Abstract
Despite causing considerable damage to host tissue at the onset of parasitism, invasive helminths establish remarkably persistent infections in both animals and plants. Secretions released by these obligate parasites during host invasion are thought to be crucial for their persistence in infection. Helminth secretions are complex mixtures of molecules, most of which have unknown molecular targets and functions in host cells or tissues. Although the habitats of animal- and plant-parasitic helminths are very distinct, their secretions share the presence of a structurally conserved group of proteins called venom allergen-like proteins (VALs). Helminths abundantly secrete VALs during several stages of parasitism while inflicting extensive damage to host tissue. The tight association between the secretion of VALs and the onset of parasitism has triggered a particular interest in this group of proteins, as improved knowledge on their biological functions may assist in designing novel protection strategies against parasites in humans, livestock, and important food crops.
Introduction
Upon infection, helminth parasites establish an intricate relationship with their host. Helminths cause considerable damage during host invasion, migration through host tissues, and feeding on host cells [1, 2], but infections by these parasites can nonetheless be very persistent and last for several decades. Helminths are masters in manipulating host defense responses [1, 3], thereby creating a suitable environment for their survival and simultaneously limiting excessive damage due to host immune responses.
Excretory/secretory (ES) products are regarded as the tools employed by helminth parasites to control host defense responses. Recently, it was shown that ES products of helminth parasites reflect their diversity in lifestyles and hosts and therefore have little in common between plant and animal parasites [4]. However, members of the alternatively named Sperm-coating protein/Tpx/antigen 5/pathogenesis-related-1/Sc7 (SCP/TAPS) or cysteine-rich secretory proteins/antigen 5/pathogenesis-related 1 (CAP) protein superfamily are ubiquitously present in ES products of helminth species that parasitize plants and animals. Although a uniform nomenclature was proposed previously [5], helminth CAP proteins still go by different names, including activation-associated secreted proteins (ASPs) or most commonly used venom allergen-like proteins (VALs or VAPs).
The expression of VALs is specifically up-regulated during parasitic phases of the life cycle of helminths, which could point to a role in host–parasite interactions [6–9]. The presence of VALs in secretions of both plant- and animal-parasitic helminths suggests that these proteins are important for the establishment of persistent infections in both plants and animals. It is possible that conserved structural properties in VALs provide a diverse group of parasites a robust platform for modulating host responses in both plant and animal kingdoms. However, a question that remains unanswered is whether VALs from plant and animal parasites could have conserved functions based on common biochemical properties of these secreted proteins.
Recent reports have shed new light on structural properties, biochemical modes of action, and functions of secreted VALs of parasitic helminths. However, most of these reports have been published in specialized journals dedicated to either medical and veterinary biology or plant pathology. Here, we present an interdisciplinary review of the latest findings from phylogenetic analyses, x-ray crystallography, and functional studies on VALs from parasitic helminths. The aim of this review is to explore conserved mechanisms underlying the role of VALs as modulators of host responses during parasitism with an emphasis on their potential impact on common concepts in plant pathology and human and/or animal parasitology.
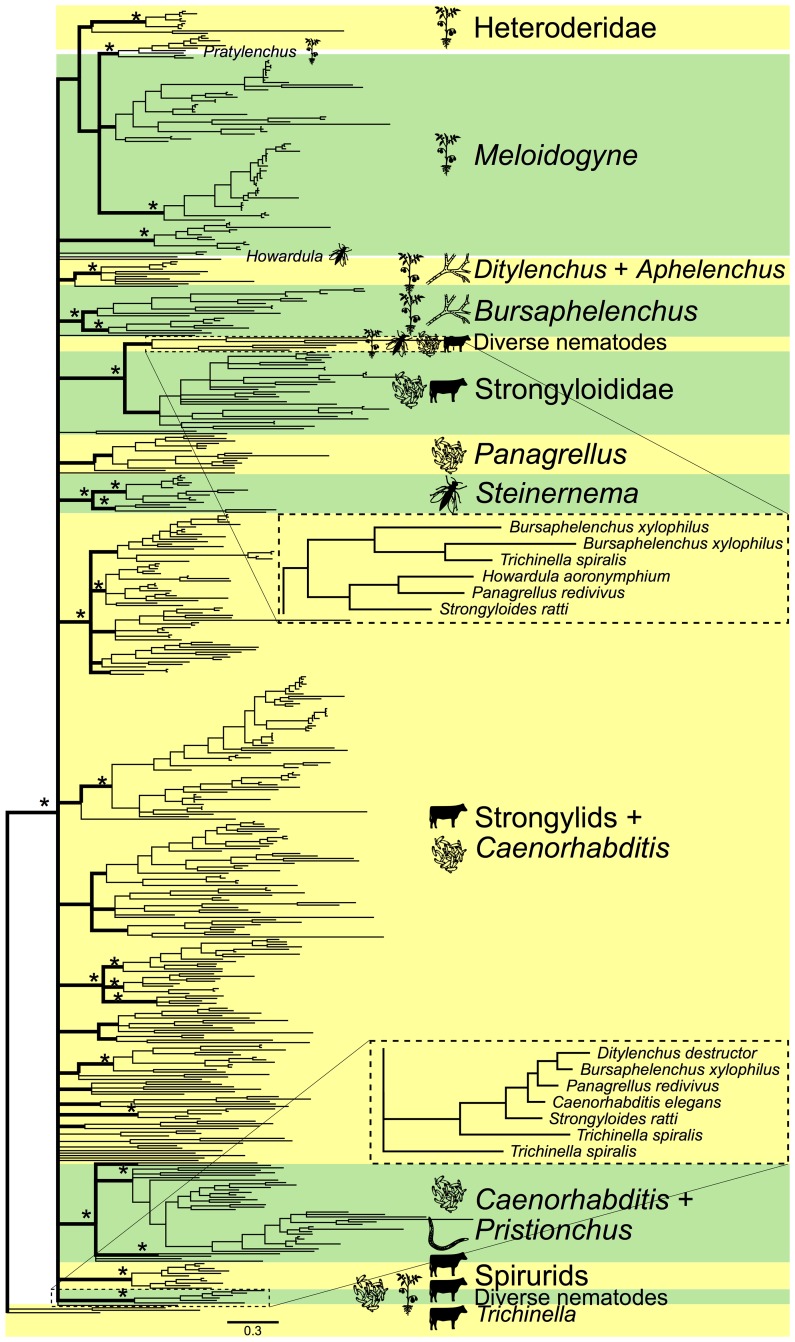
Phylogenetic analysis of nematode VALs reveals no clear links with parasitism
Multiple lineages within the nematode phylum have independently evolved the ability to parasitize plants or animals. Modelling the evolutionary history of nematode VALs, based on overall sequence diversity, may reveal links between patterns of diversification within this protein family and particular lifestyles or host organisms. To this end, we generated a Bayesian tree of available VAL sequences from plant- and animal-parasitic nematodes as well as their free-living close relatives from the distal end of the nematode tree (Fig 1 and S1 Fig, S1 Table) [10]. First of all, we observed no separate clade of VALs uniquely associated with parasitism among nematodes. So, based on overall sequence diversity in VALs, we found no clear distinction between VALs from parasitic nematodes and free-living nematodes. Similarly, we also found no evidence for a clear separation between VALs from different lineages of plant parasites and VALs from different lineages of animal parasites, which could have pointed at specific adaptations to living on either host plants or animals. Instead, several VALs from plant-parasitic nematodes (Ditylenchus destructor and Bursaphelenchus xylophilus), animal-parasitic nematodes (Howardula aoronymphium, Strongyloides ratti, and Trichinella spiralis), and free-living nematodes (Panagrellus redivivus) clustered together (Fig 1 insets). Moreover, VALs from related parasitic and free-living nematode species clustered into separate clades, which points at functional diversification in a common ancestor of these parasitic and free-living lineages. By contrast, some clusters contain large numbers of homologous VALs from the same nematode genus, which indicates extensive recent functional diversification. The large difference in numbers of VALs present in nematode genomes is striking, ranging from a few (H. aoronymphium, Brugia malayi) to over a hundred (Ancylostoma caninum). Gene family expansion also does not seem to be linked to a particular lifestyle or host organism. Altogether, our phylogenetic analysis revealed no clear link between overall sequence diversity in VAL genes in nematodes and parasitism in plant and animals.
