Abstract
Purpose:
We report a longitudinal assessment of health-related quality of life (HRQOL) in patients with glioblastoma (GBM) treated on a prospective dose escalation trial of 5-fraction stereotactic radiosurgery (25–40 Gy in 5 fractions) with concurrent and adjuvant temozolomide.
Methods:
HRQOL was assessed using the European Organization for Research and Treatment of Cancer (EORTC) quality of life questionnaire core-30 (QLQ-C30) general, the EORTC quality of life questionnaire-brain cancer specific module (QLQ-BN20), and the M.D. Anderson Symptom InventoryeBrain Tumor (MDASI-BT). Questionnaires were completed at baseline and at every follow-up visit after completion of radiosurgery. Changes from baseline for 9 predefined HRQOL measures (global quality of life, physical functioning, social functioning, emotional functioning, motor dysfunction, communication deficit, fatigue, insomnia, and future uncertainty) were calculated at every time point.
Results:
With a median follow-up time of 10.4 months (range, 0.4–52 months), 139 total HRQOL questionnaires were completed by the 30 patients on trial. Compliance with HRQOL assessment was 76% at 12 months. Communication deficit significantly worsened over time, with a decline of 1.7 points per month (PZ.008). No significant changes over time were detected in the other 8 scales of our primary analysis, including global quality of life. Although 8 patients (27%) experienced adverse radiation effects (ARE) on this dose escalation trial, it was not associated with a statistically significant decline in any of the primary HRQOL scales. Disease progression was associated with communication deficit, with patients experiencing an average worsening of 13.9 points per month after progression compared with 0.7 points per month before progression (PZ.01).
Conclusion:
On this 5-fraction dose escalation protocol for newly diagnosed GBM, overall HRQOL remained stable and appears similar to historical controls of 30 fractions of radiation therapy. Tumor recurrence was associated with worsening communication deficit, and ARE did not correlate with a decline in HRQOL.
Summary
We prospectively assessed health-related quality of life (HRQOL) in patients with newly diagnosed glioblastoma treated with 5-fraction stereotactic radiosurgery with 5-mm margins with concurrent temozolomide. Self-reported communication deficit was associated with tumor progression. Although adverse radiation effects (ARE) occurred in 27% of patients on this dose escalation trial, it was associated with improved survival compared with those without ARE, with no statistically significant decline in HRQOL.
Introduction
Survival outcomes remain poor for patients with glioblastoma (GBM), despite standard of care therapies including surgery, radiation therapy and temozolomide (1). Healthrelated quality of life (HRQOL) is increasingly recognized as an important endpoint, particularly for incurable cancers with poor prognoses (2, 3). Treatment and diseaserelated side effects may affect the HRQOL of patients with GBM, leading to fatigue, personality changes, cognitive dysfunction, and coordination and communication deficits. These symptoms can affect patients’ social relationships and ability to perform activities of daily living (4, 5) and are often exacerbated by treatment-related side effects. Thus, with any new treatment paradigm, it is critical to assess the treatment’s impact on HRQOL in addition to survival and tumor outcomes. Although HRQOL has been reported for conventional radiation therapy and dose deescalated hypofractionated radiation therapy (6, 7), few data exist for dose-escalated hypofractionated radiation therapy (8, 9).
We conducted a phase 1/2 trial of temozolomide and 5-fraction stereotactic radiosurgery (SRS) with 5-mm margins (clinicaltrials.gov: NCT01120639) for patients with newly diagnosed GBM to determine the short-term and long-term adverse effects and efficacy of this treatment regimen. Although the shortening of treatment from 6 weeks to 1 week may improve short-term HRQOL, hypofractionation as a means of dose escalation may result in increased long-term neurotoxicity and subsequent impairment of HRQOL. The primary endpoint of safety and the secondary endpoints of overall survival and patterns of failure will be reported separately. We report the HRQOL results as a secondary endpoint of this protocol, with the hypothesis that HRQOL would not be affected by this novel 1-week limitedmargin treatment regimen.
