Abstract
The identification of biological processes related to the regulation of complex traits is a difficult task. Commonly, complex traits are regulated through a multitude of genes contributing each to a small part of the total genetic variance. Additionally, some loci can simultaneously regulate several complex traits, a phenomenon defined as pleiotropy. The lack of understanding on the biological processes responsible for the regulation of these traits results in the decrease of selection efficiency and the selection of undesirable hitchhiking effects. The identification of pleiotropic key-regulator genes can assist in developing important tools for investigating biological processes underlying complex traits. A multi-breed and multi-OMICs approach was applied to study the pleiotropic effects of key-regulator genes using three independent beef cattle populations evaluated for fertility traits. A pleiotropic map for 32 traits related to growth, feed efficiency, carcass and meat quality, and reproduction was used to identify genes shared among the different populations and breeds in pleiotropic regions. Furthermore, data-mining analyses were performed using the Cattle QTL database (CattleQTLdb) to identify the QTL category annotated in the regions around the genes shared among breeds. This approach allowed the identification of a main gene network (composed of 38 genes) shared among breeds. This gene network was significantly associated with thyroid activity, among other biological processes, and displayed a high regulatory potential. In addition, it was possible to identify genes with pleiotropic effects related to crucial biological processes that regulate economically relevant traits associated with fertility, production and health, such as MYC, PPARG, GSK3B, TG and IYD genes. These genes will be further investigated to better understand the biological processes involved in the expression of complex traits and assist in the identification of functional variants associated with undesirable phenotypes, such as decreased fertility, poor feed efficiency and negative energetic balance.
Introduction
The regulation of complex traits involves multiple loci, that contribute to a small proportion of the phenotypic expression, and environmental effects [1, 2]. In addition, loci that regulate complex traits are known to be involved in the regulation of several phenotypes. This describes the primary genetic effect that leads to genetic correlation, known as pleiotropy. Currently, the interaction and regulation pattern of these loci are poorly understood, especially in livestock species. However, the use of pleiotropic markers for genomic selection may result in improvement in efficiency of selection. On the other hand, some pleiotropic markers can lead to the indirect selection of undesirable traits. The exclusion of these markers from genetic selection programs may result in the improvement of a specific trait without affecting other traits or multiple traits simultaneously.
Indirect selection for undesirable traits has been well described in livestock breeding programs. For example, variants associated with high productive performance can also reduce fertility or affect mortality rates in both beef and dairy production systems [3–6]. The identification of loci with pleiotropic effects and the dissection of the biological processes in which these loci are involved can provide useful information to improve the accuracy of genomic breeding values by providing insights on the best statistical model to be used. Thus, more accurate breeding values translates in better breeding decisions, which will lead to reduced frequency of undesirable genotypes and phenotypes in the population under selection. Additionally, the use of causal and functional variants in selection models may result in a higher prediction accuracy and improved prediction persistence over generations [7–9].
The investigation of genomic loci with pleiotropic effects involved in the regulation of economically important traits is a key step to apply the breeding strategies previously mentioned. The detection of such genomic regions enables the identification of potential causal variants mapped in key regulator genes. Multivariate trait analysis and subsequent selection indexes may then be enhanced by the identification of these variants, leading to higher genetic improvement [10–12].
The use of functional genomics in a systems biology approach is a powerful tool for the integration and analysis of biological networks using high-throughput OMICs technologies [13, 14]. The integration of results from different breeds and OMICs approaches helps unravel the relationship between candidate genes and several phenotypes to further identify genetic variants associated with changes in the expression of those genes.
Fertility and production traits are good examples of phenotypes with distinct genetic architecture, different potential to respond to selection, and with antagonistic genetic relationship. The majority of fertility traits have unfavorable genetic correlations with production traits, which may be explained by the unidirectional selection, “hitchhiking” effect or pleiotropic effects [15–17]. The biological process responsible for sexual maturity, known as puberty, involves complex pathways that are regulated by several genes involved with the development of a wide variety of phenotypes [18]. Crucial biological structures for puberty development, e.g., hypothalamus, pituitary gland and thyroid, are involved in the regulation of synthesis and secretion of several hormones directly related to production traits [19–21]. Therefore, a deep investigation of the biological processes related to genes involved in puberty may result in the identification of key regulator genes for both fertility and production traits. Within this context, the aim of this study was to integrate multi-OMICs data into studies that investigated pubertal status and fertility traits. This integration was performed using a systems biology approach to identify candidate genes with pleiotropic effects on economically relevant traits in beef cattle.
Material and methods
Ethics statement
This study was carried out analysing data from previous studies, which have been approved by respective ethics in research committees [13, 20, 22]. Therefore, additional animal welfare and use committee approval was not required.
Data collection
Candidate genes identified in three independent populations (i.e., breeds) of beef cattle were used. For the first population (Brangus cattle, n = 64), genes differentially expressed (DE) between pre- and post- puberty in eight tissues (hypothalamus, pituitary gland, liver, longissimus dorsi muscle, adipose tissue, uterine horn, endometrium, and ovary) were evaluated [13]. The candidate genes for the second population (Tropical Composite cattle, n = 866; Brahman = 843) were identified by a genome-wide association analysis (GWAS) for age at puberty, postpartum anestrous interval, and occurrence of the first postpartum ovulation before weaning in the first rebreeding period [22]. For the third population (Brahman cattle, n = 24), DE genes were identified in pre- and post- puberty stages in the pituitary gland and ovary [20].
Identification of genes near pleiotropic markers
A total of 9,194 genes identified in the three breeds (2,120 in Brangus, 3,714 in Tropical Composite, and 5,269 in Brahman) were mapped against a list of genomic markers (n = 729,068) with pleiotropic effect (P < 0.05 after false discovery rate (FDR) correction–n = 21,908 markers) reported by Bolormaa et al. (2014) [10], which analyzed 32 traits including growth, feed intake, carcass and meat quality, and reproduction. Using the list of candidate genes obtained by integrating and combining data from OMICs technologies described by Cánovas et al. (2014) [13], Hawken et al. (2012) [22] and Nguyen et al. (2017) [20], genes up to 1 Mb (downstream and upstream) from a pleiotropic marker were selected. This interval was selected based on the average recombination block and the linkage disequilibrium (LD) pattern across the cattle genome [23, 24].
Genes shared among breeds and QTL mapping
The genes identified within the pleiotropic regions were compared and those shared across the three independent populations were selected to be mapped against QTL regions using resources from the Cattle QTL database (CattleQTLdb, www.animalgenome.org/cgi-bin/QTLdb/BT/index). Using an interval of 2 Mb (1 Mb downstream and 1 Mb upstream) from each one of the shared genes, all the QTLs mapped in this interval were annotated. Those genes mapped in regions where at least five QTL categories were annotated (from six possible categories: exterior appearance, health, reproduction, production, meat and carcass and milk traits) were ranked as the genes with the highest pleiotropic potential.
Functional analyses
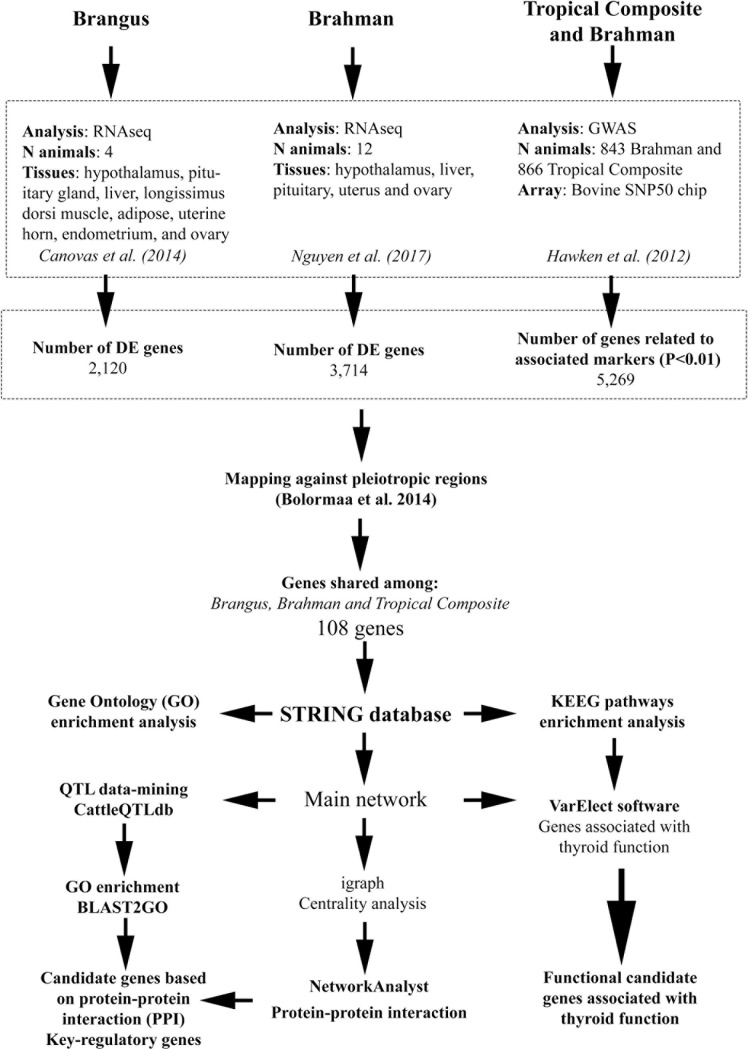
First, the relationships among pleiotropic genes were estimated using literature text-mining, co-expression, gene fusion, protein homology, gene-neighborhood and gene co-occurrence using STRING database [25]. This estimation used human database, as the available information is more complete for humans than bovine. Additionally, using STRING database, an enrichment analysis was also performed to identify associations between the pleiotropic genes with biological processes (BP) and metabolic pathways using the Kyoto Encyclopedia of Genes and Genomes (KEGG). From the gene network created using STRING database, a centrality analysis was performed in order to identify the pleiotropic genes with the highest number of connections in the network. The igraph package [26] was used to calculate the centrality coefficient, defined as the number of connections of each node in a network. The VarElect software [27] was used to match the genes present in this list to the enriched KEGG pathways. In addition, for all the genes present in the main network, BLAST2GO [28] was used to obtain a better description of the BP that these pleiotropic genes are related to. Finally, the NetworkAnalyst tool [29] was used to confirm the genes with the highest regulatory potential based on the interaction with other proteins. The identification of the interactions among the genes was performed using IMEx, based on a literature-curated comprehensive data from InnateDB [30]. All the analyses described previously are shown in Fig 1.
