Summary:
Acute perturbations of clathrin and associated proteins at synapses have provided a wealth of knowledge on the molecular mechanisms underlying clathrin-mediated endocytosis (CME). The basic approach entails presynaptic microinjection of an inhibitory reagent targeted to the CME pathway, followed by a detailed ultrastructural analysis to identify how the perturbation affects the number and distribution of synaptic vesicles, plasma membrane, clathrin-coated pits, and clathrin-coated vesicles. This chapter describes the methodology for acutely perturbing CME at the lamprey giant reticulospinal synapse, a model vertebrate synapse that has been instrumental for identifying key protein-protein interactions that regulate CME in presynaptic nerve terminals with broader extension to non-neuronal cell types.
Keywords: AP2, clathrin-coated vesicles, electron microscopy, lamprey, synapse, ultrastructure
1. Introduction:
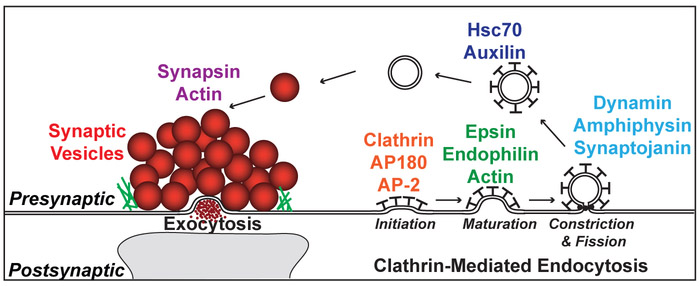
Neurotransmission depends critically upon the local recycling of synaptic vesicles at presynaptic nerve terminals. Following depolarization and calcium influx into the presynaptic nerve terminal, neurotransmitter-filled synaptic vesicles fuse with the presynaptic active zone and release their contents into the synaptic cleft (Fig. 1) (Pang and Sudhof, 2010). Vesicular membrane must then be locally recycled from the areas surrounding the active zone. One of the primary mechanisms for recycling synaptic vesicles is clathrin-mediated endocytosis (CME), though clathrin-independent mechanisms may also participate (Heuser and Reese, 1973; Saheki and De Camilli, 2012). Briefly, clathrin coat formation is initiated when clathrin is recruited to the plasma membrane by the assembly proteins, AP180 and AP2 (Fig. 1). Clathrin coat assembly then promotes invagination and maturation of the budding vesicle, a process that is assisted by epsin, endophilin and actin. Dynamin and several effector proteins drive constriction at the neck of the clathrin-coated pit (CCP), and the GTPase activity of dynamin leads to fission and generation of a free clathrin-coated vesicle (CCV). Once the CCV is uncoated by the ATPase Hsc70 and its co-chaperone, auxilin, the vesicle is then re-filled with neurotransmitter and returned to the synaptic vesicle cluster. Synapsin and actin are involved in vesicle clustering. Because CME is triggered by neuronal activity at synapses, and this is under the experimenter’s control, the neuronal synapse has proven to be a great cellular model for studying the molecular mechanisms of CME.
Figure 1.
Clathrin-mediated endocytosis at presynaptic nerve terminals. After exocytosis and neurotransmitter release, synaptic vesicles are locally recycled via CME. Shown here are the major transitions in CME and some of the proteins that participate in these transitions, which have been extensively studied using acute perturbations at synapses.
Electron microscopic (EM) studies of synapses within the frog neuromuscular junction, squid giant axon, and lamprey giant reticulospinal axon have been advantageous for studying the molecular components, morphological stages and physiological correlates of CME (Heuser and Reese, 1973; Augustine et al., 2006; Brodin and Shupliakov, 2006). These preparations allow for acute perturbations of CME and have provided a complementary approach to chronic genetic ablations or manipulations in other models. With acute perturbations, reagents such as peptides, recombinant proteins, antibodies, drugs, or toxins that are known or hypothesized to interfere with CME can be delivered via axonal microinjection directly to living, intact presynaptic terminals. Following neuronal stimulation to stimulate exo- and endocytosis at synapses, the tissue is fixed and processed for EM. Because clathrin coats are electron dense, EM can be used to determine the precise step in the clathrin pathway that is affected by the perturbation. Several advantages of acute manipulations include the opportunity to rapidly screen reagents for morphological effects and a lack of molecular compensation that may complicate data interpretation with chronic perturbations.