Fig 1. Bayesian tree of VALs in nematodes.
Asterisks indicate main branches (bold) well-supported by the Bayesian or maximum likelihood analysis. Icons represent feeding types (plant parasitism, fungal feeding, bacterial feeding, nematode predation, insect parasitism, and vertebrate parasitism). Figure insets reveal two clusters with nematode species of plant- and animal-parasitic and free-living species. Taxon names, support values, and methods for construction can be found in S1 Fig. VAL, venom allergen-like protein.
Helminth VALs bind lipids and other hydrophobic structures
Helminth VALs are classified as SCP/TAPS proteins, which include a range of structurally related proteins found in a wide range of eukaryotes [5]. Ample structural information exists on eukaryotic SCP/TAPS proteins, but so far only a handful of structures for helminth VALs have been resolved. Similar to eukaryotic SCP/TAPS proteins, the core of helminth VALs is a structurally conserved approximately 15 kDa cysteine-rich CAP domain (Pfam00188), which typically has limited sequence identity [11–26]. SCP/TAPS proteins are generally made up of single CAP domains, which are sometimes covalently linked to other functional domains [20, 27]. A higher degree of structural complexity is seen in helminth VALs as they can comprise a single CAP domain with the ability to form homodimers (like Oo-ASP-1 from Ostertagia ostertagi [15]), two covalently linked CAP domains (like Na-ASP-1 from Necator americanus [28]), or even four covalently linked CAP domains (P. redivivus gene Pan_g9869.t1, genome PRJNA186477 WormBase ParaSite). In addition, helminth VALs can be found fused to other protein family sequences such as the ShK toxin domains (Pfam01549) in Toxocara canis [29].
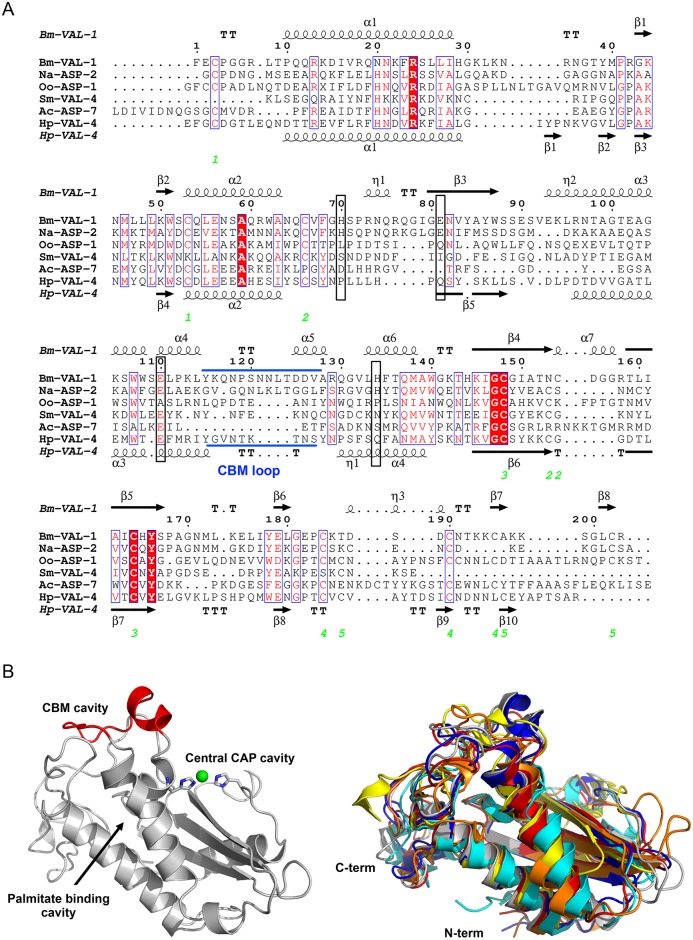
The CAP domain itself adopts a characteristic alpha-beta-alpha sandwich fold with flexible loop regions and is further stabilized by disulfide bonds [11, 13, 14]. The lengths of the flexible loops, β-strands, and α-helices vary between different proteins, making it difficult to accurately model the structures of these proteins. An alignment of the amino acid sequences from single CAP domain VALs of helminths, for which the structures have been resolved, illustrates that conservation among these sequences is mainly found in structural properties, like disulfide bonds, α-helices, and β-strands (Fig 2A). Superimposing VAL structures on top of each other nicely illustrates that loop regions in between these secondary structures as well as N- and C-termini are very distinct (Fig 2B). Importantly, these loop regions constitute up to 50% of the overall VAL structure.
Fig 2. Comparison of helminth VALs.
(A) The sequences of helminth VALs with known structure (see below) are aligned with clustalW2, and the secondary structural features are illustrated with the coordinates of Bm-VAL-1 and Hp-VAL-4 using ESPript. The different secondary structural elements shown are alpha helices as large squiggles labeled (α), 310-helices as small squiggles labeled (η), beta strands as arrows (β), and beta turns (TT). Identical residues are highlighted in solid red, and conserved residues are shown in red. The locations of the cysteine residues involved in disulfide bonds are numbered in green. The location of the CBM loop is shown in blue. The position of amino acid residues constituting the CAP tetrad are marked with a black box. (B) Bm-VAL-1 is given as a representative structure in which the different binding cavities are indicated. The CBM loop is depicted in red, and coordination of the divalent cation Zn2+ is shown in green. Superimposed structures of helminths VALs with a single CAP domain are given to illustrate structural differences among the different family members. The represented structures are Na-ASP-2 in blue [13], Oo-ASP-1 in orange [15], Ac-ASP-7 in cyan [90], Sm-VAL-4 in yellow [44], Hp-VAL-4 in red [12], and Bm-VAL-1 in gray [16]. ASP, activation-associated secreted protein; CAP, cysteine-rich secretory proteins/antigen 5/pathogenesis-related 1; CBM, caveolin-binding motif; VAL, venom allergen-like proteins.