Fig 1. Flowchart presenting the methodological pipeline performed to identify the potential key-regulatory genes for pleiotropic effect on fertility and production traits in beef cattle.
Results and discussion
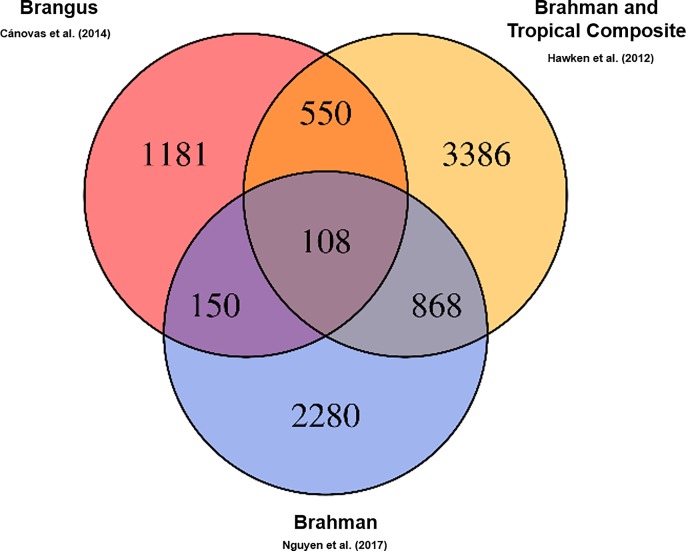
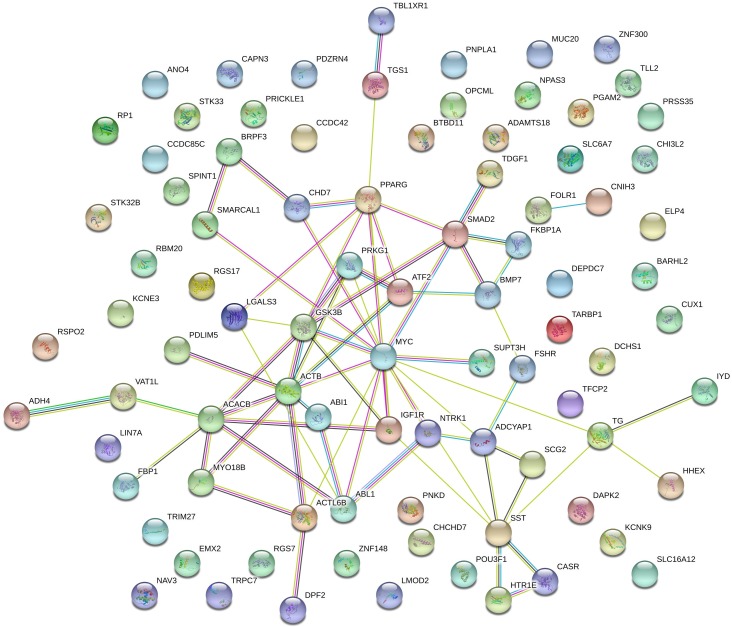
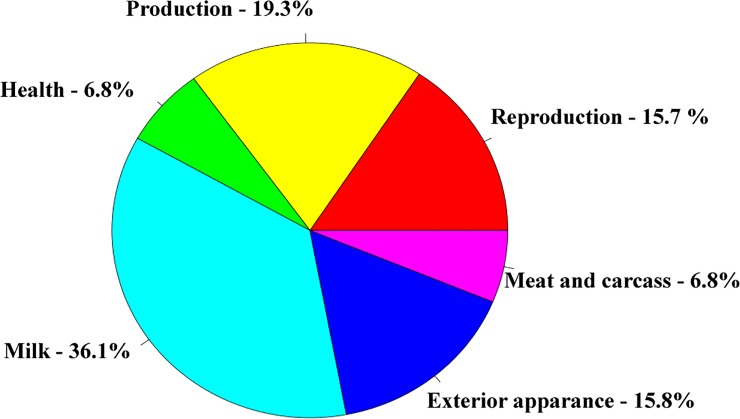
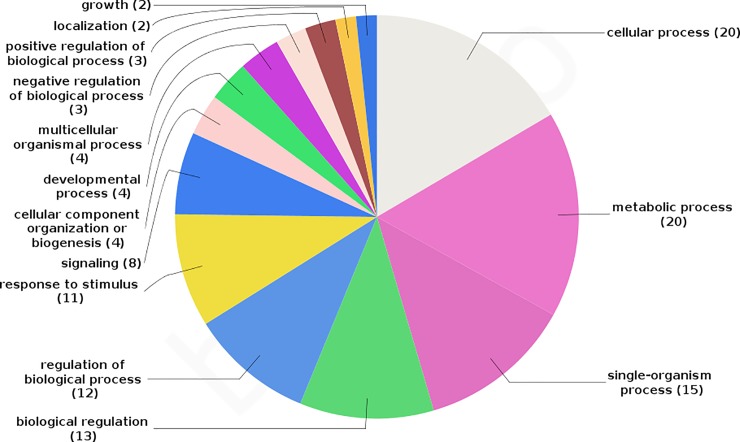
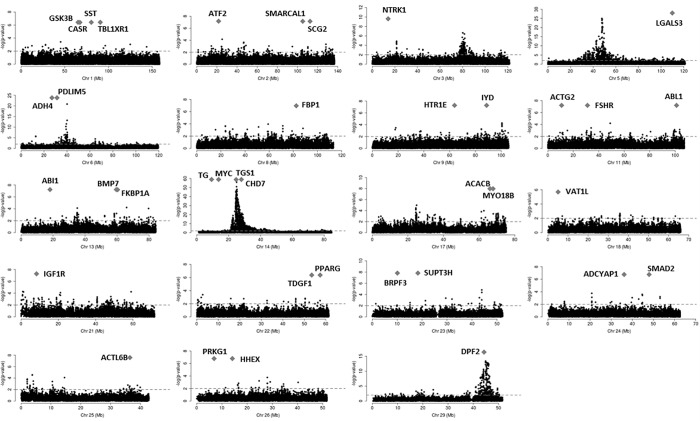
A total of 108 genes (S1 File) mapped in pleiotropic regions were shared among all three independent populations of the three beef cattle breeds considered (Fig 2). Among them, 89 genes were mapped in regions with five or six QTL categories annotated (S2 File). It is important to highlight that the approach to evaluate the post-puberty stage was different between Cánovas et al. (2014) [13] and Nguyen et al. (2017) [20]. The puberty period was defined as the second consecutive day of a circulating progesterone values >1 ng/mL by Cánovas et al. (2014) [13]. On the other hand, Nguyen et al. (2017) [20] defined the puberty period when the observation of the first corpus luteum occurred. These differences, together with the different genetic backgrounds and environmental effects between these two populations, can result in different expression profiles, even when two similar groups of phenotypes are measured (pre- and post-puberty). However, the same biological processes are expected to underlying the puberty development in both populations. Therefore, it is expected that the key-regulators of these processes maintained a similar DE profile between pre- and post-puberty phenotypes in both populations. Additionally, the consistency of expression among tissues and populations can help to identify these key-regulatory genes. The results obtained from BP and KEGG pathways enrichment analyses indicated that these genes are involved in development and maturation of the body (Table 1). From those 89 genes, 38 were grouped in the main network identified by STRING database (Fig 3 and S1 File). Fig 4 shows the percentage of each type of QTL (exterior appearance, health, reproduction, production, meat and carcass quality and milk traits) present in a 2 Mb interval (1 Mb downstream and 1 Mb upstream) around these 38 genes. The expression pattern across the tissues analyzed by Cánovas et al. (2014) [13] and Nguyen et al. (2017) [20] reinforce the functional relevance of these genes due to the DE across specific tissues (Table 2). The detailed mapping of the BP associated with these 38 genes suggested a strong relationship between these genes and the regulation of biological processes involved with cellular metabolism, cellular growth and development (Fig 5 and S3 File). Fig 6 shows the genomic context of the regions where these 38 genes were mapped as a function of the pleiotropic markers harboring these genes. The results from evaluation of the gene list composed of 38 genes suggested that the identification of key-regulatory genes was an important step to elucidate the relationship among these genes and the regulation of BP, as well as their association with economically important traits.