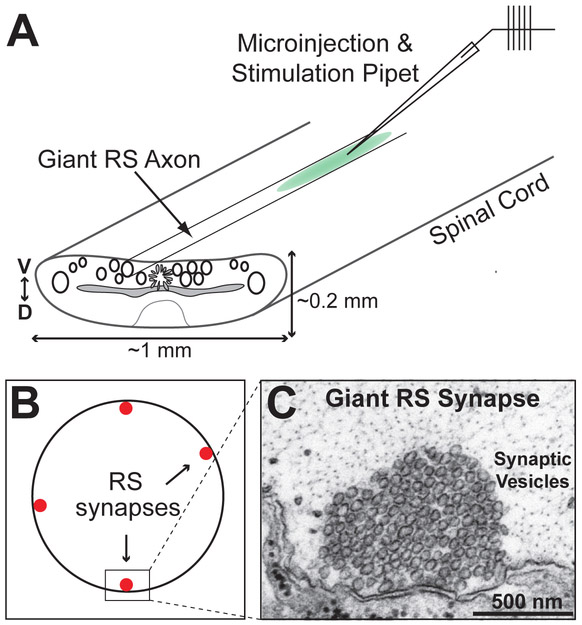
The giant reticulospinal (RS) synapses within the spinal cord of the lamprey (Fig. 2; Fig. 3) have been particularly useful for elucidating molecular mechanisms of CME (Shupliakov et al., 1997; Ringstad et al., 1999; Brodin et al., 2000; Gad et al., 2000; Shupliakov et al., 2002; Morgan et al., 2004; Bourne et al., 2006; von Kleist et al., 2011). Giant RS synapses are large en passant, glutamatergic synapses (1-2 μm diameter) located along the perimeter of giant axons (40-80 μm diameter), which are located within the ventromedial tract of the ribbon-like spinal cord (Fig. 3A-C). The large diameter and highly characteristic morphology of the giant RS axons and synapses, as well as the lack of myelin and relative transparency of the spinal cord, make the lamprey model particularly attractive for synaptic imaging, including ultrastructural analyses of the molecular mechanisms of CME. Large clusters of ~1000 synaptic vesicles are clustered at individual active zones (Fig. 3C), which are typically separated by fairly large distances, making it possible to attribute the synaptic vesicles and endocytic intermediates to a particular synapse. Since the RS synapses in the isolated spinal cord are quiescent, it is possible to trigger extended periods of exocytosis and endocytosis via action potentials that are evoked by electrical stimulation. Before, during, or after the stimulation period, perturbing reagents can be introduced to the synapses via axonal microinjection, and effects of such compounds on CME are subsequently determined.
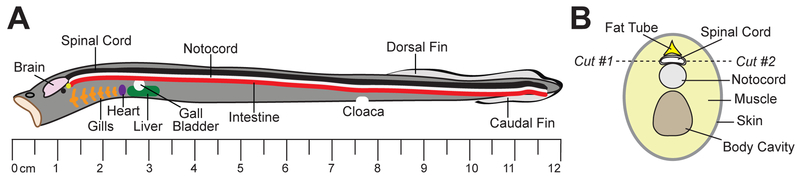
Figure 2.
Anatomy of the lamprey. A. Diagram of a late larval lamprey showing the location of the spinal cord and other major organs. B. Cross-section of a larval lamprey at the level of the gills. Note the position of the spinal cord. Dissection cuts for isolating the spinal cord are indicated.
Figure 3.
Acute perturbations of CME at lamprey giant RS synapses. A. Diagram of the lamprey spinal cord and axonal microinjection. D=dorsal; V=ventral. B. Cross-section of a giant RS axon showing the synapses along the perimeter. C. An electron micrograph of an unstimulated lamprey giant RS synapse showing a large pool of tightly-clustered synaptic vesicles.
Emphasizing its utility, acute perturbations at lamprey RS synapses have already been used successfully to identify the functions of many proteins with essential roles in CME and to define discrete stages of clathrin assembly, maturation, constriction/fission and uncoating (Fig. 1) (Shupliakov et al., 1997; Ringstad et al., 1999; Gad et al., 2000; Shupliakov et al., 2002; Morgan et al., 2004; Bourne et al., 2006; Brodin and Shupliakov, 2006; von Kleist et al., 2011; Morgan et al., 2013). Complementary studies on CME have been done at the squid giant synapse (Morgan et al., 1999; Morgan et al., 2000; Morgan et al., 2001; Morgan et al., 2003; Augustine et al., 2006). In both preparations, with low to moderate levels of activity, CME appears to be the predominant method for endocytosis (Shupliakov et al., 1997; Morgan et al., 1999; Morgan et al., 2000). This chapter describes how to acutely perturb CME at lamprey RS synapses, using microinjection of an AP2 peptide, which inhibits clathrin assembly in vitro and in vivo, as an example reagent. As previously demonstrated at the squid giant synapse (Morgan et al., 2000), and as predicted for AP2’s role in initiating clathrin assembly (Fig. 1), the AP2 peptide also causes a phenotype at lamprey RS synapses that is consistent with impairing very early stages of CME. We anticipate that acute perturbations at lamprey giant RS synapses will continue to be a useful approach for discovery-driven investigation into the molecular mechanisms of CME.