All reported SCP/TAPS protein structures have a large central CAP cavity, which are either cysteine-rich secretory protein (CRISP)-like or non-CRISP-like. The CRISP-like CAP cavity is characterized by a tetrad of residues, consisting of two histidine and two glutamic acid residues that bind divalent cations including Zn2+ and Mg2+ [14, 20, 30–38]. This tetrad was shown to be important for Zn2+ binding and the heparan-sulfate dependent inflammatory modulation mechanisms of the cobra CRISP protein natrin [38]. However, the majority of available sequences of plant and animal parasite VALs are suggestive of non-CRISP-like protein structures because they lack the histidine residues of the CAP tetrad [12]. Furthermore, our recent analysis of the Bm-VAL-1 structure reveals that the central CAP cavity is connected to other cavities by channels that can serve as pathways for water molecules, cations, and small molecules [16].
Given the shared structural topology of the CAP domain, it seems likely that CAP proteins exert a fundamentally similar biochemical function within their respective environments [20, 39, 40]. However, the exact nature of this shared biochemical function among CAP proteins remains poorly defined. Recent evidence shows that distinct pockets in the CAP domain bind lipids, such as leukotrienes, sterols, and negatively charged phospholipids [25, 41, 42]. CAP proteins from yeast (pathogen-related yeast protein [Pry1] and Pry2) bind sterols and fatty acids at two distinct lipid-binding sites [42, 43]. These two well-defined lipid-binding pockets are a flexible loop that binds sterols (called the caveolin-binding motif [CBM] loop) and a hydrophobic channel, which is formed by two parallel α-helices, the so-called palmitate-binding cavity. Furthermore, it was shown that the palmitate-binding cavity of tablysin-15 from the saliva of the horsefly Tabanus yao binds leukotrienes and thereby inhibits their pro-inflammatory effects [25].
Lipid-binding properties have recently also been demonstrated for helminth VALs using mutant yeast cells that lack their endogenous CAP proteins Pry1 and Pry2 and are deficient in sterol export. Expression of Na-ASP-2, Sm-VAL-4, Hp-VAL-4, or Bm-VAL-1 in these mutant cells restores their ability to export sterol [12, 16, 44]. These results also indicate that the CAP tetrad residues are not required for sterol export as both Sm-VAL-4 and Hp-VAL-4 lack the tetrad histidine residues. Sterol binding by Bm-VAL-1, on the other hand, can be inhibited with EDTA and therefore seems to be dependent on the binding of divalent cations. Besides sterol binding, BmVAL-1 is also able to bind palmitate in vitro with comparable affinity as tablysin-15 [16]. The two parallel α-helices that constitute the palmitate-binding cavity are structurally well conserved among other helminth VALs (Fig 2B), which suggests that these VALs likely bind palmitate or similar hydrophobic ligands as well. These studies introduce VALs as a new protein family of parasitic helminths with lipid-binding properties, just like nematode polyprotein antigens and/or allergens (NPAs), nematode fatty acid-binding proteins (nemFABPs), and fatty acid- and retinol-binding proteins (FARs) [45].
Altogether, helminth VALs share a conserved alpha-beta-alpha sandwich structure that is typical for CAP proteins. However, the length of different α-helices and β-strands and the composition of loop regions determine approximately 50% of their overall structure. This supports the idea that the CAP domain may act as a stable but versatile molecular scaffold for diversification of the entire VAL gene family. Also, evidence indicates that this stable CAP domain allows helminth VALs to sequester small hydrophobic ligands [12, 16]. This could be a potential mechanism used by VALs to modulate immune responses in their host.
Biological functions of VALs from plant parasites
Plant-parasitic nematodes use an oral stylet to perforate plant cell walls, deliver secretions into cells, and extract low molecular weight compounds from living plant cells. VALs have attracted attention because they are among the very few molecules in stylet secretions that seem to be important for persistent nematode infections in plants and animals [46]. Following the discovery of Mi-VAP-1 from the root-knot nematode Meloidogyne incognita in 2000 [17, 47], research has focussed on the role of VALs as activators and suppressors of host immune responses. For instance, while studying the function of Gr-VAP-1 from the potato cyst nematode Globodera rostochiensis, it was found that binding of this protein to the extracellular cysteine protease Rcr3pim in tomato triggers a defense-related hypersensitive response mediated by the surface-localized immune receptor Cf-2 [8]. Interestingly, tomato genotypes carrying the Rcr3pim protein but lacking the matching immune receptor Cf-2 were more susceptible to nematode infections than plants lacking both Rc3pim and Cf-2, suggesting that Gr-VAP-1 interacts with Rcr3pim to suppress host immunity.
The expression of VAL genes in plant-parasitic nematodes is regulated in association with host invasion and subsequent migration through host tissues [8, 19, 48–54]. Proteomic analyses of chemically induced secretions from infective juveniles showed the presence of VALs along with different classes of plant cell wall-degrading enzymes [8]. Plant-parasitic nematodes utilize a large repertoire of plant cell wall-degrading enzymes to break down the protective layer of cellulose and other carbohydrate polymers around cells during migration. A knockdown of stylet-secreted cell wall-degrading enzymes by RNA interference in infective juvenile nematodes can halt host invasion [55]. Similarly, a knockdown of VAL expression in migratory plant-parasitic nematodes can also reduce their ability to migrate inside host plants and successfully establish a permanent feeding site [50, 56, 57]. These findings suggest that stylet-secreted VALs are important for modulating host responses, particularly during the migration of the nematodes through host tissues and the early stages of an infection.
Ectopic expression of nematode VALs (Gr-VAP-1, and Hs-VAP-1 and Hs-VAP-2 from Heterodera schachtii) in the extracellular matrix of transgenic plants significantly increases their susceptibility to plant-parasitic nematodes [57]. Interestingly, these plants also proved to be more susceptible to infections by fungi, bacteria, and oomycetes, all of which have entirely different infection strategies [57]. Furthermore, ectopic expression of nematode VALs suppresses the growth inhibition response that normally occurs when young plants are constantly exposed to the flagellin peptide flg22. Flg22 is recognized as a pathogen-associated molecular pattern by surface-localized immune receptors in plants [58, 59]. Whole transcriptome analysis of plants ectopically expressing nematode VALs suggests that enhanced susceptibility involves plant cell wall modifications, lipid signalling, and extracellular protein processing [57]. In a different experimental setup, ectopic nematode VALs suppress a defense-related hypersensitive response in plant cells mediated by surface-localized, but not by cytoplasmic, immune receptors. Taken together, stylet-secreted VALs of plant-parasitic nematodes most likely enhance overall susceptibility of host plants to nematode infections by suppressing plant innate immunity mediated by surface-localized receptors.
Biological functions of VALs from animal parasites
Initially, the major focus of study for animal parasite VALs was their immunogenic properties. Following the discovery in the 1990s that dominant secreted proteins of hookworm larvae are the VAL family members ASP-1 and ASP-2 [22, 28], they were considered ideal candidates for vaccine development. Subsequently, VALs from a wide range of animal and human parasites have shown substantial degrees of protective immunity as vaccines [60–65]. Na-ASP-2 was explored as a potential human hookworm vaccine candidate, but despite successes in animal experiments [66–68], vaccine development was halted due to allergic reactions in previously exposed individuals in a clinical trial [69]. Although adverse, this outcome emphasized the immunogenicity of the VALs in natural helminth infection.
