Abstract
Background
Recent guidelines recommend genetic counselling and DNA testing (GCT) for patients with ovarian cancer and survivors of ovarian cancer. Finding survivors of ovarian cancer is challenging. Detecting and referring them for GCT via primary care, to allow proper screening recommendations for patients and their family, may be a solution.
Aim
To compare the effectiveness and acceptance of two pilot strategies directed at case finding women with a history of ovarian cancer for referral for GCT by their GP.
Design and setting
Non-randomised comparison of the pilot implementation of two case-finding strategies for women with a history of ovarian cancer in Dutch primary care from May 2016 to April 2017.
Method
Strategy A (unsupported) asked GPs to identify and refer eligible patients with a history of ovarian cancer. Strategy B (ICT-supported) provided GPs with information and communication technology (ICT) support to identify patients with a history of ovarian cancer electronically. The effectiveness of each strategy was assessed as the proportion of patients who were approached, referred for GCT, and seen by the clinical geneticist. Acceptance of each strategy was assessed by the intervention uptake of GP practices and GP and patient questionnaires.
Results
Nineteen out of 30 (63%) patients identified with a history of ovarian cancer were deemed eligible for referral for strategy A, and 39 out of 94 (41%) for strategy B. For each strategy, eight patients were referred and five (63%) were seen for GCT. The intervention uptake by GP practices was 31% (11 out of 36) for strategy A and 46% (21 out of 46) for strategy B. GPs considered ‘relevance’ and ‘workability’ as facilitators across both strategies whereas, for strategy B, technical barriers hindered implementation.
Conclusion
The effectiveness and acceptance of both strategies for case finding of survivors of ovarian cancer in primary care for GCT is promising, but larger studies are required before wide-scale implementation is warranted.
Keywords: case finding, DNA testing, genetic counselling, ovarian cancer, primary care, referral
INTRODUCTION
Globally, ovarian cancer is the seventh most common cancer in women, with particularly high incidence rates in Europe. Annually, approximately 65 000 European women are diagnosed with ovarian cancer,1 of whom more than 93% have epithelial ovarian cancer.2 Until recently, only patients with a high-risk profile, based on age, family history, and histology, were eligible for referral for genetic counselling and DNA testing (GCT). However, accumulating evidence suggests that BRCA1/2 mutations may be found in women with epithelial ovarian cancer irrespective of their risk profile.3 Approximately 10–15% of women with epithelial ovarian cancer carry a BRCA1 or BRCA2 mutation.4,5 Consequently, the recent guidelines recommend referring all women with epithelial ovarian cancer for GCT, irrespective of their age and family history.6,7
Women carrying a BRCA1 or BRCA2 mutation have a highly increased risk of developing epithelial ovarian cancer and breast cancer, and may require preventive surgery and tailored diagnostic monitoring.6,8–10 Therefore, according to the guidelines, women who have, or who have survived, ovarian cancer should be referred for GCT.
However, identifying women with a history of epithelial ovarian cancer is challenging, because these women are often no longer under surveillance. The need for systematic programmes to identify these women is widely recognised.9–12
GPs have extensive knowledge of their practice population, because of longstanding relationships and integrated medical data. Therefore, GPs are in the optimal position to identify and refer women with a history of epithelial ovarian cancer.13–15 For optimal case finding of epithelial ovarian cancer in primary care, all women with a history of ovarian cancer need to be referred, because detailed diagnostic information is generally not available in primary care.
So far, the case finding for ovarian cancer is suboptimally implemented in primary care. It is estimated that one in 22 women in primary care are eligible for GCT because of an increased risk of hereditary breast and ovarian cancer, but only 5% of those eligible receive these services.16 A more proactive policy, with active case finding of patients with an increased genetic risk of cancer and referral for GCT, is required.17,18 Given the limited knowledge of the genetic risks among GPs, awareness needs to be increased and support in daily practice may be required to achieve effective case finding.15,19 Because all Dutch GPs use electronic medical records (EMR) that include diagnostic codes, EMR support for identification of patients with a history of ovarian cancer may be of added value.
