Abstract
Objective:
Epileptic spasms (ES) are associated with pathological neuronal networks, which may underlie characteristic EEG patterns such as hypsarrhythmia. Here we evaluate EEG functional connectivity as a quantitative marker of treatment response, in comparison to classic visual EEG features.
Methods:
We retrospectively identified 21 ES patients and 21 healthy controls. EEG data recorded before treatment and after ≥10 days of treatment underwent blinded visual assessment, and functional connectivity was measured using cross-correlation techniques. Short-term treatment response and long-term outcome data were collected.
Results:
Subjects with ES had stronger, more stable functional networks than controls. After treatment initiation, all responders (defined by cessation of spasms) exhibited decreases in functional connectivity strength, while an increase in connectivity strength occurred only in non-responders. There were six subjects with unusually strong pre-treatment functional connectivity, and all were responders. Visually assessed EEG features were not predictive of treatment response.
Conclusions:
Changes in network connectivity and stability correlate to treatment response for ES, and high pre-treatment connectivity may predict favorable short-term treatment response. Quantitative measures outperform visual analysis of the EEG.
Significance:
Functional networks may have value as objective markers of treatment response in ES, with potential to facilitate rapid identification of personalized, effective treatments.
Keywords: Brain network, hypsarrhythmia, BASED score, West Syndrome, electroencephalography, adrenocorticotropic hormone (ACTH), infantile spasms
1. Introduction
Infantile Spasms (IS) is a form of epileptic encephalopathy that typically occurs in children less than one year old and is characterized by clusters of seizures called epileptic spasms (ES) (Pavone et al., 2014, Fisher et al., 2017). ES often leads to devastating neurocognitive consequences, and over 50% of patients with ES will develop other forms of highly refractory epilepsy (Hrachovy et al., 2003, Riikonen, 2010, Pavone et al., 2014). The impact of these outcomes, both on the patients’ families and the healthcare system, is tremendous (Beghi et al., 2005, Pellock et al., 2010). Although a majority of children suffer poor outcomes – especially those with severe underlying etiologies, early age of onset, delayed treatment, or developmental delay prior to the onset of ES – superior outcomes accompany prompt diagnosis and successful treatment (Riikonen, 2010, Yamada et al., 2014, Gaily et al., 2016).
There are significant challenges associated with standardized clinical decision making for the treatment of ES. This disease is associated with a wide range of etiologies, including focal and diffuse pathologies (Osborne et al., 2010), and it often co-occurs with a pre-existing epilepsy. It is classically accompanied by an interictal EEG pattern called hypsarrhythmia, characterized by very high voltage, irregular, asynchronous slow waves with overriding multifocal independent epileptiform discharges (Gibbs, 1952). However, multiple variants of hypsarrhythmia are commonly seen (Hrachovy et al., 1984, Donat et al., 1994, Kramer et al., 1997), and not all cases of ES exhibit hypsarrhythmia (Caraballo et al., 2016). As a result, the identification of this EEG pattern suffers from poor inter-rater reliability (Hussain et al., 2015), yet it is a standard clinical criteria used for both diagnosis and assessment of treatment response. While the presence or absence of hypsarrhythmia prior to treatment is unrelated to the likelihood of favorable shortterm response, children who exhibit hypsarrhythmia are more likely to receive first-line treatment, which is strongly associated with favorable response to treatment (Demarest et al., 2017). Therefore, there is a need for objective interictal EEG markers of ES that are independent from visually-dominating patterns such as hypsarrhythmia.
Recent clinical studies demonstrate that functional network characteristics associated with ES are good candidates for this marker. Multiple neurophysiologic approaches, including EEG source analysis (Japaridze et al., 2013), fMRI (Siniatchkin et al., 2007), PET (Chugani et al., 1992), and SPECT (Chiron et al., 1993), all find that hypsarrhythmia is likely generated subcortically, with predominant cortical expression in the parietal and occipital cortices. This suggests that a common network may underlie EEG patterns associated with ES, despite a seemingly chaotic appearance on standard clinical review. This is supported by the fact that hypsarrhythmia is associated with increased EEG coherence in long-distance connections (Burroughs et al., 2014), and nonlinear time series analysis demonstrates that hypsarrhythmia contains only weakly nonlinear structures and is not strictly chaotic (Van Putten et al., 2001). However, studies of functional connectivity in ES have focused only on patients exhibiting hypsarrhythmia, and the changes in functional networks following treatment have never been systematically evaluated (Siniatchkin et al., 2007, Japaridze et al., 2013, Burroughs et al., 2014). Therefore, we set out to measure EEG-based functional networks associated with ES both before and after treatment, and we compared the characteristics of these networks to clinical EEG findings, short-term treatment response, and long-term neurocognitive outcomes.
2. Methods
2.1. Subject identification
We retrospectively identified patients with new-onset epileptic spasms by querying an EEG database for studies that immediately preceded initial treatment of epileptic spasms with ACTH (H.P. Acthar gel) and/or vigabatrin. We included consecutive patients between August 2011 and December 2014 who underwent video-EEG both at diagnosis and after at least 10 days of treatment. We also retrospectively identified 21 controls who (1) carried no known neurological diagnoses, (2) underwent routine EEG for evaluation of clinical “spells”, and (3) whose EEGs were interpreted as normal by a board-certified pediatric epileptologist. Control subjects were selected such that the group’s overall distribution of ages was similar to the ES cohort (similar median value and IQR).
2.2. Data collection
Relevant clinical and demographic data were abstracted from the medical record. For each subject, digital scalp EEG recordings were retrospectively collected. All studies were acquired using the Nihon Kohden EEG acquisition system, with 19 electrodes placed according to the international 10–20 standard. All but four studies were originally recorded with 200Hz sampling rate; the remaining four studies were originally recorded at 500 Hz and downsampled to 200Hz using the MATLAB “resample” function prior to any analysis. For the ES subjects, two separate interictal epochs during wakefulness, each lasting twenty minutes or longer, were extracted; the first was isolated from the study performed at the time of the epileptic spasms diagnosis (prior to treatment) and the second was from the subsequent follow-up EEG. No ictal events were included in our analysis. The selection of EEG data segments was performed without reviewer knowledge of treatment status or outcome, as each specific EEG study was de-identified and assigned a code prior to epoch abstraction. Epochs of awake EEG data were similarly extracted from the control subjects’ recordings. Approval for this study was obtained from the Institutional Review Board of the Children’s Hospital Orange County, and the requirement for informed consent was waived.
While sleep is known to enhance some characteristics of the hypsarrhythmia pattern, including amplitude and oscillatory behavior (Hrachovy et al., 1981), many of the pre-treatment ES subjects in this study exhibited hypsarrhythmia, defined as BASED score of 4 or greater (Mytinger et al., 2015) during wakefulness. We analyzed awake EEG data for two methodological reasons. First, wakefulness is easily recorded in nearly every routine clinical EEG performed on infants, whereas sleep cannot be consistently captured without long epochs and relies upon extended EEG monitoring. This is particularly true of ES patients, who sleep less than healthy infants of the same age (Hrachovy et al., 1981). Second, the features of hypsarrhythmic EEG vary with stage of sleep (Kellaway, 1985), necessitating accurate sleep staging prior to quantitative analysis. However, ES and hypsarrhythmia are associated with altered structure and progression of sleep stages, with notably diminished/absent REM sleep (Hrachovy et al., 1981) and sleep spindles in stage 2 sleep (Altunel A. et al., 2015), and EEG characteristics change during the course of treatment, making it infeasible to employ standardized sleep staging procedures to ensure the data were analyzed consistently.