Fig 2. Venn diagram displaying the comparison of genes among the different independent populations analysed.
In yellow, the genes identified in Brahman and Tropical Composite breeds (Hawken et al., 2012) [22]. In blue, the genes identified in Brahman breed (Nguyen et al., 2017) [20]. In red, the genes identified in Brangus breed (Cánovas et al., 2014) [13].
Table 1. Top 10 enriched biological processes (BP) and enriched KEGG pathways identified by STRING database using the 89 genes shared among the reported results from Cánovas et al. (2014) [13], Hawken et al. (2012) [22] and Nguyen et al. (2017) [20] and mapped in regions with 5 or more categories of QTL.
| Top 10 enriched GO (Biological Function) | |||
|---|---|---|---|
| ID | Description | Bonferroni P-value | Implicated genes |
| GO:0048513 | Organ development | 8.67E-05 | ABI1, ABL1, ACTB, ACTL6B, ADAMTS18, ATF2, CAPN3, CCDC85C, CHD7, CUX1, DCHS1, EMX2, FKBP1A, FOLR1, FSHR, GSK3B, HHEX, IGF1R, LIN7A, MYC, NTRK1, POU3F1, PPARG, PRKG1, RBM20, RP1, RSPO2, SPINT1, TBL1XR1, TG, ZNF148 |
| GO:0048731 | System development | 0.0002 | ABI1, ACTB, ACTL6B, ADAMTS18, ADCYAP1, ATF2, BARHL2, BMP7, CAPN3, CCDC85C, CHD7, EMX2, FKBP1A, FOLR1, FSHR, GSK3B, HHEX, IGF1R, LGALS3, LIN7A, MYC, OPCML, PDLIM5, PPARG, PRICKLE1, PRKG1, RBM20, RP1, RSPO2, SCG2, SPINT1, TBL1XR1, TG, TRPC7, ZNF148 |
| GO:0007275 | Multicellular organismal development | 0.0003 | ABI1, ACTB, ACTL6B, ADAMTS18, ADCYAP1, ATF2, BARHL2, BMP7, CAPN3, CCDC85C, CHD7, EMX2, FKBP1A, FOLR1, FSHR, GSK3B, HHEX, IGF1R, LGALS3, LIN7A, MYC, OPCML, PDLIM5, PPARG, PRICKLE1, PRKG1, RBM20, RP1, RSPO2, SCG2, SMAD2, SPINT1, TBL1XR1, TG, TLL2, TRPC7, ZNF148 |
| GO:0048856 | Anatomical structure development | 0.0003 | ABI1, ACTB, ACTL6B, ADAMTS18, ADCYAP1, ATF2, BARHL2, BMP7, CAPN3, CASR, CCDC85C, CHD7, EMX2, FKBP1A, FOLR1, FSHR, GSK3B, HHEX, IGF1R, LIN7A, LMOD2, MYC, OPCML, PDLIM5, PPARG, PRICKLE1, PRKG1, RBM20, RP1, RSPO2, SCG2, SMAD2, SPINT1, TBL1XR1, TG, TRPC7, ZNF148 |
| GO:0044707 | Single-multicellular organism process | 0.0004 | ABI1, ACTB, ACTL6B, ADAMTS18, ADCYAP1, ATF2, BARHL2, BMP7, BRPF3, CAPN3, CASR, CCDC85C, CHD7, EMX2, FKBP1A, FOLR1, FSHR, GSK3B, HHEX, IGF1R, LGALS3, LIN7A, LMOD2, MYC, NPAS3, OPCML, PDLIM5, PGAM2, PNKD, PPARG, PRICKLE1, RBM20, RP1, RSPO2, SCG2, SMAD2, SPINT1, SST, TBL1XR1, TG, TLL2, TRPC7, ZNF148 |
| GO:0009888 | Tissue development | 0.0007 | ABI1, ATF2, CHD7, CUX1, DCHS1, FKBP1A, FOLR1, GSK3B, HHEX, IGF1R, LGALS3, MYC, NTRK1, POU3F1, PPARG, PRICKLE1, RP1, RSPO2, SPINT1, TBL1XR1, TDGF1 |
| GO:0032502 | Developmental process | 0.001 | ABI1, ACTB, ACTL6B, ADAMTS18, ADCYAP1, ATF2, BARHL2, BMP7, CASR, CCDC85C, CHD7, EMX2, FKBP1A, FOLR1, FSHR, GSK3B, HHEX, IGF1R, LIN7A, LMOD2, MYC, NPAS3, OPCML, PDLIM5, PPARG, PRICKLE1, PRKG1, RBM20, RP1, RSPO2, SCG2, SMAD2, SPINT1, TBL1XR1, TG, TLL2, TRPC7, ZNF148 |
| GO:0060429 | Epithelium development | 0.001 | ABI1, CHD7, CUX1, DCHS1, FOLR1, HHEX, IGF1R, LGALS3, MYC, NTRK1, POU3F1, PPARG, PRICKLE1, RSPO2, SMAD2, SPINT1 |
| GO:0035239 | Tube morphogenesis | 0.003 | BMP7, CHD7, DCHS1, FOLR1, HHEX, MYC, PRICKLE1, RSPO2, SMAD2, SPINT1 |
| GO:0006468 | Receptor activity | 0.003 | ABI1, ABL1, ATF2, DAPK2, GSK3B, IGF1R, MYC, NTRK1, PRKG1, SCG2, SMAD2, STK32B, STK33, TDGF1 |
| Enriched KEGG Pathways | |||
| KEGG: 5202 | Transcriptional misregulation in cancer | 0.00183 | HHEX, IGF1R, MYC, NTRK1, PPARG, SPINT1, SUPT3H |
| KEGG: 5200 | Pathways in cancer | 0.00943 | ABL1, DAPK2, GSK3B, IGF1R, MYC, NTRK1, PPARG, SMAD2 |
| KEGG: 5216 | Thyroid cancer | 0.0193 | MYC, NTRK1, PPARG |
| KEGG: 4390 | Hippo signaling pathway | 0.0286 | ACTB, BMP7, GSK3B, MYC, SMAD2 |
Fig 3. Gene network displaying the connections between the markers shared among the three independent populations and mapped in pleiotropic regions.
The nodes represent individual genes. The coloured lines linking the nodes represents the interactions between the genes. The interactions between the genes can be divided in three types: 1) Known interaction: from curated databases (light blue) and experimentally determined (purple); 2) Predicted interactions: gene neighbourhood (green), gene fusions (red) and gene co-occurrence (dark blue); 3) Others: text mining (yellow), co-expression (black) and protein homology (violet). The interactions were based on human data, since the human database is more curate and complete than the bovine database.
Fig 4. Proportion of each QTL category (reproduction, production, milk, meat and carcass quality, exterior appearance, and health) mapped in a 2 Mb interval (1Mb upstream and 1 Mb downstream) from each gene present in the main network (38 genes).
Table 2. Inclusion criteria and/or expression pattern (differentially expressed), in Cánovas et al. (2014) [13] and Nguyen et al. (2017) [20], for the 38 genes mapped in the main network identified by STRING database.
| Gene Symbol | Coordinate | Brangus (Cánovas et al., 2014) [13] | Tropical composite and Brahman (Nguyen et al., 2017) [20] |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SNP | TS | TF | Hyp | Pit | Ov | Ut | End | Lv | Ad | ldm | Pit | Ov | ||
| GSK3B | 1:65265851–65292071 | X | X | |||||||||||
| CASR | 1:67255165–67344655 | X | X | |||||||||||
| SST | 1:80250205–80251648 | X | X | X | X | X | X | X | ||||||
| TBL1XR1 | 1:90428333–90609529 | X | X | |||||||||||
| ATF2 | 2:21725894–21830562 | X | X | |||||||||||
| SMARCAL1 | 2:105135339–105189135 | X | X | |||||||||||
| SCG2 | 2:112549869–112555375 | X | X | |||||||||||
| NTRK1 | 3:14019229–14037583 | X | X | X | ||||||||||
| ADH4 | 6:26853175–26885436 | X | X | X | X | X | ||||||||
| PDLIM5 | 6:31333660–31568270 | X | X | |||||||||||
| FBP1 | 8:82460863–82491694 | X | X | |||||||||||
| HTR1E | 9:63760625–63852410 | X | X | |||||||||||
| IYD | 9:88612923–88624698 | X | X | X | ||||||||||
| LGALS3 | 10:67843328–67861114 | X | ||||||||||||
| FSHR | 11:31110744–31305197 | X | X | |||||||||||
| ABL1 | 11:101011169–101152856 | X | X | |||||||||||
| ABI1 | 13:18094943–18182433 | X | X | |||||||||||
| BMP7 | 13:59424987–59510393 | X | X | |||||||||||
| FKBP1A | 13:60276502–60303717 | X | X | X | ||||||||||
| TG | 14:9253697–9263933 | X | X | X | ||||||||||
| MYC | 14:13769242–13775688 | X | X | |||||||||||
| TGS1 | 14:24747192–24772996 | X | X | |||||||||||
| CHD7 | 14:28043739–28172246 | X | X | |||||||||||
| ACACB | 17:66101179–66217542 | X | X | |||||||||||
| MYO18B | 17:67768278–68002792 | X | X | |||||||||||
| VAT1L | 18:5045212–5211278 | X | X | |||||||||||
| IGF1R | 21:7967701–8268340 | X | X | |||||||||||
| TDGF1 | 22:53432382–53437166 | X | X | X | X | X | X | X | ||||||
| PPARG | 22:57367072–57432321 | X | X | X | ||||||||||
| BRPF3 | 23:10096561–10132302 | X | X | |||||||||||
| SUPT3H | 23:18223167–18623659 | X | X | |||||||||||
| ADCYAP1 | 24:36114443–36121104 | X | X | X | ||||||||||
| SMAD2 | 24:47963393–48022086 | X | X | |||||||||||
| ACTL6B | 25:36459674–36469794 | X | X | |||||||||||
| ACTB | 25:39343633–39347047 | X | ||||||||||||
| PRKG1 | 26:6901760–8343635 | X | X | |||||||||||
| HHEX | 26:14120258–14126069 | X | X | |||||||||||
| DPF2 | 29:44205003–44217694 | X | X | X | ||||||||||
SNP: Genes expressed in at least one tissue among the two physiological states (pre- and post-puberty) and mapped near the markers associated with fertility traits by GWAS; TF: transcriptional factor; TS: genes identified with high probability to show a binding site for TF differentially expressed; Hyp: hypothalamus; Pit: pituitary; Ov: ovary; Ut: uterus; End: endometrium; Lv: liver; Ad: adipose tissue; ldm: longissimus dorsi muscle.