The ubiquitious presence and frequent dominance of VALs in helminth ES products—and their up-regulation during parasitic phases of the life cycle—point to a major role in host–parasite interactions. VALs are among the most abundant secreted proteins in the intestinal cattle parasites Cooperia oncophora and O. ostertagi, where they are prevalently upregulated in parasitic stages [70], and in the rodent model nematodes Heligmosomoides polygyrus [6] and Nippostrongylus brasiliensis [9]. A fascinating example is presented by Strongyloides nematodes, which can follow either a free-living life cycle or a parasitic cycle through mammals; multiple VAL family members show preferential expression in the parasitic adult worm compared to the free-living form of the same species [7]. Furthermore, predicted VALs from the trematode Schistosoma mansoni are up-regulated during parasite infective stages, indicating a role in invasion of the human host [71]. Sm-VAL-4 has been detected in human skin following invasion of S. mansoni cercariae [72]. In the filarial nematode B. malayi, Bm-VAL-1 is highly expressed in the mosquito-borne L3 stage prior to entry into the mammalian host, and is re-expressed subsequently by later stages [73]. ASP/VAL genes of A. caninum and T. canis, the dog hookworm and roundworm respectively, are abundantly expressed in invasive and migratory larval stages [74, 75], as are VALs in the adult stages of A. caninum, Haemonchus contortus, and N. americanus [76–78]. Environmental niche is also linked to VAL expression, which is significantly higher in mucosal-dwelling larvae of Teladorsagia circumcincta compared to larvae from the lumen [79]. VALs therefore seem to be expressed at stages of the parasite life cycle where maximal contact occurs between parasite and host, whether this is transmission, tissue migration, or feeding.
Location of expression in the parasite also hints at function, with many helminth VALs being expressed in secretory glands. Staining for the glycan found on H. polygyrus VAL-1 and VAL-2 identified a series of structures on the exterior cuticle in contact with host tissues [6]. Several A. caninum ASP proteins localize to the pharyngeal glands and glandular esophagus, which produce hookworm ES secretions [26, 80]. S. mansoni VALs also localize to the esophageal gland [81]. Ov-ASP-1, an ASP/VAL transcript from the human parasite Onchocerca volvulus—causal agent of river blindness—is localized in the glandular esophagus of L2 and L3 larvae and secreted via degranulation following the invasion of the host [63].
Though both timing and location of expression of VALs point to roles in parasite–host interactions, relatively few of these proteins have a well-defined physiological function. Na-ASP-2 binds to human B cells via CD79A, triggering downregulation of receptor signaling pathways [82]. Na-ASP-2 also induces neutrophil migration and accumulation within tissues, a potentially pro-inflammatory activity that may aid tissue migrating larvae through increased tissue permeability [83]. This contrasts with other VALs that suppress immune responses once a parasite is tissue-dwelling. For example, neutrophil inhibitor factor (NIF) from A. caninum binds to neutrophils via the integrin CD11b/CD18 and blocks their adhesion to vascular endothelial cells and oxidative bursts [84–86]. Hookworm platelet inhibitor (HPI) is secreted from adult A. caninum at the site of intestinal attachment, where it inhibits platelet aggregation and adhesion, allowing continuous feeding without blood clotting [87, 88]. Sm-VAL-9 from S. mansoni induces differential expression of matrix metalloproteinases in macrophages and modulates host extracellular matrix remodeling gene expression in both its vertebrate and snail hosts [89].
Importantly, care should be taken when results obtained from in vitro studies with recombinant helminth proteins are extrapolated to the in vivo situation. First of all, these proteins are often studied at seemingly supraphysiological concentrations. Furthermore, differences in protein folding and post-translational modifications (e.g., N-glycosylation) between native and recombinant helminth proteins could significantly influence binding to target cells or biological activity.
Taken together, the pattern of animal-parasitic VAL evolution and expression suggests that most family members fulfill core functions at the host–parasite interface, with rapid diversification and adaptation to each host species.
Concluding paragraph
The expression pattern of VALs during invasion and migration of host tissues by both plant- and animal-parasitic helminths suggests that these proteins might have conserved mechanisms of immune modulation in their respective host. However, sequence analysis suggests an independent evolution of these functions. In recent years, several VAL family members have been functionally characterized (summarized in S2 Table) and revealed modulation of similar biological processes in plant and animal hosts, like suppression of innate immune responses and remodeling of the extracellular matrix. Furthermore, VALs reveal a conserved CAP domain that allows them to sequester small hydrophobic ligands (e.g., cholesterol and palmitate), but little is known about endogenous ligands that are bound during parasitism. Therefore, future research should focus on the specificity for different lipid ligands and how these ligands can be involved in the immunomodulatory properties of helminth VALs.
Supporting information
For multidomain proteins, each domain was included separately. Trichinella spiralis served as an outgroup. The alignment was created in BioEdit version 7.2.5 using ClustalW 1,4 and was further manually refined. The Bayesian tree was created using MrBayes version 3.2.6 and run for 10 million generations with 4 chains in 4 parallel runs using a mixed amino acid substitution model. Runs converged after a burnin of 2 million generations and used the WAG substitution model. Posterior probabilities are given above the branches. Displayed below the branches are the bootstrap percentages of a fast maximum likelihood tree run on the same dataset with RAxML version 8.2.10 using the WAG substitution model with 1000 bootstraps. All sequences that were used to constrict this tree are listed in S1 Table. VAL, venom allergen-like protein; WAG, Whelan and Goldman.
(PDF)
Sequences were gathered by blasting published genomes, EST databases and GenBank with VAL sequences or taken from literature references. Genomes were blasted on Wormbase Parasite (http://parasite.wormbase.org) with the exception of B. xylophilus (GeneDB: http://www.genedb.org), S. ratti (https://www.sanger.ac.uk/cgi-bin/blast/submitblast/strongyloides), P. coffeae (https://www.ncbi.nlm.nih.gov), and H. aoronymphium (http://nematodes.org/downloads/959nematodegenomes/blast/db/Howardula_aoronymphium_clc_1.fna). EST databases were blasted on Nematode.net (http://nematode.net). The G. rostochienis, P. coffeae, and H. aoronymphium sequences were extracted from unannotated genome data. Identical sequences were merged in a consensus sequence. EST, expressed sequence tag.
(XLSX)
Collection of helminth VALs as discussed in this review with current nomenclature, structural information (including RCSB PDB reference [https://www.rcsb.org]) and information on lipid binding, interactions and function are given when available. PDB, Protein Data Bank; RCSB, Research Collaboratory for Structural Bioinformatics; VAL, venom allergen-like protein.