Methods
Patients and treatment
The primary outcomes of this institutional review board approved prospective phase 1/2 trial are pending (10). All patients gave written informed consent. Briefly, patients 18 years or older with newly diagnosed, histologically confirmed supratentorial GBM were trial candidates. Eligibility criteria included adequate organ function for temozolomide chemotherapy, expected survival of 12 weeks, and a planning target volume (PTV) defined as residual T1 postecontrast medium enhancing tumor and/or resection cavity plus 0.5-cm margin of ≤150 cm3. Patients were enrolled on 2 treatment arms, stratified by PTV size (<60 cm3 vs 60–150 cm3), and treated with escalated doses of hypofractionated SRS at 4 radiation dose levels per standard 3 þ 3 design: 25 Gy, 30 Gy, 35 Gy, and 40 Gy in 5 fractions delivered on consecutive days. Oral temozolomide (75 mg/m2/day) was administered daily during radiosurgery and continued at the discretion of the treating neuro-oncologist after radiosurgery. The primary objective was to determine the maximum tolerated dose, defined as grade 3 to 5 acute or late central nervous system toxicity, of this 1-week, 5-mm dose escalated radiation therapy. Assessment of HRQOL was one of the secondary endpoints.
HRQOL assessment
The HRQOL was assessed using 3 instruments: the European Organization for Research and Treatment of Cancer (EORTC) quality of life questionnaire core-30 (QLQ-C30) general (11), the EORTC quality of life questionnaire brain cancerespecific module (QLQ-BN20) (12, 13), and the M.D. Anderson Symptom InventoryeBrain Tumor (MDASI-BT). Questionnaires were completed at baseline (after surgery and before radiation treatment), at 1 month, at 6 months, and subsequently every 6 months after radiosurgery until death.
The EORTC QLQ-C30 is a core measure consisting of the following scales: function (physical, role, emotional, cognitive, social), symptom (fatigue, nausea, vomiting, pain), single-item (dyspnea, insomnia, appetite loss, constipation, diarrhea, and financial effect of tumor and treatment), and overall quality of life. A high functional scale score represents a high level of functioning, and a high symptom scale score represents a high burden of symptoms.
The validated EORTC QLQ-BN20 was developed specifically for patients with brain cancer undergoing treatment. The QLQ-BN20 consists of 4 multi-item scales: future uncertainty, visual disorder, motor dysfunction, and communication deficit. Additionally, 7 single items assess headaches, seizures, drowsiness, hair loss, itchy skin, weakness of legs, and bladder control.
The items on both EORTC measures were scored per EORTC guidelines (14). Raw scores are transformed to a linear scale (0–100), with a higher score representing a higher level of functioning or a higher level of symptoms. Differences of at least 10 points are considered the minimum clinically meaningful change in an HRQOL parameter (15).
Symptom burden and interference were additionally assessed using the MD Anderson Symptom Inventory Brain Tumor Module (MDASI-BT), which has been validated for patients with both primary and metastatic brain tumors (16, 17). It consists of 23 symptoms rated on an 11-point scale to indicate the presence and severity of the symptom rated at its worst level in the past 24 hours, with 0 as “not present” and 10 “as bad as you can imagine.” The symptom items on the MDASI-BT were averaged and grouped into previously identified symptom burden scores (affective, cognitive, neurologic, treatment-related, generalized/disease, and gastrointestinal-related) and symptom interference scores (activity-related and mood) (17, 18).
Statistical analysis
Nine scales (EORTC global health status, physical functioning, social functioning, emotional functioning, motor dysfunction, communication deficit, fatigue, insomnia, and future uncertainty) were selected a priori for primary analysis to be consistent with prior studies (6, 7, 19, 20), and the remaining HRQOL measures were analyzed on an exploratory basis (Appendix A; available online at www.redjournal.org).
Mixed-effects linear regression models were used to estimate the change of HRQOL outcomes over time since first evaluation. Patient and time were defined as random variables, and adverse radiation affect (ARE) and disease progression were added as time-varying, fixed-effect covariates to the specified models. All patients with at least 1 HRQOL assessment were included in the analyses. In terms of missing data, the EORTC scale scores were calculated with only items for which data were available, provided that at least half the items in a scale were complete. Patients with missing data were included in our mixed-model analysis because the model uses likelihoodbased estimation.
Given multiple outcomes of primary interest, the Benjamini-Hochberg method was used to control the false discovery rate at 20% (21, 22). The Benjamini-Hochberg method was first applied to the 9 outcomes of primary interest and then applied to the remaining 24 outcomes in the exploratory analysis. A sample size of 30 patients provided 70% power to detect a change of 10 units over the median follow-up time in the study, assuming a variance and covariance between time points of 500 and 307, respectively, based on the variance and covariance in the global quality of life scale in our study.
Finally, median overall survival was determined using Kaplan-Meier statistics, and cumulative incidences of ARE and disease progression were determined using competingrisk methods, with death treated as a competing risk.
All statistical analyses were performed using SAS, version 9.4 (SAS Institute, Cary, NC) or STATA, version 13.1 (STATA Corp LP, College Station, TX).