2. Materials:
The following materials and methods have been adapted from previous references (Pieribone et al., 1995; Shupliakov et al., 1997; Ringstad et al., 1999; Gad et al., 2000; Shupliakov et al., 2002; Morgan et al., 2004; Bourne et al., 2006; Morgan et al., 2013).
2.1. Spinal cord dissections:
1. Late stage larval sea lampreys (Petromyzon marinus; 10-13 cm). As lampreys are vertebrates, all procedures must be approved by the local institutional animal care and use committee prior to experimentation.
2. Anesthesia: MS-222 (0.1 g/L) buffered to pH 7.4 with sodium bicarbonate.
3. Sylgard® 184. Using the manufacturer’s protocol, make ~100 mL of Sylgard mixture. Pour Sylgard into two large petri dishes (100 mm) to a thickness of 5-7 mm. With the remaining Sylgard, pour 5-10 small petri dishes (35 mm) to a thickness of 3-4 mm. Cure on a level surface at room temperature 1-3 days (see Note 1).
4. Lamprey Ringer’s solution: 100 mM NaCl, 2.1 mM KCl, 2.6 mM CaCl2, 1.8 mM MgCl2, 4 mM glucose, 0.5 mM glutamine, 2 mM HEPES pH 7.4. Make up to 2L. Adjust pH to 7.4 with NaOH. Store at 4 °C up to 2 weeks. Immediately prior to use, aerate for 20 minutes with oxygen.
5. Dissecting tools: 2 pairs of Dumont Dumoxel #5 forceps, Student Vannas spring scissors, standard or carbon steel razor blades, minutien pins. Cut 4-6 minutien pins in half, and place the pointed ends in the small Sylgard dish. Discard the blunt ends.
2.2. Buffers and Peptides:
1. AP2 peptide: Peptides should be custom synthesized by commercial suppliers such as AnaSpec, Inc. (Fremont, CA) or similar company to >95% purity by HPLC. The amino acid sequence is shown in Table 1. A control AP2 peptide, in which the second DLL motif is mutated to AAA (e.g. mutant AP2 peptide), should also be synthesized (Morgan et al., 2000). Separate the lyophilized peptides into 1-2 mg aliquots and store at −80 °C until use.
Table 1.
List of reagents used to acutely perturb CME at synapses and their effects. Details of their effects on synaptic morphology and function, as well as control reagents, are described in the references.
| STAGE OF CME AFFECTED |
REAGENT | BIOCHEMICAL ACTIONS |
SEQUENCE | REFS |
|---|---|---|---|---|
| CLATHRIN ASSEMBLY | AP2 peptide | Binds clathrin N-terminal domain and inhibits clathrin assembly in vitro and in vivo | QGDLLGDLLNLDLGPPVNVPQ | Morgan et al., 2000; Zhou et al., 2015;Fig. 3 |
| AP180 peptide | Inhibits clathrin assembly in vitro and in vivo | SGGATAWGDLLGEDSLAALSS | Morgan et al., 2000 | |
| Pitstop 1 | Inhibits ligand interactions with clathrin N-terminal domain | n.a. | von Kleist et al., 2011 | |
| CLATHRIN COAT MATURATION | Endophilin Antibody | Inhibits CME and vesicle recycling in vivo; build up of shallow CCPs | Antibody raised against a peptide corresponding to a.a. 255-274 of rat endophilin (YQPKPRMSLEFATGDGTQPN) | Ringstad et al., 1999 |
| Actin Toxins (Phalloidin, Bot C2; Lat B, Swinholide) | Inhibit actin dynamics in vivo | n.a. | Bourne et al., 2006; Shupliakov et al., 2002 | |
| PIP kinase peptide | Inhibits actin polymerization in vivo | TDERSWVYSPLHYS | Morgan et al., 2004 | |
| CONSTRICTION & FISSION | Dynamin peptide | Inhibits dynamin-amphiphysin SH3 interaction | PPPQVPSRPNRAPPG | Shupliakov et al., 1997 |
| Amphiphysin SH3 domain | Binds dynamin in vitro and prevents recruitment to CCP | GST-tagged SH3 domain of human amphiphysin (a.a. 623-694) | Shupliakov et al., 1997 | |
| FISSION & UNCOATING | Synaptojanin peptide | Inhibits endophilin interactions with dynamin and synaptojanin in vitro | VAPPARPAPPQRPPPPSGA | Gad et al., 2000 |
| Auxilin ΔHPD mutant | Inhibits Hsc70-auxilin interaction and clathrin uncoating in vitro | Recombinant bovine auxilin truncation (a.a. 547-910) with mutated HPD motif (a.a. 874-876) | Morgan et al., 2001 | |
| α-Synuclein | Binds Hsc70 | Recombinant full-length human α-synuclein (a.a. 1-140) | Banks et al., 2015 |
2. Lamprey internal solution: 180 mM KCl, 10 mM HEPES, pH 7.4. Make up to 50 mL. Syringe filter into 0.5-1 mL aliquots. Store at −20 °C until use.
