Abstract
Objective:
Recent studies describe refeeding in anorexia nervosa (AN) using various methods to deliver higher calorie loads than is currently recommended. We systematically examined approaches to refeeding in hospitalized patients with AN
Methods:
Systematic review of PubMed, PsycINFO, Scopus, and Clinical Trials databases from 1960–2015 using the terms refeeding, weight restoration, hypophosphatemia, anorexia nervosa, anorexia, and anorexic.
Results:
948 abstracts were retrieved using the search criteria and were included if they described a refeeding protocol in hospitalized patients with AN with sufficient detail to allow replication. Twenty-two papers were included in the final review. Most studies were in adolescents and were observational or retrospective; the majority of studies since 2010 have reported on refeeding approaches beginning with higher calories or advancing caloric prescriptions faster than current treatment recommendations.
Discussion:
The available evidence supported seven conclusions, summarized here: 1) In moderately malnourished patients with AN, higher calorie feeding is feasible; 2) Meal-only approaches or combined nasogastric plus meal-feeding approaches can deliver higher calorie feeds in hospital; 3) In severely malnourished patients with AN, there is insufficient evidence to support any change to current standards of care for refeeding hospitalized patients; 4) Higher calorie approaches to refeeding appear safe under close medical supervision and with correction of electrolyte abnormalities; 5) The impact of differing approaches to refeeding on long-term outcomes is unknown; 6) TPN is not recommended unless no other form of refeeding is possible; and 7) Meals and liquid formulas with nutrient compositions within recommended ranges are appropriate for refeeding.
Keywords: Anorexia nervosa, refeeding, weight restoration, nutritional rehabilitation, refeeding syndrome, hypophosphatemia, medical complications, medical stability, length of stay
Refeeding is the first important step to recovery from anorexia nervosa (AN). The primary goal of refeeding hospitalized patients is to reverse malnutrition and its complications. Adequate weight gain in hospital is crucial and predicts weight recovery at one year (1–3). However, the need for weight gain must be balanced against the potentially fatal medical complications of the refeeding syndrome, which can manifest in cardiac arrhythmia, cardiac failure or arrest, hemolytic anemia, delirium, seizures, coma, and sudden death (4–8). These clinical sequelae are thought to occur in response to intracellular movement of glucose, fluid, and electrolytes that is caused by surges in insulin after nutrients are reintroduced following starvation (9).
Until recently, the standard of care for refeeding in AN has been to “start low and go slow” to minimize the risk of refeeding syndrome. Recommendations in the United States begin with calorie prescriptions around 1200 per day with slow advancement by about 100 calories per day (kcal/d) (10–12). Caloric levels as low as 200–600 kcal/d have been recommended in Europe and the United Kingdom (13). Lower calorie refeeding has recently been linked to poor weight gain and prolonged hospitalization (14). Growing recognition of the so-called “underfeeding syndrome” (8) has spurred renewed interest in more aggressive approaches to refeeding (15). These approaches are varied, but typically begin with higher calorie and/or more rapid advancement and may be delivered through meals alone or a combination of meals with supplemental nasogastric (NG) feeding.
Patients who are physiologically or psychologically unstable are admitted to hospital for refeeding. Criteria for admission to hospital vary by region, age (adolescents vs. adults), and the type of treatment facility. However, published guidelines for adolescent and adult care have suggested that hospital admission is warranted in the presence of vital sign abnormalities (bradycardia, hypotension, orthostatic heart rate and blood pressure, and hypothermia), failure to respond to lower levels of care, suicidality, or other severe psychiatric symptoms (11, 15, 16). Severe malnutrition alone, defined as Body Mass Index (BMI) < 15 kg/m2 in adults (13) or <70% of the median BMI (%mBMI) (16) per CDC data (17) in adolescents, may also warrant hospitalization.
The initial focus of refeeding is to restore physiological stability through weight gain. Bradycardia normalizes more quickly during refeeding than other signs, such as orthostatic changes, which may take weeks (18). In adolescents in the United States, normalization of vital signs is commonly used as a discharge criterion from medical inpatient units, which is why some studies of refeeding have used length of hospital stay as a proxy for the time required to achieve medical stability. In addition to physiological instability at presentation, patients are at risk of developing complications during the refeeding process itself. Refeeding hypophosphatemia (RH) is used as a sensitive marker of risk for the refeeding syndrome (19) and is more likely to occur in severely malnourished patients (20). Monitoring of serum electrolytes every 24–48 hours is typically recommended during the first week of hospitalization, when risk for the development of the refeeding syndrome is highest (21). However, there are no recommendations in place for electrolyte correction with supplements, resulting in wide variations in clinical practice (22). For these reasons, both degree of malnutrition and electrolyte supplementation must be considered when evaluating studies of refeeding.
Not only is weight gain in hospital required for medical stabilization, but it may also set the stage for long-term recovery. Studies examining predictors of recovery have identified weight gain in hospital (higher discharge weight and faster gain) as a positive predictor of weight recovery one year post-hospitalization (1–3). Weight gain in the outpatient setting is also crucial for long-term recovery. Randomized controlled trials (RCTs) of outpatient psychotherapy in adolescents have demonstrated that weight at the end of treatment is the best predictor of recovery (23), and faster weight gain during the first 3–4 weeks (0.43–0.86 kg/week) of outpatient treatment predicts full remission at 12 months (24). Finally, weight restoration is central to reversing the long-term medical complications, including amenorrhea, which usually resumes or begins at a weight above 95% mBMI (25, 26).
These collective findings underscore the need to identify approaches to refeeding that simultaneously maximize weight recovery and minimize the associated risks. In addition, they highlight the need to consider how relatively short-term approaches to inpatient refeeding may support the long-term goals of recovery, which include cognitive recovery and eating disorder psychopathology. Thus, the purpose of this review was to systematically examine published studies on approaches to refeeding in hospitalized adolescents and adults with AN, with particular attention to the approach to refeeding (including caloric level, methods of delivery, and nutrient content) and characteristics of the study population (including age and degree of malnutrition). Pertinent medical outcomes were weight gain, length of stay (as a proxy for time to achieve medical stability), and RH; cognitive outcomes included neurocognitive functioning and eating disorder thoughts and behaviors.
METHODS
Literature search
We performed a comprehensive database search for abstracts published in English from 1960 to March 15, 2015, in Pubmed, PsycINFO, Scopus, and Clinical Trials databases. Search strategies combined controlled vocabulary terms (MeSH, Thesaurus) with keywords and phrases for the following concepts: refeeding, weight restoration, hypophosphatemia, and anorexia nervosa. Exclusion terms were: neoplasm(s) and cancer(s) and tumor(s). References were exported to Endnote (version X7), and duplicates were removed using Author, Year, Title, and Reference Type as the comparison criteria.
Screening for eligibility
Inclusion and exclusion panel are described in Panel 1. Abstracts identified from the initial search were screened in a secondary review process, and abstracts not meeting inclusion criteria were excluded. Abstracts of review papers and case studies were removed from this review but cataloged for further bibliography and referencing. In cases of uncertainty (e.g. the abstract was unavailable or contained insufficient detail to determine if inclusion criteria were met), the full-text of the paper was obtained and screened.
Panel 1: Inclusion and exclusion criteria applied during screening
REQUIRED FOR INCLUSION
Studies of approaches to refeeding in hospitalized adolescents and adults with AN, where:
#1: feeding protocol is defined such that it could be reproduced, by either caloric level or mode of delivery including rate and or concentration.
--AND--
#2: Outcomes are related to short and/or long-term restoration of medical stability (including vital signs, or length of stay as a proxy, and weight recovery) or cognitive improvements (including neurocognitive functioning and/or long-term eating disorder cognitions and behaviors).
EXCLUSION CRITERIA
#1: Studies comparing malnourished to refed patients (therefore the feeding protocol was undefined and varied)
#2: Studies with no medical and/or cognitive outcomes
#3: Studies of refeeding where the protocol was not described*
* In cases where the paper met inclusion criteria with the exception that the refeeding protocol was not described, details of the refeeding protocols were procured by author communication.
