tooth morphogenesis. J Cell Sci. 2003;116:1647–1648. doi: 10.1242/jcs.00410. [DOI] [PubMed] [Google Scholar]
- 24.Nogawa H, Takahashi Y. Substitution for mesenchyme by basement-membrane-like substratum and epidermal growth factor in inducing branching morphogenesis of mouse salivary epithelium. Development. 1991;112:855–861. doi: 10.1242/dev.112.3.855. [DOI] [PubMed] [Google Scholar]
- 25.Chen DY, Yoon J, Chandra D, Crosby AJ, Hayward RC. Stimuli-Responsive Buckling Mechanics of Polymer Films. Journal of Polymer Science Part B-Polymer Physics. 2014;52:1441–1461. [Google Scholar]
- 26.Klein Y, Efrati E, Sharon E. Shaping of elastic sheets by prescription of non-Euclidean metrics. Science. 2007;315:1116–1120. doi: 10.1126/science.1135994. [DOI] [PubMed] [Google Scholar]
- 27.Guvendiren M, Burdick JA, Yang S. Kinetic study of swelling-induced surface pattern formation and ordering in hydrogel films with depth-wise crosslinking gradient. Soft Matter. 2010;6:2044–2049. [Google Scholar]
- 28.Kim J, Hanna JA, Byun M, Santangelo CD, Hayward RC. Designing responsive buckled surfaces by halftone gel lithography. Science. 2012;335:1201–1205. doi: 10.1126/science.1215309. [DOI] [PubMed] [Google Scholar]
- •29.Na JH, Bende NP, Bae J, Santangelo CD, Hayward RC. Grayscale gel lithography for programmed buckling of non-Euclidean hydrogel plates. Soft Matter. 2016;12:4985–4990. doi: 10.1039/c6sm00714g. Develops a new method to introduce finely spaced metrics that induce programmable, temperature-responsive 3D buckles on initially flat hydrogels. [DOI] [PubMed] [Google Scholar]
- 30.Guo HY, Cheng J, Wang JY, Huang P, Liu YJ, Jia Z, Chen XY, Sui KY, Li T, Nie ZH. Reprogrammable ultra-fast shape-transformation of macroporous composite hydrogel sheets. Journal of Materials Chemistry B. 2017;5:2883–2887. doi: 10.1039/C6TB02198K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang X, Pint CL, Lee MH, Schubert BE, Jamshidi A, Takei K, Ko H, Gillies A, Bardhan R, Urban JJ, et al. Optically- and thermally-responsive programmable materials based on carbon nanotube-hydrogel polymer composites. Nano Lett. 2011;11:3239–3244. doi: 10.1021/nl201503e. [DOI] [PubMed] [Google Scholar]
- •32.Kapyla E, Delgado SM, Kasko AM. Shape-Changing Photodegradable Hydrogels for Dynamic 3D Cell Culture. ACS Appl Mater Interfaces. 2016;8:17885–17893. doi: 10.1021/acsami.6b05527. This work reports deformable cell culture systems following the transformation of a 2D hydrogel into a folded 3D tubular structure through light-induced activation. [DOI] [PubMed] [Google Scholar]
- 33.Wang S, Sekiguchi R, Daley WP, Yamada KM. Patterned cell and matrix dynamics in branching morphogenesis. J Cell Biol. 2017;216:559–570. doi: 10.1083/jcb.201610048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larsen M, Wei C, Yamada KM. Cell and fibronectin dynamics during branching morphogenesis. J Cell Sci. 2006;119:3376–3384. doi: 10.1242/jcs.03079. [DOI] [PubMed] [Google Scholar]
- 35.Wei C, Larsen M, Hoffman MP, Yamada KM. Self-organization and branching morphogenesis of primary salivary epithelial cells. Tissue Eng. 2007;13:721–735. doi: 10.1089/ten.2006.0123. [DOI] [PubMed] [Google Scholar]
- 36.Daley WP, Kohn JM, Larsen M. A focal adhesion protein-based mechanochemical checkpoint regulates cleft progression during branching morphogenesis. Dev Dyn. 2011;240:2069–2083. doi: 10.1002/dvdy.22714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pankov R, Cukierman E, Katz BZ, Matsumoto K, Lin DC, Lin S, Hahn C, Yamada KM. Integrin dynamics and matrix assembly: tensin-dependent translocation of alpha(5)beta(1) integrins promotes early fibronectin fibrillogenesis. J Cell Biol. 2000;148:1075–1090. doi: 10.1083/jcb.148.5.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patel VN, Hoffman MP. Salivary gland development: a template for regeneration. Semin Cell Dev Biol. 2014;25–26:52–60. doi: 10.1016/j.semcdb.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hotulainen P, Lappalainen P. Stress fibers are generated by two distinct actin assembly mechanisms in motile cells. J Cell Biol. 2006;173:383–394. doi: 10.1083/jcb.200511093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kadoya Y, Yamashina S. Cellular dynamics of epithelial clefting during branching morphogenesis of the mouse submandibular gland. Dev Dyn. 2010;239:1739–1747. doi: 10.1002/dvdy.22312. [DOI] [PubMed] [Google Scholar]