3. Alexa Fluor® 488 dextran (3000 MW, anionic) (see Note 2).
2.3. Microinjection and Electrophysiology Components
1. Premium standard wall borosilicate capillary glass with filament (see Note 3).
2. Electrode puller (e.g. P-97 or P-2000 from Sutter Instrument Company; Novato, CA)
3. 10 μl Glass Hamilton syringe with standard beveled 32G needle
4. Compound upright microscope with 10× air and 40× water dipping objectives (e.g. Zeiss Axioskop 2FS or similar)
5. Motorized micromanipulator with capabilities to drive fine movements in the nanometer to micrometer range and a diagonal axis, such as a Sutter MP-285 or Burleigh PCS-6000 (ThorLabs).
6. Straight electrode holder with side port for attaching tubing from pressure injector (see #9).
7. Intracellular amplifier, such as Axon Instruments Axoclamp 2B or Molecular Devices Multiclamp 700B. Mount an amplifier headstage (1× or 10×) onto the motorized micromanipulator, and plug in electrode holder from step 6. Plug headstage into the amplifier.
8. Computer or oscilloscope: Connect the output of the correct amplifier channel from Step 7 into a computer running pClamp software (or equivalent), or alternatively into an oscilloscope so that the neuronal signals can be monitored during injections and stimulation.
9. Pressure injector: Picospritzer III or similar. Set up the injector according to manufacturer’s instructions. The small diameter tubing should be firmly sealed onto the side port of the electrode holder (see Note 4).
2.4. Electron microscopy
Make all solutions in a fume hood and follow appropriate safety precautions.
1. Cacodylate Buffer: Stock solution of 200 ml of 0.2M sodium cacodylate, pH 7.4. Dilute 1:1 with distilled water to make 100 ml of 0.1M Na cacodylate for buffer washes.
2. Glutaraldehyde fixative: Make 3% glutaraldehyde / 2% paraformaldehyde in 0.1M sodium cacodylate buffer, pH 7.4. Heat 50 ml of 0.2M sodium cacodylate buffer, pH 7.4, to 70 °C, and stir on a heating stirplate. Add a few (~4) drops of 5N NaOH to increase the pH. Add 2g of paraformaldehyde prills. Stir until paraformaldehyde is completely in solution. pH to 7.4. Adjust volume to 70 ml with distilled water. Filter 23.3 ml of the paraformaldehyde fixative into a 50 ml conical tube using a 0.22 μm syringe filter. Add 10 ml of 10% aqueous glutaraldehyde using a glass Pasteur pipet (see Note 5). Confirm that pH is still at 7.4. Prepare on the same day as the experiments.
3. Osmium fixative: Use extreme caution! Immediately prior to post-fixing (Section 3.3, step 3), prepare 4 ml of 2% osmium tetroxide / 2% K+ ferrocyanide in 0.1M sodium cacodylate, pH 7.4. Dissolve 0.2g K+ ferrocyanide in 5mL of 0.2M Na cacodylate, pH 7.4. Mix 2mL of this solution with a 2mL ampoule of 4% aqueous OsO4. Store solution in a designated refrigerator only used for osmium tetroxide.
4. Glass scintillation vials – 20 ml capacity, with caps.
5. 2 bottles of 100% ethanol. Using one bottle, prepare 50-100 ml stocks of 50%, 70%, and 95%.
6. Propylene oxide, EM grade.
7. Embedding supplies: EMbed-812 resin kit, Wooden sticks, Dental wax sheets; Flat embedding mold; Dry oven set to 60 °C.
8. Ultramicrotome, such as Leica EM UC7 or Reichert-Jung Ultracut.
9. Glass knife maker, such as Leica EM KMR3 or similar. Make 4-6 glass knives, and store in a lint-free environment until use. This video shows how to make glass knives: https://www.youtube.com/watch?v=NJcLhM3sfEM. The more experienced EM microscopist will use a diamond knife for thin sectioning.
10. Sectioning supplies: glass knife boats; hot pen; formvar-coated copper slot grids; grid mats.
11. 1% Toluidine blue: Mix 1g Borax and 1g toluidine blue in 100 ml ddH2O water, and stir overnight.
12. Counterstaining solutions: Uranyl acetate: Make 50 ml of a 2% solution. Store at 4 °C in the dark until use. Lead citrate: Make 50 ml of a 0.4% solution. Store at 4 °C until use. Make 50 ml of 0.2N NaOH, and store at room temperature.
13. Electron microscope, such as an FEI Technai Spirit BioTwin T12.
3. Methods:
3.1. Spinal cord dissection.
1. Anesthetize a lamprey in buffered 0.1 g/L tricaine methanesulfonate diluted in tank water. Complete anesthesia takes ~10-15 minutes and is determined by lack of spontaneous muscle contractions, no response to tail pinch, and slowed gill contractions.