2.3. Blinded assessment of EEG characteristics
Two board-certified pediatric epileptologists (OK and RR) reviewed each pre- and posttreatment EEG in a blinded fashion. For each EEG, reviewers were asked to (1) determine whether or not classically defined hypsarrhythmia was present, (2) assign a BASED score (Mytinger et al., 2015), and (3) subjectively describe the degree of interhemispheric synchrony using a 5-point Likert scale.
2.4. Classification of hypsarrhythmia
When evaluating the association between hypsarrhythmia and functional EEG connectivity measures, hypsarrhythmia was defined as an average BASED score ≥ 4, based on the scores assigned by the two blinded reviewers.
2.5. EEG data pre-processing
A board-certified pediatric epileptologist (DS) reviewed all de-identified EEG recordings and marked artifacts caused by eye blinks, muscle activity, movement, and poor electrode contact. EEG channels with constant artifact were excluded from analysis (n=1 channel in 21 control datasets, n=4 channels in 42 spasms datasets), and all included channels were re-referenced to the common average (Chu et al., 2012). A broadband filter (3rd order Butterworth, 0.5–55 Hz) was applied to all data before analysis.
2.6. Functional connectivity analysis
Data were divided into 1-second epochs, and any epochs containing marked artifacts were excluded. The remaining windows were normalized to have zero mean and unit variance for each channel. Then functional connectivity between all pairs of electrodes was assessed by identifying the maximum cross correlation within each 1-second window of data, with a maximum lag of 200ms. Maximum cross-correlation values occurring at zero time lag were removed, as this removal has been shown to counteract the effects of volume conduction (Chu et al., 2012). A partial correlation between the two EEG channels and the common average signal was used to identify cases where the use of a common reference may have inflated the correlation value. For each pair of channels, we required that the difference between the measured cross-correlation and the partial correlation, accounting for the common reference, was less than 0.25, indicating that the reference did not cause a spuriously high correlation value.
Significance was assessed by standardizing the cross-correlation value, calculated as the Fisher transformation of the correlation coefficient divided by the estimated standard deviation, taking into account the variance of the sample autocorrelation for each channel in the pair (Kramer et al., 2009, Chu et al., 2012). We then compared this standardized value to a baseline distribution generated via permutation resampling, under the null hypothesis of no connectivity between the two electrodes (Nichols et al., 2001, Raz et al., 2003). To create the baseline distribution, we randomly shifted one channel in time by a minimum of 1 second, chose a random 1-second epoch of data, and then calculated the standardized maximum cross-correlation with all channels as described above. The aforementioned procedure represented one iteration. After 1000 iterations, the resulting standardized correlation values were sorted, and the threshold for significance (denoted as T) was defined as any value greater than or equal to the 95th percentile. This process was repeated for all channels. Significance was determined for each 1-second epoch of data by comparing the measured maximum cross-correlation to the significance threshold for that channel pair. This was a binary decision; each 1-second epoch was defined as significant or not.
We then defined the connection strength for each electrode pair and each subject based on these results. Let Ci j,n be the strength of the connection between electrodes i and j for subject n, defined as the fraction of one-second epochs that were significant, ranging from zero (never significant) to one (always significant). Then define Q i j,n to be an indicator that is equal to 1 if the connection strength exceeds the threshold, C i j,n>T; it takes the value of 0 otherwise.
Finally, define the overall connection strength Sn for subject n to be the sum of Qi j,n over all pairs (i,j). In other words, Sn is a count of the number of individual connections with strength Ci j,n >T.
2.7. Assessment of network stability
We define a “stable” functional network to be one in which the connection strengths are consistent when measured on independent datasets. We created independent datasets by dividing the full EEG dataset into successive windows of a fixed duration, and we calculated the strength of the connections within each window. We then compared the functional network in each window of time to the successive window by calculating the 2D correlation (MATLAB function “corr2”) between the connectivity matrices. This procedure was repeated for windows of time ranging from 25 seconds to 200 seconds, in increments of 25 seconds. A maximum window size of 200 seconds was chosen to ensure that the results from a majority of patients contained two or more correlation values (calculated from at least three windows of data). The mean and 95% confidence intervals were calculated based on the distribution of correlation values across all subjects. Note that four control subjects were excluded from this calculation, as their EEG recordings contained less than 10 minutes of wakefulness following artifact removal.
2.8. Statistical methods
To compare the strength of functional connections between groups of subjects, we created two distributions across subjects for each pair of electrodes, e.g. Fp1-Fp2 connectivity across 21 control subjects and Fp1-Fp2 connectivity across 21 pre-treatment spasms subjects. We then applied a Wilcoxon rank-sum test for each electrode pair and corrected for multiple comparisons using the Benjamini-Hochberg procedure. Significant channel pairs had FDR-corrected p-values < 0.05.
3. Results
3.1. Subject characteristics
We identified 21 subjects with epileptic spasms whose records contained both pre- and posttreatment EEG evaluation. Eleven subjects were taking anti-seizure medications at the time of diagnosis. The pre-treatment group (n=21) represents subjects with clinical spasms before treatment was initiated, either with (n=13) or without (n=8) hypsarrhythmia (mean BASED score ≥ 4). Their ages ranged from 4 – 19 months (median 6.3, IQR 5.2–8.1 months), and the median time between spasms onset and diagnosis was 8 days (IQR 4.75–30 days). The median time between the two recordings performed before and after treatment was 29 days (IQR 19–42.25 days). We saw no correlation between the strength of the functional connections and subject age (Supplementary Figure S1) or duration of ES at the time of the first EEG. Following treatment, 11 (52%) exhibited freedom from both ES and hypsarrhythmia at the time of the second EEG recording and were classified as responders. Ten (48%) exhibited continued epileptic spasms, either with or without hypsarrhythmia, and were classified as non-responders. No patients exhibited resolution of spasms with persistent hypsarrhythmia. Sixteen (76%) of the ES subjects were treated with ACTH alone, four (19%) were treated with vigabatrin alone, and one (5%) was treated with both ACTH and vigabatrin. Etiologies were known in 71% of cases (n=14), consisting of structural (n=8), genetic (n=2), structural and genetic (n=3), and metabolic (n=1) causes. Other relevant clinical information is described in Table 1. For comparison, we identified 21 control subjects aged 1 – 26 months (median 7, IQR 5.75–11.25) with normal EEG recordings and no known neurological diagnoses.