Results
Patients
Between August 2010 and October 2015, 30 patients were treated with hypofractionated SRS on trial. All patients completed at least 1 HRQOL assessment. Table 1 shows baseline characteristics of these patients.
Table 1.
Baseline characteristics of all patients
| Characteristic | n (%) |
|---|---|
| Total | 30 (100) |
| Age (median, range) | 66 (51–86) |
| Sex | |
| Male | 15 (50) |
| Female | 15 (50) |
| KPS (median, range) | 80 (50–100) |
| Extent of resection | |
| Gross total resection | 12 (40) |
| Subtotal resection | 15 (50) |
| Biopsy | 3 (10) |
| MGMT status | |
| Methylated | 15 (50) |
| Nonmethylated | 13 (43) |
| Unknown | 2(7) |
| PTV in cc (median, range) | 60 (15–137) |
| Dose | |
| 25 Gy in 5 fractions | 6 (20) |
| 30 Gy in 5 fractions | 6 (20) |
| 35 Gy in 5 fractions | 6 (20) |
| 40 Gy in 5 fractions | 12 (40) |
Abbreviations: KPS = Karnofsky performance status; MGMT = O6-methylguanin-DNA-methyltransferase; PTV = planning treatment volume.
Compliance with HRQOL assessment
Although baseline compliance with HRQOL assessment was high, compliance decreased over time. Of patients surviving, compliance was 97% at baseline and declined to 76% at month 12 and to 56% at 18 months. Because of the small number of patients with HRQOL data still available at 18 months (nZ5), data were analyzed up to 12 months for all patients. Table 2 shows compliance at each followup time point, defined as within 6 weeks of the intended assessment time.
Table 2.
Patient compliance with questionnaires
| Timepoint | Questionnaires received | Patients alive | Compliance (%) |
|---|---|---|---|
| Baseline | 29 | 30 | 97 |
| 1 month | 28 | 29 | 97 |
| 6 month | 25 | 28 | 89 |
| 12 months | 13 | 17 | 76 |
| 18 months | 5 | 9 | 56 |
| 24 months | 5 | 7 | 71 |
| 36 months | 1 | 3 | 33 |
HRQOL results
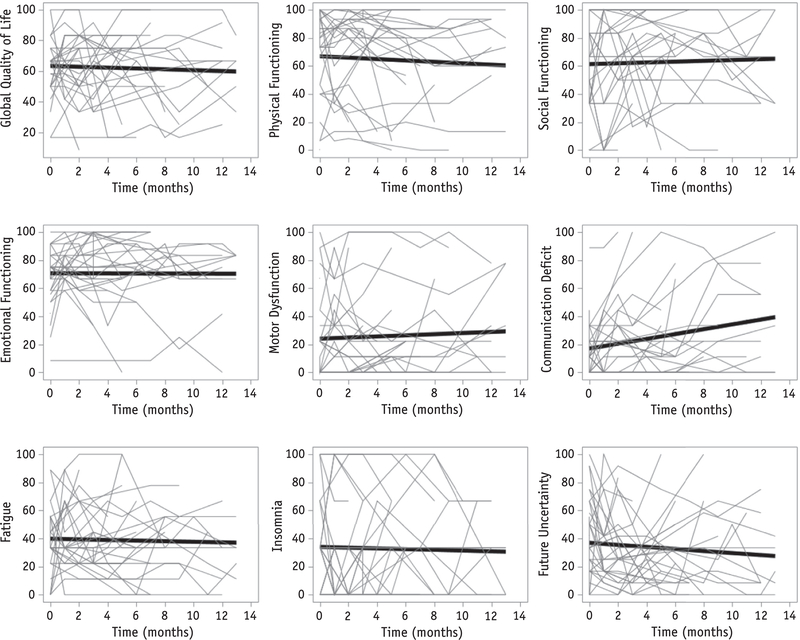
Table 3 shows mean baseline HRQOL scores for all patients. HRQOL as measured by QLQ-C30 scores was impaired at baseline compared with reference data (23). There was a statistically significant difference in patients’ changes over time in raw scores for the communication deficit scale, with a worsening of 1.72 points a month (PZ.008) from baseline evaluation. The false discovery rate associated with this outcome was 7%, which was below the predetermined rate of 20%, and thus we accepted this result as a true discovery. No significant changes over time were detected in the 8 other scales in our primary analysis (Fig. 1) (Appendix A; available online at www.redjournal.org).
Table 3.