(XLSX)
Funding Statement
This work was supported by ALW Grant 84713008 from the Netherlands Organisation for Scientific Research. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Goverse A, Smant G. The activation and suppression of plant innate immunity by parasitic nematodes. Annu Rev Phytopathol. 2014;52:243–65. 10.1146/annurev-phyto-102313-050118 [DOI] [PubMed] [Google Scholar]
- 2.Allen J, Wynn T. Evolution of Th2 immunity: a rapid repair response to tissue destructive pathogens. PLoS Pathog. 2011;7(5):e1002003 10.1371/journal.ppat.1002003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maizels R, McSorley H. Regulation of the host immune system by helminth parasites. J Allergy Clin Immunol. 2016;138(3):666–75. 10.1016/j.jaci.2016.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cuesta-Astroz Y, Oliveira F, Nahum L, Oliveira G. Helminth secretomes reflect different lifestyles and parasitized hosts. Int J Parasitol. 2017;47(9):529–44. 10.1016/j.ijpara.2017.01.007 [DOI] [PubMed] [Google Scholar]
- 5.Cantacessi C, Campbell B, Visser A, Geldhof P, Nolan M, Nisbet A, et al. A portrait of the "SCP/TAPS" proteins of eukaryotes—Developing a framework for fundamental research and biotechnological outcomes. Biotechnol Adv. 2009;27(4):376–88. 10.1016/j.biotechadv.2009.02.005 [DOI] [PubMed] [Google Scholar]
- 6.Hewitson J, Harcus Y, Murray J, van Agtmaal M, Filbey K, Grainger J, et al. Proteomic analysis of secretory products from the model gastrointestinal nematode Heligmosomoides polygyrus reveals dominance of venom allergen-like (VAL) proteins. J Proteomics. 2011;74(9):1573–94. 10.1016/j.jprot.2011.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunt V, Tsai I, Coghlan A, Reid A, Holroyd N, Foth B, et al. The genomic basis of parasitism in the Strongyloides clade of nematodes. Nat Genet. 2016;48(3):299–307. 10.1038/ng.3495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lozano-Torres J, Wilbers R, Gawronski P, Boshoven J, Finkers-Tomczak A, Cordewener J, et al. Dual disease resistance mediated by the immune receptor Cf-2 in tomato requires a common virulence target of a fungus and a nematode. Proc Natl Acad Sci U S A. 2012;109(25):10119–24. 10.1073/pnas.1202867109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sotillo J, Sanchez-Flores A, Cantacessi C, Harcus Y, Pickering D, Bouchery T, et al. Secreted proteomes of different developmental stages of the gastrointestinal nematode Nippostrongylus brasiliensis. Mol Cell Proteomics. 2014;13(10):2736–51. 10.1074/mcp.M114.038950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Megen H, van den Elsen S, Holterman M, Karssen G, Mooyman P, Bongers T, et al. A phylogenetic tree of nematodes based on about 1200 full-length small subunit ribosomal DNA sequences. Nematology. 2009;11:927–S27. [Google Scholar]
- 11.Asojo O. Structure of a two-CAP-domain protein from the human hookworm parasite Necator americanus. Acta Crystallogr D Biol Crystallogr. 2011;67(Pt 5):455–62. 10.1107/S0907444911008560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asojo O, Darwiche R, Gebremedhin S, Smant G, Lozano-Torres J, Drurey C, et al. Heligmosomoides polygyrus Venom Allergen-like Protein-4 (HpVAL-4) is a sterol binding protein. Int J Parasitol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asojo O, Goud G, Dhar K, Loukas A, Zhan B, Deumic V, et al. X-ray structure of Na-ASP-2, a pathogenesis-related-1 protein from the nematode parasite, Necator americanus, and a vaccine antigen for human hookworm infection. J Mol Biol. 2005;346(3):801–14. 10.1016/j.jmb.2004.12.023 [DOI] [PubMed] [Google Scholar]
- 14.Asojo O, Koski R, Bonafe N. Structural studies of human glioma pathogenesis-related protein 1. Acta Crystallogr D Biol Crystallogr. 2011;67(Pt 10):847–55. 10.1107/S0907444911028198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borloo J, Geldhof P, Peelaers I, Van Meulder F, Ameloot P, Callewaert N, et al. Structure of Ostertagia ostertagi ASP-1: insights into disulfide-mediated cyclization and dimerization. Acta Crystallogr D Biol Crystallogr. 2013;69(Pt 4):493–503. 10.1107/S0907444912050019 [DOI] [PubMed] [Google Scholar]
- 16.Darwiche R, Lugo F, Drurey C, Varossieau K, Smant G, Wilbers R, et al. Crystal structure of Brugia malayi venom allergen-like protein-1 (BmVAL-1), a vaccine candidate for lymphatic filariasis. Int J Parasitol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ding X, Shields J, Allen R, Hussey R. Molecular cloning and characterisation of a venom allergen AG5-like cDNA from Meloidogyne incognita. Int J Parasitol. 2000;30(1):77–81. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez C, Szyperski T, Bruyere T, Ramage P, Mosinger E, Wuthrich K. NMR solution structure of the pathogenesis-related protein P14a. J Mol Biol. 1997;266(3):576–93. 10.1006/jmbi.1996.0772 [DOI] [PubMed] [Google Scholar]
- 19.Gao B, Allen R, Maier T, Davis E, Baum T, Hussey R. Molecular characterisation and expression of two venom allergen-like protein genes in Heterodera glycines. Int J Parasitol. 2001;31(14):1617–25. [DOI] [PubMed] [Google Scholar]
- 20.Gibbs G, Roelants K, O’Bryan M. The CAP superfamily: cysteine-rich secretory proteins, antigen 5, and pathogenesis-related 1 proteins—roles in reproduction, cancer, and immune defense. Endocr Rev. 2008;29(7):865–97. 10.1210/er.2008-0032 [DOI] [PubMed] [Google Scholar]
- 21.Guo M, Teng M, Niu L, Liu Q, Huang Q, Hao Q. Crystal structure of the cysteine-rich secretory protein stecrisp reveals that the cysteine-rich domain has a K+ channel inhibitor-like fold. J Biol Chem. 2005;280(13):12405–12. 10.1074/jbc.M413566200 [DOI] [PubMed] [Google Scholar]
- 22.Hawdon J, Narasimhan S, Hotez P. Ancylostoma secreted protein 2. cloning and characterization of a second member of a family of nematode secreted proteins from Ancylostoma caninum. Mol Biochem Parasit. 1999;99(2):149–65. [DOI] [PubMed] [Google Scholar]
- 23.Shikamoto Y, Suto K, Yamazaki Y, Morita T, Mizuno H. Crystal structure of a CRISP family Ca2+-channel blocker derived from snake venom. J Mol Biol. 2005;350(4):735–43. 10.1016/j.jmb.2005.05.020 [DOI] [PubMed] [Google Scholar]
- 24.Wang J, Shen B, Guo M, Lou X, Duan Y, Cheng X, et al. Blocking effect and crystal structure of natrin toxin, a cysteine-rich secretory protein from Naja atra venom that targets the BKCa channel. Biochemistry. 2005;44(30):10145–52. 10.1021/bi050614m [DOI] [PubMed] [Google Scholar]