Table 1:
Subject clinical characteristics. Abbreviations: PHB phenobarbital, TPM topiramate, VPA valproic acid, LRZ lorazepam, DZP diazepam, CLB clobazam, PHT phenytoin, LEV levetiracetam, ACTH adrenocorticotrophic hormone (H.P. Acthar gel, Questcor/Mallinckrodt), VGB vigabatrin
| Subj. | Age at first EEG (mos/Sex) | Etiology (Etiology Category) | Treatment (Prior AEDs) | Pre-BASED score | Post-BASED score | Spasms Resolved |
|---|---|---|---|---|---|---|
| 1 | 12.0/F | Cortical Malformation (Structural) | VGB (PHB) | 3.5 | 4.5 | No |
| 2 | 5.5/F | Neonatal HIE (Structural) | ACTH | 4.5 | 2 | Yes |
| 3 | 8.7/F | Unknown, Prematurity, Diffuse Cerebral Atrophy (Unknown) | ACTH | 5 | 2 | Yes |
| 4 | 6.8/M | Tuberous Sclerosis (Structural/Genetic) | VGB | 2 | 2 | Yes |
| 5 | 4.5/F | Unknown | ACTH (PHB,TPM) | 4 | 2.5 | No |
| 6 | 6.0/M | Neurofibromatosis Type 1 (Genetic) | ACTH | 3 | 2 | No |
| 7 | 4.5/F | Unknown | ACTH (PHB) | 5 | 3 | No |
| 8 | 7.9/F | Paroxysomal Bifunctional Protein Deficiency (Metabolic) | ACTH (PHB) | 4.5 | 2 | Yes |
| 9 | 3.7/F | GBS Ventriculitis, hydrocephalus (Structural) | VGB (LEV) | 5 | 5 | No |
| 10 | 6.6/F | CDKL5 Mutation (Genetic) | ACTH & VGB (VPA,TPM,CLB) | 2.5 | 2 | No |
| 11 | 18.3/M | Unknown | ACTH | 3.5 | 2 | Yes |
| 12 | 4.9/F | Neonatal HIE (Structural) | ACTH (PHB) | 3.5 | 2 | Yes |
| 13 | 6.3/F | Unknown | ACTH | 4.5 | 3 | Yes |
| 14 | 7.7/M | Unknown | ACTH | 5 | 2 | Yes |
| 15 | 7.7/M | Tuberous Sclerosis (Structural/Genetic) | VGB (PHB) | 4 | 2.5 | No |
| 16 | 6.0/F | Chromosome 8 Abnormality & Stroke (Structural & Genetic) | ACTH (PHB) | 3.5 | 2 | Yes |
| 17 | 5.8/M | Pachygyria (Structural) | ACTH (PHT,LRZ) | 5 | 2 | Yes |
| 18 | 5.3/M | Lissencephaly (Structural) | ACTH | 3 | 5 | No |
| 19 | 19.4/F | Bacterial Meningoencephalitis (Structural) | ACTH (PHB,DZP) | 5 | 2 | No |
| 20 | 9.0/F | Prematurity & Left-sided IVH (Structural) | ACTH | 4.5 | 2 | Yes |
| 21 | 4.9/F | Unknown | ACTH | 4.5 | 3.5 | No |
3.2. Brain networks associated with epileptic spasms are stable and exhibit strong crosshemispheric functional connections
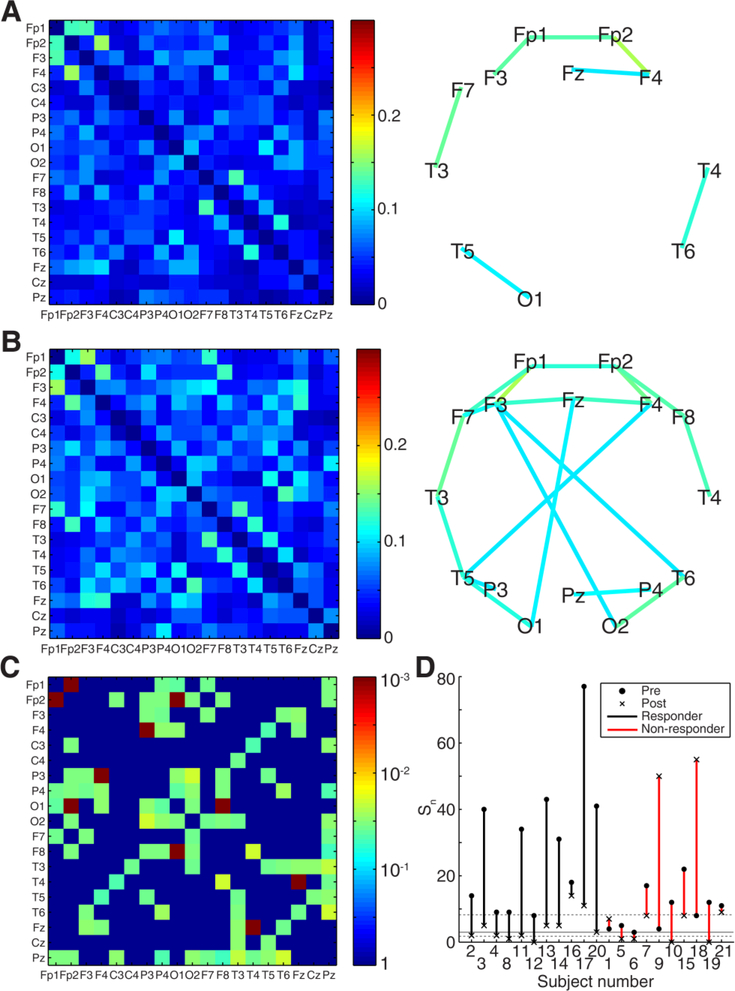
When the connectivity matrices were averaged across all control subjects, a core network of strong functional connections emerged (Figure 1A). These connections were located in two bilaterally symmetric clusters: 1) occipital and posterior temporal head regions (electrodes T5, T6, O1, and O2), and 2) frontopolar, frontal, and anterior/mid-temporal head regions (electrodes Fp1, Fp2, Fz, F3, F4, F7, F8, T3, and T4). The average connectivity matrix for pre-treatment epileptic spasms subjects contained this same core network, with additional strong crosshemispheric connections between frontal and parietal regions (Figure 1B).
Figure 1:
Average functional connection strengths Cij and network maps for (A) Controls (n=21) and (B) Pre-treatment epileptic spasms (n=21). Values in the connectivity matrices represent the proportion of 1second epochs for which the connectivity between two channels was statistically significant. Network maps show all connections with strength > 0.15. (C) Statistical significance for differences in connection strength between controls and pre-treatment spasms, with significant pairs colored according to the FDRcorrected p-value. Non-significant pairs are given a value of one. (D) Stability of functional connectivity measurements for control subjects (gray) and pre-treatment epileptic spasms subjects (green). Each solid colored line represents the mean, while the shaded areas denote the 95% confidence interval across all subjects in the group.
Functional connections in pre-treatment ES subjects were significantly elevated compared to control subjects (n=21, p<0.05 pre-specified threshold FDR, corrected for multiple comparisons using the Benjamini-Hochberg procedure; pFDR = 0.006, q-value = 0.006). Of the 171 electrode pairs, 73 pairs had connection strengths Ci j,n that were statistically different between pretreatment spasms subjects and controls, with 72 pairs (all except Cz-O2) showing higher median levels of connectivity in the spasms group (Figure 1C).
The stability of the functional networks within each subject group was assessed as a function of the length of data used to calculate the connection strength (Figure 1D). Analysis of the control subject data showed levels of stability that were similar to those previously reported for adult data (Chu et al., 2012). The stability of networks for pre-treatment ES subjects was significantly higher than the control group for segments of data up to ~400 seconds long (Figure 1D).