Fig 5. Biological processes (BP) significantly enriched in the main network identified by STRING database.
The number inside the parenthesis indicated the number of genes associated with each BP.
Fig 6. Chromosome specific plots displaying pleiotropic effect around the genes shared among all breeds.
The x-axis corresponds to the genomic position in each chromosome and the y-axis to the -log(p-value). The -log(p-value) showed in the y-axis corresponds to the p-values adjusted to multiple-testing (FDR<0.01) obtained by Bolormaa et al. (2014) [10] for the pleiotropic analysis. The grey diamond corresponds to the start coordinate of each gene. The horizontal dashed lines indicate the nominal threshold of -log(p-value)>2. All the genes mapped in an interval of 1 Mb of a marker with significant signal for pleiotropic effect were considered as a genes in pleiotropic regions.
Identification of key-regulatory genes through gene-network analysis
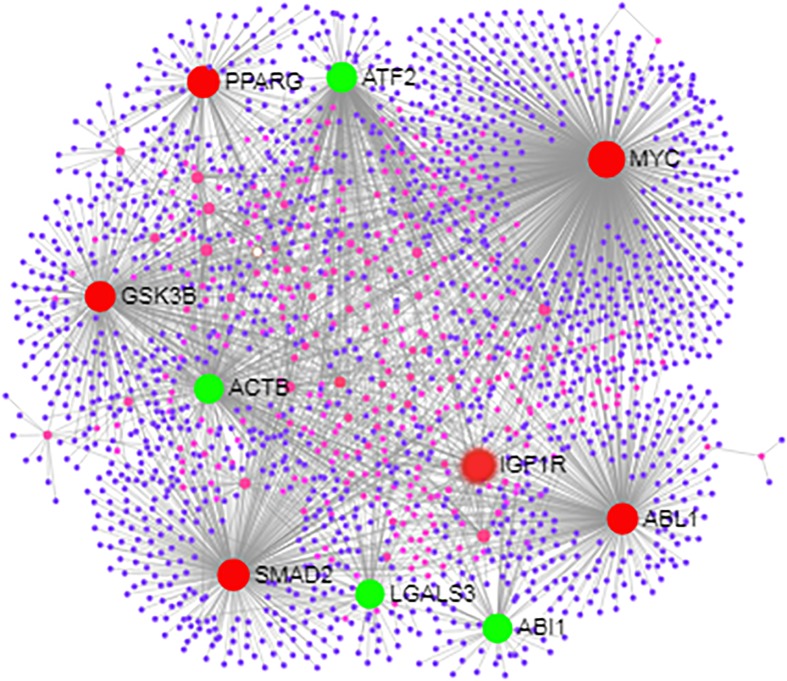
The centrality analysis for the main gene network, composed by 38 genes, identified the genes with the largest number of connections in the network. Table 3 shows the number of connections for the top 10 genes with the largest number of interactions. In order to confirm and reinforce the identification of genes with the highest regulatory potential, the interaction pattern of these 38 genes with other proteins was evaluated using IMEx. From this analysis, it was observed that there was an overlap between the top 10 genes with more interactions in the STRING database network and the top 10 genes from the IMEx interactions. In the IMEx analyses, it was also possible to identify, from the top 10 genes, six genes directly related to positive and negative regulation of cellular metabolic processes (red circles in Fig 7). Interestingly, these 6 genes were also present in the list of top connected genes in the main network identified by STRING database. These results confirmed the regulatory potential of these genes and highlight the biological processes in which these genes were involved. These genes were: MYC proto-oncogene (MYC) Peroxisome Proliferator Activated Receptor Gamma (PPARG), Glycogen Synthase Kinase 3 Beta (GSK3B), SMAD Family Member 2 (Smad2), ABL Proto-Oncogene 1, Non-Receptor Tyrosine Kinase (ABL1) and Insulin-like growth factor 1 receptor (IGF1R).
Table 3. Top 10 genes based on the number of interactions identified in the STRING database and NetworkAnalyst analyses.
In bold are shown the genes present in both top 10 lists.
| Top 10 genes for numbers of interactions with other genes | |||
|---|---|---|---|
| STRING database | NetworkAnalyst | ||
| Gene symbol | Number of interaction | Gene symbol | Number of interaction |
| MYC | 14 | MYC | 714 |
| ACTB | 9 | SMAD2 | 313 |
| GSK3B | 8 | ABL1 | 276 |
| PPARG | 8 | GSK3B | 243 |
| SST | 7 | ATF2 | 241 |
| ACACB | 7 | ACTB | 198 |
| ABL1 | 5 | PPARG | 124 |
| SMAD2 | 5 | ABI1 | 64 |
| ADCYAP1 | 5 | IGF1R | 58 |
| IGF1R | 5 | LGALS3 | 56 |
Fig 7. Interactome displaying the protein-protein interactions for the genes present in the main gene network identified by STRING database with other proteins across the genome.
Larger nodes (highlighted in red and green) represent the genes with the highest number of connections. Genes in red are the genes associated with positive and negative regulation of cellular metabolic processes.
Three of these genes are involved in cell proliferation: MYC, SMAD2 and ABL1. MYC was the gene with the highest number of interaction with other genes in both network analyses. This gene codes for a transcription factor responsible for regulating transcription of several genes. Consequently, MYC plays a multifunctional action involved with the control of crucial biological processes, such as cell cycle control and cellular transformation [31]. The expression of MYC was decreased in the muscle tissue of orchidectomized testosterone-treated male mice, indicating that this gene might be involved with the promotion of muscle mass by maintaining myoblasts in the proliferative state; and with the differentiation and growth of muscle tissue in a process mediated by androgen receptors [32]. Additionally, MYC was identified as playing a crucial role in the regulation of gene networks during development of the lactation cycle and meat and carcass traits in cattle [33–35].
SMAD2 is a member of the TGF-beta-SMAD signaling pathway and it is involved with the regulation of several processes associated with female reproduction and embryonic development in cattle [36]. In male rats, SMAD2 is DE between non-sexually mature and sexually mature rats, indicating a relationship with puberty progression [37]. The expression of myostatin, a protein involved with muscle proliferation, is directly regulated by SMAD2 activity [38–40]. Therefore, SMAD2 is a crucial regulator of processes involved with fertility and production traits.
ABL1 is a proto-oncogene associated with the regulation of several biological processes related to cellular division and differentiation. ABL1 was observed as DE in mouse testis after heat shock, indicating a regulatory activity in this tissue [41]. Additionally, homozygous disruptions of ABL1 are associated with neonatal lethality in mice [42]. To our best knowledge, the association between ABL1 and production traits in cattle has been poorly investigated. However, a significant peak associated with angularity in Brown Swiss cattle was identified close to ABL1 region. These results suggested a potential association between ABL1 with fertility and production and conformation traits, reinforcing the necessity to improve our understanding of these relationships.
Two of the other six genes identified in this analysis are involved in energy conservation metabolism: PPARG, GSK3B. PPARG belongs to a subfamily of nuclear receptors involved in several crucial biological processes, such as adipogenesis and immune cell activation [43]. Alterations in the expression pattern of PPARG, usually decrease in expression, were already associated with puberty progression and fertility traits in several species, including humans and cattle [44–46]. Additionally, PPARG has also been associated with meat quality and, more specifically, intramuscular fat percentage, and milk synthesis in cattle, which are economically important traits in beef and dairy cattle production [47–49], respectively.