- 25.Xu X, Francischetti I, Lai R, Ribeiro J, Andersen J. Structure of protein having inhibitory disintegrin and leukotriene scavenging functions contained in single domain. J Biol Chem. 2012;287(14):10967–76. 10.1074/jbc.M112.340471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhan B, Liu Y, Badamchian M, Williamson A, Feng J, Loukas A, et al. Molecular characterisation of the Ancylostoma-secreted protein family from the adult stage of Ancylostoma caninum. Int J Parasitol. 2003;33(9):897–907. [DOI] [PubMed] [Google Scholar]
- 27.Gibbs G, O’Bryan M. Cysteine rich secretory proteins in reproduction and venom. Soc Reprod Fertil Suppl. 2007;65:261–7. [PubMed] [Google Scholar]
- 28.Hawdon J, Jones B, Hoffman D, Hotez P. Cloning and characterization of Ancylostoma-secreted protein—A novel protein associated with the transition to parasitism by infective hookworm larvae. J Biol Chem. 1996;271(12):6672–8. [DOI] [PubMed] [Google Scholar]
- 29.Tetteh K, Loukas A, Tripp C, Maizels R. Identification of abundantly expressed novel and conserved genes from the infective larval stage of Toxocara canis by an expressed sequence tag strategy. Infect Immun. 1999;67(9):4771–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kratzschmar J, Haendler B, Eberspaecher U, Roosterman D, Donner P, Schleuning W. The human cysteine-rich secretory protein (CRISP) family. Primary structure and tissue distribution of CRISP-1, CRISP-2 and CRISP-3. Eur J Biochem. 1996;236(3):827–36. [DOI] [PubMed] [Google Scholar]
- 31.Magdaleno L, Gasset M, Varea J, Schambony A, Urbanke C, Raida M, et al. Biochemical and conformational characterisation of HSP-3, a stallion seminal plasma protein of the cysteine-rich secretory protein (CRISP) family. FEBS Lett. 1997;420(2–3):179–85. [DOI] [PubMed] [Google Scholar]
- 32.Mason L, Tribolet L, Simon A, von Gnielinski N, Nienaber L, Taylor P, et al. Probing the equatorial groove of the hookworm protein and vaccine candidate antigen, Na-ASP-2. Int J Biochem Cell B. 2014;50:146–55. [DOI] [PubMed] [Google Scholar]
- 33.Milne T, Abbenante G, Tyndall J, Halliday J, Lewis R. Isolation and characterization of a cone snail protease with homology to CRISP proteins of the pathogenesis-related protein superfamily. J Biol Chem. 2003;278(33):31105–10. 10.1074/jbc.M304843200 [DOI] [PubMed] [Google Scholar]
- 34.Udby L, Johnsen A, Borregaard N. Human CRISP-3 binds serum alpha B-1-glycoprotein across species. Bba-Gen Subjects. 2010;1800(4):481–5. [DOI] [PubMed] [Google Scholar]
- 35.Udby L, Lundwall A, Johnsen A, Fernlund P, Valtonen-Andre C, Blom A, et al. beta-Microseminoprotein binds CRISP-3 in human seminal plasma. Biochem Bioph Res Co. 2005;333(2):555–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Urayama S, Harada Y, Nakagawa Y, Ban S, Akasaka M, Kawasaki N, et al. Ascidian sperm glycosylphosphatidylinositol-anchored CRISP-like protein as a binding partner for an allorecognizable sperm receptor on the vitelline coat. J Biol Chem. 2008;283(31):21725–33. 10.1074/jbc.M802631200 [DOI] [PubMed] [Google Scholar]
- 37.Volpert M, Mangum J, Jamsai D, D’Sylva R, O’Bryan M, McIntyre P. Eukaryotic expression, purification and structure/function analysis of native, recombinant CRISP3 from human and mouse. Sci Rep-Uk. 2014;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y, Kuo J, Lee S, Liu J, Hsieh Y, Shih Y, et al. Cobra CRISP functions as an inflammatory modulator via a novel Zn2+- and heparan sulfate-dependent transcriptional regulation of endothelial cell adhesion molecules. J Biol Chem. 2010;285(48):37872–83. 10.1074/jbc.M110.146290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cantacessi C, Gasser R. SCP/TAPS proteins in helminths—where to from now? Mol Cell Probes. 2012;26(1):54–9. 10.1016/j.mcp.2011.10.001 [DOI] [PubMed] [Google Scholar]
- 40.Schneiter R, Di Pietro A. The CAP protein superfamily: function in sterol export and fungal virulence. Biomol Concepts. 2013;4(5):519–25. 10.1515/bmc-2013-0021 [DOI] [PubMed] [Google Scholar]
- 41.Van Galen J, Van Balkom B, Serrano R, Kaloyanova D, Eerland R, Stuven E, et al. Binding of GAPR-1 to negatively charged phospholipid membranes: unusual binding characteristics to phosphatidylinositol. Mol Membr Biol. 2010;27(2–3):81–91. 10.3109/09687680903507080 [DOI] [PubMed] [Google Scholar]
- 42.Choudhary V, Schneiter R. Pathogen-Related Yeast (PRY) proteins and members of the CAP superfamily are secreted sterol-binding proteins. P Natl Acad Sci USA. 2012;109(42):16882–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Darwiche R, Mene-Saffrane L, Gfeller D, Asojo O, Schneiter R. The pathogen-related yeast protein Pry1, a member of the CAP protein superfamily, is a fatty acid-binding protein. J Biol Chem. 2017;292(20):8304–14. 10.1074/jbc.M117.781880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kelleher A, Darwiche R, Rezende W, Farias L, Leite L, Schneiter R, et al. Schistosoma mansoni venom allergen-like protein 4 (SmVAL4) is a novel lipid-binding SCP/TAPS protein that lacks the prototypical CAP motifs. Acta Crystallogr D Biol Crystallogr. 2014;70(Pt 8):2186–96. 10.1107/S1399004714013315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Franchini G, Porfido J, Shimabukuro M, Burusco M, Belgamo J, Smith B, et al. The unusual lipid binding proteins of parasitic helminths and their potential roles in parasitism and as therapeutic targets. Prostag Leukotr Ess. 2015;93:31–6. [DOI] [PubMed] [Google Scholar]
- 46.Jasmer D, Goverse A, Smant G. Parasitic nematode interactions with mammals and plants. Annu Rev Phytopathol. 2003;41:245–70. 10.1146/annurev.phyto.41.052102.104023 [DOI] [PubMed] [Google Scholar]
- 47.Ding X, Shields J, Allen R, Hussey R. Molecular cloning and characterisation of a venom allergen AG5-like cDNA from Meloidogyne incognita. Int J Parasitol. 2000;30(1):77–81. [DOI] [PubMed] [Google Scholar]
- 48.Duarte A, Curtis R, Maleita C, Tiago I, Abrantes I. Characterization of the venom allergen-like protein (vap-1) and the fatty acid and retinol binding protein (far-1) genes in Meloidogyne hispanica. Eur J Plant Pathol. 2014;139(4):825–36. [Google Scholar]
- 49.Duarte A, Maleita C, Abrantes I, Curtis R. Tomato root exudates induce transcriptional changes of Meloidogyne hispanica genes. Phytopathol Mediterr. 2015;54(1):104–8. [Google Scholar]
- 50.Kang J, Koh Y, Moon Y, Lee S. Molecular properties of a venom allergen-like protein suggest a parasitic function in the pinewood nematode Bursaphelenchus xylophilus. Int J Parasitol. 2012;42(1):63–70. 10.1016/j.ijpara.2011.10.006 [DOI] [PubMed] [Google Scholar]