Quality assessment
The quality of the selected studies was independently assessed by three authors (AKG, SS, and GR). We used the diagram for flow of information from the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement for systematic reviews as well as the organizational structure from the PRISMA checklist, including a detailed description of the eligibility criteria (Panel 1) and the search and selection criteria for studies (27). The limited designs of the available studies did not allow for a formal quality assessment or statistical methods to evaluate the results (e.g. to compare or evaluate collective effect sizes). The bulk of the evidence on approaches to refeeding in AN utilizes retrospective and/or observational designs, which have no empirically validated method for quality evaluation (28). These study designs are subject to several types of bias, which are described and discussed in the text where relevant.
RESULTS
Study Selection
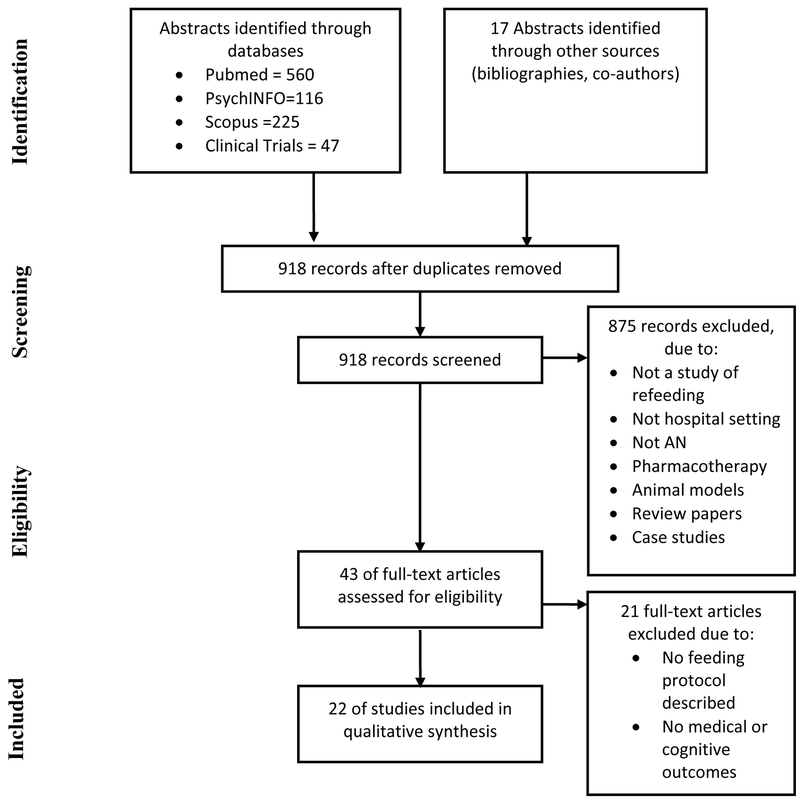
A flow chart depicting the study selection process is shown in Figure 1. The initial search of electronic databases yielded 948 references. An additional 17 abstracts were identified from bibliographies of relevant review papers. After removing 47 duplicates, 918 abstracts were included for screening. Initial screening using the inclusion and exclusion criteria led to the exclusion of 875 abstracts. Studies were most often excluded because: they were not a study of refeeding (e.g. a cross-sectional study comparing malnourished to refed patients with AN), did not take place in a hospital setting, did not include patients with AN, focused on pharmacotherapy, or utilized animal models. This process yielded 43 abstracts. The full texts of these abstracts were obtained and evaluated again according to the inclusion and exclusion criteria. Of these, 21 studies were excluded primarily because no refeeding protocol was described and/or the outcomes were not pertinent to medical or cognitive/behavioral recovery. Six studies (21, 29–33) met eligibility based on initial screening, but details of the refeeding protocols were missing and subsequently obtained via author communication.
Figure 1:
Flow chart of study selection
Quality assessment
More than 80% of the studies meeting our selection criteria were published in the last 10 years, and 90% of the study designs were retrospective and/or observational. The most common types of bias were selection bias and bias due to differential loss to follow-up. Selection bias may have resulted in patients who were perceived as high risk (due to medical complications, severe malnutrition, and/or chronic illness) being assigned to lower calorie refeeding groups (34). Although these studies attempted to address this by enrolling participants on their first hospital admission and adjusting for %mBMI at presentation, this source of bias could result in poor outcomes being overly attributed to the lower calorie refeeding approach. Selection bias could also result in disproportionate phosphate supplementation in patients perceived as high risk (27) and must be considered when interpreting rates of RH as an outcome. Differential loss to follow-up can be a problem in long-term studies with open follow-up, whereby the characteristics of the remaining patient population become skewed as patients are lost to follow-up along the way. This is a recognized problem in studies of long-term outcomes in AN (35). It is unclear whether it affects the one long-term study included here, since the authors report that “all” patients returned for follow-up (36). However, it is a possible source of bias in the two studies (34, 37) using length of stay as an outcome. Length of stay is a proxy for time to restore medical stability, however it is subject to bias because medically complicated patients may remain in hospital longer for a variety of reasons regardless of refeeding approach.
Meal-based refeeding
Eleven studies described meal-based approaches to refeeding where the caloric level was divided into meals and snacks, and any liquid supplements were taken orally; NG feeding was only used for acute food refusal. Eight of these eleven studies reported weight gain as an outcome. The first five studies in this group examined lower calorie diets. Arii et al. (38) compared lower calorie meals to oral liquid formula in 12 adolescents and adults with severe malnutrition and found that the liquid formula group consumed more calories and gained significantly more weight. Solanto et al. (1994) and Garber et al. (2012) (14, 32) reported that adolescents who were refed starting at 1,000–1200 kcal/day and advancing by about 100 kcal/day initially lost weight and then slowly gained at a rate of 0.68–0.88 kg/week. Garber et al. (2012) (14) further demonstrated an association between lower caloric prescription, poorer weight gain, and longer hospital stay. Ornstein et al. (2003) (21) reported a mean hospital stay of more than 25 days in adolescents refed on a similar protocol. While these studies indicate that refeeding according to the current recommendations (10–13) contributes to poor weight gain and long hospital stays in moderately malnourished adolescents, Gaudiani et al. (2012) demonstrated the utility of lower calorie refeeding in restoring medical, biochemical, and hematological stability in severely malnourished patients.
A group of six studies, beginning with Whitelaw et al. (2010) (39), examined higher calorie meal-based approaches to refeeding hospitalized adolescents with AN. These approaches started between 1500–2400 kcal/day and advanced by 67–250 kcal/day. Rates of weight gain were reported in all studies except El Ghoch et al. 2014 (40) and ranged from 1.3–1.98 kg/week. Maximal caloric prescriptions achieved before discharge ranged from 2800–4350 kcal/day. These studies established the feasibility of meal-based refeeding to facilitate weight gain in hospitalized adolescents with AN. Only two studies compared lower versus higher meal-based refeeding protocols (34, 37). In a prospective observational study of 56 adolescents, Garber et al. (2013) (34) reported faster weight gain and a mean 5.7 days shorter hospital stay; in a retrospective study of 310 adolescents, Golden et al. (2013) (37) reported a mean shorter stay of 3.6 days.
Eight studies examined RH as an outcome. Ornstein et al. (2003) (21) were the first to explore the rate of RH with caloric intake. This retrospective chart review found that 27.5% of hospitalized adolescents with AN, who were started on 1200–1400 kcal/day and increased by 200 kcal every 24–48 hours, developed RH requiring phosphate supplementation. This study showed that the most severely malnourished patients were at greater risk for developing hypophosphatemia, which became an important outcome for subsequent studies of refeeding. Golden et al. (2013) (37) observed that 15.8% of adolescents developed RH, with no differences in the rates according to whether they received higher or lower calorie refeeding. Garber et al. (2013) (34) similarly reported no differences in electrolyte abnormalities between patients refed using higher and lower calorie protocols. In that study, 36% of adolescents received electrolyte correction (34), similar to the rate reported by Whitelaw et al. (2010) (39). RH occurred in 18.5% of patients in the study by Redgrave et al. (2015) (31) and was associated with admission BMI rather than the rate of weight gain. In contrast, Le Clerc et al. (2013) reported that only one of thirty patients required electrolyte replacement for low serum phosphorus (41). These highly variable rates of RH likely reflect different strategies for electrolyte correction: some programs initiated prophylactic phosphate supplementation (29, 42–44), others treated low or declining levels (21, 31, 34, 37, 39, 41), one used supplementation at a BMI< 12 kg/m2 (36), and another supplemented patients on NG but not meals-only feeding (45).