How this fits in
Recent guidelines recommend genetic counselling and DNA testing (GCT) for all women newly diagnosed with ovarian cancer. GCT is also warranted for survivors of ovarian cancer, most of whom have not received GCT, to allow family members at increased cancer risk to receive proper screening recommendations. Two case-finding strategies were piloted and assessed in primary care, with the aim of identifying survivors of ovarian cancer and referring them for GCT. The results suggest that both information technology-supported and unsupported case-finding strategies enable the identification of women at increased risk of carrying a BRCA1/2 gene mutation.
This pilot implementation study in primary care compared the effectiveness and acceptance of two strategies directed at case finding women with a history of ovarian cancer and referring them for GCT.
METHOD
Study design
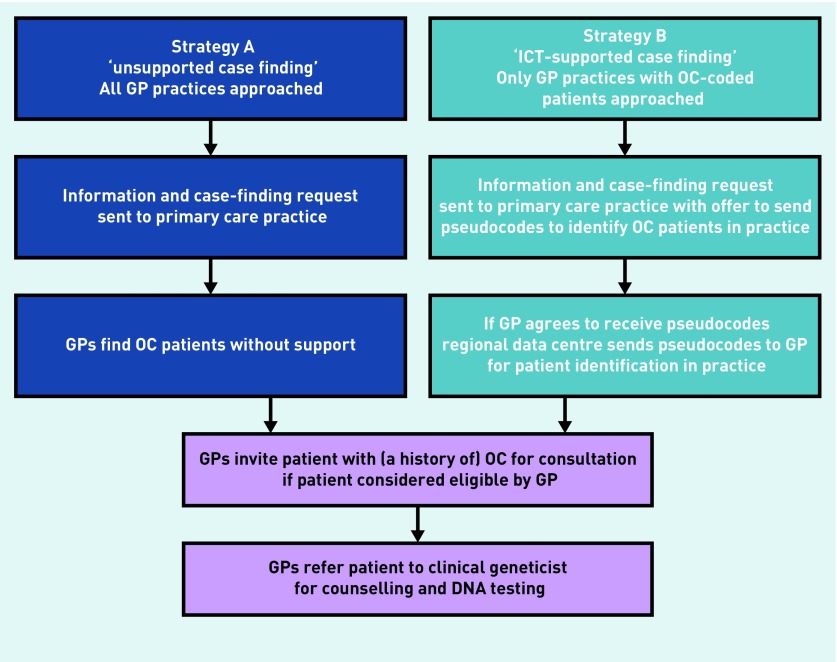
In a non-randomised setting, two case-finding strategies were piloted in primary care in separate regions in the Netherlands, from May 2016 to April 2017, and their effectiveness and acceptance were compared. Both strategies aimed to identify and refer patients with a history of ovarian cancer for GCT. Strategy A was called ‘unsupported case finding’ and strategy B was called ‘information and communication technology (ICT) supported case finding’. Flowcharts for both strategies are provided in Figure 1.
Figure 1.
Overview of two strategies aimed at case finding women with a history of ovarian cancer (OC) in primary care for referral for genetic counselling and DNA testing.
ICT = information and communication technology.
Interventions
Strategy A, ‘unsupported case finding’, informed GPs about the revised national guideline concerning hereditary and familial ovarian cancer7 by letter, and asked them to identify eligible patients in their practice and refer them for GCT. For this strategy, all 56 GPs from 36 practices who were members of a regional primary care network for continuing medical education were contacted. The letter included information about the revised guideline and the need to refer patients for GCT, and a video was provided. In the video, a geneticist explicitly recommended the identification and referral of patients with ovarian cancer in their population who had not previously undergone genetic testing. Assistance was offered by the genetics department to check whether patients had already been seen by the genetics department. GPs received contact information of the research coordinator. For patients, an explanatory video including information about the ovarian cancer guideline and the design of the study was available. GPs identified patients, without support, approached them for consultation, and suggested referral if it was considered appropriate. GPs asked patients providing consent to be contacted by the research team. Referral followed regular referral pathways to the genetics department of the University Medical Center Utrecht.