Summary of health-related quality of life scores at baseline for all patients
| Scale | Mean(SD) | Median (IQR range) |
|---|---|---|
| QLQ-C30 | ||
| Global health status* | 63.1 (18.5) | 66.7 (50.0–75.0) |
| Physical functioning* | 67.7 (30.4) | 73.3 (46.7–93.3) |
| Social functioning* | 58.6 (29.7) | 66.7 (33.3–83.3) |
| Role functioning* | 50.0 (30.4) | 50.0 (16.7–66.7) |
| Emotional functioning* | 68.7 (17.9) | 75.0 (58.3–79.2) |
| Cognitive functioning* | 69.6 (24.5) | 75.0 (58.3–83.3) |
| Fatigue† | 35.7 (22.3) | 33.3 (22.2–50.0) |
| Nausea and vomiting† | 1.8 (5.2) | 0.0 (0.0–0.0) |
| Pain† | 15.5 (19.2) | 8.3 (0.0–33.3) |
| Dyspnea† | 13.1 (21.0) | 0.0 (0.0–33.3) |
| Insomnia† | 35.7 (35.1) | 33.3 (0.0–66.7) |
| Appetite loss† | 8.3 (17.3) | 0.0 (0.0–0.0) |
| Constipation† | 22.6 (31.5) | 0.0 (0.0–33.3) |
| Diarrhea† | 10.7 (22.3) | 0.0 (0.0–16.7) |
| Financial difficulties† | 29.6 (28.2) | 33.3 (0.0–33.3) |
| QLQ-BN20 | ||
| Future uncertainty† | 43.1 (27.7) | 37.5 (20.8–62.5) |
| Visual disorder† | 16.7 (27.6) | 0.0 (0.0–22.2) |
| Motor dysfunction† | 21.8 (29.4) | 11.1 (0.0–27.8) |
| Communication deficit† | 16.3 (19.7) | 11.1 (0.0–27.8) |
| Headache† | 19.0 (21.1) | 16.7 (0.0–33.3) |
| Seizure† | 3.7 (10.7) | 0.0 (0.0–0.0) |
| Drowsiness† | 21.4 (26.0) | 16.7 (0.0–33.3) |
| Hair loss† | 6.0 (13.0) | 0.0 (0.0–0.0) |
| Itchy skin† | 9.5 (22.0) | 0.0 (0.0–0.0) |
| Difficulty with bladder control† | 10.7 (27.3) | 0.0 (0.0–0.0) |
| Weakness of both legs† | 8.3 (14.7) | 0.0 (0.0–16.7) |
| MDASI-BT symptom | ||
| Affective† | 2.6 (1.6) | 2.7 (1.2–3.6) |
| Cognitive† | 2.3 (2.1) | 1.6 (0.8–3.5) |
| Neurologic† | 1.4 (1.7) | 0.6 (0.0–3.3) |
| Treatment-related† | 1.5 (1.8) | 0.8 (0.3–2.3) |
| Generalized/disease† | 1.7 (1.8) | 1.5 (0.5–2.0) |
| Gastrointestinal-related† | 0.3 (0.8) | 0.0 (0.0–0.0) |
| MDASI-BT interference | ||
| Activity-related† | 2.6 (2.5) | 2.0 (1.0–3.0) |
| Mood† | 3.6 (2.7) | 3.7 (1.3–5.5) |
Abbreviations: IQR = interquartile range; MDASI-BT = MD Anderson Symptom Inventory Brain Tumor Module; QLQBN20 = quality of life questionnaire-brain cancer specific module; QLQ-C30 = quality of life questionnaire core 30; SD = standard deviation.
Higher scores indicate better HRQOL.
Lower scores indicate better HRQOL.
Fig. 1.
In the 9 health-related quality of life scores selected for primary analysis, all except communication deficit (P =.008) remained stable over time. The trend lines are the predicted values from our repeated measures model and are superimposed on the spaghetti plot to show how the trend relates to the lines for the individual patients.