Two studies of higher calorie meal-based approaches examined sub-samples of severely malnourished patients. In a study of 461 admissions for refeeding, Redgrave et al. (2015) (31) analyzed 135 adolescents and adults with BMI < 15 kg/m2 refed with meals starting between 1200–1500 kcal, advancing rapidly by 500 kcal every 2–3 days, supported by continuous intravenous (IV) 5% dextrose at 75 ml/hr and close medical monitoring until postprandial blood glucose normalized. Weight gain was comparable to the higher BMI participants, but rates of RH were higher (32.4% vs. 18.5% in the whole sample). Also using a higher calorie refeeding approach with careful medical supervision but without IV dextrose, Golden et al. (2013) (37) compared a sub-sample of severely malnourished adolescents (mBMI 65%) who were fed higher (N=31) vs. lower (N=18) calorie diets. Similar weight gain was reported in both groups. The rate of RH was higher in the severely malnourished subsample than in the total sample but did not differ by caloric prescription.
Only one study (40) reported on psychological outcomes, using a meals-only approach to refeeding. That study assessed 50 adults with chronic AN (mean length of illness of 8.7±6.7 years) and severe malnutrition. The refeeding intervention took place over 20 weeks (13 weeks inpatient and 7 weeks partial hospitalization) and began with 1500 kcal to 2500 kcal/day for three weeks with subsequent dietary intake aimed at achieving a weight gain goal of 0.45–0.68 kg/week. Participants also received intensive psychological treatment and cognitive behavioral therapy for eating disorders. At the mean maximal BMI achieved [19.6(0.8) kg/m2], eating disorder thoughts and behaviors [measured by the Eating Disorder Examination (46)] had improved significantly.
Nasogastric refeeding
Nine studies meeting inclusion criteria described approaches to refeeding using NG feeding in combination with oral feeding. Five of these studies began with a combination of supplemental NG feeding and oral intake upon admission (36, 43, 44, 47, 48); three studies began with NG feeding only and then introduced meals (29, 30, 45); and one study began with meals for 3 days, followed by 3 days of exclusive NG tube feeding, followed by transition back to meals only (49).
NG feeding approaches were used to deliver both lower and higher calorie loads. Four studies reported lower calorie approaches. Gentile et al. (2010; 2012) focused on lower calorie refeeding in severely underweight adolescents and adults with AN, with purposefully low weight gain goals (0.5–1 kg/week) to minimize potential complications in these higher risk patients (43, 44). Robb et al. (2002) and Silber et al. (2004) compared supplemental nocturnal NG feeding in hospitalized adolescents to historical controls fed with meals alone (47, 48). The rate of NG feeding was set to bring the total starting kcal level (including meals) to 1200 kcal/day and was then increased to support a consistent weight gain rate of 1–2 kg/week. With this method, Robb et al. (2002) reported that the total mean calorie intake was greater and weight gain was faster in the supplemental NG group (3255 kcal/day; 1.7 kg/week) compared to those fed meals alone (2508 kcal/day; 0.76kg/week), with no difference in the length of stay among 100 females (48). Silber et al. (2004) confirmed this finding in 14 males (47). The remaining studies reported higher calorie approaches. Hatch et al. (2010) and Madden et al. (2015) (29, 30) started with 24–72 hours of continuous NG feeding that began at 2400 kcal/day and then transitioned to a combination of NG feeding and meals for a total of 2400–3000 kcals/day to achieve a target rate of weight gain of 1 kg/week (author communication) (29, 30). In this group, Hatch et al. (2010) (29) was the only study to examine changes in cognition and eating disorder psychopathology. At the end of the admission (12.7 weeks), participants showed significant improvements in cognitive processing speed in sensorimotor tasks and cognitive inhibition and less distractibility on memory tasks. The study did not report improvement in eating disorder cognitions measured by the Eating Disorder Inventory III or obsessionality as measured by the Maudsley Obsessive Compulsive scale following weight restoration, however there were significant improvements in depression, anxiety, and stress as measured by the Depression, Anxiety and Stress Scale. Agostino et al. (2013) reported a similar approach beginning with a slightly lower caloric level of 1500 or 1800 kcal/day, depending on age. Weight gain was greater in the group that received continuous NG feeding for seven days and then transitioned to include meals, as compared to historical controls who received only meals (45).
The only RCT comparing different approaches to refeeding is from Rigaud et al. (2007) (36), who compared 70 days of NG feeding twice daily plus meals to meals alone in chronically ill young adults with a duration of illness of 3–4 years, at least one prior hospitalization, and BMI greater than 11 kg/m2 (36). The groups were separated in time, so that patients on the unit all received the same treatment; patients randomized to a treatment that was not active at the time of randomization waited up to three months for admission. Calorie levels in both groups were adjusted based on resting energy expenditure (REE) to produce a 1 kg/week weight gain. Patients were not restricted to bed, and there was little monitoring of activity levels during treatment. Consistent with the other studies described here (45, 47, 48), the NG group consumed more calories and gained more weight. Assessment of tolerability of the NG feeds indicated that they caused the most emotional distress during the early phase of refeeding. Patients required an average of 2 tube changes over the 70-day course of treatment, but no serious physical complications were reported. There was no difference in weight and eating disorder psychopathology at one-year follow-up, however relapse was delayed by seven weeks in the NG-fed group.
Parenteral refeeding
Only one study that met our inclusion criteria examined total parenteral nutrition (TPN). Diamanti et al. (2008) reported that TPN in combination with oral feeding resulted in a greater rate of weight gain than oral feeding alone (50). These authors recommended that the rate of initial parenteral refeeding be limited to 600–800 kcal per day and the amount of protein be limited to 2 g per kg of body weight to avoid development of refeeding syndrome. Weekly weight gain rates were low in both groups (<1 kg/week), and TPN treated patients gained on average only 183 g/week faster than those fed orally. Various complications of parenteral nutrition were reported, including elevated transaminases, lower extremity edema, and hypophosphatemia.
Refeeding with altered nutrient content
Only one study described a refeeding protocol with a nutrient content that differed significantly from current dietary guidelines [such as the 2010 US Dietary Guidelines for Americans (51) and the 2006 Nutrient Reference Values in Australia and New Zealand (52). Rigaud et al. (2010) compared a low-sodium (1600–2000 mg) to a normal-sodium diet (4000–4800 mg) in a non-randomized study among severely malnourished adults with AN (53). Weight gain and peripheral edema were greater on the normal- compared to low-sodium diet, suggesting that reducing the sodium content of the refeeding diet may be useful in managing fluid shifts, especially in adults with a BMI < 15 kg/m2. We re-examined the studies included in the meal-based group (above) to determine if any conclusions could be drawn about the effect of nutrient content on refeeding outcomes. Six of the eleven studies reported nutrient content of the refeeding diet (14, 34, 37, 39–41); the nutrient information for an additional four studies was obtained by author communication (21, 31–33). Of these 10 studies, the macronutrient distribution of the diets was consistent with currently recommended ranges (around 25–35% of calories from fat, 15–20% protein and 50–60% carbohydrate). Therefore no associations between differing nutrient content and refeeding outcome could be determined. Finally, some refeeding reported using IV glucose (31, 43, 44) due to concerns of post-prandial hypoglycemia and the risk for RH during the initial phase of refeeding (9, 54). Gentile et al. (2012) described a protocol for refeeding severely malnourished adults (mean BMI 11.3 kg/m2) that initially supplemented oral refeeding with 10% glucose intravenously; Redgrave et al. (2015) used a similar approach, with 5% dextrose in severely malnourished patients (31).