Strategy B, ‘ICT-supported case finding’, was implemented in GP practices participating in the Julius GP Network, led by the Julius Center, the academic primary care network of the University Medical Centre Utrecht, which includes pseudonymised routine care data with up to 20 years’ follow-up of 164 GPs from 64 practices with approximately 300 000 patients in the Utrecht region. The Julius GP Network is described more elaborately elsewhere.17
To identify patients with ovarian cancer in their history, Julius GP Network data analysts screened the Julius GP Network database for the presence of the International Classification of Primary Care (ICPC) code X77.02 (ovarian cancer), and the practice in which the patients were listed. For strategy B only practices were approached that were identified as having a patient with ovarian cancer in the history. These GPs received a similar letter as those in strategy A, including the information and video about the revised referral guideline. Furthermore, the letter included information about the presence of patients with a history of ovarian cancer in their practice, identified through the Julius GP Network selection. In this letter, GPs were asked to indicate the wish for further participation. If the GP indicated willingness to participate, pseudonyms of patients with ovarian cancer were sent to the corresponding participating GP practice, using a secure software system installed at the GP’s practice. Simultaneously, researchers sent the GPs a second letter that included information about the new national guideline, the request to contact eligible patients with ovarian cancer and offer referral, technical support to trace back the code to the corresponding patient in the EMR, and contact details of one of the researchers for assistance. As in strategy A, videos for patients and GPs were available. The consent procedure was also similar to that used for strategy A: GPs were asked to obtain all ovarian cancer patients’ permission for sending questionnaires and to list the identification, approach, and referral of patients with ovarian cancer.
Outcomes and data collection
The effectiveness of the two interventions was assessed based on the proportion of patients with a history of ovarian cancer who were deemed eligible by the GP, approached by the GP, referred for GCT, and seen by the clinical geneticist. The proportion of patients with non-epithelial ovarian cancer who are not eligible for GCT among the referred patients with ovarian cancer was reported separately. The number of identified patients with ovarian cancer was registered by GPs in strategy A, and by the Julius GP Network in strategy B. The number of patients who were eligible, approached, and referred for GCT was reported by GPs. The actual number of clinical geneticist consultations and any genetic predisposition detected were registered by the clinical geneticist.
Acceptance of the strategies was assessed by the intervention uptake by GP practices, defined as ‘GP practices that actively follow up ovarian cancer patients’. Before performing the pilot, the local thresholds for satisfactory effectiveness were set at over 25% active follow-up of intervention by GP and more than 50% of referred patients seen by clinical geneticist. In addition, acceptance was explored with questionnaires for GPs and patients.
Questionnaires included closed and open-ended questions to explore motives for (contradicting) feasibility/desirability (further details are available from the authors on request). GPs were provided the opportunity to suggest ways to improve the strategies. All eligible patients were sent questionnaires, irrespective of GP approach and GCT referral. All GPs who agreed to participate in the study were sent questionnaires.
Data collection and analyses were performed in SPSS (version 21.0).
RESULTS
Effectiveness
As shown in Table 1, the number of GPs approached in strategy A was 36. For strategy B, 46 out of 64 (72%) GP practices were approached, because of the presence of a diagnostic code for ovarian cancer in the EMR of their practice. Strategies A and B identified 30 and 94 patients with ovarian cancer, respectively (Table 2). Of those identified, 19 (63%) and 39 (41%) were registered as ‘eligible for GCT referral’ by GPs, in strategy A and B, respectively. Reasons for non-eligibility and ‘not approached by GP’ are provided in Table 2. The main reasons were ‘already consulted clinical geneticist’ (Strategy A) and ‘patient no longer in GP practice’ (Strategy B). The number of patients approached by the GP for consultation was 15 (79% of those eligible for GCT) in strategy A and 33 (85% of those eligible) in Strategy B. Eight patients in each strategy accepted referral to the clinical geneticist and five patients in each strategy actually visited the clinical geneticist.
Table 1.
Acceptance of two strategies aimed at case finding women with a history of ovarian cancer in primary care for genetic counselling and DNA testing
| Acceptance | Case-finding strategy, n (%) | ||
|---|---|---|---|
|
| |||
| A | B | ||
| Measurement data | |||
|
| |||
| GP practices approached for participation | 36 | 46 | |
|
| |||
| Uptake: GP practices’ active follow-up of patients with OC | 11 (31) | 21 (46) | |
|
| |||
| Questionnaire data | |||
|
| |||
| GP practices sent questionnairesa,b | 36 | 46 | |
|
| |||
| GP practices returning questionnaires | 10 (28) | 17 (37) | |
|
| |||
| Number of questionnaires returnedb | 10 from 10 practices | 30 from 17 practices | |
|
| |||
| Feasible in daily practice | – Yes | 7 (70) | 18 (60) |
| – No | 2 (20) | 5 (17) | |
| – No answer | 1 (10) | 7 (23) | |
|
| |||
| Desirable in daily practice | – Yes | 8 (80) | 21 (70) |
| – No | 1 (10) | 4 (13) | |
| – No answer | 1 (10) | 5 (17) | |
Only practices that expressed an interest in participating in the study were sent a questionnaire.