2. After anesthesia is complete, move the lamprey to a paper towel. Using a razor blade, decapitate near the level of the 2nd gill (Fig. 2A) and pith. Make a second cut at the level of the dorsal fin to remove the tail.
3. Move the lamprey body piece to one of the large Sylgard dishes. Pin on both ends using syringe needles, and submerge in fresh, oxygenated lamprey Ringer’s solution.
4. Using dissecting scissors, make two horizontal cuts through the cartilaginous casing at the lateral edges of the spinal cord (Fig. 2B). Extend the cuts through the muscle and skin on both sides of the animal. As these two cuts are extended rostrally, the top portion of the body can be lifted up with forceps to reveal the dorsal surface of the spinal cord. Continue in this manner until the entire spinal cord is revealed (see Note 6).
5. Isolate a piece (2-3 cm) of the spinal cord. Hold onto one end of the spinal cord with the forceps, and gently peel it up out of the cartilage encasing until it is free on both ends (see Note 7).
6. Move the spinal cord to a small Sylgard dish and submerge in fresh, oxygenated lamprey Ringer’s solution. Pin the spinal cord ventral side up by placing 1-2 minutien pins in each end of the spinal cord (see Note 8).
7. Using fine forceps, remove the thin layer of meninx primativa from the ventral surface of the spinal cord (see Note 9).
8. Store spinal cord in lamprey Ringer’s solution at 4 °C while the peptides are being prepared.
3.2. Preparation of peptides and microinjection.
1. Pull 5-10 sharp microelectrodes using the electrode puller (see Note 10). These will be used for peptide microinjections and for stimulating the axons.
2. Dilute an aliquot of the AP2 peptide (or control peptide) in lamprey internal solution to a concentration of 20 mM or higher (see Note 11). pH to 7.4 using 5M KOH. The final volume of the peptide aliquot typically ranges from 10-30 μL depending on the molecular weight of the peptide.
3. Add Alexa Fluor® 488 dextran to the peptide aliquot to a final dye concentration of 100 μM. Mix by pipetting up and down.
4. Centrifuge the peptide at 13,000 rpm in a tabletop microfuge for 10 min at 4 °C to remove any aggregates.
5. Immediately load 1-2 μl of the peptide solution into several microelectrodes using the Hamilton syringe. Store the remaining peptide solution on ice. Mount a peptide-containing microelectrode onto the electrode holder and secure it tightly.
6. Place the small dish containing the lamprey spinal cord onto the stage of an appropriate upright microscope, making sure it is securely fastened. Place a minutien pin in or beside the lateral edge of the spinal cord, away from the area of the large RS axons, in a position parallel to the injection site. The pin will remain in place throughout the fixation and will later serve as a landmark to identify the injection site during the EM analysis.
7. Using the coarse movements on the micromanipulator, position the tip of the microelectrode just above the axon to be injected. Make sure the microelectrode is oriented along the same axis as the selected axon (i.e. parallel with the longitudinal axis of the spinal cord), as this will allow for easier axon impalements (Fig. 3A).
8. Switch the micromanipulator to the fine movements, and slowly advance the microelectrode until the tip just enters the axon, registering a membrane potential of at least −58 mV (see Note 12). Wait 1-2 minutes to ensure that the resting membrane potential is stable before injecting.
10. Inject the peptide using brief pulses of N2 delivered through the pressure injector (5-40 ms; 0.1-0.3 Hz; 30-50 psi). Start with the lowest settings, and then increase as needed until each pulse produces a small burst of fluorescence in the axon.
11. Continue injecting pulses of peptide for 5-30 minutes, until the desired concentration is achieved (see Note 13).
12. While continuing to inject, stimulate the axon at the desired rate by delivering current pulses (1 ms; 20-80 nA; 0.5-20 Hz) to evoke action potentials (see Note 14). Start by injecting a 1 nA current, and then increase the amplitude until the axon reliably fires action potentials. The most common stimulation paradigms for eliciting CME at lamprey synapses are 5 Hz for 30 min (Shupliakov et al., 1997; Ringstad et al., 1999; Gad et al., 2000; Busch et al., 2014) or 20 Hz for 5 min (Morgan et al., 2004; Bourne et al., 2006; Morgan et al., 2013; Busch et al., 2014), though the latter may also evoke bulk endocytosis (Busch et al., 2014).
13. At the end of the stimulation period, while continuing to stimulate, carefully remove the lamprey Ringer’s solution from the chamber and replace with freshly prepared Glutaraldehyde fixative. Triturate 10-20 times with a disposable transfer pipet. The action potentials will disappear within 20-30 sec, as the tissue fixes. Gently remove the microelectrode. Immediately transfer the fixed spinal cord to a fume hood. Wash 3 × 5 minutes with fresh fixative, triturating each time. Continue fixing the spinal cords for 2-5 hours at room temperature, replacing with fresh fixative approximately once per hour. Store overnight at 4 °C (see Note 15).