DISCUSSION
For years, a lower calorie approach to refeeding hospitalized patients with AN has been recommended, beginning around 1200 kcal/day (10–13) or lower (13) and advancing slowly. The purpose of these conservative approaches was to minimize risk for the development of the refeeding syndrome. In this respect, it could be argued that these approaches have been successful, as only a few cases of the refeeding syndrome have been reported during the decades that lower calorie approaches have been the standard of care for refeeding in AN (5, 55, 56). However, lower calorie refeeding has also been linked to poor weight gain (14, 32, 34, 37) and prolonged hospital stay (34, 37). Increasing recognition that underfeeding leads to poor outcomes in AN (8) is contributing to a shift in clinical practice and research toward higher calorie refeeding. These changes are reflected in the results of the present systematic review. Since 2010, ten studies have reported approaches to refeeding beginning with 1400 kcal/day or more through meals alone (31, 34, 37, 39–41) or combined NG and oral feeding (29, 30, 45). Only two recent studies reported using lower calorie approaches, both in higher-risk (severely malnourished) patients (33, 44).
We also found that there is marked heterogeneity in approaches to higher calorie refeeding, with large variations in starting calorie levels, rates of advancement, and modes of delivery. The outcomes of interest were likely influenced by these differences, in addition to the effects of variability in length of stay, electrolyte correction, type of program (medical or psychiatric), and level and duration of psychotherapy provided. For example, the two available studies comparing groups on lower and higher meal-based refeeding come from comparable adolescent medical in-patient units. However, the feeding protocols differed by both starting calories and rates of advancement, which may explain why one study reported a faster rate of weight gain in the higher calorie group (34), and the other did not (37). For these reasons, we did not attempt to quantify the relationship between calories and weight gain across studies statistically. Nevertheless, our systematic review supports the following evidence-based conclusions:
In moderately malnourished patients with AN, lower calorie refeeding is too conservative.
The large proportion of adolescents hospitalized in medical stabilization units across the United States (57), Canada (41), Australia (30, 39), and the United Kingdom (58) is moderately malnourished (mBMI 75–85%), with relatively acute onset of AN. Studies have linked lower calorie refeeding to poor outcomes in this patient population, including poor weight gain and prolonged hospital stay. Subsequent studies of higher calorie refeeding in moderately malnourished adolescents, using either meals-only (31, 34, 37, 39, 41) or NG feeding in combination with meals (29, 30, 45), report similarly good weight gain with wide variability in RH (see #4 below for a discussion of safety).
Meal-based approaches and combined approaches using NG feeding with meals can be used for higher calorie refeeding in hospitalized patients with AN.
There is insufficient evidence to determine whether one of these approaches confers any advantage over the other. This is largely due to limitations in study design: studies comparing supplemental enteral feeding to a meals-only approach were not designed to determine whether there was an independent effect of the method of delivery. Instead, these studies collectively demonstrate that supplemental NG feeding is useful for increasing the total caloric load (29, 30, 47, 48). However, equally good weight gain has been reported using meals-only approaches (31, 34, 37, 39–41). Tolerance and/or acceptability could tip the balance in favor of one approach over the other, however this has not been sufficiently examined. One study of higher calorie meal-based feeding reported that patients could complete the higher calorie meals to the same extent as those on the lower calorie meals, and none required NG feeding (34); other studies have reported that 8% (39) and 15% (41) of adolescents on higher calorie meal-based refeeding required supplemental NG feeding to meet caloric prescriptions. In a study of supplemental NG feeding, patients reported that it was acceptable when presented in the context of a standardized protocol that is uniformly applied within a specialty program for the treatment of AN (36). This is consistent with studies beyond this review (59).
In severely malnourished patients with AN (BMI <15 kg/m2 in adults or mBMI <70% in adolescents), there is insufficient evidence to support changing the current standard of care for refeeding.
Greater caution continues to be used with more severely malnourished, chronically ill, and/or adult patients, who, due to more severe malnutrition, are at greater risk for refeeding syndrome (20). The few recent studies that have explicitly focused on this patient population have used lower calorie approaches with slow advancement under medical monitoring (43, 44). Three studies also administered intravenous glucose to mitigate risks of post-prandial hypoglycemia (31, 43, 44). There is currently insufficient evidence to indicate whether such patients might tolerate more aggressive approaches (31, 37, 40). Two studies (31, 37) of higher calorie meal-based approaches examined sub-samples of severely malnourished patients and reported comparable weight gain and RH that was manageable with medical monitoring. Further studies of approach to refeeding in this higher-risk patient population are needed.
Higher calorie approaches to refeeding in hospitalized patients with AN appear safe in the presence of medical monitoring and electrolyte correction.
No cases of refeeding syndrome were reported within the studies of higher calorie refeeding. Variable rates of RH, a sensitive marker of risk for the refeeding syndrome (19), were reported without incident. However, these aspects have not been compared within a controlled trial, and there are several limitations to the available evidence that preclude a comparison of safety across approaches to refeeding. First, sample sizes have been generally small. A very large, multicenter trial would be required to comprehensively examine the full range of clinical features associated with the refeeding syndrome using various refeeding approaches, since only a handful of cases of cardiac arrest and death have been reported in the AN literature (5, 55, 56). Second, while patients with severe malnutrition are at highest risk (20) they have not been well represented in the available studies. Third, the incidence of RH may in fact be underrepresented by the current studies. This is due in part to the variety of approaches to electrolyte correction that are currently used in clinical practice (22). RH has been indicated by the lowest serum phosphorus level during hospitalization, and it is possible that this measure was obtained in participants who had already begun receiving supplements (and therefore an actual nadir was never reached). Selection bias could also contribute to the underestimation of RH if more severely malnourished participants (39) and/or those on higher calories were more likely to receive phosphate supplementation (34). Fourth, the relative safety of differing methods of delivery has not been compared. The hypothesis that NG feeding ameliorates RH (29, 30, 60) warrants examination. These limitations, together with the gravity of the risk of the refeeding syndrome, underscore the need for close medical monitoring when higher calorie approaches are undertaken. Prospective studies using well-described protocols (for refeeding and electrolyte replacement as well as admission and discharge criteria) are needed.
The impact of approaches to refeeding on long-term outcomes is unknown.
Rapid and early weight gain in hospital (1–3) and during outpatient psychotherapy (24) improves long-term recovery in AN. These findings imply that higher calorie refeeding would improve long-term weight recovery. However there is currently no evidence to support this. The only study comparing long-term outcomes of refeeding approaches was an RCT reporting no difference in one-year weight recovery in a combined enteral and meal feeding group as compared to a meals-only group (36). While this study reported earlier relapse in the meals-only group, more calories were delivered in the enteral group. Thus the only conclusion that can be drawn is that higher calorie refeeding may reduce long-term relapse rates. Tempering the strength of this RCT slightly is the open follow-up design (discussed earlier) and the ‘wait-list’ randomization scheme, whereby one group of patients may have become sicker during the three-month period while awaiting treatment. Nevertheless, recovery from AN is plagued by high relapse rates (61), and therefore the possibility that refeeding in hospital could improve long-term outcomes such as relapse warrants investigation.
There is little and inconsistent evidence to indicate that the approach to refeeding in hospital improves long-term cognitive and behavioral recovery. Hatch et al. (2015) reported no reduction of eating disorder thoughts and behaviors in acutely malnourished adolescents after 12 weeks of higher calorie combined NG feeding plus meals (29). In contrast, El Ghoch et al. (2014) reported significant improvements in eating disorder and general psychopathology after 20 weeks of higher calorie refeeding in severely and chronically malnourished adults. Differing lengths of follow-up (12 vs. 20 weeks) and variable types and intensity of psychotherapeutic interventions might explain these discrepant findings (40). Nevertheless, both of these studies demonstrate that refeeding in hospital impacts cognitive recovery. There is a pressing need to examine how refeeding that takes place over a relatively short-period can support the long-term goals of recovery, since weight recovery in the absence of concomitant behavioral recovery does not bode well for complete or sustained recovery and renders patients vulnerable to relapse (62).
TPN is not recommended unless no other form of refeeding is possible.