The number of questionnaires sent to GP practices corresponds with the number of GPs working in the practice. Therefore, multiple questionnaires could be returned from one GP practice. OC = ovarian cancer.
Table 2.
Effectiveness of two strategies of case finding women with a history of ovarian cancer in primary care for genetic counselling and DNA testing
| Effectiveness | Case finding strategy, n (%) | |
|---|---|---|
| A | B | |
| Patients with OC identified | 30 | 94 |
| Eligible for CG referral according to GPa | 19 (63) | 39 (41) |
| Approached for referral by GPb | 15 (79) | 33 (85) |
| Consulted GP and referredc | 8 (53) | 8 (24) |
| EOC patients seen by CGd | 5 (63) | 5 (63) |
| Genetic predisposition detected | 1 | 0 |
In strategy A, 11 patients were considered not eligible for CG referral because they had ‘already consulted CG’. In strategy B, reasons that 55 patients were ineligible for referral included: ‘already consulted CG’ (10 patients), ‘no longer in GP practice’ (13 patients), ‘deceased’ (25 patients), and ‘other reason’ (7 patients), including false-positive diagnostic codes.
In strategy A, 4 patients who were eligible were not approached by the GP because it would be emotionally too burdensome, patient had dementia, patient had wrong tumour pathology (mucinous borderline, mucinous cystadenoma, reason unknown: all 1 patient). In strategy B, 6 patients who were eligible were not approached because the GP stated they had already been referred for CG.
Of the 48 patients approached for GP consultation and referral, 32 did not comply or follow-up was not registered; for 16 patients the GP did not report on their acceptance or decline of invitation and referral, 12 declined the invitation for unknown reasons, 3 patients were previously referred for GCT, 1 left the GP practice.
Reasons for accepted referral but not seen by CG (6 patients): 3 patients were not eligible for follow-up by CG because of borderline ovarian cancer and granulosa cell tumour; 3 patients cancelled the CG consultation for unknown reasons. CG = clinical geneticist. EOC = epithelial ovarian cancer. OC = ovarian cancer.
In one referred patient, a 87-year-old woman who had had epithelial ovarian cancer 31 years previously, a BRCA2 gene mutation was detected. Consequently, 20 of her relatives received GCT and opted for a predictive DNA test. One female mutation carrier opted for risk-reducing salpingo-oophorectomy and was diagnosed with early ovarian cancer for which she received chemotherapy.
Acceptance
As shown in Table 1, the intervention uptake by GP practices was 31% (11 of 36) for strategy A, and 46% (21 of 46) for strategy B. For strategy A, 10 (28%) of the 36 approached GP practices returned questionnaires. For strategy B, 17 (37%) of 46 GP practices sent in 30 questionnaires. The reasons reported by GPs in both strategies for ‘eligible but not approached’ were comparable, and included ‘older age’ and ‘too stressful for patient’ (Table 2).
The strategy was considered ‘feasible in daily care’ by seven out of 10 GP practices in strategy A, and not feasible by two. For strategy B, ‘feasible in daily care’ was supported in 18 out of 30 GP questionnaires and five GPs considered the strategy not feasible. Motives for supporting and for contradicting feasibility were similar for both strategies. Supportive motives can be summarised as: ‘easy to perform’, ‘I know my population well’, and ‘low numbers of patients with resulting little effort required’. Barriers to feasibility included ‘technical obstacles for patient identification in the EMR’ and ‘time consuming’. Opportunities to improve strategies were mainly mentioned by GPs in strategy B, and included: ‘improving external technical support’ and ‘facilitating the identification process’. These comments referred to the technical barriers hampering the use of the newly introduced EMR support. This support system had not been used before by most GPs. Getting accustomed to the steps required to convert and trace back the pseudonymised patient identifiers was sometimes experienced by GPs as complicated.