- 41.Hieda Y, Nakanishi Y. Epithelial morphogenesis in mouse embryonic submandibular gland: its relationships to the tissue organization of epithelium and mesenchyme. Dev Growth Differ. 1997;39:1–8. doi: 10.1046/j.1440-169x.1997.00001.x. [DOI] [PubMed] [Google Scholar]
- 42.Nogawa H. Determination of the curvature of epithelial cell mass by mesenchyme in branching morphogenesis of mouse salivary gland. J Embryol Exp Morphol. 1983;73:221–232. [PubMed] [Google Scholar]
- 43.Kim HY, Pang MF, Varner VD, Kojima L, Miller E, Radisky DC, Nelson CM. Localized Smooth Muscle Differentiation Is Essential for Epithelial Bifurcation during Branching Morphogenesis of the Mammalian Lung. Developmental Cell. 2015;34:719–726. doi: 10.1016/j.devcel.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moore KA, Polte T, Huang S, Shi B, Alsberg E, Sunday ME, Ingber DE. Control of basement membrane remodeling and epithelial branching morphogenesis in embryonic lung by Rho and cytoskeletal tension. Dev Dyn. 2005;232:268–281. doi: 10.1002/dvdy.20237. [DOI] [PubMed] [Google Scholar]
- 45.Thomson AA, Timms BG, Barton L, Cunha GR, Grace OC. The role of smooth muscle in regulating prostatic induction. Development. 2002;129:1905–1912. doi: 10.1242/dev.129.8.1905. [DOI] [PubMed] [Google Scholar]
- 46.Bi DP, Lopez JH, Schwarz JM, Manning ML. A density-independent rigidity transition in biological tissues. Nature Physics. 2015;11:1074. [Google Scholar]
- 47.Biot MA. Surface instability of rubber in compression. Applied Scientific Research, Section A. 1963;12:168–182. [Google Scholar]
- 48.Hohlfeld E, Mahadevan L. Unfolding the sulcus. Phys Rev Lett. 2011;106:105702. doi: 10.1103/PhysRevLett.106.105702. [DOI] [PubMed] [Google Scholar]
- 49.Chen D, Yoon J, Chandra D, Crosby AJ, Hayward RC. Stimuli-responsive buckling mechanics of polymer films. Journal of Polymer Science Part B: Polymer Physics. 2014;52:1441–1461. [Google Scholar]
- 50.Kim J, Yoon J, Hayward RC. Dynamic display of biomolecular patterns through an elastic creasing instability of stimuli-responsive hydrogels. Nature Materials. 2010;9:159–164. doi: 10.1038/nmat2606. [DOI] [PubMed] [Google Scholar]
- 51.Chen DY, Hyldahl RD, Hayward RC. Creased hydrogels as active platforms for mechanical deformation of cultured cells. Lab on a Chip. 2015;15:1160–1167. doi: 10.1039/c4lc01296h. [DOI] [PubMed] [Google Scholar]
- •52.Trujillo V, Kim J, Hayward RC. Creasing instability of surface-attached hydrogels. Soft Matter. 2008;4:564–569. doi: 10.1039/b713263h. This work reports free-swelling gels with mechanical mismatches that permit the combined presentation of creasing and buckling instabilities. [DOI] [PubMed] [Google Scholar]
- 53.Takahashi R, Ikura Y, King DR, Nonoyama T, Nakajima T, Kurokawa T, Kuroda H, Tonegawa Y, Gong JP. Coupled instabilities of surface crease and bulk bending during fast free swelling of hydrogels. Soft Matter. 2016;12:5081–5088. doi: 10.1039/c6sm00578k. [DOI] [PubMed] [Google Scholar]
- 54.Spurlin JW, 3rd, Nelson CM. Building branched tissue structures: from single cell guidance to coordinated construction. Philos Trans R Soc Lond B Biol Sci. 2017:372. doi: 10.1098/rstb.2015.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goodwin K, Nelson CM. Generating tissue topology through remodeling of cell-cell adhesions. Exp Cell Res. 2017;358:45–51. doi: 10.1016/j.yexcr.2017.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]