Given the extent of weight gain that can be achieved by other methods, the small incremental increase in weight gain reportedly attributable to supplemental TPN (50) does not outweigh the potential risks. TPN is associated with high rates of complications and requires more intensive medical monitoring, which adds to its expense. In a large nation-wide database study from Japan (which was beyond the scope of this review because it included diagnoses other than AN), Michihata et al. (63) compared TPN alone (N=278) to NG feeding (N=634). They found significantly higher rates of sepsis, disseminated intravascular coagulation, and death in the TPN group (63). Also beyond the scope of this systematic review are case reports of both successful weight restoration (64) as well as deaths from TPN refeeding in AN (56). Given the medical risks, further research is not indicated around this topic. The only indication for administering TPN in AN is when there are no alternatives for achieving weight restoration, such as in cases where gastrointestinal complications preclude enteral refeeding (e.g. superior mesenteric artery syndrome, following gastroenterological surgery, or in the setting of severe recurrent vomiting when nasojejunal feeding has also proven unsuccessful).
Meals and liquid formulas with nutrient compositions within recommended ranges are appropriate for refeeding.
The macronutrient composition of the diets used in the studies included here fell within range of the standard recommendations for the general population (approximately 25–35% of calories from fat, 15–20% protein and 50–60% carbohydrate) (51, 52, 65). In theory, lowering the glucose load of the diet or formula could attenuate risk for the refeeding syndrome (60). This may be accomplished by changing the nutrient content of the diets, such as lower carbohydrate or lower glycemic load meals or liquids. This has also been postulated as a benefit of NG feeding (29, 30, 60), since delivering a formula at a lower, continuous rate could avoid the wide glucose and insulin excursions associated with bolus-feeds. Another strategy employed to ameliorate the post-prandial hypoglycemia associated with risk for the refeeding syndrome is to continuously infuse intravenous glucose during the initial phase of refeeding (31, 43, 44). The only study that focused on micronutrient content was Rigaud et al. (2010) (53), which indicated that a lower sodium diet reduces fluid shifts during refeeding in severely malnourished patients. Beyond this review, Schebendach and colleagues reported that dietary variety and energy density may predict recovery outcomes in AN (66, 67). Further study is needed to build on these lines of evidence and determine if there is an optimal nutrient profile for refeeding in AN.
Conclusion
This systematic review of approaches to refeeding reflects a recent shift in clinical practice from previously conservative approaches to higher calorie and/or faster approaches to increasing calories in hospitalized patients with AN. While lower calorie approaches with slow advancement may have a role in severely malnourished and more chronically ill patients, higher calorie approaches are feasible for the larger proportion of moderately malnourished and acutely ill patients with AN. Meal based approaches and combined approaches using NG feeding to supplement oral intake can achieve similar weight gain. Other than the possible advantage of lower sodium diets in severely malnourished patients, we can only conclude that higher calories can be delivered in macronutrient ranges consistent with the current dietary recommendations. The many research gaps described above highlight the need for prospective studies to directly compare different approaches to refeeding. Such studies should examine both short- and long-term outcomes, since any benefit of higher calorie refeeding, including faster weight gain or shorter hospital stay, could be easily outweighed by unintended consequences, such as higher rates of relapse.
Table 1.
Summary of in-hospital refeeding studies, by refeeding approach
| Reference | Study design | Na | Ageb | Admission BMIc (SD/SE) |
Approach | Start Kcal/day |
Caloric Advancement (Kcal/day)d |
Weight Gain Rate (Kg/wk)e |
IP LOSf, days (SD/SE) |
Rate of RHg |
|---|---|---|---|---|---|---|---|---|---|---|
| Meal-based Refeeding | ||||||||||
| Solanto et al. 1994 | Observational | 22 L 31 H |
16.0 L 16.2 H |
15.0 (1.9) Ah 14.3 (1.6) B |
Meals; behavioral contracts distinguish groups | 1000-1200 | 67-100 (200 q 2-3 days) |
0.64 (0.32) A 1.15 (0.48) B |
≥28 days | NRi |
| Arii et al. 1996 | Prospective | 12 | 19.2 | 13.61 | Meals for 1 wk, then one group to oral liquid only for 2-6 wks, then back to meals | 1000 | Variablej | NR | NR | NR |
| Ornstein et al. 2003 | Retrospective | 69 | 15.5 | 15.0 (1.5)k | Meals | 1200-1400 | 100-200 (200 q 1-2 days) |
NR | 25.6 (11.6) | 27.5% |
| Whitelaw et al. 2010 | Retrospective | 29 | 15.7 | NRl | Target 2200 by day 3, 2700 by day 5 | 1900 | 100-300 | 1.3m | NR | 38% |
| Garber et al. 2012 | Observational | 35 | 16.2 | 16.3 (2.3)n | Meals | 1205 | 100 | 0.88 | 16.7 (16.4) | 20% |
| Gaudiani et al. 2012 | Retrospective | 25 | 26.0 | 13.1o | Target 2000 kcal/day | 990 | NR | NR | 19 | 45% |
| Garber et al. 2013 | Observational | 56 | 16.2 | 15.8 (0.5) Lp 16.6 (0.4) Hq |
Meals | 1093 L 1764 H |
98(6) L 122(8) H |
0.98 L 1.90 H |
17.6 (1.2) L 11.9 (1.0) H |
45% |
| Golden et al. 2013 | Retrospective | 310 | 16.1 | 15.9 (2.2) L 16.1 (1.7) Hr |
Meals | 1163 L 1557 H |
100-200 | 1.52 L 1.56 H |
16.6 (9.0) L 13.0 (7.3) H |
18.2% L 14.9% H |
| Le Clerc et al. 2013 | Retrospective | 29 | 14.7 | 16.4 (1.7)s | Target weight gain 1 kg/week | 1500 | 75-250 (250 q 2 days) |
1.7 | NR | 3.5% |
| El Ghoch et al. 2014 | Prospective | 50 | 26.5 | 15.4 (1.6) | Target weight gain 1-1.5kg/week | 1500 | NR | NRt | 91u | NR |
| Redgrave et al. 2015 | Observational | 361 | 28.5 | 16.2 (2.2) | Target 3500-4000kcals/day with IV glucose in BMI<14 |
1200-1500 | 250 (500 q 2-3 days) |
1.98 (0.86) | 27.68 (18.14) | 18.5% |
| Combined nasogastric feeding with oral intake | ||||||||||
| Bufano et al. 1990 | Retrospective | 9 | NR | NRv | Mean maximum of 2311 kcals/day | NG at 25% of REEw | 25% of target kcals on Day 1, 50% on Day 2, 75% on Day 3 | 1.92 | 9-51x | 33.3% |
| Robb et al. 2002 | Retrospective | 52 NG+Oy 48 O |
14.8 NG+O 15.0 O |
15.5 (1.7) NG+O 16.0 (1.8) O |
Compare nocturnal NG + oral to oral; mean maximum kcals: 3255 (NG+O), 2508 (O) | NR | 600, 1080, 1200 on first three days (NG), oral kcals NR | 1.70 NG+O 0.76 O |
22.3 NG+O 22.1 O |
NR |
| Silber et al. 2004 | Retrospective | 6 NG+O 8 O |
13.8 NG+O 14.9 O |
15.3 (1.7) NG+O 17.4 (2.3) O |