Impact of ARE and disease progression on HRQOL
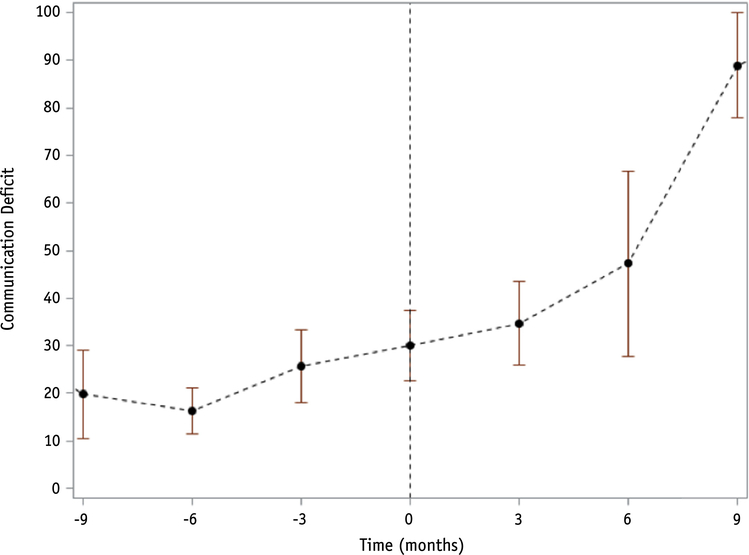
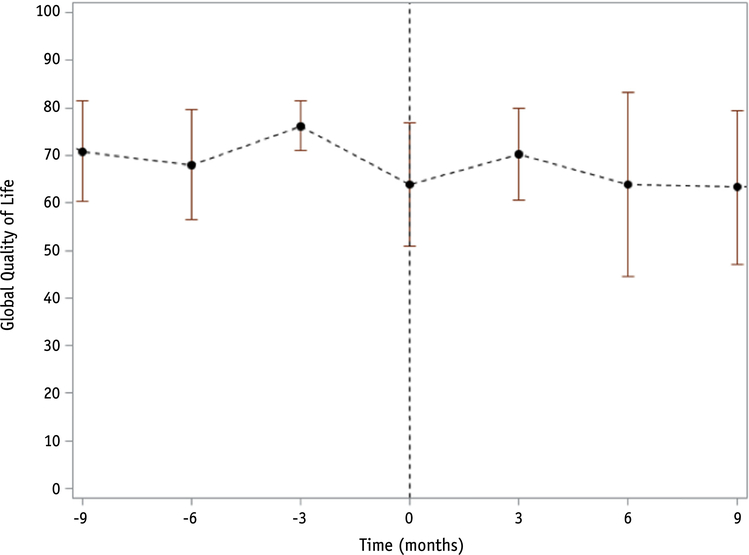
With a median clinical follow-up time of 13.5 months (range, 0.4–62.4 months) and 10.4 months (range, 0.4–52 months) for QOL assessment, median overall survival was 14.2 months (95% confidence interval [CI] 8.7–20.9 months) from the end of radiosurgery. Twenty-six patients experienced progression, with 12-month cumulative incidence of progression of 64% (95% CI 43%−79%). When progression was entered into the model as a timedependent covariate, it was significantly associated with communication deficit (PZ.01), with patients experiencing an average worsening of 13.9 points per month after progression compared with 0.7 points per month before progression (Fig. 2). Eight patients experienced ARE, with a 12-month cumulative incidence of 28% (95% CI 13%−45%). Six (75%) of those with ARE were symptomatic (all G2 according to the Common Terminology Criteria for Adverse Events) and were initially treated with corticosteroids in 2 patients and bevacizumab in 4. All were ultimately treated with bevacizumab. ARE was not significantly associated with any of the 9 scales in our primary analysis (Fig. 3).
Fig. 2.
Mean communication deficit score over time with 95% confidence bars in the 26 patients who experienced progression (time of progression at 0 months). The communication deficit score was based on the following questions: (1) Did you have trouble finding the right words to express yourself? (2) Did you have difficulty speaking? (3) Did you have trouble communicating your thoughts?
Fig. 3.
Mean global quality of life score over time with 95% confidence bars in the 8 patients who experienced adverse radiation effects (ARE) (time of ARE at 0 months). The development of ARE was not associated with worsening of any of the health-related quality of life scales in our primary analysis.
HRQOL in long-term survivors
Because of our concern for late effects of the doseescalated hypofractionated SRS treatment, we looked separately at long-term survivors, defined as those with more than 2 years of HRQOL follow-up. We identified 5 long-term survivors with a median follow-up time of 34.7 months (range, 31.7–44.7 months). Of note, 4 of 5 experienced ARE at a median of 9.7 months (range, 4.4–12.6 months) from the end of radiosurgery. Similar to the primary analysis of all patients, the HRQOL scales for these long-term survivors were relatively stable over an extended time period of 2 years, with the exception of the communication deficit scale (Appendix B; available online at www.redjournal.org).