- 51.Peng H, Gao BL, Kong L, Yu Q, Huang W, He X, et al. Exploring the host parasitism of the migratory plant-parasitic nematode Ditylenchus destuctor by expressed sequence tags analysis. PLoS ONE. 2013;8(7):e69579 10.1371/journal.pone.0069579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Petitot A, Dereeper A, Agbessi M, Da Silva C, Guy J, Ardisson M, et al. Dual RNA-seq reveals Meloidogyne graminicola transcriptome and candidate effectors during the interaction with rice plants. Mol Plant Pathol. 2016;17(6):860–74. 10.1111/mpp.12334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang X, Li H, Hu Y, Fu P, Xu J. Molecular cloning and analysis of a new venom allergen-like protein gene from the root-knot nematode Meloidogyne incognita. Exp Parasitol. 2007;117(2):133–40. 10.1016/j.exppara.2007.03.017 [DOI] [PubMed] [Google Scholar]
- 54.Yan X, Cheng X, Wang Y, Luo J, Mao Z, Ferris V, et al. Comparative transcriptomics of two pathogenic pinewood nematodes yields insights into parasitic adaptation to life on pine hosts. Gene. 2012;505(1):81–90. 10.1016/j.gene.2012.05.041 [DOI] [PubMed] [Google Scholar]
- 55.Rehman S, Butterbach P, Popeijus H, Overmars H, Davis E, Jones J, et al. Identification and Characterization of the Most Abundant Cellulases in Stylet Secretions from Globodera rostochiensis. Phytopathology. 2009;99(2):194–202. 10.1094/PHYTO-99-2-0194 [DOI] [PubMed] [Google Scholar]
- 56.Duarte A, Maleita C, Egas C, Abrantes I, Curtis R. Significant effects of RNAi silencing of the venom allergen-like protein (Mhi-vap-1) of the root-knot nematode Meloidogyne hispanica in the early events of infection. Plant Pathol. 2017;66(8):1329–37. [Google Scholar]
- 57.Lozano-Torres J, Wilbers R, Warmerdam S, Finkers-Tomczak A, Diaz-Granados A, van Schaik C, et al. Apoplastic Venom Allergen-like Proteins of Cyst Nematodes Modulate the Activation of Basal Plant Innate Immunity by Cell Surface Receptors. PLoS Pathog. 2014;10(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chinchilla D, Bauer Z, Regenass M, Boller T, Felix G. The Arabidopsis receptor kinase FLS2 binds flg22 and determines the specificity of flagellin perception. Plant Cell. 2006;18(2):465–76. 10.1105/tpc.105.036574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gomez-Gomez L, Boller T. FLS2: an LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol Cell. 2000;5(6):1003–11. [DOI] [PubMed] [Google Scholar]
- 60.Gonzalez-Hernandez A, Van Coppernolle S, Borloo J, Van Meulder F, Paerewijck O, Peelaers I, et al. Host protective ASP-based vaccine against the parasitic nematode Ostertagia ostertagi triggers NK cell activation and mixed IgG1-IgG2 response. Sci Rep-Uk. 2016;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hewitson J, Filbey K, Esser-von Bieren J, Camberis M, Schwartz C, Murray J, et al. Concerted activity of IgG1 antibodies and IL-4/IL-25-dependent effector cells trap helminth larvae in the tissues following vaccination with defined secreted antigens, providing sterile immunity to challenge infection. PLoS Pathog. 2015;11(3):e1004676 10.1371/journal.ppat.1004676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kalyanasundaram R, Balumuri P. Multivalent vaccine formulation with BmVAL-1 and BmALT-2 confer significant protection against challenge infections with Brugia malayi in mice and jirds. Res Rep Trop Med. 2011;2011(2):45–56. 10.2147/RRTM.S13679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.MacDonald A, Tawe W, Leon O, Cao L, Liu J, Oksov Y, et al. Ov-ASP-1, the Onchocerca volvulus homologue of the activation associated secreted protein family is immunostimulatory and can induce protective anti-larval immunity. Parasite Immunol. 2004;26(1):53–62. 10.1111/j.0141-9838.2004.00685.x [DOI] [PubMed] [Google Scholar]
- 64.Meyvis Y, Geldhof P, Gevaert K, Timmerman E, Vercruysse J, Claerebout E. Vaccination against Ostertagia ostertagi with subfractions of the protective ES-thiol fraction. Vet Parasitol. 2007;149(3–4):239–45. 10.1016/j.vetpar.2007.08.014 [DOI] [PubMed] [Google Scholar]
- 65.Schallig H, vanLeeuwen M, Cornelissen A. Protective immunity induced by vaccination with two Haemonchus contortus excretory secretory proteins in sheep. Parasite Immunol. 1997;19(10):447–53. [DOI] [PubMed] [Google Scholar]
- 66.Loukas A, Bethony J, Brooker S, Hotez P. Hookworm vaccines: past, present, and future. Lancet Infect Dis. 2006;6(11):733–41. 10.1016/S1473-3099(06)70630-2 [DOI] [PubMed] [Google Scholar]
- 67.Mendez S, D’Samuel A, Antoine A, Ahn S, Hotez P. Use of the air pouch model to investigate immune responses to a hookworm vaccine containing the Na-ASP-2 protein in rats. Parasite Immunol. 2008;30(1):53–6. 10.1111/j.1365-3024.2007.00994.x [DOI] [PubMed] [Google Scholar]
- 68.Xiao S, Zhan B, Xue J, Goud G, Loukas A, Liu Y, et al. The evaluation of recombinant hookworm antigens as vaccines in hamsters (Mesocricetus auratus) challenged with human hookworm, Necator americanus. Exp Parasitol. 2008;118(1):32–40. 10.1016/j.exppara.2007.05.010 [DOI] [PubMed] [Google Scholar]
- 69.Diemert D, Pinto A, Freire J, Jariwala A, Santiago H, Hamilton R, et al. Generalized urticaria induced by the Na-ASP-2 hookworm vaccine: implications for the development of vaccines against helminths. J Allergy Clin Immunol. 2012;130(1):169–76 e6. 10.1016/j.jaci.2012.04.027 [DOI] [PubMed] [Google Scholar]
- 70.Heizer E, Zarlenga D, Rosa B, Gao X, Gasser R, De Graef J, et al. Transcriptome analyses reveal protein and domain families that delineate stage-related development in the economically important parasitic nematodes, Ostertagia ostertagi and Cooperia oncophora. BMC Genomics. 2013;14:118 10.1186/1471-2164-14-118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chalmers I, McArdle A, Coulson R, Wagner M, Schmid R, Hirai H, et al. Developmentally regulated expression, alternative splicing and distinct sub-groupings in members of the Schistosoma mansoni venom allergen-like (SmVAL) gene family. BMC Genomics. 2008;9:89 10.1186/1471-2164-9-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hansell E, Braschi S, Medzihradszky K, Sajid M, Debnath M, Ingram J, et al. Proteomic analysis of skin invasion by blood fluke larvae. PLoS Negl Trop Dis. 2008;2(7):e262 10.1371/journal.pntd.0000262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Murray J, Gregory W, Gomez-Escobar N, Atmadja A, Maizels R. Expression and immune recognition of Brugia malayi VAL-1, a homologue of vespid venom allergens and Ancylostoma secreted proteins. Mol Biochem Parasitol. 2001;118(1):89–96. [DOI] [PubMed] [Google Scholar]