Compare nocturnal NG + oral to oral; maximum kcals were 4350 (NG+O) and 3400 (O) | NR | 600, 1080, 1200 on first three days (NG), oral kcals NR | 2.12 NG+O 0.53 O |
36.0 NG+O 39.9 O |
NRz |
| Riguad et al. 2007 | RCT | 41 NG+O 40 O |
22.5 NG+O 24.2 O |
12.1 (1.5) NG+O 12.8 (2.0) O |
Compare 2-mo NG to NG + oral; target weight gain rate 1 kg/week | Based on REEaa | Variable, to target weight gain rate | 1.36 NG+O 0.88 O |
70 days | 0%bb |
| Gentile et al. 2010 | Retrospective | 33 | 22.8 | 11.3 (0.7) | 30 with NG+O; 3 with oral supplements and IV glucose | 1402 | 1895 by Day 7; 2212 by Day 15; 2510 by Day 30; 2683 by Day 45; 3000 by Day 60 | 0.63 | 60 | 0%cc |
| Hatch et al. 2010 | Prospective (test-retest) | 37 | 15.1 | 16.1 (0.93)dd | 24-72 hr continuous NG only, then nighttime NG + meals to target weight gain rate 1 kg/week | 24-72 hr NG (100 ml/hr), then NG + 1200-1400 meals | Variable, to attain 2400-3000 kcal/day | NR | 2.5 wks | NR |
| Gentile et al. 2012 | Retrospective | 10 | 23.9 | 11.2 (0.7) | Concentrated continuous NG feed with IV glucose for 24 hr and oral intake, started at 1199 kcal increase to 2871 by day 90 | 1199 | 2508 by day 15; 2784 by day 30; 2935 by day 60; 2871 by day 90 | 1.30 | 90 | 0%ee |
| Agostino et al. 2013 | Retrospective | 31 NG+O 134 O |
14.9 | 16.6 (2.2) NG+O 16.7 (2.3) O |
Compare continuous NG transitioning to oral alone to oral alone (historical controls) | 1500-1800 NG+O 1000-1200 O |
200 | 0.66 L 0.92 H |
50.9 (24) L 33.8 (11) H |
3.6%ff |
| Madden et al. 2015 | Prospective cohort |
78 | 14.8 | 24-72 hr continuous NG only, then nighttime NG + meals to target 2400-3000 kcals/day | 72 hr NG (100 ml/hr), then NG + 1200-1400 meals | Variable, to attain 2400-3000 kcal/day | 2.79 (wk 1) 1.34 (over 2.5 wk) |
28.6 | 0%gg | |
| Combined total parenteral nutrition with oral intake | ||||||||||
| Diamanti et al. 2008 | Retrospective | 104 PNhh+O 94 O |
14.9 PN+O 15.2 O |
14.3 (0.2) PN+O 16.0 (0.2) O |
Compare oral to oral+TPN; both groups start 40 kcal/kg/d for 5 d then increase to 60 kcal/kg/d over 1 week | 40 kcal/kg/day | Target 60 kcal/kg/day at day 7 | 0.71 PN+O 0.53 O |
30.7 (2.2) PN+O 15.6 (1.0) O |
3.8% (PN+O only) |
| Altered nutrient content | ||||||||||
| Rigaud et al. 2010 | Retrospective | 42 Nii 176 L |
22.1 N 23.3 L |
13.8 (1.7) N 13.2 (1.2) L |
Compare normal (4000-4800mg) vs. low (1600-2000 mg) Na diet. Both groups got NG+O feeding. | Ad lib except for Na | Ad lib except for Na | 1.09 L; N was NR | 2 months | NR |
For studies that included comparison groups, we report Ns separately for low-weight-gain (L) and high-weight-gain (H) interventions.
Age is presented as mean
BMI=body mass index (kg/m2).
Rates of caloric advancement are calculated for some programs, who may not advance calories every day.
In some cases these rates are calculated rather than derived directly from the published reports, and not all patients stayed at least a week in all studies.
IP LOS=inpatient length of stay.
RH=refeeding hypophosphatemia.
A and B refer to differing behavioral contracts (all participants started on same range of calories).
NR=not reported.
Per text, “daily calorie intake was increased only following agreement between the therapist and the patient.” (p 56)
Mean (SD) percent of ideal body weight was 72.7% (7.1).
Mean (SD) percent of ideal body weight was 72.9% (9.1).
Over the first two weeks of admission.
Mean (SD) percent median BMI was 80.1 (11.5).
Mean (SD) percent ideal body weight was 62% (10).
For studies that included comparison groups, Ns are reported separately for low-calorie (L) and high-calorie (H) interventions.
Mean (SD) percent median BMI was 77.6 (2.6) in the lower calorie group, 81.9 (1.8) in the higher calorie group.
Mean (SD) percent median BMI was 77.9 (9.6) in the lower calorie group, 78.7 (7.8) in the higher calorie group.
Mean (SD) percent ideal body weight was 75.8% (5.4).
Weight gain goal was 0.45-0.68 kg/wk
Program included 13 weeks inpatient treatment plus 7 weeks partial hospitalization.
Mean (SD) body weight was 34.51 (4.48) kg.
REE=resting energy expenditure.
Patients were discharged from the program to outpatient follow-up when they requested to switch back from enteral to meal-based refeeding and could demonstrate they had “food intake exceeding the basal metabolic rate” (p 405).
NG=nasogastric refeeding; O=oral refeeding.
“There were no cases of refeeding syndrome.” (p 417)
REE=resting energy expenditure.
Patients with BMI < 12 received prophylactic phosphorous supplementation.
Most patients received prophylactic phosphorous supplementation.
Mean (SD) percent ideal body weight was 79.1% (5.8).
Patients received prophylactic phosphorous supplementation.
All 6 patients with hypophosphatemia were in the oral-only group; however, 90.3% of patients in the enteral feeding group were on prophylactic phosphorous supplementation.
Patients received prophylactic phosphorous supplementation.
PN=parenteral nutrition; O=oral.
N=Normal sodium diet; L=low sodium diet.
Acknowledgements
We would like to thank Victoria G. Riese, MLIS, AHIP, Clinical Informationist at the Welch Medical Library of Johns Hopkins Medical Institutions for executing the search strategy.
Footnotes
Disclosure of Conflicts
The authors have no conflicts of interest, financial or otherwise, to disclose.
Contributor Information
Andrea K. Garber, Division of Adolescent & Young Adult Medicine, University of California, San Francisco Benioff Children’s Hospital.
Susan M Sawyer, Centre for Adolescent Health, Royal Children’s Hospital; , Department of Paediatrics, The University of Melbourne, Faculty of Medicine, Dentistry, Health Sciences, The University of Melbourne, and Murdoch Childrens Research Institute.
Neville H. Golden, Division of Adolescent Medicine The Marron and Mary Elizabeth Kendrick; Stanford University.
Angela S. Guarda, Johns Hopkins School of Medicine; Johns Hopkins Eating Disorders Program The Johns Hopkins Hospital.
Debra K. Katzman, Division of Adolescent Medicine, Department of Pediatrics The Hospital for Sick Children and University of Toronto.
Michael R Kohn, Adolescent Medicine, Sydney Children’s Hospital Network, Westmead; The University of Sydney.
Daniel Le Grange, Eating Disorders Program Departments of Psychiatry and Pediatrics University of California, San Francisco.
Sloane Madden, Eating Disorder Coordinator Sydney Children’s Hospital Network.
Melissa Whitelaw, Department of Nutrition and Food Services Centre for Adolescent Health The Royal Children’s Hospital Melbourne.
Graham W. Redgrave, Johns Hopkins School of Medicine, Johns Hopkins Eating Disorders Program Department of Psychiatry and Behavioral Sciences Johns Hopkins University School of Medicine.