Discussion
Health-related QOL assessment in brain cancer can help capture a patient’s unique perspective of his or her disease course, and it should be included in the evaluation of treatment effectiveness. In our trial of 5-fraction SRS with 5-mm margins with concurrent temozolomide, we found that HRQOL was stable and did not deteriorate after SRS in any of the 9 preselected HRQOL scales except for communication deficit. Although we found that patients who had disease progression experienced worsening of this HRQOL scale, the development of ARE was not associated with worsening of any of the HRQOL scales in our primary analysis.
Our results are comparable with reported HRQOL outcomes with standard fractionation. Keime-Guibert et al (24) found that for elderly patients treated with either conventionally fractionated radiation or supportive care alone, the global score for HRQOL did not change significantly. However, they found that mean scores were significantly worse over time on the physical, cognitive, social, fatigue, and motor dysfunction scales. By contrast, Taphoorn and Bottomley (2) reported that in patients treated with radiation therapy and temozolomide on the trial by Stupp et al (1), outcomes in their 7 selected scales for primary analyses (fatigue, overall HRQOL, social function, emotional function, future uncertainty, insomnia, and communication deficit) showed either stability or mild-to-moderate improvement over time. Although they noted an increase in communication deficit over time as we did, this was not statistically significant in their study. Similarly, HRQOL results from the phase 3 AVAglio trial did not demonstrate clinically significant changes in mean scores from baseline in either arm for most of the EORTC QLQ-C30 and QLQBN20 scales (19). However, the investigators noted that local progression was associated with a decline in HRQOL.
The main concern with high doses per fraction is the potential for ARE and neurotoxicity, which can have a potential impact on HRQOL. Other groups have used hypofractionated radiation therapy, with or without chemotherapy, for treatment of high-grade glioma and have shown treatment to be well tolerated (25–32). Minniti et al (7) found that 40 Gy in 15 fractions with concurrent temozolomide was associated with stable or improved HRQOL in elderly patients. However, they captured HRQOL data only until the time of disease progression, and thus they did not report longer than 6 months after treatment because of the low number of patients. Reddy et al (33) also found that hypofractionated radiation therapy (60 Gy in 10 fractions) with concurrent temozolomide did not appear to have a negative impact on overall HRQOL, noting significant improvement over time in insomnia, future uncertainty, motor dysfunction, and drowsiness. They found significant worsening over time in communication deficit, similar to our results, and also in cognitive functioning, social functioning, and appetite loss. Furthermore, they too found no significant impact of HRQOL in patients with ARE. In our study, 8 patients experienced ARE, but this was not associated with a decline in HRQOL outcomes. All patients with ARE were treated with bevacizumab, which can promote improvement in symptoms and neurocognitive function (8, 34) and could explain the lack of negative impact of ARE on HRQOL. Omuro et al (8) showed stable HRQOL among patients treated with 6 Gy in 6 fractions with concomitant and adjuvant temozolomide and bevacizumab.
Finally, we found that communication deficit worsened over time after treatment, primarily influenced by the development of disease progression. Communication disorders can result in social isolation, depression, and psychologic distress (35, 36), and thus it is important for providers to be aware of these risks inasmuch as patients may benefit from early intervention with speech therapy, rehabilitation, and additional social support.
The limitations of our study include the small patient number associated with a phase 1/2 trial. However, the study had 70% power to be able to detect a clinically relevant change of 10 units over the study period. Similarly, we had to restrict our analysis to the first year of treatment because the remaining patients were few, and thus we could not reliably assess the longer-term impact of treatment on HRQOL. With similar questionnaire compliance as ours, other studies have also restricted their analyses to 1 year or less from treatment (6, 19, 24). However, in the 5 long-term survivors in our trial, we found similar trends in their HRQOL scores as our primary analysis even at 2 years of follow-up. Additionally, although a potential concern is that compliance with HRQOL questionnaires may decrease as patients become symptomatic, leading to missing data when a patient experiences ARE, 7/8 patients had QOL assessment after the development of ARE. Finally, it is possible that transient declines in QOL resulting from ARE that occur in between scheduled QOL assessments may have been missed. However, it appears that these declines did not persist at subsequent QOL assessment.
In conclusion, on this prospective trial of 5-fraction SRS with 5-mm margins with temozolomide for newly diagnosed GBM, other than a decline in communication deficit associated with tumor progression, overall HRQOL was maintained throughout patients’ lives. Although ARE occurred in 27% of patients on this dose escalation trial, it was associated with improved survival in comparison with patients without ARE, with no statistically significant decline in HRQOL; asymptomatic ARE, especially in the bevacizumab era, may be a clinically desirable outcome rather than a dose-limiting “toxicity.”