Muscle glycogen is absolutely fundamental as energy reservoir. The amount of liver and muscle glycogen available determines the use of fat and, as a last resource, amino acids, for providing energy. GSK3B is a serine-threonine kinase member of a subfamily of glycogen synthase kinase and its corresponding function is known to be associated with energy metabolism, body pattern formation and neuronal cell development [50]. Additionally, GSK3B has been associated with age at puberty, sperm motility and decrease in spermatogenesis. Therefore, its function is directly related to fertility traits [51–53]. Interestingly, GSK3B has also been associated with several economically relevant traits such as skeletal muscle hypertrophy, intramuscular fat, meat quality, milk synthesis and mammary gland proliferation [54–57].
The last gene in this list, IGF1R, is the receptor of the Insulin-like growth factor 1 (IGF1), which also binds IGF2 and insulin with lower affinity. IGF1 and IGF2 have remarkable functions in the steroidogenic activity and regulation of body growth and maturation. In female cattle, IGF1 and IGF2 are associated with the regulation of the steroidogenic activity in the glanulosa cells, as well as, the regulation of the mitosis (IGF1 and IGF2) and apoptosis during the follicular development (IGF1) [58, 59]. The bovine testis is an important source of IGF1 production [60]. Both circulating and locally produced (testis) IGF1 may play a crucial role in the testicular size and testosterone secretion [61, 62]. The IGF1 pathway influence gonadotrophin‐releasing hormone (GnRH) neurons during the puberty and it is directly associated with the puberty progression and the development of reproductive traits [52, 63, 64]. Additionally, IGF1 was associated with the regulation of several production traits, for example, feed intake, feed conversion, body weight, milk protein yield, milk fat yield, milk fat concentration and somatic cell score [65–67]. Variants mapped on IGF1R may result in a change of affinity between IGF1 and its receptor, resulting in a different response to the circulating levels of this hormone. The crucial roles of IGF1 in the regulation of body development, in addition to the results obtained in the present study, highlights the potential of IGF1R to act like a key-regulator of pleiotropic effects associated with fertility and production traits.
All the six genes described above are directly related to fertility and economically relevant traits. Additionally, these genes were identified as potential key-regulatory genes due to the number of interactions with other genes and its biological functions. Consequently, these genes are important candidate genes for pleiotropic effect on multiple production and fertility traits.
Genes that do not appeared in both analyses described in Table 3 were not included in the discussion. However, some of these genes are fundamental to the metabolic processes discussed here. For example, somatostatin (SST), also known as growth hormone inhibiting hormone (GHIH), affects energy conservation metabolism by inhibiting insulin and glucagon secretions. In addition, somatostatin produced in the hypothalamus is transported to anterior pituitary, where it inhibits the release of growth hormone (GH), TSH and prolactin [68]. This gene also encodes neuronostatin, which is also implicated in the releasing of pituitary hormones [69]. In addition, somatostatin is involved in the MAPK, cAMP, PKA- pathways, among many other ones [70]. As a consequence, somatostatin has also a role in energy conservation metabolism and cell proliferation, the two main biological processes detected in the current study.
ACACB and ADCYAP1 are involved in the energy conservation metabolism. ACACB encodes acetil-CoA carboxilase, the enzyme converting acetil-CoA in malonyl-CoA, fundamental for fatty acids biosynthesis (KEGG ID: 04931; ID: 00061). ADCYAP1 encodes adelylate-clycase interacting protein 1, which through the cAMP and PKA pathways is involved in the insulin upregulation and in the regulation of insulin levels in insulin secretory granules, respectively, among other functions (KEGG ID: 04911).
ABI1 and ATF2 are cell proliferation regulators. ABI1 encodes ABL interacting protein 1, which facilitates the ABL cell proliferation signal. ATF2 encodes activating transcription factor 2, also known as CREB2. CREB2 regulates cell cycle proteins, pro-apoptotic proteins, cell adhesion molecules, and membrane and cytoplasm signaling proteins. In additions, CREB2 is the final step in many pathways, including cAMP, PKA, estradiol 17-beta (KEGG ID: 04915) and glucagon (KEGG ID: 04922). Therefore, CREB2 is also involved in the energy conservation metabolism.
LGALS3 encodes galectin 3, a carbohydrate binding protein with affinity for beta-galactosides. As a consequence, galectin 3 is involved in cell proliferation, adhesion, differentiation, angiogenesis and apoptosis [71]. It has been shown that galectin 3 is expressed by trophoblast cells in response to 17b-estradiol, progesterone, and human chorionic gonadotropin (hCG). Among many other effects, galectin 3 induces apoptosis in endometrial cells, which would allow embryo implantation [72].
Thyroid function and genes with pleiotropic effect
Among the genes present in the main network identified by STRING database, there are two crucial genes for the synthesis of thyroid hormones. These genes are thyroglobulin (TG) and iodotyrosine deiodinase (IYD). TG is metabolized through the addition of iodine molecules to produce mono- and di-iodotyrosine and the thyroid hormones triiodothyronine (T3) and thyroxine (T4). Consequently, TG is one of the main storage molecules of iodine in the body [73]. IYD is responsible to conserve iodide, recycling iodine, during synthetization of T3 and T4 hormones, from mono- and diiodotyrosine. Due to the low availability of iodine in the nature, IYD dysfunctions reduce the amount of available iodine for T3 and T4 synthesis [74–76]. In target cells, IYD converts T4 to T3, the active thyroid hormone, and converts T3 to di-iodotyrosine, inactivating T3. These processes also provide iodine to peripheral tissues. The thyroid hormones are related to the control of several crucial biological processes involved with the regulation of basal metabolism. In cattle, genes related to thyroid hormone regulation have been identified as DE in studies evaluating feed efficiency, lactation stage, fat deposition and early embryonic development [77–83]. Additionally, some studies suggest a pleiotropic effect for TG polymorphisms in production traits [84, 85]. Additionally, changes in the levels of thyroid hormones during pregnancy, mainly in the initial development of the embryos, are associated with adverse pregnancy outcomes and embryonic losses [86]. Moreover, alterations in thyroid activity may result in male and female infertility [19, 87].
An interesting link between the thyroid hormones and the selection for productive traits in cattle is that TG (BTA14:9,253,697–9,263,933) is mapped in the same core selective sweep (CSS) region of DGAT1 (BTA14: 1,795,425–1,804,838) [88]. The QTL related to DGAT1 is considered to have a major effect on production traits and has been associated with several phenotypes in both dairy and beef cattle breeds [89–91]. Due to this major effect, molecular markers associated with the DGAT1 effect are intensively exploited in genetic improvement programs. Generally, the use of molecular markers in selection programs does not consider the relationship among the aimed marker and the surrounding mapped markers. However, the intensive selection may increase the extent and the overall LD in a region [92]. This phenomenon may result in an indirect selection (hitchhiking effect) of markers mapped in different genes and with unpredictable effects. It is important to note that, in the same CSS, the highest signal for pleiotropic effect was observed by Bolormaa et al. (2014) [10], as shown in Fig 6. In the same position, three additional genes shared among the three independent populations mapped to regions with 5 or more previously reported QTLs and in the main network identified by STRING database, i.e. MYC, TGS1 and CHD7.
The thyroid cancer pathway (KEEG ID: 05216) was one of the enriched pathways identified by STRING database (Table 1). In this pathway, thyroid hormones and precursors are not involved, but both MYC and PPARG are implicated, reinforcing the association between this main-network and the regulation of thyroid activity. In addition to the presence of TG and IYD in the main network identified by STRING database (Fig 3), these results suggest that this network is enriched by genes related to thyroid function. The VarElect software was used to confirm this hypothesis through an association processes between the genes that composed the main network and the keywords “thyroid” and “thyroid hormones”. Through data-mining using information available on GeneCards and MalaCards, it was possible to identify that from the 38 genes (S4 File) present in the main network, 31 are directly related to those keywords. Therefore, indicating that this gene network was enriched by genes related to thyroid function.
As previously described, thyroid function is related to the regulation of several biological processes associated with economically important traits. Additionally, due to this association with several processes and traits, the genes involved in thyroid activity are excellent functional candidate genes for pleiotropic effects. Further functional analyses will be performed in order to elucidate the relationship between the different traits affected by pleiotropic effects, as well as, to identify candidate variants associated with the function of these candidate genes.
It is important to highlight that during the analyses performed here, some differences were observed in the gene expression profile among populations, even when similar groups were compared (Pre- and post-puberty). A very common phenomenon observed in biological analyses that can help to address this issue is the Simpson’s paradox. The Simpson’s paradox is observed when results from aggregated data contradict those from separate analyses. There are several reports in the literature discussing the impact of Simpson’s paradox in different fields, such as network analysis and gene expression [93, 94, 95]. The biological bases for the Simpson’s paradox in biological analyses are still poorly understood. However, some points can be raised to help in the discussion of this phenomenon. For example, in the data analysed in the present manuscript, as addressed in the previous commentary, the differences in the genetic background, environmental effects and evaluation of the puberty, and number of tissues evaluated between populations can help to explain these differences. It is important to highlight that these populations (Brangus, Brahman and Tropical composition) shared the Brahman genetic component. However, these proportions are different in each population. For example, in the Brangus population the animals have 3/8 of Brahman component and 5/8 of Angus component. All these differences can be taken together in order to discussion the possible causes of the phenomenon observed here. Additionally, it is important to highlight that the genes shown in Table 2 are a specific group of genes, which are the genes with the highest potential to perform pleiotropic effect. Additionally, the consistency of expression among tissues and populations can help to identify these key-regulatory genes. The present study aims exactly on those genes that even with all these possible confounding factors maintain the expression profile, which can be an additional evidence of crucial regulatory role.