3.3. Strong pre-treatment connectivity is associated with favorable treatment response
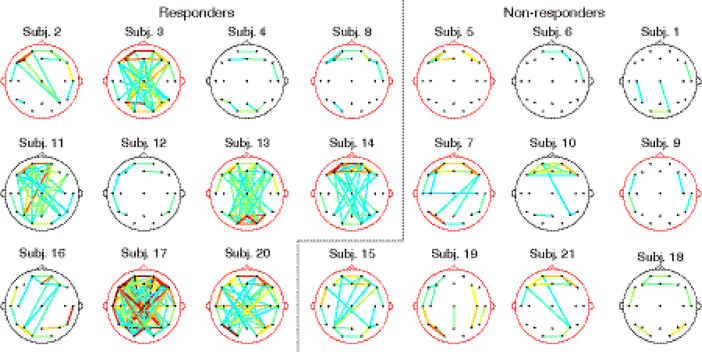
When the pre-treatment functional networks were grouped by treatment response (Figure 2), we found that responders were associated with higher levels of pre-treatment connectivity. In particular, six subjects (3, 11, 13, 14, 17, 20) had an unusually large number of strong connections. These six subjects, representing over half of all responders (n=11), had diverse clinical attributes, including BASED score and etiology (see Section 3.4 and Discussion for details). In contrast, all non-responders had relatively weak pre-treatment connectivity.
Figure 2:
Pre-treatment functional connectivity maps for all epileptic spasms subjects. Only connections with Cij,n > 0.15 are shown, to aid visualization. The color of each connection indicates its strength, with the strongest connections displayed in orange and red. The color of the head model designates mean BASED score ≥4 (red) or <4 (black). The overall strength of a subject’s functional connections is not related to the presence or absence of hypsarrhythmia at the subject level.
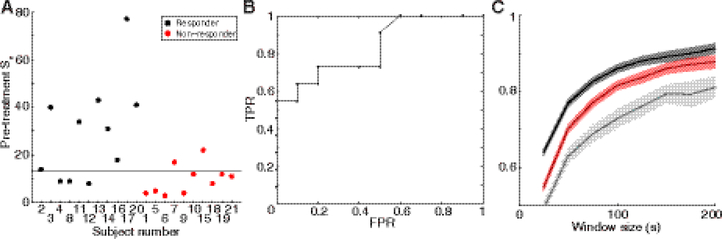
Across all subjects, strong pre-treatment functional networks were predictive of good outcome (Figure 3). Statistically, the pre-treatment Sn for responders had a higher median value than the pre-treatment Sn for non-responders (Figure 3A, Wilcoxon rank sum, p = 0.011). A receiver operating characteristic (ROC) curve demonstrated that pre-treatment Sn could be used to classify responders and non-responders (Figure 3B). The ROC curve had an area under the curve of 0.83; a value of one indicates a perfect test and a value of 0.5 indicates a test operating at chance levels.
Figure 3:
Pre-treatment connectivity strength predicts treatment response at the individual subject level. (A) Number of strong connections, Sn, with threshold T= 0.15, in the pre-treatment functional networks of all epileptic spasms subjects. Color indicates treatment response, and the dashed line is the threshold that provides optimal separation between responders and non-responders based on the ROC curve. (B) The ROC curve for pre-treatment Sn has an area under the curve of 0.83. Sensitivity is measured as true positive rate (TPR) and is plotted versus false positive rate (FPR, 1-specificity). (C) Stability of functional connectivity measurements for pre-treatment data from epileptic spasms subjects. Subjects are grouped into responders (black) and non-responders (red). Control subjects (gray) are shown for comparison. Each solid colored line represents the mean and the shaded areas denote the 95% confidence interval.
Differences between treatment response groups were reflected in the stability of functional connectivity as well. Prior to treatment, the functional networks of responders had higher levels of stability than non-responders (Figure 3C), making network stability another potential pretreatment predictor of response.
3.4. Change in functional connectivity strength correlates to treatment response
Following treatment, the strength of functional connections in responders (Figure 4A) was consistently reduced. When averaged across the subjects in this group, the functional networks following treatment had structures similar to the core network seen in control subjects (compare Figure 4A to Figure 1A), with the strongest connections found in the posterior head regions (occipital and posterior temporal) as well as in the frontotemporal head regions, bilaterally. In contrast, some cross-hemispheric functional connections remained pathologically elevated in non-responders after treatment (Figure 4B). In a paired statistical test, responders had 54 connections that were significantly lower after treatment initiation (Figure 4C, permutation test, p<0.0005, q<0.0005, pre-specified threshold FDR, corrected for multiple comparisons using the Benjamini-Hochberg procedure), while non-responders showed no significant differences between pre- and post-treatment connection strengths.
Figure 4:
Average post-treatment functional connection strengths Cij,n and network maps for (A) Responders (n=11), (B) Non-responders (n=10). Network maps show all connections with strength Cij,n >0.15 for visualization purposes. At the group level, both responders and non-responders exhibit decreases in functional connectivity strength following treatment, but non-responders retain some of the strong cross-hemispheric connections seen in pre-treatment subjects. (C) Significance of pre- and posttreatment connection differences for responders (n=11). (D) Number of strong connections, for pre- and post-treatment data from all epileptic spasms subjects (threshold T=0.15). Subjects are grouped based on treatment response (line color), and both pre-treatment (dots) and post-treatment (x’s) values are shown. The gray solid line represents the median value for control subjects, and the gray dashed lines represent the 25th and 75th percentiles.
These changes in functional connectivity, from pre- to post-treatment, were related to treatment response at an individual subject level (Figure 4D). Responders, who exhibited resolution of hypsarrhythmia and cessation of spasms, all had a decrease in connectivity strength following treatment initiation (Figure 4D, black). Three non-responders demonstrated an increase in connection strength following treatment initiation, while the other non-responders exhibited small decreases (Figure 4D, red). Statistically, the change in Sn from pre- to post-treatment was unequal between responders and non-responders (Wilcoxon rank sum, p = 0.011); however, it is likely that this is largely due to the differing pre-treatment values. These results suggest that the change in connection strength is related to treatment response, particularly for large increases or decreases.
The six subjects with the strongest pre-treatment functional connectivity (Subjects 3, 11, 13, 14, 17, and 20) were all responders and had the largest decreases in connectivity strength when comparing pre- and post-treatment values. This was not likely due to chance. If all 21 ES subjects were randomly assigned to responder (n=11) and non-responder groups (n=10), the likelihood of these six subjects all being assigned to the responder group is p < 0.009. This result is robust to changes in the threshold T=0.15 which was used to define “strong” connections in Figure 2. These same six subjects have the highest values of Sn for thresholds ranging from T =0.09 (all six subjects have Sn≥60) to T=0.09 (all six subjects have Sn≥7). Because the strength of individual connections typically ranges from zero to 0.3 (see Figures 1 and 4), this represents 47% of all possible thresholds.
3.5. Pre-treatment functional connectivity strength is related to long-term outcome
Long-term outcome data were collected for ES subjects, including the degree of cognitive delay and seizure control (Table 2). The average time between initial treatment and collection of follow-up data was 37 months. Of the 21 subjects, five subjects (24%) had either no delay or mild cognitive developmental delay, four (19%) had moderate delay, three (14%) had moderate to severe delay, and nine (43%) had severe delay. Six subjects (29%) were seizure free and not taking antiepileptic medications, three subjects (14%) were seizure free while taking antiepileptic medications, and twelve (57%) experienced continued seizures while taking antiepileptic medications.