References
- 1.Lock J, Litt I. What predicts maintenance of weight for adolescents medically hospitalized for anorexia nervosa? Eat Disord. 2003;11(1):1–7. [DOI] [PubMed] [Google Scholar]
- 2.Lund BC, Hernandez ER, Yates WR, Mitchell JR, McKee PA, Johnson CL. Rate of inpatient weight restoration predicts outcome in anorexia nervosa. The International journal of eating disorders. 2009;42(4):301–5. [DOI] [PubMed] [Google Scholar]
- 3.Baran SA, Weltzin TE, Kaye WH. Low discharge weight and outcome in anorexia nervosa. Am J Psychiatry. 1995;152(7):1070–2. [DOI] [PubMed] [Google Scholar]
- 4.Fisher M, Simpser E, Schneider M. Hypophosphatemia secondary to oral refeeding in anorexia nervosa. The International journal of eating disorders. 2000;28(2):181–7. [DOI] [PubMed] [Google Scholar]
- 5.Kohn MR, Golden NH, Shenker IR. Cardiac arrest and delirium: presentations of the refeeding syndrome in severely malnourished adolescents with anorexia nervosa. J Adolesc Health. 1998;22(3):239–43. [DOI] [PubMed] [Google Scholar]
- 6.Beumont PJ, Large M. Hypophosphataemia, delirium and cardiac arrhythmia in anorexia nervosa. Med J Aust. 1991;155(8):519–22. [DOI] [PubMed] [Google Scholar]
- 7.Hall DE, Kahan B, Snitzer J. Delirium associated with hypophosphatemia in a patient with anorexia nervosa. J Adolesc Health. 1994;15(2):176–8. [DOI] [PubMed] [Google Scholar]
- 8.Miller SJ. Death resulting from overzealous total parenteral nutrition: the refeeding syndrome revisited. Nutrition in clinical practice : official publication of the American Society for Parenteral and Enteral Nutrition. 2008;23(2):166–71. [DOI] [PubMed] [Google Scholar]
- 9.O’Connor G, Goldin J. The refeeding syndrome and glucose load. The International journal of eating disorders. 2011;44(2):182–5. [DOI] [PubMed] [Google Scholar]
- 10.Practice guideline for the treatment of patients with eating disorders (revision). American Psychiatric Association Work Group on Eating Disorders. Am J Psychiatry. 2000;157(1 Suppl):1–39. [PubMed] [Google Scholar]
- 11.Treatment of patients with eating disorders, third edition. American Psychiatric Association. Am J Psychiatry. 2006;163(7 Suppl):4–54. [PubMed] [Google Scholar]
- 12.Position of the American Dietetic Association: Nutrition intervention in the treatment of anorexia nervosa, bulimia nervosa, and other eating disorders. J Am Diet Assoc 2006;106(12):2073–82. [DOI] [PubMed] [Google Scholar]
- 13.NICE. Eating Disorders: Core interventions in the treatment and management of anorexia nervosa, bulimia nervosa, and related eating disorders 2004. [cited 2012 Sep 12]. Available from: http://www.nice.org.uk/nicemedia/pdf/CG9FullGuideline.pdf. [PubMed]
- 14.Garber AK, Michihata N, Hetnal K, Shafer MA, Moscicki AB. A prospective examination of weight gain in hospitalized adolescents with anorexia nervosa on a recommended refeeding protocol. The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 2012;50(1):24–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hay P, Chinn D, Forbes D, Madden S, Newton R, Sugenor L, et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for the treatment of eating disorders. Australian and New Zealand Journal of Psychiatry. 2014;48(11):977–1008. [DOI] [PubMed] [Google Scholar]
- 16.Society for Adolescent H, Medicine, Golden NH, Katzman DK, Sawyer SM, Ornstein RM, et al. Position Paper of the Society for Adolescent Health and Medicine: medical management of restrictive eating disorders in adolescents and young adults. J Adolesc Health. 2015;56(1):121–5. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. Clinical Growth Charts. 2000. [cited 2015 Jan 20]. Available from: http://www.cdc.gov/growthcharts/clinical_charts.htm.
- 18.Shamim T, Golden NH, Arden M, Filiberto L, Shenker IR. Resolution of vital sign instability: an objective measure of medical stability in anorexia nervosa. J Adolesc Health. 2003;32(1):73–7. [DOI] [PubMed] [Google Scholar]
- 19.Khan LU, Ahmed J, Khan S, Macfie J. Refeeding syndrome: a literature review. Gastroenterol Res Pract 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Refeeding hypophosphatemia in hospitalized adolescents with anorexia nervosa: a position statement of the society for adolescent health and medicine. J Adolesc Health. 2014;55(3):455–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ornstein RM, Golden NH, Jacobson MS, Shenker IR. Hypophosphatemia during nutritional rehabilitation in anorexia nervosa: implications for refeeding and monitoring. The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 2003;32(1):83–8. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz BI, Mansbach JM, Marion JG, Katzman DK, Forman SF. Variations in admission practices for adolescents with anorexia nervosa: a North American sample. The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 2008;43(5):425–31. [DOI] [PubMed] [Google Scholar]
- 23.Lock J, Agras WS, Le Grange D, Couturier J, Safer D, Bryson SW. Do end of treatment assessments predict outcome at follow-up in eating disorders? Int J Eat Disord. 2013;46(8):771–8. [DOI] [PubMed] [Google Scholar]
- 24.Le Grange D, Accurso EC, Lock J, Agras S, Bryson SW. Early weight gain predicts outcome in two treatments for adolescent anorexia nervosa. Int J Eat Disord. 2014;47(2):124–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Golden NH, Jacobson MS, Schebendach J, Solanto MV, Hertz SM, Shenker IR. Resumption of menses in anorexia nervosa. Archives of pediatrics & adolescent medicine. 1997;151(1):16–21. [DOI] [PubMed] [Google Scholar]
- 26.Faust JP, Goldschmidt AB, Anderson KE, Glunz C, Brown M, Loeb KL, et al. Resumption of menses in anorexia nervosa during a course of family-based treatment. Journal of eating disorders. 2013;1:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Journal of clinical epidemiology. 2009;62(10):1006–12. [DOI] [PubMed] [Google Scholar]
- 28.Russell R, Chung M, Balk EM, Atkinson S, Giovannucci EL, Ip S, et al. Issues and Challenges in Conducting Systematic Reviews to Support Development of Nutrient Reference Values: Workshop Summary: Nutrition Research Series, Vol 2 AHRQ Technical Reviews; Rockville (MD)2009. [PubMed] [Google Scholar]
- 29.Hatch A, Madden S, Kohn MR, Clarke S, Touyz S, Gordon E, et al. In first presentation adolescent anorexia nervosa, do cognitive markers of underweight status change with weight gain following a refeeding intervention? The International journal of eating disorders. 2010;43(4):295–306. [DOI] [PubMed] [Google Scholar]
- 30.Madden S, Miskovic-Wheatley J, Clarke S, Touyz S, Hay P, Kohn MR. Outcomes of a rapid refeeding protocol in Adolescent Anorexia Nervosa. J Eat Disord. 2015;3:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Redgrave GW, Coughlin JW, Schreyer CC, Martin LM, Leonpacher AK, Seide M, et al. Refeeding and weight restoration outcomes in anorexia nervosa: Challenging current guidelines. The International journal of eating disorders. 2015. [DOI] [PubMed] [Google Scholar]
- 32.Solanto MV, Jacobson MS, Heller L, Golden NH, Hertz S. Rate of weight gain of inpatients with anorexia nervosa under two behavioral contracts. Pediatrics. 1994;93(6 Pt 1):989–91. [PubMed] [Google Scholar]
- 33.Gaudiani JL, Sabel AL, Mascolo M, Mehler PS. Severe anorexia nervosa: outcomes from a medical stabilization unit. The International journal of eating disorders. 2012;45(1):85–92. [DOI] [PubMed] [Google Scholar]
- 34.Garber AK, Mauldin K, Michihata N, Buckelew SM, Shafer MA, Moscicki AB. Higher calorie diets increase rate of weight gain and shorten hospital stay in hospitalized adolescents with anorexia nervosa. The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 2013;53(5):579–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Le Grange D, Lock J, Accurso EC, Agras WS, Darcy A, Forsberg S, et al. Relapse from remission at two- to four-year follow-up in two treatments for adolescent anorexia nervosa. J Am Acad Child Adolesc Psychiatry. 2014;53(11):1162–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rigaud D, Brondel L, Poupard AT, Talonneau I, Brun JM. A randomized trial on the efficacy of a 2-month tube feeding regimen in anorexia nervosa: A 1-year follow-up study. Clinical nutrition (Edinburgh, Scotland). 2007;26(4):421–9. [DOI] [PubMed] [Google Scholar]