Acknowledgments
Supported by KL2 Mentored Career Development Award of the Stanford Clinical and Translational Science Award to Spectrum (NIH KL2 TR 001083) to E.L.P.
Footnotes
Conflict of interest: none.
Supplementary material for this article can be found at www.redjournal.org.
References
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987–996. [DOI] [PubMed] [Google Scholar]
- 2.Taphoorn MJ, Bottomley A. Health-related quality of life and symptom research in glioblastoma multiforme patients. Expert Rev Pharmacoecon Outcomes Res 2005;5:763–774. [DOI] [PubMed] [Google Scholar]
- 3.Efficace F, Osoba D, Gotay C, et al. Has the quality of health-related quality of life reporting in cancer clinical trials improved over time? Towards bridging the gap with clinical decision making. Ann Oncol 2007;18:775–781. [DOI] [PubMed] [Google Scholar]
- 4.Moore G, Collins A, Brand C, et al. Palliative and supportive care needs of patients with high-grade glioma and their carers: A systematic review of qualitative literature. Patient Educ Couns 2013; 91:141–153. [DOI] [PubMed] [Google Scholar]
- 5.Russell B, Collins A, Dally M, et al. Living longer with adult highgrade glioma: Setting a research agenda for patients and their caregivers. J Neurooncol 2014;120:1–10. [DOI] [PubMed] [Google Scholar]
- 6.Taphoorn MJ, Stupp R, Coens C, et al. Health-related quality of life in patients with glioblastoma: A randomised controlled trial. Lancet Oncol 2005;6:937–944. [DOI] [PubMed] [Google Scholar]
- 7.Minniti G, Scaringi C, Baldoni A, et al. Health-related quality of life in elderly patients with newly diagnosed glioblastoma treated with short-course radiation therapy plus concomitant and adjuvant temozolomide. Int J Radiat Oncol Biol Phys 2013;86:285–291. [DOI] [PubMed] [Google Scholar]
- 8.Omuro A, Beal K, Gutin P, et al. Phase II study of bevacizumab, temozolomide, and hypofractionated stereotactic radiotherapy for newly diagnosed glioblastoma. Clin Cancer Res 2014;20:5023–5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Minniti G, De Sanctis V, Muni R, et al. Hypofractionated radiotherapy followed by adjuvant chemotherapy with temozolomide in elderly patients with glioblastoma. J Neurooncol 2009;91:95–100. [DOI] [PubMed] [Google Scholar]
- 10.Azoulay M, Ho CK, Fujimoto DK, et al. : A Phase I/II Trial of 5 Fraction Stereotactic Radiosurgery With 5-mm Margins With Concurrent and Adjuvant Temozolomide in Newly Diagnosed Supratentorial Glioblastoma Multiforme. Presented at American Society for Radiation Oncology, Annual Meeting 2016, Boston, MA. [DOI] [PubMed] [Google Scholar]
- 11.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A qualityof-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365–376. [DOI] [PubMed] [Google Scholar]
- 12.Osoba D, Aaronson NK, Muller M, et al. The development and psychometric validation of a brain cancer quality-of-life questionnaire for use in combination with general cancer-specific questionnaires. Qual Life Res 1996;5:139–150. [DOI] [PubMed] [Google Scholar]
- 13.Taphoorn MJ, Claassens L, Aaronson NK, et al. An international validation study of the EORTC brain cancer module (EORTC QLQBN20) for assessing health-related quality of life and symptoms in brain cancer patients. Eur J Cancer 2010;46:1033–1040. [DOI] [PubMed] [Google Scholar]
- 14.Fayers P, Aaronson N, Bjordal K, et al. 63 EORTC QLQ-C30 In: Scoring Manual. 3rd ed. Belgium: EORTC Publications; 2001. [Google Scholar]
- 15.Osoba D, Rodrigues G, Myles J, et al. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol 1998;16: 139–144. [DOI] [PubMed] [Google Scholar]
- 16.Armstrong TS, Gning I, Mendoza TR, et al. Clinical utility of the MDASI-BT in patients with brain metastases. J Pain Symptom Manage 2009;37:331–340. [DOI] [PubMed] [Google Scholar]
- 17.Armstrong TS, Mendoza T, Gning I, et al. Validation of the M.D. Anderson Symptom Inventory Brain Tumor Module (MDASI-BT). J Neurooncol 2006;80:27–35. [DOI] [PubMed] [Google Scholar]