14. Repeat steps 5-13 until the desired number of preparations has been fixed.
3.3. Fixation and processing for electron microscopy.
The general steps for EM processing are: fixation, dehydration, resin infiltration, and curing of preparation in resin molds. Perform all incubations in a fume hood and wear appropriate personal protective gear.
1. Remove the pins from the spinal cord, and transfer it to a scintillation vial containing 0.1M sodium cacodylate buffer, pH 7.4.
2. Wash 3 × 10 minutes in 0.1M sodium cacodylate buffer.
3. Postfix for 1.5 hours in freshly-prepared K+ ferrocyanide/OsO4 on ice and in the dark (see Note 16).
4. Wash 3 × 15 min in 0.1 M sodium cacodylate buffer, pH 7.4
5. Wash 3 × 15 min in ddH2O water.
6. Perform en bloc staining with 2% aqueous uranyl acetate for 2 hours in the dark at room temperature (see Note 17).
7. Wash 3 × 5 min in ddH2O water.
8. Dehydrate spinal cord in graded ethanol series, while rotating, as follows: 50% for 10 min; 70% for 15 min; 95% for 2 × 10 min; 100% for 2 × 15 min. Then, wash 2 × 30 min longer in 100% ethanol (EM grade) from a freshly opened bottle (or absolute EtOH stored over molecular sieves).
9. Wash in propylene oxide 2 × 15 minutes in a scintillation vial with the cap on (see Note 18).
10. Infiltrate the spinal cord in a 1:1 mixture of propylene oxide:Embed 812 rotating overnight in the fume hood. Leave the cap off of the scintillation vial.
11. Move the spinal cord to a new scintillation vial, and cover with fresh EMbed 812. Infiltrate the preparation with EMbed 812 at room temperature for 4-5 hours.
12. During step 11, print small computer labels with the experiment date and condition, and place in the appropriate EM molds.
13. At the end of the infiltration, use a wooden stick to move the fixed spinal cord onto a piece of dental wax. Under a dissecting microscope, trim the spinal cord to a length of 2-3 mm, centered around the injection site, as identified by the pin mark (see 3.2.6). Place one end of the spinal cord flush with the tapered end of the embedding block, and cover with fresh EMbed 812 until the mold is filled.
14. Polymerize the blocks in a dry oven at 60 °C for >48 hrs until completely hardened.
3.4. Electron microscopy and image analysis.
1. Mount the spinal cord block in an ultramicrotome chuck. Using a fresh razor blade, trim the block face into a trapezoid shape around the spinal cord, keeping the longest edge at a length of ~1mm.
2. Using a glass knife, with a ddH2O-filled boat and mounted on the ultramicrotome, cut thick sections (1 μm) until the entire spinal cord fills the section. To determine this, collect several sections onto a microscope slide, stain the section with 1-2% toluidine blue for 2-3 minutes on a hot plate, destain by gently washing the sections with water, and visualize on a standard upright compound microscope (see Note 19). The toluidine blue stained sections are used to assess the quality of the tissue after fixation and for identifying the target injected axons.
3. When within 200 μm of the injection site, start collecting ultrathin, silver sections (~70 nm), which can be obtained using a glass or diamond knife.
4. Collect 4-8 ultrathin serial sections, and place them on a formvar-coated copper slot grid. Use a hot pen to smooth out the sections before moving them onto the grid. Continue collecting sections at 5-20 μm intervals up to the injection site (marked by a large hole in the lateral edge of the spinal cord), until you have the desired number of grids (see Note 20).
5. Counterstain the sections: Using a syringe filter, place one drop of each solution onto a piece of parafilm for each grid. Grids can be moved between droplets using a wire loop or fine-pointed forceps. First, wash the grids by placing them on ddH2O droplets for 1 min. To do so, place the grids section-side-down onto the drops of ddH2O. Following the same procedure, stain the sections with 2% uranyl acetate for 7 min in the dark. Wash grids for 5 × 1 min in ddH2O. Stain sections with 0.4% lead citrate for 4 min (see Note 21). Wash grids in 0.2N NaOH for 2 min. Finally, wash the grids in ddH2O for 1 min. Carefully blot the edge of the grids dry with a tissue and place them to dry on a grid mat.
6. Load a grid into the specimen holder of the electron microscope. Identify the injected, stimulated axon, and acquire images of all synapses around the perimeter of that axon at the desired magnification (see Note 22). We typically use 26,500-37,000× magnification. Repeat until every section of every synapse from every grid has been imaged.