Conclusions
The present study described a multi-breed and multi-OMICs approach to identify key-regulatory candidate genes for pleiotropic effects in beef cattle using the results generated by previous studies. Our findings confirm the feasibility of using a systems biology approach to unravel candidate genes regulating complex traits. Genes identified in this study are mainly involved in two biological processes: energy conservation metabolism and cell proliferation, probably the most theoretically plausible processes to unify the phenotypes investigated in this study: exterior appearance, health, reproduction, production, meat and carcass, and milk traits. This study contributes to the understanding of the cause-consequence relationships between variants mapped on candidate pleiotropic genes affecting complex traits. Additionally, the results obtained here will be useful for better defining statistical models to improve the accuracy of genomic prediction of breeding values and avoid the simultaneous selection for unfavorable genetically correlated traits in beef cattle and other livestock species.
Supporting information
(XLSX)
(TXT)
(TXT)
(XLSX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was supported by the Beef Farmers of Ontario, OMAFRA (Ontario Ministry of Agriculture, Food and Rural Affairs), Beef Cattle Research Council (BCRC), NSERC (Natural Sciences and Engineering Research Council) and Ontario Centres of Excellence (OCE). MRSC is supported by a fellowship from the Brazilian National Research Council (CNPq 312068/2015-8) and grants from the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG, APQ APQ-01377-17 and APQ-01377-17). PASF is supported by a fellowship from the Brazilian National Research Council. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Antonarakis SE, Chakravarti A, Cohen JC, Hardy J. Mendelian disorders and multifactorial traits: the big divide or one for all? Nature Reviews Genetics. 2010;11(5):380 10.1038/nrg2793 [DOI] [PubMed] [Google Scholar]
- 2.Moser G, Lee SH, Hayes BJ, Goddard ME, Wray NR, Visscher PM. Simultaneous discovery, estimation and prediction analysis of complex traits using a Bayesian mixture model. PLoS genetics. 2015;11(4):e1004969 10.1371/journal.pgen.1004969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Camargo GMF, Porto-Neto LR, Kelly MJ, Bunch RJ, McWilliam SM, Tonhati H, et al. Non-synonymous mutations mapped to chromosome X associated with andrological and growth traits in beef cattle. BMC genomics. 2015;16(1):384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kadri NK, Sahana G, Charlier C, Iso-Touru T, Guldbrandtsen B, Karim L, et al. A 660-Kb deletion with antagonistic effects on fertility and milk production segregates at high frequency in Nordic Red cattle: additional evidence for the common occurrence of balancing selection in livestock. PLoS genetics. 2014;10(1):e1004049 10.1371/journal.pgen.1004049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saatchi M, Schnabel RD, Taylor JF, Garrick DJ. Large-effect pleiotropic or closely linked QTL segregate within and across ten US cattle breeds. BMC genomics. 2014;15(1):442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsuruta S, Lourenco D, Misztal I, Lawlor T. Genomic analysis of cow mortality and milk production using a threshold-linear model. Journal of dairy science. 2017;100(9):7295–305. 10.3168/jds.2017-12665 [DOI] [PubMed] [Google Scholar]
- 7.Hayes BJ, MacLeod IM, Daetwyler HD, Phil BJ, Chamberlain AJ, Vander Jagt C, et al., editors. Genomic prediction from whole genome sequence in livestock: the 1000 bull genomes project. 10th World Congress on Genetics Applied to Livestock Production (WCGALP); 2014.
- 8.MacLeod IM, Hayes BJ, Goddard ME. The effects of demography and long-term selection on the accuracy of genomic prediction with sequence data. Genetics. 2014;198(4):1671–84. 10.1534/genetics.114.168344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meuwissen TH, Goddard ME. Prediction of identity by descent probabilities from marker-haplotypes. Genetics Selection Evolution. 2001;33(6):605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bolormaa S, Pryce JE, Reverter A, Zhang Y, Barendse W, Kemper K, et al. A multi-trait, meta-analysis for detecting pleiotropic polymorphisms for stature, fatness and reproduction in beef cattle. PLoS genetics. 2014;10(3):e1004198 10.1371/journal.pgen.1004198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bolormaa S, Swan AA, Brown DJ, Hatcher S, Moghaddar N, Van Der Werf JH, et al. Multiple-trait QTL mapping and genomic prediction for wool traits in sheep. Genetics Selection Evolution. 2017;49(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scutari M, Howell P, Balding DJ, Mackay I. Multiple quantitative trait analysis using bayesian networks. Genetics. 2014;198(1):129–37. 10.1534/genetics.114.165704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cánovas A, Reverter A, DeAtley KL, Ashley RL, Colgrave ML, Fortes MR, et al. Multi-tissue omics analyses reveal molecular regulatory networks for puberty in composite beef cattle. PloS one. 2014;9(7):e102551 10.1371/journal.pone.0102551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suravajhala P, Kogelman LJ, Kadarmideen HN. Multi-omic data integration and analysis using systems genomics approaches: methods and applications in animal production, health and welfare. Genetics Selection Evolution. 2016;48(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Awda B, Miller S, Montanholi Y, Voort GV, Caldwell T, Buhr M, et al. The relationship between feed efficiency traits and fertility in young beef bulls. Canadian Journal of Animal Science. 2013;93(2):185–92. [Google Scholar]
- 16.Mu Y, Voort GV, Abo-Ismail M, Ventura R, Jamrozik J, Miller S. Genetic correlations between female fertility and postweaning growth and feed efficiency traits in multibreed beef cattle. Canadian Journal of Animal Science. 2016;96(3):448–55. [Google Scholar]
- 17.Walsh S, Williams E, Evans A. A review of the causes of poor fertility in high milk producing dairy cows. Animal reproduction science. 2011;123(3–4):127–38. 10.1016/j.anireprosci.2010.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dorn LD, Biro FM. Puberty and its measurement: A decade in review. Journal of research on adolescence. 2011;21(1):180–95. [Google Scholar]
- 19.Fernández M, Loaiza Echeverri A, Henry M, Drummond M, Andrade de Oliveira D, Demyda Peyrás S, et al. Bovine thyroglobulin gene polymorphisms and their association with sexual precocity in Guzerat bulls. Reproduction in Domestic Animals. 2017;52(5):911–3. 10.1111/rda.12989 [DOI] [PubMed] [Google Scholar]
- 20.Nguyen L, Reverter A, Cánovas A, Venus B, Islas-Trejo A, Porto-Neto L, et al. Global differential gene expression in the pituitary gland and the ovaries of pre-and postpubertal Brahman heifers. Journal of animal science. 2017;95(2):599–615. 10.2527/jas.2016.0921 [DOI] [PubMed] [Google Scholar]
- 21.Viitala S, Szyda J, Blott S, Schulman N, Lidauer M, Mäki-Tanila A, et al. The role of the bovine growth hormone receptor and prolactin receptor genes in milk, fat and protein production in Finnish Ayrshire dairy cattle. Genetics. 2006;173(4):2151–64. 10.1534/genetics.105.046730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hawken R, Zhang Y, Fortes M, Collis E, Barris W, Corbet N, et al. Genome-wide association studies of female reproduction in tropically adapted beef cattle. Journal of Animal Science. 2012;90(5):1398–410. 10.2527/jas.2011-4410 [DOI] [PubMed] [Google Scholar]
- 23.O’Brien AMP, Mészáros G, Utsunomiya YT, Sonstegard TS, Garcia JF, Van Tassell CP, et al. Linkage disequilibrium levels in Bos indicus and Bos taurus cattle using medium and high density SNP chip data and different minor allele frequency distributions. Livestock Science. 2014;166:121–32. [Google Scholar]
- 24.Villa-Angulo R, Matukumalli LK, Gill CA, Choi J, Van Tassell CP, Grefenstette JJ. High-resolution haplotype block structure in the cattle genome. BMC genetics. 2009;10(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szklarczyk D, Morris JH, Cook H, Kuhn M, Wyder S, Simonovic M, et al. The STRING database in 2017: quality-controlled protein–protein association networks, made broadly accessible. Nucleic acids research. 2016:gkw937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Csardi G, Nepusz T. The igraph software package for complex network research. InterJournal, Complex Systems. 2006;1695(5):1–9. [Google Scholar]