- 37.Golden NH, Keane-Miller C, Sainani KL, Kapphahn CJ. Higher caloric intake in hospitalized adolescents with anorexia nervosa is associated with reduced length of stay and no increased rate of refeeding syndrome. The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 2013;53(5):573–8. [DOI] [PubMed] [Google Scholar]
- 38.Arii I, Yamashita T, Kinoshita M, Shimizu H, Nakamura M, Nakajima T. Treatment for inpatients with anorexia nervosa: comparison of liquid formula with regular meals for improvement from emaciation. Psychiatry and clinical neurosciences. 1996;50(2):55–9. [DOI] [PubMed] [Google Scholar]
- 39.Whitelaw M, Gilbertson H, Lam PY, Sawyer SM. Does aggressive refeeding in hospitalized adolescents with anorexia nervosa result in increased hypophosphatemia? The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 2010;46(6):577–82. [DOI] [PubMed] [Google Scholar]
- 40.El Ghoch M, Milanese C, Calugi S, Pellegrini M, Battistini NC, Dalle Grave R. Body composition, eating disorder psychopathology, and psychological distress in anorexia nervosa: a longitudinal study. The American journal of clinical nutrition. 2014;99(4):771–8. [DOI] [PubMed] [Google Scholar]
- 41.Leclerc A, Turrini T, Sherwood K, Katzman DK. Evaluation of a nutrition rehabilitation protocol in hospitalized adolescents with restrictive eating disorders. The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 2013;53(5):585–9. [DOI] [PubMed] [Google Scholar]
- 42.Madden S, Miskovic-Wheatley J, Wallis A, Kohn M, Lock J, Le Grange D, et al. A randomized controlled trial of in-patient treatment for anorexia nervosa in medically unstable adolescents. Psychological medicine. 2014:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gentile MG, Pastorelli P, Ciceri R, Manna GM, Collimedaglia S. Specialized refeeding treatment for anorexia nervosa patients suffering from extreme undernutrition. Clinical nutrition (Edinburgh, Scotland). 2010;29(5):627–32. [DOI] [PubMed] [Google Scholar]
- 44.Gentile MG. Enteral nutrition for feeding severely underfed patients with anorexia nervosa. Nutrients. 2012;4(9):1293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Agostino H, Erdstein J, Di Meglio G. Shifting paradigms: continuous nasogastric feeding with high caloric intakes in anorexia nervosa. The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 2013;53(5):590–4. [DOI] [PubMed] [Google Scholar]
- 46.Cooper Z, Cooper PJ, Fairburn CG. The validity of the eating disorder examination and its subscales. Br J Psychiatry. 1989;154:807–12. [DOI] [PubMed] [Google Scholar]
- 47.Silber TJ, Robb AS, Orrell-Valente JK, Ellis N, Valadez-Meltzer A, Dadson MJ. Nocturnal nasogastric refeeding for hospitalized adolescent boys with anorexia nervosa. Journal of developmental and behavioral pediatrics : JDBP. 2004;25(6):415–8. [DOI] [PubMed] [Google Scholar]
- 48.Robb AS, Silber TJ, Orrell-Valente JK, Valadez-Meltzer A, Ellis N, Dadson MJ, et al. Supplemental nocturnal nasogastric refeeding for better short-term outcome in hospitalized adolescent girls with anorexia nervosa. The American journal of psychiatry. 2002;159(8):1347–53. [DOI] [PubMed] [Google Scholar]
- 49.Bufano G, Bellini C, Cervellin G, Coscelli C. Enteral nutrition in anorexia nervosa. JPEN Journal of parenteral and enteral nutrition. 1990;14(4):404–7. [DOI] [PubMed] [Google Scholar]
- 50.Diamanti A, Basso MS, Castro M, Bianco G, Ciacco E, Calce A, et al. Clinical efficacy and safety of parenteral nutrition in adolescent girls with anorexia nervosa. The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 2008;42(2):111–8. [DOI] [PubMed] [Google Scholar]
- 51.Bowers WA, Ansher LS. The effectiveness of cognitive behavioral therapy on changing eating disorder symptoms and psychopathology of 32 anorexia nervosa patients at hospital discharge and one year follow-up. Annals of clinical psychiatry : official journal of the American Academy of Clinical Psychiatrists. 2008;20(2):79–86. [DOI] [PubMed] [Google Scholar]
- 52.Mehler PS. Eating disorders: 1. Anorexia nervosa. Hospital practice (1995). 1996;31(1):109–13, 17. [PubMed] [Google Scholar]
- 53.Rigaud D, Boulier A, Tallonneau I, Brindisi MC, Rozen R. Body fluid retention and body weight change in anorexia nervosa patients during refeeding. Clinical nutrition (Edinburgh, Scotland). 2010;29(6):749–55. [DOI] [PubMed] [Google Scholar]
- 54.Kinzig KP, Coughlin JW, Redgrave GW, Moran TH, Guarda AS. Insulin, glucose, and pancreatic polypeptide responses to a test meal in restricting type anorexia nervosa before and after weight restoration. American journal of physiology Endocrinology and metabolism. 2007;292(5):E1441–6. [DOI] [PubMed] [Google Scholar]
- 55.Norris ML, Pinhas L, Nadeau PO, Katzman DK. Delirium and refeeding syndrome in anorexia nervosa. Int J Eat Disord. 2011. [DOI] [PubMed] [Google Scholar]
- 56.Weinsier RL, Krumdieck CL. Death resulting from overzealous total parenteral nutrition: the refeeding syndrome revisited. The American journal of clinical nutrition. 1981;34(3):393–9. [DOI] [PubMed] [Google Scholar]
- 57.Forman SF, Grodin LF, Graham DA, Sylvester CJ, Rosen DS, Kapphahn CJ, et al. An eleven site national quality improvement evaluation of adolescent medicine-based eating disorder programs: predictors of weight outcomes at one year and risk adjustment analyses. J Adolesc Health. 2011;49(6):594–600. [DOI] [PubMed] [Google Scholar]
- 58.Hudson LD, Nicholls DE, Lynn RM, Viner RM. Medical instability and growth of children and adolescents with early onset eating disorders. Arch Dis Child. 2012;97(9):779–84. [DOI] [PubMed] [Google Scholar]
- 59.Zuercher JN, Cumella EJ, Woods BK, Eberly M, Carr JK. Efficacy of voluntary nasogastric tube feeding in female inpatients with anorexia nervosa. JPEN Journal of parenteral and enteral nutrition. 2003;27(4):268–76. [DOI] [PubMed] [Google Scholar]
- 60.Kohn MR, Madden S, Clarke SD. Refeeding in anorexia nervosa: increased safety and efficiency through understanding the pathophysiology of protein calorie malnutrition. Current opinion in pediatrics. 2011;23(4):390–4. [DOI] [PubMed] [Google Scholar]
- 61.Steinhausen HC, Grigoroiu-Serbanescu M, Boyadjieva S, Neumarker KJ, Winkler Metzke C. Course and predictors of rehospitalization in adolescent anorexia nervosa in a multisite study. Int J Eat Disord. 2008;41(1):29–36. [DOI] [PubMed] [Google Scholar]
- 62.Lock J, Le Grange D, Agras WS, Moye A, Bryson SW, Jo B. Randomized clinical trial comparing family-based treatment with adolescent-focused individual therapy for adolescents with anorexia nervosa. Arch Gen Psychiatry. 2010;67(10):1025–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Michihata N, Matsui H, Fushimi K, Yasunaga H. Comparison between enteral nutrition and intravenous hyperalimentation in patients with eating disorders: results from the Japanese diagnosis procedure combination database. Eat Weight Disord. 2014;19(4):473–8. [DOI] [PubMed] [Google Scholar]
- 64.Mehler PS, Weiner KL. Use of total parenteral nutrition in the refeeding of selected patients with severe anorexia nervosa. The International journal of eating disorders. 2007;40(3):285–7. [DOI] [PubMed] [Google Scholar]
- 65.Thouzeau C, Le Maho Y, Larue-Achagiotis C. Refeeding in fasted rats: dietary self-selection according to metabolic status. Physiology & behavior. 1995;58(6):1051–8. [DOI] [PubMed] [Google Scholar]
- 66.Schebendach JE, Mayer LE, Devlin MJ, Attia E, Contento IR, Wolf RL, et al. Dietary energy density and diet variety as predictors of outcome in anorexia nervosa. The American journal of clinical nutrition. 2008;87(4):810–6. [DOI] [PubMed] [Google Scholar]
- 67.Schebendach JE, Mayer LE, Devlin MJ, Attia E, Contento IR, Wolf RL, et al. Food choice and diet variety in weight-restored patients with anorexia nervosa. J Am Diet Assoc. 2011;111(5):732–6. [DOI] [PMC free article] [PubMed] [Google Scholar]