Desirability of the corresponding strategies was generally confirmed: by eight out of 10 GP practices in strategy A, and by 21 out of 30 practices in strategy B. Reasons provided for desirability were similar for both strategies, and can be summarised as: ‘clinical relevance of case finding and referral’, ‘easy to perform’, ‘GP the right person for case finding’, and ‘workability of the strategies’. Reasons for opposing desirability all referred to the technical aspect of the strategies, including ‘too much work’, ‘ICT support should improve’, and ‘no ICT support required’.
Fifteen patients used the opportunity to report on desirability of ovarian cancer case finding by the GP for GCT. In these patient-reported data, 13 patients confirmed desirability, mainly because: ‘knowing the increased risk is important for offspring’. One patient marked ‘does not apply’ and one contradicted desirability because: ‘I was open to such diagnostic procedures during sickness, but now I want to move on.’
DISCUSSION
Summary
Evaluation of the pilot implementation of unsupported and ICT-supported case finding of patients with ovarian cancer at risk of a genetic mutation in primary care suggests that both strategies are effective in identifying women with a history of epithelial ovarian cancer and referring them for GCT.
Implementation in almost 100 general practices resulted in referral of 10 patients with a history of epithelial ovarian cancer who were eligible for genetic counselling and DNA testing. Consequently, a BRCA2 gene mutation was found in one patient, leading to treatment of a not previously diagnosed early ovarian cancer in one of her family members. Patients and GPs generally considered both strategies desirable, mainly based on relevance and workability. According to GPs, technical barriers are the main obstacles to their success.
Strengths and limitations
This pilot study has several strengths. First, the pilot implementation of the case-finding strategies in daily practice provides a realistic rather than theoretical assessment of the effects and acceptance. Furthermore, the simultaneously executed strategies showed similar findings, particularly relating to acceptance, which supports the reliability of the findings.
However, several methodological and practical limitations should be kept in mind when considering these findings. To attain optimal certainty about the effect on case finding and referral, a randomised controlled design using an intervention and control arm would have been preferable. The research design may have led to an overestimation of the effect of the strategies, because it was assumed that all new referrals for GCT of eligible patients with a history of ovarian cancer were due to the intervention strategies. GPs may also have referred eligible patients without intervention, in line with the recent guideline recommending referral of women with a history of epithelial ovarian cancer. However, given the novelty of the guideline and GPs’ lack of familiarity with the guidelines of the Netherlands Comprehensive Cancer Organization (IKNL) in primary care, which are not part of the primary guidelines generally used by GPs, it may be assumed that potential overestimation of effectiveness would be minimal at most.
In contrast, the effect of the strategies may have been underestimated, mainly because of the limited possibilities of the research team to stimulate the response rate by GPs. Only identification, eligibility, approach, and referral reported by GPs to the research team were included in the analyses. If the GP acted on these strategies without reporting to the research team during or outside the follow-up period, these patients were not included in analyses. Corresponding underestimation of effect is also suggested by the observation that, out of the 48 patients who were approached for GP consultation and referral, for 16 patients the GP did not report on their acceptance or decline of the invitation and referral, whereas there were 16 registered acceptances of referral and 12 patients who declined.
The ovarian cancer screening method in strategy B, based on diagnostic codes in the EMR with subsequent reporting to GPs using pseudonymised patient identifiers, had not been used before. This led to technical barriers that hampered optimal execution of the ICT-supported strategy. This is likely to have counteracted the effectiveness and acceptance of this strategy. When implementing a new ICT supported system, similar barriers are likely to be experienced by GPs. Preventing or overcoming these problems will presumably enhance the future effectiveness and acceptance of this strategy. The encountered technical barriers might also explain the higher number of GPs returning the questionnaire in strategy B compared with strategy A.
A limitation of strategy B is the result of the limitations of registration systems that use diagnostic codes. This should be kept in mind when interpreting the results and when considering implementation in other healthcare systems using diagnostic codes in the EMR. It should also be considered that completeness and reliability of diagnostic codes may be affected by incomplete diagnostic codes (false negatives); misclassification as the diagnostic code used in this strategy (X77.02 — ovarian cancer) is a subcode of X77 — malignancy female genitals;17 and the inclusion of deceased patients and patients who have moved but remain in the EMR system.
These limitations in diagnostic codes also result in limited comparability of the number of ‘patients with ovarian cancer identified’ provided in Table 2, because the population represented by diagnostic codes in this denominator differs from the ‘ovarian cancer patients identified’ based on GPs’ knowledge in strategy A.