7. Using Image J or similar image analysis software, perform quantitative analyses on the synapses for your features of interest (see Note 23). First, select a section at or near the center of the synapse, as determined by the longest length of the active zone. Next, count the number of synaptic vesicles within a 1 μm distance from the active zone. Measure the size of the plasma membrane evaginations, as described in Busch et al., 2014. Briefly, draw a straight line (1 μm) from the edge of the active zone to the nearest point on the axolemma. Then, measure the curved distance between the two points. Repeat for the other side of the synapse, and determine the average. Next, identify the clathrin-coated pits (CCPs) and clathrin-coated vesicles (CCVs) by the appearance of an electron dense fuzzy coat (see Fig. 4G-I), and stage them accordingly. Stages are as follows: Stage 1 – CCP with little invagination; Stage 2 – invaginated, unconstricted CCP; Stage 3 – invaginated, constricted CCP, sometimes exhibiting a dense ring of protein (e.g. dynamin); Stage 4 – free CCVs (Bourne et al., 2006; Morgan et al., 2013; Busch et al., 2014).
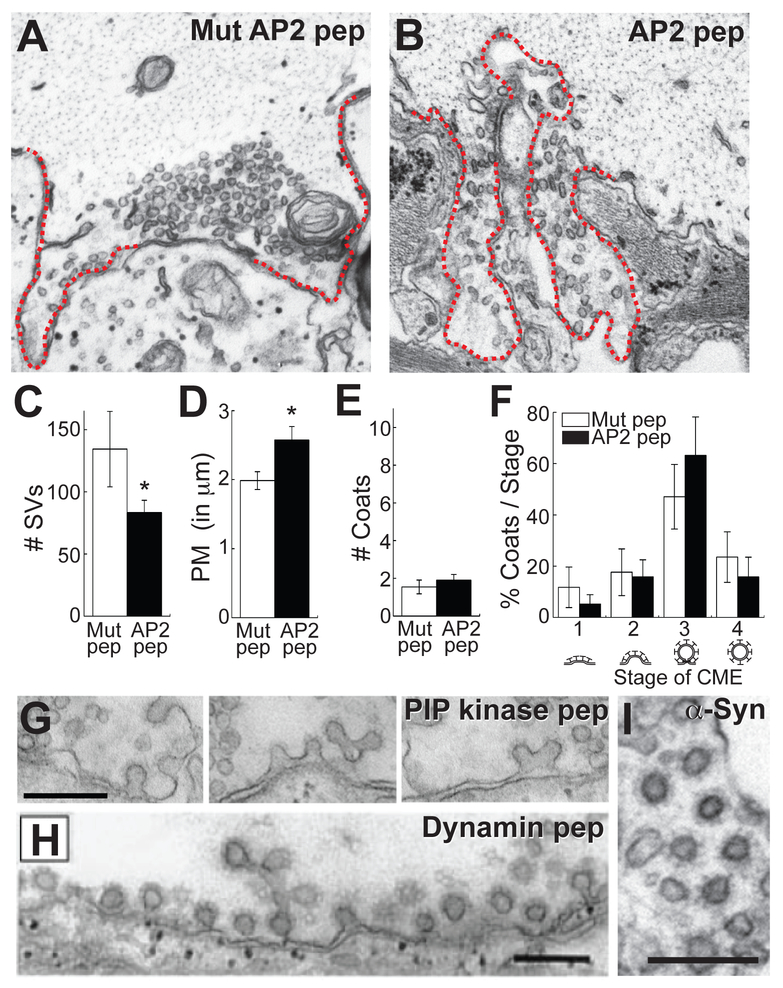
Figure 4.
Acute perturbations of CME at lamprey RS synapses with an AP2 peptide. A-B. Electron micrographs of stimulated synapses (20 Hz, 5 min) treated either with a mutant AP2 peptide (control) or the AP2 peptide. Note the smaller synaptic vesicle cluster and larger plasma membrane evaginations (dotted line) with AP2 peptide, which is a characteristic hallmark of inhibiting CME at synapses. C-F. Quantification of the phenotype produced by the AP2 peptide. AP2 peptide causes a loss of synaptic vesicles (C), increased plasma membrane evaginations (D), but no change in either the number or distribution of CCPs or CCVs (E-F). Bars represent mean ± SEM from n=10-20 synapses; Asterisks indicate significance (T-test in C-E; ANOVA in F). G-I. Phenotypes observed when later stages of CME are acutely perturbed. As examples, disruption of presynaptic actin cytoskeleton, using a PIP kinase peptide, causes a large increase in CCPs that are invaginated, but unconstricted (G), while disruption of dynamin inhibits fission and increases the number of constricted CCPs (H). An interesting new finding is that excess human α-synuclein increases CCVs (I), suggesting effects on uncoating (Banks et al., 2015). Scale bars = 0.2 μm. Panel H from Shupliakov et al., 1997. Reprinted with permission from AAAS.