Table 2:
Long-term developmental outcome and seizure outcome for epileptic spasms subjects.
| Subject | Follow-up (time since initial treatment) | Cognitive developmental delay | Seizure outcome (other seizure types, not spasms) |
|---|---|---|---|
| 1 | 3 yrs, 3 mos | Severe | Continued seizures, on meds |
| 2 | 8 mos | None | Seizure free, off meds |
| 3 | 4 yrs, 9 mos | None/mild | Seizure free, off meds |
| 4 | 5 yrs, 8 mos | Moderate | Seizure free, on meds |
| 5 | 3 yrs, 8 mos | Severe | Continued seizures, on meds |
| 6 | 1 yrs, 1 mos | Moderate | Continued seizures, on meds |
| 7 | 2 yrs, 1 mos | Severe (deceased) | Continued seizures, on meds |
| 8 | 4 yrs, 9 mos | Severe | Continued seizures, on meds |
| 9 | 4 yrs, 10 mos | Severe | Continued seizures, on meds |
| 10 | 7 mos | Severe (deceased) | Continued seizures, on meds |
| 11 | 1 yrs, 7 mos | Mild | Seizure free, off meds |
| 12 | 1 yrs, 0 mos | Moderate/Severe | Seizure free, off meds |
| 13 | 2 yrs, 2 mos | Moderate | Seizure free, off meds |
| 14 | 10 mos | None | Seizure free, off meds |
| 15 | 4 yrs, 9 mos | Moderate/Severe | Continued seizures, on meds |
| 16 | 6 yrs, 3 mos | Moderate/Severe | Seizure free, on meds |
| 17 | 6 mos | Moderate | Continued seizures, on meds |
| 18 | 2 yrs, 0 mos | Severe | Continued seizures, on meds |
| 19 | 4 yrs, 2 mos | Severe | Continued seizures, on meds |
| 20 | 5 yrs, 5 mos | Mild | Seizure free, on meds |
| 21 | 4 yrs, 1 mos | Severe | Continued seizures, on meds |
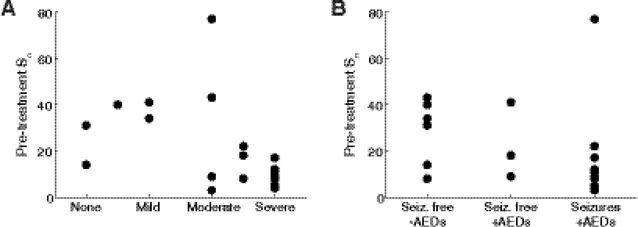
While the relationships between functional connectivity strength and long-term outcomes are not strong, particularly for long-term seizure control, there are some promising trends that warrant further investigation. Of the six subjects with the strongest pre-treatment connectivity, four had no developmental delay or mild delay and two had moderate delay (Figure 5A). This is noteworthy, considering that only five subjects in the entire dataset were categorized as having no or mild developmental delay. Moreover, all subjects with severe delay had low pre-treatment connectivity (Sn<20). In general, the relationship between functional connectivity strength and long-term seizure control was weak (Figure 5B), but as with developmental delay, the worst outcome (continued seizures while on medication) was associated with low pre-treatment connectivity, with the exception of one subject, who had a cortical malformation. Here, we forego statistical analysis due to the small sample size, the ordinal nature of the long-term outcome data, and the lack of established criteria for assigning patients to each category. This is a limitation of the current study that can be addressed in future work.
Figure 5:
Relationships of functional connectivity strength to long-term outcomes for 21 epileptic spasms subjects. Pre-treatment functional connectivity strength Sn versus (A) Long-term developmental delay and (B) Long-term seizure outcome. Long-term seizure outcomes were grouped into three categories: (1) seizure free off antiepileptic drugs (AEDs), (2) seizure free on AEDs, and (3) continued seizures on AEDs.
3.6. Functional connectivity measurements are not surrogates of visual EEG features
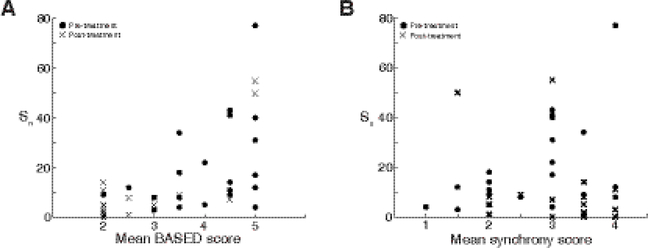
The strength of functional connectivity in each subject was not directly related to any visuallydiscernable characteristic of the EEG data. For example, subjects with relatively high levels of connectivity (Sn > 20) appear to have average BASED scores of 3.5 or higher (Figure 6A, R2 = 0.41, p = 4.07e-6, linear regression model). However, subjects with BASED scores ≥ 4, indicative of hypsarrhythmia, had a wide range of connectivity levels, from very low to very high. This suggests that the high levels of connectivity seen in some subjects may be due, in part, to the presence of hypsarrhythmia, yet hypsarrhythmia is not always associated with elevated levels of functional connectivity. Blinded ratings of synchrony (Figure 6B) were not correlated with overall connectivity strength at a clinically meaningful level (R2 = 0.001 for synchrony; linear regression).
Figure 6:
(A) Overall strength of functional connectivity, Sn, versus average BASED score. Sn indicates the number of connections in each subject n with strength greater than threshold T=0.15. While most subjects with high levels of connectivity have a high BASED score, there are many subjects with high BASED score and weak connectivity. (B) Mean synchrony scores are not related to the strength of functional connectivity Sn, with threshold T=0.15; both pre- and post-treatment values are shown.
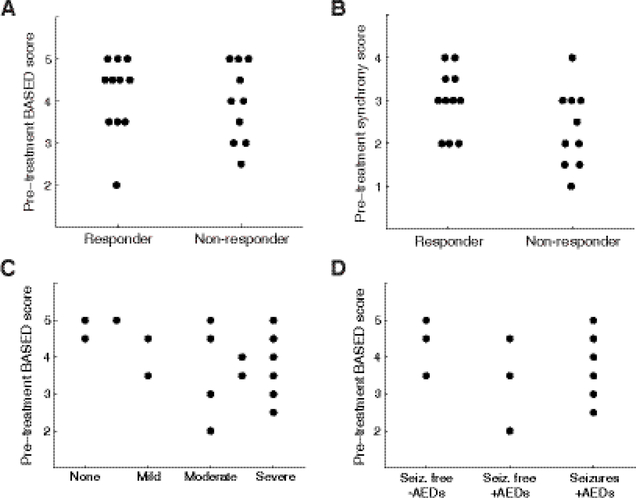
3.7. Visual EEG features are not predictive of treatment response nor long-term outcome
Whereas pre-treatment functional connectivity predicted treatment outcome in a subset of patients, the mean pre-treatment BASED score, used as a more reliable indicator of hypsarrhythmia, was unrelated to treatment response (Figure 7A). The lack of prognostic value for hypsarrhythmia echoes the observation of Demarest et al. in a contemporary large-scale prospective cohort study (Demarest et al., 2017). Similarly, the subjective visual assessment of synchrony in pre-treatment EEG data did not predict response to treatment (Figure 7B).
Figure 7:
Relationship between visual EEG features, treatment response, and long-term outcomes. (A) Mean pre-treatment BASED score is not related to treatment response. (B) Mean pre-treatment synchrony score, assessed visually, is not related to treatment response. Bottom subfigures show relationships of visual EEG assessment to long-term outcomes for 21 epileptic spasms subjects. Pre-treatment BASED scores versus (C) Long-term developmental delay and (D) Long-term seizure outcome.