When comparing referral rates, it should also be considered that the check by the research team, whether patients had already been referred to the clinical geneticist, was only offered in strategy A.
A final limitation is that the modest questionnaire response rate may have resulted in selective responses concerning desirability and feasibility. Questionnaires may be more likely to be returned in case of particular concerns or approval concerning an intervention. This could lead to overestimation of positive and negative trends in responses. The higher questionnaire response rate in strategy B may be a reflection of the technical barriers experienced by GPs in strategy B.
Comparison with existing literature
Although the need for systematic efforts to identify patients with a history of epithelial ovarian cancer is widely supported in the literature, to the authors’ knowledge no studies addressing the effectiveness of case finding of these patients in primary care have been published previously.9–12 The aforementioned suggestion that the GP is in a suitable position to identify and refer women with a history of ovarian cancer and that GPs are willing to take that role is confirmed by the study’s findings.13–15,20
Information concerning cost-effectiveness is also valuable if implementation is considered. Recently, it has been shown that implementing secondary care germline BRCA testing in all patients newly diagnosed with ovarian cancer is extremely likely (99.9%) to be cost-effective.21 Given the low incremental cost-effectiveness ratio of this secondary care strategy (£4339 per quality-adjusted life year), and the low additional costs of the intervention (sending a letter and/or retrieval of diagnostic code) and minimal additional clinical burden (GP consultation), the intervention described in the current study seems likely to be cost-effective. However, it would be useful to include a cost-effectiveness analysis in a larger-scale randomised controlled trial or implementation study.
The literature has shown that the main barrier to integrating genetics in primary care is the perceived complexity of genetic risk exploration and the absence of clear and accessible guidelines.13–15 The fact that these barriers can easily be overcome by an intervention that provides the succinct and simple message to GPs to ‘refer all patients with a history of ovarian cancer’ explains the effectiveness and acceptance demonstrated by this study.
Implications for research and practice
An unsupported strategy and an ICT-supported strategy for case finding women with a history of ovarian cancer in primary care for genetic counselling and testing both show promising effectiveness and acceptance. Although these pilot results are promising, a large-scale implementation study is required to confirm and elaborate on the findings, for example, by including a cost-effectiveness analysis of the case-finding strategies.
By the standards for satisfactory effectiveness that were set before the pilot (over 25% active follow-up of intervention by GP and more than 50% of referred patients seen by clinical geneticist), the findings support primary care-based case finding of women with a history of ovarian cancer using either strategy. Nevertheless, acceptability of yield and preferred strategy are subject to preference and regional standards and setting. For any regional strategy, local opportunities and barriers should be recognised before designing and implementing an ovarian cancer case-finding strategy.
Acknowledgments
Charles W Helsper and Liesbeth M Van Vliet contributed equally and share first authorship. Thanks to Julia Velikopolskaia and Hugo Smeets from the Julius Huisartsen Network for all their help in retrieving the reports for GPs in strategy B and providing assistance to GPs in accessing this report; to the patient organisation Stichting Olijf for its assistance in designing the strategies and the supporting materials; and to Dr RB van der Luijt of the University Medical Center Utrecht for the interpretation of DNA test results.
Funding
This study was made possible through an unconditional research grant from AstraZeneca. The sponsor had no role in the design or execution of the study, nor in interpreting the data or the decision to submit this article for publication.
Ethical approval
The Medical Research Ethical Committee of the Utrecht Medical Center concluded that the Medical Research Human Subject Acts (WMO) did not apply to this study.