- 27.Stelzer G, Plaschkes I, Oz-Levi D, Alkelai A, Olender T, Zimmerman S, et al. VarElect: the phenotype-based variation prioritizer of the GeneCards Suite. BMC genomics. 2016;17(2):444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conesa A, Götz S, García-Gómez JM, Terol J, Talón M, Robles M. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005;21(18):3674–6. 10.1093/bioinformatics/bti610 [DOI] [PubMed] [Google Scholar]
- 29.Xia J, Benner MJ, Hancock RE. NetworkAnalyst-integrative approaches for protein–protein interaction network analysis and visual exploration. Nucleic acids research. 2014;42(W1):W167–W74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Breuer K, Foroushani AK, Laird MR, Chen C, Sribnaia A, Lo R, et al. InnateDB: systems biology of innate immunity and beyond—recent updates and continuing curation. Nucleic acids research. 2012;41(D1):D1228–D33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bretones G, Delgado MD, León J. Myc and cell cycle control. Biochimica et Biophysica Acta (BBA)-Gene Regulatory Mechanisms. 2015;1849(5):506–16. [DOI] [PubMed] [Google Scholar]
- 32.Rana K, Lee NK, Zajac JD, MacLean HE. Expression of androgen receptor target genes in skeletal muscle. Asian journal of andrology. 2014;16(5):675 10.4103/1008-682X.122861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bionaz M, Loor JJ. Gene networks driving bovine milk fat synthesis during the lactation cycle. BMC genomics. 2008;9(1):366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oh J, Kim E, Lee H, Song K. Effect of a c-MYC Gene Polymorphism (g. 3350G> C) on Meat Quality Traits in Berkshire. Asian-Australasian journal of animal sciences. 2015;28(11):1545 10.5713/ajas.15.0425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang X, Chu Q, Guo G, Dong G, Li X, Zhang Q, et al. Genome-wide association studies identified multiple genetic loci for body size at four growth stages in Chinese Holstein cattle. PloS one. 2017;12(4):e0175971 10.1371/journal.pone.0175971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang K, Rajput SK, Lee K-B, Wang D, Huang J, Folger JK, et al. Evidence supporting a role for SMAD2/3 in bovine early embryonic development: potential implications for embryotropic actions of follistatin. Biology of reproduction. 2015;93(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu J, Beyer AR, Walker WH, McGee EA. Developmental and Stage‐Specific Expression of Smad2 and Smad3 in Rat Testis. Journal of andrology. 2003;24(2):192–200. [DOI] [PubMed] [Google Scholar]
- 38.Sartori R, Milan G, Patron M, Mammucari C, Blaauw B, Abraham R, et al. Smad2 and 3 transcription factors control muscle mass in adulthood. American Journal of Physiology-Cell Physiology. 2009;296(6):C1248–C57. 10.1152/ajpcell.00104.2009 [DOI] [PubMed] [Google Scholar]
- 39.Welle SL. Myostatin and muscle fiber size. Focus on “Smad2 and 3 transcription factors control muscle mass in adulthood” and “Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size”. American Journal of Physiology-Cell Physiology. 2009;296(6):C1245–C7. 10.1152/ajpcell.00154.2009 [DOI] [PubMed] [Google Scholar]
- 40.Zhu X, Topouzis S, Liang L-f, Stotish RL. Myostatin signaling through Smad2, Smad3 and Smad4 is regulated by the inhibitory Smad7 by a negative feedback mechanism. Cytokine. 2004;26(6):262–72. 10.1016/j.cyto.2004.03.007 [DOI] [PubMed] [Google Scholar]
- 41.Rockett JC, Mapp FL, Garges JB, Luft JC, Mori C, Dix DJ. Effects of hyperthermia on spermatogenesis, apoptosis, gene expression, and fertility in adult male mice. Biology of reproduction. 2001;65(1):229–39. [DOI] [PubMed] [Google Scholar]
- 42.Tybulewicz VL, Crawford CE, Jackson PK, Bronson RT, Mulligan RC. Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-abl proto-oncogene. Cell. 1991;65(7):1153–63. [DOI] [PubMed] [Google Scholar]
- 43.Janani C, Kumari BR. PPAR gamma gene–a review. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2015;9(1):46–50. [DOI] [PubMed] [Google Scholar]
- 44.Fortes MR, Reverter A, Zhang Y, Collis E, Nagaraj SH, Jonsson NN, et al. Association weight matrix for the genetic dissection of puberty in beef cattle. Proceedings of the National Academy of Sciences. 2010;107(31):13642–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stueve TR, Wolff MS, Pajak A, Teitelbaum SL, Chen J. CYP19A1 promoter methylation in saliva associated with milestones of pubertal timing in urban girls. BMC pediatrics. 2014;14(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vitti M, Di Emidio G, Di Carlo M, Carta G, Antonosante A, Artini PG, et al. Peroxisome proliferator-activated receptors in female reproduction and fertility. PPAR research. 2016;2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goszczynski DE, Mazzucco JP, Ripoli MV, Villarreal EL, Rogberg-Muñoz A, Mezzadra CA, et al. Genetic characterisation of PPARG, CEBPA and RXRA, and their influence on meat quality traits in cattle. Journal of animal science and technology. 2016;58(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guo B, Kongsuwan K, Greenwood PL, Zhou G, Zhang W, Dalrymple BP. A gene expression estimator of intramuscular fat percentage for use in both cattle and sheep. Journal of animal science and biotechnology. 2014;5(1):35 10.1186/2049-1891-5-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moisá SJ, Shike DW, Faulkner DB, Meteer WT, Keisler D, Loor JJ. Central role of the PPARγ gene network in coordinating beef cattle intramuscular adipogenesis in response to weaning age and nutrition. Gene regulation and systems biology. 2014;8:GRSB. S11782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu D, Pan W. GSK3: a multifaceted kinase in Wnt signaling. Trends in biochemical sciences. 2010;35(3):161–8. 10.1016/j.tibs.2009.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bhattacharjee R, Goswami S, Dudiki T, Popkie AP, Phiel CJ, Kline D, et al. Targeted disruption of glycogen synthase kinase 3A (GSK3A) in mice affects sperm motility resulting in male infertility. Biology of reproduction. 2015;92(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fortes MR, Li Y, Collis E, Zhang Y, Hawken RJ. The IGF1 pathway genes and their association with age of puberty in cattle. Animal genetics. 2013;44(1):91–5. 10.1111/j.1365-2052.2012.02367.x [DOI] [PubMed] [Google Scholar]
- 53.Kim ST, Omurtag K, Moley KH. Decreased spermatogenesis, fertility, and altered Slc2A expression in Akt1−/− and Akt2−/− testes and sperm. Reproductive Sciences. 2012;19(1):31–42. 10.1177/1933719111424449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Esmailizadeh A, Morris C, Cullen N, Kruk Z, Lines D, Hickey S, et al. Genetic mapping of quantitative trait loci for meat quality and muscle metabolic traits in cattle. Animal genetics. 2011;42(6):592–9. 10.1111/j.1365-2052.2011.02197.x [DOI] [PubMed] [Google Scholar]
- 55.Miretti S, Martignani E, Accornero P, Baratta M. Functional effect of mir-27b on myostatin expression: a relationship in piedmontese cattle with double-muscled phenotype. BMC genomics. 2013;14(1):194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Underwood KR, Tong J, Zhu MJ, Shen QW, Means WJ, Ford SP, et al. Relationship between kinase phosphorylation, muscle fiber typing, and glycogen accumulation in longissimus muscle of beef cattle with high and low intramuscular fat. Journal of agricultural and food chemistry. 2007;55(23):9698–703. 10.1021/jf071573z [DOI] [PubMed] [Google Scholar]
- 57.Zhang X, Zhao F, Si Y, Huang Y, Yu C, Luo C, et al. GSK3β regulates milk synthesis in and proliferation of dairy cow mammary epithelial cells via the mTOR/S6K1 signaling pathway. Molecules. 2014;19(7):9435–52. 10.3390/molecules19079435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mani AM, Fenwick MA, Cheng Z, Sharma MK, Singh D, Wathes DC. IGF1 induces up-regulation of steroidogenic and apoptotic regulatory genes via activation of phosphatidylinositol-dependent kinase/AKT in bovine granulosa cells. Reproduction. 2010;139(1):139–51. 10.1530/REP-09-0050 . [DOI] [PubMed] [Google Scholar]
- 59.Spicer LJ, Aad PY. Insulin-like growth factor (IGF) 2 stimulates steroidogenesis and mitosis of bovine granulosa cells through the IGF1 receptor: role of follicle-stimulating hormone and IGF2 receptor. Biol Reprod. 2007;77(1):18–27. 10.1095/biolreprod.106.058230 [DOI] [PubMed] [Google Scholar]
- 60.Lee C, Hunt D, Gray S, Henricks D. Secretory patterns of growth hormone and insulin-like growth factor-I during peripubertal period in intact and castrate male cattle. Domestic Animal Endocrinology. 1991;8(4):481–9. [DOI] [PubMed] [Google Scholar]
- 61.Brito LF, Barth AD, Rawlings NC, Wilde RE, Crews DH Jr, Mir PS, et al. Effect of nutrition during calfhood and peripubertal period on serum metabolic hormones, gonadotropins and testosterone concentrations, and on sexual development in bulls. Domestic animal endocrinology. 2007;33(1):1–18. 10.1016/j.domaniend.2006.04.001 [DOI] [PubMed] [Google Scholar]