To discern whether the long-term outcome was related to the presence or absence of hypsarrhythmia, we examined the relationship between the pre-treatment BASED score and long-term outcomes of cognitive delay (Figure 7C) and seizure control (Figure 7D). There were no direct relationships between these variables, particularly for long-term seizure control. It is possible that low pre-treatment BASED scores are associated with poor developmental outcome, as four subjects had BASED scores ≤ 3, and all experienced moderate or severe developmental delay. However, assessing the significance of this trend was limited by sample size. High pretreatment BASED scores were linked to all possible developmental outcomes.
4. Discussion
This study is noteworthy in that it is the first to correlate functional networks in ES with treatment response and long-term outcome. We found that subjects with ES had elevated EEGbased functional connectivity compared to healthy control subjects. However, analysis of individual subject networks demonstrated a high level of intragroup heterogeneity with regards to both network strength and structure. A subset of subjects with ES exhibited very high levels of connectivity, and these subjects all responded favorably to initial treatment. Following treatment initiation, all responders showed decreased connectivity, whereas minimally decreased or increased connectivity was noted in non-responders. Additionally, the functional networks of untreated ES subjects exhibited unusually high degrees of stability which returned to levels similar to healthy controls following successful treatment. With validation in a larger, prospective dataset, the characterization of functional connectivity and network stability may become a valuable tool for the prediction and assessment of treatment response in ES.
We defined connectivity strength Sn as the number of connections exceeding a threshold, rather than using a measure of central tendency, such as the mean or median connectivity value. We did this because the distribution of all connectivity values was skewed toward low values, which were likely to occur by chance. Here we assumed that the most important connections in the network were the strongest ones (high C i j,n), indicating consistent significant correlations between electrode pairs. Therefore, the measure of strength Sn is robust against potential bias from the large number of weaker and less important connections.
Our report of high functional connectivity in subjects with ES is not altogether surprising. Diverse methodological approaches, including EEG source analysis (Japaridze et al., 2013), PET (Chugani et al., 1992), and simultaneous EEG and fMRI (Siniatchkin et al., 2007), have all implicated a variety of deep brain structures in the generation and propagation of epileptic spasms and hypsarrhythmia. It is likely that subcortical pathology mediates the high functional connectivity discussed in this study. Analogous to our findings, elevated EEG coherence has been observed in association with hypsarrhythmia (Burroughs et al., 2014). However, we chose to use a relatively simple approach, cross-correlation, because it produces robust and stable measurements of functional connectivity for scalp EEG (Chu et al., 2012). Cross-correlation also produced more stable measurements than coherence and autoregressive modeling in a testretest experiment (Fiecas et al., 2013).
In control subjects, we found symmetric functional networks involving connections within the posterior temporal/occipital head region as well as within the frontotemporal head region bilaterally. Although eye blink artifacts were removed prior to analysis, it is possible that eye movements contributed to the high levels of connectivity seen in the frontal head regions bilaterally, specifically in the Fp1-Fp2 electrode pairing. The increased connectivity we saw in the bilateral posterior temporal and occipital head regions is consistent with previous literature supporting the existence of infantile resting-state networks involving the primary visual and auditory cortices (Fransson et al., 2007), though contributions from posteriorly dominant rhythms of control subjects may have played a role as well.
Although pre-treatment ES subjects exhibited higher levels of connectivity than controls in known physiologic networks (i.e. fronto-temporal and temporo-occipital), a more striking discovery was the presence of long-range connections from multiple brain regions observed in the pre-treatment spasms group. This suggests that long-range connections are important substrates of the pathological network responsible for generating epileptic spasms, consistent with data previously reported (Burroughs et al., 2014).
The strength of functional connectivity in each subject was not closely related to the subject’s BASED score. More specifically, subjects with BASED scores ≥ 4, indicative of hypsarrhythmia, had significant variation in their levels of connectivity. On the other hand, subjects with relatively high levels of connectivity (Sn > 20) were noted to have average BASED scores of 3.5 or higher. As mentioned above, this supports the notion that the presence of hypsarrhythmia imparts some degree of elevated connectivity to the subject’s EEG, in certain instances. This is logical, as hypsarrhythmia is a unique electroencephalographic pattern seen nearly exclusively in the infant brain, and is most often seen diffusely throughout the cortex, even in cases where it is caused by an underlying focal lesion. This further suggests the involvement of a deep network of subcortical brain structures that propagates the abnormal activity seen in hypsarrhythmia in a hyper-connected fashion, as previously discussed.
Another visually apparent component of the EEG related to functional connectivity is interhemispheric synchrony (Rasanen et al., 2013, Koolen et al., 2014). We found no correlation between visually assessed interhemispheric synchrony and functional connectivity (R2 = 0.001), as shown in Figure 6B. This suggests that functional connectivity is not discernable with standard clinical EEG review, and that strong functional connectivity should not be equated with hypsarrhythmia (BASED score ≥ 4) or hypersynchrony, as identified by electroencephalographers.
Of the ES subjects, 11 were responders and 10 were non-responders. Among the responders, six subjects exhibited the highest levels of pre-treatment connectivity of the entire cohort (Sn > 25), as can be seen in Figure 2 and Figure 3A. These six subjects did not stand out in any way based on clinical data or visual features of the EEG. Blinded reviewers noted nothing unusual about these particular studies. Three of these subjects had an unknown etiology with normal MRI; one was premature with diffuse atrophy on MRI; one was premature with left-sided hemorrhage; one had pachygyria. Five out of the six subjects were aged 5.8–9.0 months at the time of the first EEG study, while the sixth subject was 18 months old. Five out of the six subjects were not on any medication at the time of the first EEG study; in total, 10 out of 21 subjects fell into this category. The time between onset and treatment ranged from four days to greater than one month. The only characteristic that was common to all six subjects was that none of them had prior seizures; note that, in total, 10 out of the 21 ES subjects did not have prior seizures. Although the size of the cohort is small, these findings suggest that high pre-treatment Sn values indicates a state of susceptibility to treatment and may therefore be a predictor of favorable treatment response. Additionally, a clear relationship between change in connectivity and treatment response was seen. All responders showed decreases in connectivity toward control values following treatment initiation, whereas non-responders demonstrated either mild decreases or increases in connectivity.
To our knowledge, this is the first study to systematically evaluate the effect of treatment on cortical connectivity in ES. We found that high levels of pre-treatment connectivity, unique to a subset of responders, were indicative of favorable treatment response. These elevated connectivity levels are likely multifactorial in nature – they may be partially due to the seizurenaïve state of the brain, as all six subjects were seizure-free prior to the onset of ES.
Hyperconnectivity, while associated with the pathological condition of ES, may also indicate a transient susceptibility to treatment. For example, it is unknown how the strength of these pathological networks changes over time after the onset of spasms. Perhaps the connectivity is high early in the course of ES and diminishes over time. Because ES comprises a diverse group of patients with similarly diverse functional networks, further study is needed to explore this phenomenon.
Additionally, the connectivity of responders decreased to levels comparable to control subjects following treatment initiation. The normalization of this measurement suggests that connectivity may also be an objective way to assess treatment response. However, the majority of nonresponders also exhibited decreased connectivity following treatment, with only three nonresponders showing increased post-treatment connectivity. It was unclear why these nonresponders showed an increase in connectivity, but all three belonged to the group of nonresponders with the worst long-term outcomes, as defined by severe cognitive impairment and intractable seizures. The development of other seizure types, as occurs in 50–70% of patients with ES (Pavone et al., 2014), is one possible explanation for the increase in connectivity; however, this group of subjects is too small to draw concrete conclusions.