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
REFERENCES
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.The Netherlands Cancer Registry. Cancer figures. (In Dutch). Dutch Cancer Registry. https://www.cijfersoverkanker.nl/selecties/Dataset_1/img5bab9248cc12d (accessed 2 Oct 2018)
- 3.Arts-de Jong M, de Bock GH, van Asperen CJ, et al. Germline BRCA1/2 mutation testing is indicated in every patient with epithelial ovarian cancer: a systematic review. Eur J Cancer. 2016;61:137–145. doi: 10.1016/j.ejca.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Alsop K, Fereday S, Meldrum C, et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study Group. J Clin Oncol. 2012;30(21):2654–2663. doi: 10.1200/JCO.2011.39.8545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang S, Royer R, Li S, et al. Frequencies of BRCA1 and BRCA2 mutations among 1,342 unselected patients with invasive ovarian cancer. Gynecol Oncol. 2011;121(2):353–357. doi: 10.1016/j.ygyno.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 6.Daly MB, Axilbund JE, Buys S, et al. Genetic/familial high-risk assessment: breast and ovarian. J Natl Compr Canc Netw. 2010;8(5):562–594. doi: 10.6004/jnccn.2010.0043. [DOI] [PubMed] [Google Scholar]
- 7.Comprehensive Cancer Centre the Netherlands (IKNL). National guideline: hereditary and familial ovarian carcinoma. [In Dutch]. 2015. http://www.oncoline.nl/erfelijk-en-familiair-ovariumcarcinoom (accessed 2 Oct 2018)
- 8.Kuchenbaecker KB, Hopper JL, Barnes DR, et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA. 2017;317(23):2402–2416. doi: 10.1001/jama.2017.7112. [DOI] [PubMed] [Google Scholar]
- 9.Eccles DM, Balmana J, Clune J, et al. Selecting patients with ovarian cancer for germline BRCA mutation testing: findings from guidelines and a systematic literature review. Adv Ther. 2016;33(2):129–150. doi: 10.1007/s12325-016-0281-1. [DOI] [PubMed] [Google Scholar]
- 10.Samimi G, Bernardini MQ, Brody LC, et al. Traceback: a proposed framework to increase identification and genetic counseling of BRCA1 and BRCA2 mutation carriers through family-based outreach. J Clin Oncol. 2017;35(20):2329–2337. doi: 10.1200/JCO.2016.70.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyer LA, Anderson ME, Lacour RA, et al. Evaluating women with ovarian cancer for BRCA1 and BRCA2 mutations: missed opportunities. Obstet Gynecol. 2010;115(5):945–952. doi: 10.1097/AOG.0b013e3181da08d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karakasis K, Burnier JV, Bowering V, et al. Ovarian cancer and BRCA1/2 testing: opportunities to improve clinical care and disease prevention. Front Oncol. 2016;6:119. doi: 10.3389/fonc.2016.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trinidad SB, Fryer-Edwards K, Crest A, et al. Educational needs in genetic medicine: primary care perspectives. Community Genet. 2008;11(3):160–165. doi: 10.1159/000113878. [DOI] [PubMed] [Google Scholar]
- 14.Wood ME, Stockdale A, Flynn BS. Interviews with primary care physicians regarding taking and interpreting the cancer family history. Fam Pract. 2008;25(5):334–340. doi: 10.1093/fampra/cmn053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carroll JC, Brown JB, Blaine S, et al. Genetic susceptibility to cancer. Family physicians’ experience. Can Fam Physician. 2003;49(1):45–52. [PMC free article] [PubMed] [Google Scholar]
- 16.Quillin JM, Krist AH, Gyure M, et al. Patient-reported hereditary breast and ovarian cancer in a primary care practice. J Community Genet. 2014;5(2):179–183. doi: 10.1007/s12687-013-0161-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sollie A, Roskam J, Sijmons RH, et al. Do GPs know their patients with cancer? Assessing the quality of cancer registration in Dutch primary care: a cross-sectional validation study. BMJ Open. 2016;6(9):e012669. doi: 10.1136/bmjopen-2016-012669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Riel E, van Dulmen S, Ausems MG. Who is being referred to cancer genetic counseling? Characteristics of counselees and their referral. J Community Genet. 2012;3(4):265–274. doi: 10.1007/s12687-012-0090-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baars MJ, Henneman L, Ten Kate LP. Deficiency of knowledge of genetics and genetic tests among general practitioners, gynecologists, and pediatricians: a global problem. Genet Med. 2005;7(9):605–610. doi: 10.1097/01.gim.0000182895.28432.c7. [DOI] [PubMed] [Google Scholar]
- 20.Burke S, Martyn M, Thomas H, Farndon P. The development of core learning outcomes relevant to clinical practice: identifying priority areas for genetics education for non-genetics specialist registrars. Clin Med (Lond) 2009;9(1):49–52. doi: 10.7861/clinmedicine.9-1-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eccleston A, Bentley A, Dyer M, et al. A cost-effectiveness evaluation of germline BRCA1 and BRCA2 testing in UK women with ovarian cancer. Value Health. 2017;20(4):567–576. doi: 10.1016/j.jval.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]