- 18.Armstrong TS, Wefel JS, Wang M, et al. Net clinical benefit analysis of radiation therapy oncology group 0525: A phase III trial comparing conventional adjuvant temozolomide with dose-intensive temozolomide in patients with newly diagnosed glioblastoma. J Clin Oncol 2013;31:4076–4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taphoorn MJ, Henriksson R, Bottomley A, et al. Health-related quality of life in a randomized phase iii study of bevacizumab, temozolomide, and radiotherapy in newly diagnosed glioblastoma. J Clin Oncol 2015;33:2166–2175. [DOI] [PubMed] [Google Scholar]
- 20.Osoba D, Brada M, Yung WK, et al. Health-related quality of life in patients with anaplastic astrocytoma during treatment with temozolomide. Eur J Cancer 2000;36:1788–1795. [DOI] [PubMed] [Google Scholar]
- 21.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol 1995;57:289–300. [Google Scholar]
- 22.Benjamini Y, Hochberg Y. On the adaptive control of the false discovery rate in multiple testing with independent statistics. J Educ Behav Stat 2000;5:60–83. [Google Scholar]
- 23.Michelson H, Bolund C, Nilsson B, et al. Health-related quality of life measured by the EORTC QLQ-C30: Reference values from a large sample of Swedish population. Acta Oncol 2000;39:477–484. [DOI] [PubMed] [Google Scholar]
- 24.Keime-Guibert F, Chinot O, Taillandier L, et al. Radiotherapy for glioblastoma in the elderly. N Engl J Med 2007;356:1527–1535. [DOI] [PubMed] [Google Scholar]
- 25.Thomas R, James N, Guerrero D, et al. Hypofractionated radiotherapy as palliative treatment in poor prognosis patients with high grade glioma. Radiother Oncol 1994;33:113–116. [DOI] [PubMed] [Google Scholar]
- 26.Floyd NS, Woo SY, Teh BS, et al. Hypofractionated intensitymodulated radiotherapy for primary glioblastoma multiforme. Int J Radiat Oncol Biol Phys 2004;58:721–726. [DOI] [PubMed] [Google Scholar]
- 27.Hulshof MC, Schimmel EC, Andries Bosch D, et al. Hypofractionation in glioblastoma multiforme. Radiother Oncol 2000; 54:143–148. [DOI] [PubMed] [Google Scholar]
- 28.Kleinberg L, Slick T, Enger C, et al. Short course radiotherapy is an appropriate option for most malignant glioma patients. Int J Radiat Oncol Biol Phys 1997;38:31–36. [DOI] [PubMed] [Google Scholar]
- 29.Sultanem K, Patrocinio H, Lambert C, et al. The use of hypofractionated intensity-modulated irradiation in the treatment of glioblastoma multiforme: Preliminary results of a prospective trial. Int J Radiat Oncol Biol Phys 2004;58:247–252. [DOI] [PubMed] [Google Scholar]
- 30.Chang EL, Yi W, Allen PK, et al. Hypofractionated radiotherapy for elderly or younger low-performance status glioblastoma patients: Outcome and prognostic factors. Int J Radiat Oncol Biol Phys 2003; 56:519–528. [DOI] [PubMed] [Google Scholar]
- 31.Roa W, Brasher PM, Bauman G, et al. Abbreviated course of radiation therapy in older patients with glioblastoma multiforme: A prospective randomized clinical trial. J Clin Oncol 2004;22:1583–1588. [DOI] [PubMed] [Google Scholar]
- 32.Ernst-Stecken A, Ganslandt O, Lambrecht U, et al. Survival and quality of life after hypofractionated stereotactic radiotherapy for recurrent malignant glioma. J Neurooncol 2007;81:287–294. [DOI] [PubMed] [Google Scholar]
- 33.Reddy K, Gaspar LE, Kavanagh BD, et al. Prospective evaluation of health-related quality of life in patients with glioblastoma multiforme treated on a phase II trial of hypofractionated IMRT with temozolomide. J Neurooncol 2014;114:111–116. [DOI] [PubMed] [Google Scholar]
- 34.Gutin PH, Iwamoto FM, Beal K, et al. Safety and efficacy of bevacizumab with hypofractionated stereotactic irradiation for recurrent malignant gliomas. Int J Radiat Oncol Biol Phys 2009;75:156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baylor C, Burns M, Eadie T, et al. A qualitative study of interference with communicative participation across communication disorders in adults. Am J Speech Lang Pathol 2011;20:269–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hetu R, Jones L, Getty L. The impact of acquired hearing impairment on intimate relationships: Implications for rehabilitation. Audiology 1993;32:363–381. [DOI] [PubMed] [Google Scholar]