Connectivity strength and network stability appeared to be related to one another, as five of the six subjects with the highest connectivity strengths Sn also had the highest levels of stability. This is not surprising; strong connections that are well above chance levels of connectivity will stand out from the noisy background and are more likely to be consistently detected over time.
However, these two quantities were not directly correlated to one another. For example, subject 11 had high Sn but relatively low levels of stability, and it was noted by one epileptologist that this EEG contained intermittent bursts of hypsarrhythmia. This suggests that the temporal properties of hypsarrhythmia may be important to consider when developing quantitative measurements.
Long-term developmental and seizure outcomes were compared to both visually assessed EEG characteristics and pre-treatment Sn values. It should be emphasized that these outcome data are significantly limited by several factors, as described below, possibly confounding this analysis. In Figure 5A, it is noteworthy that four of the five subjects with the best developmental outcomes are from the six subjects with the highest pre-treatment Sn values, and that none of highest six subjects were found to have severe or moderate/severe developmental delay. This suggests that higher pre-treatment connectivity levels are associated with more favorable longterm cognitive outcomes. Likewise, in Figure 5B, the vast majority of subjects in the worst seizure outcome group (continued seizures while taking AEDs) exhibited lower pre-treatment Sn values; the one exception was subject 17, who has an underlying diagnosis of pachygyria. Lastly, four of the six most highly connected subjects reside in the best long-term seizure outcome category, again suggesting that higher pre-treatment connectivity levels may indicate more favorable long-term outcomes.
Recent literature has suggested that pre-treatment hypsarrhythmia is not indicative of treatment response in patients with ES (Demarest et al., 2017). This is consistent with our results, as BASED scores ≥ 4 were seen in responders and non-responders, with no clear correlation noted (Figure 7A). Visually scored synchrony was also not strongly correlated to treatment response (Figure 7B), and as depicted in Figures 7C and 7D, there were no clear correlations between BASED score and long-term developmental outcome or long-term seizure outcome. This highlights the need for more robust biomarkers of treatment response and long-term outcome in ES.
The current study has several important limitations. First, this was a retrospective analysis of EEG data with a relatively small sample size, especially considering the diversity of etiologies associated with ES. The retrospective nature of the data collection prevented more rigorous standardization of the interval between the pre- and post-treatment EEG recordings. Four subjects had very long time intervals between recordings (> 180 days); however, this subgroup contained an equal number of responders and non-responders, and those that responded did so immediately following treatment initiation. Exclusion of these four subjects did not change any of the conclusions presented here. A major limitation that arose from using retrospective data was measuring long-term developmental and seizure outcomes. Two of the subjects had passed away within six months of ES onset, and several others were lost to follow-up within one year. Additionally, both developmental status and seizure control were not recorded in a standardized fashion, requiring significant extrapolation of these measures. This undoubtedly imparted inconsistencies into the analysis, and a more rigorous, prospective data collection may show significant correlations between long-term outcomes and the various measurements we evaluated.
While complete treatment response in ES remains clinically defined as resolution of both spasms and hypsarrhythmia, various studies have suggested different levels of importance for the presence of hypsarrhythmia in the post-treatment EEG (Koo et al., 1993, Yamada et al., 2014, Altunel et al., 2015). On one hand, this supports the need for new, objective, and robust measurements for ES; however, using post-treatment hypsarrhythmia, or even BASED score, may not be the most accurate way to classify treatment response. While the EEG data segments were clipped in a blinded fashion, without knowledge of treatment status or outcome, they were not selected randomly. Segments of data with minimal artifact were chosen for inclusion in this study, which may potentially be a source of selection bias. Lastly, while the effect of volume conduction is always a concern when assessing EEG connectivity, we addressed this by eliminating zero time lag cross-correlation values, which has been shown to be an effective and conservative approach (Chu et al., 2012).
Due to these limitations, more rigorous validation is needed to further assess the true clinical significance of functional connectivity and its relationship to other clinical measurements and outcomes. We have shown that strong functional connections are related to the presence of hypsarrhythmia over a wide range of etiologies and that high Sn values may be indicative of favorable treatment response. Additionally, the change in functional connectivity following treatment initiation is a promising classifier of responders versus non-responders. Given hypsarrhythmia’s limited clinical value, further study is needed to identify metrics that can assess true treatment response and predict long-term outcomes in patients with ES. Therefore, future work will include validation of these results in large-scale and prospective studies, with the goal of developing an index to measure the disease burden and degree of response to therapy for individual patients. Both short-term electroclinical outcomes and long-term developmental outcomes will be considered, and the quantitative EEG analysis will be expanded to include calculation of frequency-specific networks. Once validated, there is nothing to prevent these methods from being implemented in clinical practice; clinicians would simply select a segment of awake EEG data for analysis and provide it as input into a piece of software that would calculate the connectivity and relevant metrics. Overall, the use of functional connectivity as an objective, robust tool for the assessment of ES has the potential to (1) impact clinical care by enabling personalized treatment programs and expediting successful treatment for children affected by this disease, and (2) increase the efficiency of clinical trials by enabling the use of an objective measure of treatment response.
Supplementary Material
Highlights.
Subjects with epileptic spasms had strong functional connectivity in EEG; those with the strongest networks responded to treatment.
Post-treatment, responders had weaker networks; increased strength was only seen in non-responders.
Visual EEG measures (hypsarrhythmia, synchrony) were not predictive of treatment response.
Acknowledgements
The authors would like to thank Mary Zupanc, MD, for her mentorship and critical review of the manuscript, as well as Vaibhav Bajaj and Rachel Smith, who contributed preliminary data analysis. This work was supported by a Children’s Hospital of Orange Country (CHOC) PSF Tithe grant and an ICTS CHOC-UC Irvine Collaborative Pilot grant.