- 74.Datu B, Gasser R, Nagaraj S, Ong E, O’Donoghue P, McInnes R, et al. Transcriptional changes in the hookworm, Ancylostoma caninum, during the transition from a free-living to a parasitic larva. PLoS Negl Trop Dis. 2008;2(1):e130 10.1371/journal.pntd.0000130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stroehlein A, Young N, Hall R, Korhonen P, Hofmann A, Sternberg P, et al. CAP protein superfamily members in Toxocara canis. Parasite Vector. 2016;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mohandas N, Young N, Jabbar A, Korhonen P, Koehler A, Amani P, et al. The barber’s pole worm CAP protein superfamily—A basis for fundamental discovery and biotechnology advances. Biotechnol Adv. 2015;33(8):1744–54. [DOI] [PubMed] [Google Scholar]
- 77.Morante T, Shepherd C, Constantinoiu C, Loukas A, Sotillo J. Revisiting the Ancylostoma Caninum Secretome Provides New Information on Hookworm–Host Interactions. PROTEOMICS. 2017;17(23–24):1700186. [DOI] [PubMed] [Google Scholar]
- 78.Tang Y, Gao X, Rosa B, Abubucker S, Hallsworth-Pepin K, Martin J, et al. Genome of the human hookworm Necator americanus. Nat Genet. 2014;46:261 10.1038/ng.2875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McNeilly T, Frew D, Burgess S, Wright H, Bartley D, Bartley Y, et al. Niche-specific gene expression in a parasitic nematode; increased expression of immunomodulators in Teladorsagia circumcincta larvae derived from host mucosa. Sci Rep. 2017;7(1):7214 10.1038/s41598-017-07092-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bethony J, Loukas A, Smout M, Brooker S, Mendez S, Plieskatt J, et al. Antibodies against a secreted protein from hookworm larvae reduce the intensity of hookworm infection in humans and vaccinated laboratory animals. Faseb J. 2005;19(9):1743–+. [DOI] [PubMed] [Google Scholar]
- 81.Rofatto H, Parker-Manuel S, Barbosa T, Tararam C, Wilson R, Leite L, et al. Tissue expression patterns of Schistosoma mansoni Venom Allergen-Like proteins 6 and 7. Int J Parasitol. 2012;42(7):613–20. 10.1016/j.ijpara.2012.04.008 [DOI] [PubMed] [Google Scholar]
- 82.Tribolet L, Cantacessi C, Pickering D, Navarro S, Doolan D, Trieu A, et al. Probing of a Human Proteome Microarray With a Recombinant Pathogen Protein Reveals a Novel Mechanism by Which Hookworms Suppress B-Cell Receptor Signaling. J Infect Dis. 2015;211(3):416–25. 10.1093/infdis/jiu451 [DOI] [PubMed] [Google Scholar]
- 83.Bower M, Constant S, Mendez S. Necator americanus: The Na-ASP-2 protein secreted by the infective larvae induces neutrophil recruitment in vivo and in vitro. Experimental Parasitology. 2008;118(4):569–75. 10.1016/j.exppara.2007.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moyle M, Foster D, Mcgrath D, Brown S, Laroche Y, Demeutter J, et al. A Hookworm Glycoprotein That Inhibits Neutrophil Function Is a Ligand of the Integrin Cd11b Cd18. J Biol Chem. 1994;269(13):10008–15. [PubMed] [Google Scholar]
- 85.Rieu P, Sugimori T, Griffith D, Arnaout M. Solvent-accessible residues on the metal ion-dependent adhesion site face of integrin CR3 mediate its binding to the neutrophil inhibitory factor. J Biol Chem. 1996;271(27):15858–61. [DOI] [PubMed] [Google Scholar]
- 86.Schnyder-Candrian S, Maillet I, Le Bert M, Brault L, Jacobs M, Ryffel B, et al. Neutrophil Inhibitory Factor Selectively Inhibits the Endothelium-Driven Transmigration of Eosinophils In Vitro and Airway Eosinophilia in OVA-Induced Allergic Lung Inflammation. J Allergy (Cairo). 2012;2012:245909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chadderdon R, Cappello M. The hookworm platelet inhibitor: functional blockade of integrins GPIIb/IIIa (alphaIIbbeta3) and GPIa/IIa (alpha2beta1) inhibits platelet aggregation and adhesion in vitro. J Infect Dis. 1999;179(5):1235–41. 10.1086/314724 [DOI] [PubMed] [Google Scholar]
- 88.Del Valle A, Jones B, Harrison L, Chadderdon R, Cappello M. Isolation and molecular cloning of a secreted hookworm platelet inhibitor from adult Ancylostoma caninum. Mol Biochem Parasit. 2003;129(2):167–77. [DOI] [PubMed] [Google Scholar]
- 89.Yoshino T, Brown M, Wu X, Jackson CJ Ocadiz-Ruiz R, Chalmers I, et al. Excreted/secreted Schistosoma mansoni venom allergen-like 9 (SmVAL9) modulates host extracellular matrix remodelling gene expression. Int J Parasitol. 2014;44(8):551–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Osman A, Wang C, Winter A, Loukas A, Tribolet L, Gasser R, et al. Hookworm SCP/TAPS protein structure—A key to understanding host-parasite interactions and developing new interventions. Biotechnol Adv. 2012;30(3):652–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For multidomain proteins, each domain was included separately. Trichinella spiralis served as an outgroup. The alignment was created in BioEdit version 7.2.5 using ClustalW 1,4 and was further manually refined. The Bayesian tree was created using MrBayes version 3.2.6 and run for 10 million generations with 4 chains in 4 parallel runs using a mixed amino acid substitution model. Runs converged after a burnin of 2 million generations and used the WAG substitution model. Posterior probabilities are given above the branches. Displayed below the branches are the bootstrap percentages of a fast maximum likelihood tree run on the same dataset with RAxML version 8.2.10 using the WAG substitution model with 1000 bootstraps. All sequences that were used to constrict this tree are listed in S1 Table. VAL, venom allergen-like protein; WAG, Whelan and Goldman.
(PDF)
Sequences were gathered by blasting published genomes, EST databases and GenBank with VAL sequences or taken from literature references. Genomes were blasted on Wormbase Parasite (http://parasite.wormbase.org) with the exception of B. xylophilus (GeneDB: http://www.genedb.org), S. ratti (https://www.sanger.ac.uk/cgi-bin/blast/submitblast/strongyloides), P. coffeae (https://www.ncbi.nlm.nih.gov), and H. aoronymphium (http://nematodes.org/downloads/959nematodegenomes/blast/db/Howardula_aoronymphium_clc_1.fna). EST databases were blasted on Nematode.net (http://nematode.net). The G. rostochienis, P. coffeae, and H. aoronymphium sequences were extracted from unannotated genome data. Identical sequences were merged in a consensus sequence. EST, expressed sequence tag.
(XLSX)
Collection of helminth VALs as discussed in this review with current nomenclature, structural information (including RCSB PDB reference [https://www.rcsb.org]) and information on lipid binding, interactions and function are given when available. PDB, Protein Data Bank; RCSB, Research Collaboratory for Structural Bioinformatics; VAL, venom allergen-like protein.
(XLSX)