- 62.Brito LF, Barth AD, Rawlings NC, Wilde RE, Crews DH Jr, Mir PS, et al. Effect of improved nutrition during calfhood on serum metabolic hormones, gonadotropins, and testosterone concentrations, and on testicular development in bulls. Domestic animal endocrinology. 2007;33(4):460–9. 10.1016/j.domaniend.2006.09.004 [DOI] [PubMed] [Google Scholar]
- 63.Fortes M, Reverter A, Kelly M, McCulloch R, Lehnert S. Genome‐wide association study for inhibin, luteinizing hormone, insulin‐like growth factor 1, testicular size and semen traits in bovine species. Andrology. 2013;1(4):644–50. 10.1111/j.2047-2927.2013.00101.x [DOI] [PubMed] [Google Scholar]
- 64.Nogueira GdP, Beltran MP. Influence of plasmatic leptin and IGF-1 on puberty and precocious fertility of Nellore heifers (Bos taurus indicus). Biology of Reproduction. 2008:146–7. [Google Scholar]
- 65.do Amaral Grossi D, Buzanskas ME, Grupioni NV, de Paz CCP, de Almeida Regitano LC, de Alencar MM, et al. Effect of IGF1, GH, and PIT1 markers on the genetic parameters of growth and reproduction traits in Canchim cattle. Molecular biology reports. 2015;42(1):245–51. 10.1007/s11033-014-3767-4 [DOI] [PubMed] [Google Scholar]
- 66.Mullen MP, Berry DP, Howard DJ, Diskin MG, Lynch CO, Giblin L, et al. Single nucleotide polymorphisms in the insulin-like growth factor 1 (IGF-1) gene are associated with performance in Holstein-Friesian dairy cattle. Frontiers in genetics. 2011;2:3 10.3389/fgene.2011.00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Siadkowska E, Zwierzchowski L, Oprzadek J, Strzalkowska N, Bagnicka E, Krzyzewski J. Effect of polymorphism in IGF-1 gene on production traits in Polish Holstein-Friesian cattle. Anim Sci Pap Rep. 2006;24(3):225–37. [Google Scholar]
- 68.Rorsman P, Huising MO. The somatostatin-secreting pancreatic δ-cell in health and disease. Nature Reviews Endocrinology. 2018:1 10.1038/nrendo.2017.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Luque RM, Kineman RD. Neuronostatin exerts actions on pituitary that are unique from its sibling peptide somatostatin. Journal of Endocrinology. 2018;237(3):217–27. 10.1530/JOE-18-0135 [DOI] [PubMed] [Google Scholar]
- 70.Rai U, Thrimawithana TR, Valery C, Young SA. Therapeutic uses of somatostatin and its analogues: current view and potential applications. Pharmacology & therapeutics. 2015;152:98–110. [DOI] [PubMed] [Google Scholar]
- 71.Song L, Tang J-w, Owusu L, Sun M-Z, Wu J, Zhang J. Galectin-3 in cancer. Clinica chimica acta. 2014;431:185–91. [DOI] [PubMed] [Google Scholar]
- 72.Yang H, Taylor HS, Lei C, Cheng C, Zhang W. Hormonal regulation of galectin 3 in trophoblasts and its effects on endometrium. Reproductive Sciences. 2011;18(11):1118–27. 10.1177/1933719111407212 [DOI] [PubMed] [Google Scholar]
- 73.Ma ZF, Skeaff SA. Thyroglobulin as a biomarker of iodine deficiency: a review. Thyroid. 2014;24(8):1195–209. 10.1089/thy.2014.0052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moreno JC. Identification of novel genes involved in congenital hypothyroidism using serial analysis of gene expression. Hormone Research in Paediatrics. 2003;60(Suppl. 3):96–102. [DOI] [PubMed] [Google Scholar]
- 75.Moreno JC, Pauws E, van Kampen AH, Jedlicková M, de Vijlder JJ, Ris-Stalpers C. Cloning of tissue-specific genes using serial analysis of gene expression and a novel computational substraction approach. Genomics. 2001;75(1):70–6. [DOI] [PubMed] [Google Scholar]
- 76.Moreno JC, Visser TJ. Genetics and phenomics of hypothyroidism and goiter due to iodotyrosine deiodinase (DEHAL1) gene mutations. Molecular and cellular endocrinology. 2010;322(1–2):91–8. 10.1016/j.mce.2010.03.010 [DOI] [PubMed] [Google Scholar]
- 77.Ashkar FA, Revay T, Rho N, Madan P, Dufort I, Robert C, et al. Thyroid hormones alter the transcriptome of in vitro-produced bovine blastocysts. Zygote. 2016;24(2):266–76. 10.1017/S0967199415000167 [DOI] [PubMed] [Google Scholar]
- 78.Capuco A, Wood D, Elsasser T, Kahl S, Erdman R, Van Tassell C, et al. Effect of Somatotropin on Thyroid Hormones and Cytokines in Lactating Dairy Cows During Ad Libitum and Restricted Feed Intake1. Journal of dairy science. 2001;84(11):2430–9. 10.3168/jds.S0022-0302(01)74693-0 [DOI] [PubMed] [Google Scholar]
- 79.Meyerholz MM, Mense K, Linden M, Raliou M, Sandra O, Schuberth H-J, et al. Peripheral thyroid hormone levels and hepatic thyroid hormone deiodinase gene expression in dairy heifers on the day of ovulation and during the early peri-implantation period. Acta Veterinaria Scandinavica. 2015;58(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pezzi C, Accorsi P, Vigo D, Govoni N, Gaiani R. 5′-Deiodinase activity and circulating thyronines in lactating cows. Journal of Dairy Science. 2003;86(1):152–8. 10.3168/jds.S0022-0302(03)73595-4 [DOI] [PubMed] [Google Scholar]
- 81.Schering L, Albrecht E, Komolka K, Kühn C, Maak S. Increased expression of thyroid hormone responsive protein (THRSP) is the result but not the cause of higher intramuscular fat content in cattle. International journal of biological sciences. 2017;13(5):532 10.7150/ijbs.18775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang YH, Bower N, Reverter A, Tan S, De Jager N, Wang R, et al. Gene expression patterns during intramuscular fat development in cattle. Journal of Animal Science. 2009;87(1):119–30. 10.2527/jas.2008-1082 [DOI] [PubMed] [Google Scholar]
- 83.Weber KL, Welly BT, Van Eenennaam AL, Young AE, Porto-Neto LR, Reverter A, et al. Identification of gene networks for residual feed intake in Angus cattle using genomic prediction and RNA-seq. PLoS One. 2016;11(3):e0152274 10.1371/journal.pone.0152274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fontanesi L, Calò D, Galimberti G, Negrini R, Marino R, Nardone A, et al. A candidate gene association study for nine economically important traits in Italian Holstein cattle. Animal genetics. 2014;45(4):576–80. 10.1111/age.12164 [DOI] [PubMed] [Google Scholar]
- 85.Tait R, Cushman R, McNeel A, Casas E, Smith T, Freetly H, et al. Estimates of epistatic and pleiotropic effects of () and () genetic markers on beef heifer performance traits enhanced by selection. Journal of animal science. 2016;94(3):920–6. 10.2527/jas.2015-9860 [DOI] [PubMed] [Google Scholar]
- 86.Twig G, Shina A, Amital H, Shoenfeld Y. Pathogenesis of infertility and recurrent pregnancy loss in thyroid autoimmunity. Journal of autoimmunity. 2012;38(2–3):J275–J81. 10.1016/j.jaut.2011.11.014 [DOI] [PubMed] [Google Scholar]
- 87.Dittrich R, Beckmann MW, Oppelt PG, Hoffmann I, Lotz L, Kuwert T, et al. Thyroid hormone receptors and reproduction. Journal of reproductive immunology. 2011;90(1):58–66. 10.1016/j.jri.2011.02.009 [DOI] [PubMed] [Google Scholar]
- 88.Gutiérrez-Gil B, Arranz JJ, Wiener P. An interpretive review of selective sweep studies in Bos taurus cattle populations: identification of unique and shared selection signals across breeds. Frontiers in genetics. 2015;6:167 10.3389/fgene.2015.00167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hayes BJ, Bowman PJ, Chamberlain A, Goddard M. Invited review: Genomic selection in dairy cattle: Progress and challenges. Journal of dairy science. 2009;92(2):433–43. 10.3168/jds.2008-1646 [DOI] [PubMed] [Google Scholar]
- 90.Kühn C, Thaller G, Winter A, Bininda-Emonds OR, Kaupe B, Erhardt G, et al. Evidence for multiple alleles at the DGAT1 locus better explains a quantitative trait locus with major effect on milk fat content in cattle. Genetics. 2004;167(4):1873–81. 10.1534/genetics.103.022749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Thaller G, Kühn C, Winter A, Ewald G, Bellmann O, Wegner J, et al. DGAT1, a new positional and functional candidate gene for intramuscular fat deposition in cattle. Animal genetics. 2003;34(5):354–7. [DOI] [PubMed] [Google Scholar]
- 92.Sargolzaei M, Schenkel F, Jansen G, Schaeffer L. Extent of linkage disequilibrium in Holstein cattle in North America. Journal of Dairy Science. 2008;91(5):2106–17. 10.3168/jds.2007-0553 [DOI] [PubMed] [Google Scholar]
- 93.Trapnell Cole. Defining cell types and states with single-cell genomics. Genome research, v. 25, n. 10, p. 1491–1498, 2015. 10.1101/gr.190595.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Petri Tobias et al. Addressing false discoveries in network inference. Bioinformatics, v. 31, n. 17, p. 2836–2843, 2015. 10.1093/bioinformatics/btv215 [DOI] [PubMed] [Google Scholar]
- 95.Wu Timothy H. et al. Meta-analytical biomarker search of EST expression data reveals three differentially expressed candidates. In: BMC genomics. BioMed Central, 2012. p. S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(TXT)
(TXT)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.