Footnotes
Conflict of Interest Statement
Dr. Hussain has received research support from the Epilepsy Therapy Project, the Milken Family Foundation, the Hughes Family Foundation, the Elsie and Isaac Fogelman Endowment, Eisai, Lundbeck, Insys Therapeutics, GW Pharmaceuticals, and the NIH (R34MH089299). He has served on the scientific advisory boards of Mallinckrodt, Upsher-Smith Laboratories, and Insys Therapeutics, on the speakers bureau of Mallinckrodt, and as a consultant to Eisai, UCB, and Mallinckrodt. The remaining authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altunel A, Sever A, Altunel EO. Hypsarrhythmia paroxysm index: A tool for early prediction of infantile spasms. Epilepsy Res. 2015;111:54–60. [DOI] [PubMed] [Google Scholar]
- Altunel A, Altunel EO, Sever A. The Utility of the Hypsarrhythmia Paroxysm Index and Sleep Spindles in EEG for Predicting Cognitive Outcomes in a Case Series of Infantile Spasms. J Neurol Neurophysiol. 2015;6:319. [Google Scholar]
- Beghi E, Frigeni B, Beghi M, De Compadri P, Garattini L. A review of the costs of managing childhood epilepsy. Pharmacoeconomics. 2005;23:27–45. [DOI] [PubMed] [Google Scholar]
- Burroughs SA, Morse RP, Mott SH, Holmes GL. Brain connectivity in West syndrome. Seizure. 2014;23:576–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraballo RH, Fortini S, Reyes G, Carpio Ruiz A, Sanchez Fuentes SV, Ramos B. Epileptic spasms in clusters and associated syndromes other than West syndrome: A study of 48 patients. Epilepsy Res. 2016;123:29–35. [DOI] [PubMed] [Google Scholar]
- Chiron C, Dulac O, Bulteau C, Nuttin C, Depas G, Raynaud C, et al. Study of regional cerebral blood flow in West syndrome. Epilepsia. 1993;34:707–15. [DOI] [PubMed] [Google Scholar]
- Chu CJ, Kramer MA, Pathmanathan J, Bianchi MT, Westover MB, Wizon L, et al. Emergence of stable functional networks in long-term human electroencephalography. J Neurosci. 2012;32:2703–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugani HT, Shewmon DA, Sankar R, Chen BC, Phelps ME. Infantile spasms: II. Lenticular nuclei and brain stem activation on positron emission tomography. Ann Neurol. 1992;31:212–9. [DOI] [PubMed] [Google Scholar]
- Demarest ST, Shellhaas RA, Gaillard WD, Keator C, Nickels KC, Hussain SA, et al. The impact of hypsarrhythmia on infantile spasms treatment response: Observational cohort study from the National Infantile Spasms Consortium. Epilepsia. 2017;58:2098–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donat JF, Lo WD. Asymmetric hypsarrhythmia and infantile spasms in west syndrome. J Child Neurol. 1994;9:290–6. [DOI] [PubMed] [Google Scholar]
- Fiecas M, Ombao H, van Lunen D, Baumgartner R, Coimbra A, Feng D. Quantifying temporal correlations: a test-retest evaluation of functional connectivity in resting-state fMRI. Neuroimage. 2013;65:231–41. [DOI] [PubMed] [Google Scholar]
- Fisher RS, Cross JH, French JA, Higurashi N, Hirsch E, Jansen FE, et al. Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58:522–30. [DOI] [PubMed] [Google Scholar]
- Fransson P, Skiold B, Horsch S, Nordell A, Blennow M, Lagercrantz H, et al. Resting-state networks in the infant brain. Proc Natl Acad Sci U S A. 2007;104:15531–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaily E, Lommi M, Lapatto R, Lehesjoki AE. Incidence and outcome of epilepsy syndromes with onset in the first year of life: A retrospective population-based study. Epilepsia. 2016;57:1594–601. [DOI] [PubMed] [Google Scholar]
- Gibbs F Atlas of Electroencephalography. Cambridge, MA: Addison-Wesley; 1952. [Google Scholar]
- Hrachovy RA, Frost JD, Jr., Infantile epileptic encephalopathy with hypsarrhythmia (infantile spasms/West syndrome). J Clin Neurophysiol. 2003;20:408–25. [DOI] [PubMed] [Google Scholar]
- Hrachovy RA, Frost JD, Jr., Kellaway P Sleep characteristics in infantile spasms. Neurology. 1981;31:688–93. [DOI] [PubMed] [Google Scholar]
- Hrachovy RA, Frost JD, Jr., Kellaway P Hypsarrhythmia: variations on the theme. Epilepsia. 1984;25:317–25. [DOI] [PubMed] [Google Scholar]
- Hussain SA, Kwong G, Millichap JJ, Mytinger JR, Ryan N, Matsumoto JH, et al. Hypsarrhythmia assessment exhibits poor interrater reliability: a threat to clinical trial validity. Epilepsia. 2015;56:77–81. [DOI] [PubMed] [Google Scholar]
- Japaridze N, Muthuraman M, Moeller F, Boor R, Anwar AR, Deuschl G, et al. Neuronal networks in west syndrome as revealed by source analysis and renormalized partial directed coherence. Brain Topogr. 2013;26:157–70. [DOI] [PubMed] [Google Scholar]
- Sleep Kellaway P. and epilepsy. Epilepsia. 1985;26 Suppl 1:S15–30. [DOI] [PubMed] [Google Scholar]
- Koo B, Hwang PA, Logan WJ. Infantile spasms: outcome and prognostic factors of cryptogenic and symptomatic groups. Neurology. 1993;43:2322–7. [DOI] [PubMed] [Google Scholar]
- Koolen N, Dereymaeker A, Rasanen O, Jansen K, Vervisch J, Matic V, et al. Interhemispheric synchrony in the neonatal EEG revisited: activation synchrony index as a promising classifier. Front Hum Neurosci. 2014;8:1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer MA, Eden UT, Cash SS, Kolaczyk ED. Network inference with confidence from multivariate time series. Phys Rev E Stat Nonlin Soft Matter Phys. 2009;79:061916. [DOI] [PubMed] [Google Scholar]
- Kramer U, Sue WC, Mikati MA. Hypsarrhythmia: frequency of variant patterns and correlation with etiology and outcome. Neurology. 1997;48:197–203. [DOI] [PubMed] [Google Scholar]
- Mytinger JR, Hussain SA, Islam MP, Millichap JJ, Patel AD, Ryan NR, et al. Improving the inter-rater agreement of hypsarrhythmia using a simplified EEG grading scale for children with infantile spasms. Epilepsy Res. 2015;116:93–8. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: A primer with examples. Hum Brain Mapp. 2001;15:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne JP, Lux AL, Edwards SW, Hancock E, Johnson AL, Kennedy CR, et al. The underlying etiology of infantile spasms (West syndrome): information from the United Kingdom Infantile Spasms Study (UKISS) on contemporary causes and their classification. Epilepsia. 2010;51:2168–74. [DOI] [PubMed] [Google Scholar]
- Pavone P, Striano P, Falsaperla R, Pavone L, Ruggieri M. Infantile spasms syndrome, West syndrome and related phenotypes: what we know in 2013. Brain Dev. 2014;36:739–51. [DOI] [PubMed] [Google Scholar]
- Pellock JM, Hrachovy R, Shinnar S, Baram TZ, Bettis D, Dlugos DJ, et al. Infantile spasms: a U.S. consensus report. Epilepsia. 2010;51:2175–89. [DOI] [PubMed] [Google Scholar]
- Rasanen O, Metsaranta M, Vanhatalo S. Development of a novel robust measure for interhemispheric synchrony in the neonatal EEG: activation synchrony index (ASI). Neuroimage. 2013;69:256–66. [DOI] [PubMed] [Google Scholar]
- Raz J, Zheng H, Ombao H, Turetsky B. Statistical tests for fMRI based on experimental randomization. Neuroimage. 2003;19:226–32. [DOI] [PubMed] [Google Scholar]
- Riikonen RS. Favourable prognostic factors with infantile spasms. Eur J Paediatr Neurol. 2010;14:13–8. [DOI] [PubMed] [Google Scholar]
- Siniatchkin M, van Baalen A, Jacobs J, Moeller F, Moehring J, Boor R, et al. Different neuronal networks are associated with spikes and slow activity in hypsarrhythmia. Epilepsia. 2007;48:2312–21. [DOI] [PubMed] [Google Scholar]
- Van Putten MJAM Stam CJ. Is the EEG really “chaotic” in hypsarrhythmia. IEEE Eng Med Biol Mag. 2001;20:72–9. [DOI] [PubMed] [Google Scholar]
- Yamada K, Toribe Y, Kimizu T, Kimura S, Ikeda T, Mogami Y, et al. Predictive value of EEG findings at control of epileptic spasms for seizure relapse in patients with West syndrome. Seizure. 2014;23:703–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.