8. Plot the average data from all synapses, and select representative images that show the resulting phenotype (Fig. 4A-F). Compared to the mutant AP2 peptide (control), the AP2 peptide significantly reduced the number of synaptic vesicles (Mut pep: 134 ± 30 SVs, n=10 synapses; AP2 pep: 83 ± 10 SVs, n=20 synapses; T-test, p=0.05) (Fig. 4A-C). The AP2 peptide also increased the size of the plasma membrane evaginations (Mut pep: 2.0 ± 0.1 μm, n=10 synapses; AP2 pep: 2.6 ± 0.2 μm, n=20 synapses; T-test, p=0.05) (Fig. 4D). However, no change was observed in the total number of clathrin-coated pits and vesicles (Mut pep: 1.5 ± 0.4 CCP/Vs, n=10 synapses; AP2 pep: 1.9 ± 0.3 CCP/Vs, n=20 synapses; T-test, p=0.48) (Fig. 4E). The distribution of CCPs and CCVs was also unchanged (Fig. 4F). Similarly, at lamprey and squid synapses, a loss of synaptic vesicles compensated by an expanded plasma membrane has also been observed in other studies where various stages of CME have been acutely perturbed, including clathrin assembly, clathrin coat maturation, constriction and fission, and clathrin uncoating (see Note 24) (Morgan et al., 1999; Ringstad et al., 1999; Gad et al., 2000; Morgan et al., 2000; Morgan et al., 2001; Bourne et al., 2006; von Kleist et al., 2011; Morgan et al., 2013). However, one major difference is that reagents that affect later stages of CME cause a >10-fold increase in the number of CCPs and CCVs at synapses. For example, actin-disrupting reagents impair clathrin coat maturation and cause a build-up of Stage 2 CCPs, while reagents that perturb dynamin function inhibit vesicle fission and cause a build-up of Stage 3 CCPs (Fig. 4G-H) (Shupliakov et al., 1997; Shupliakov et al., 2002; Morgan et al., 2004; Bourne et al., 2006; Morgan et al., 2013). Reagents that affect uncoating induce a build-up of Stage 4 CCVs (Fig. 4I) (Morgan et al., 2001). Table 1 is a non-exhaustive list of some of the reagents that perturb CME at lamprey giant RS synapses. Note the diversity of reagents, including peptides, toxins, recombinant proteins and antibodies, illustrating the many ways that CME can be acutely manipulated at synapses.
ACKNOWLEDGMENTS:
This work was supported by NIH grant RO1 NS078165 to JRM. The authors would like to thank Paul Oliphint and Kara Marshall for technical assistance.
4 Notes:
Sylgard dishes are re-usable, even after exposed to fixative, as long as they are washed thoroughly between experiments.
A fluorescent dextran is co-injected along with the peptide in order to track the diffusion and concentration of the peptide throughout the axon. The 3000 MW fluorescent dextran provides a good proxy for most peptides, which have relatively low molecular weights. However, peptides may also be synthesized with a covalently attached fluorophore, such as FITC, for direct tracking of the peptide diffusion. For larger proteins or antibodies, a higher molecular weight dextran should be injected, one that is closely matched to the molecular weight of the protein. Alternatively, the protein or antibody can be directly conjugated with a fluorescent dye using an Alexa Fluor® labeling kit from Thermo Fisher, Inc.
The outer diameter (OD) of the capillary glass should be matched to the size of the port on the microelectrode holder, typically 1.0 or 1.2 mm.
Make sure the seal is tight so that each puff of air escapes only from the front end of the electrode holder. Be sure to secure any loose tubing, so that there is little to no movement with each puff of air. This will significantly lower the risk of mechanical damage to the axon during the microinjections.
Equilibrate the 10% aqueous glutaraldehyde to room temperature prior to use. Otherwise, it may precipitate in the tissue. For best results, the 3% glutaraldehyde / 2% paraformaldehyde fixative should be made on the day of the experiment.
During the dissection, keep the scissors as horizontal as possible, and make small cuts. This will help to prevent nicking the spinal cord. In some cases, a thick layer of spongy connective tissue can be seen on top of the spinal cord, and if present it should be removed.
After removing the experimental piece of spinal cord, the rest of the animal can be stored in lamprey Ringer’s at 4 °C up to 24 hours. If the dissection went well, there should be enough remaining spinal cord for 1-2 additional experiments.
Stretch the spinal cord to the same length it was in the animal. Do not use the blunt ends of the minutien pins for pinning, as these create large holes that can tear or otherwise damage the tissue.
It is essential to remove the meninx primativa because the microinjection pipettes cannot easily penetrate it. Starting at one end of the spinal cord, use the fine forceps to pick at the top layer of the tissue until you can pull up a thin, translucent sheet of tissue. This is the meninx. If done carefully, the meninx can be removed in a single sheet from the entire ventral surface of the spinal cord. At the very least, remove the meninx from the middle portion of the spinal cord where the giant axons are located.
The Pipette Cookbook 2015 published by Sutter Instruments is an excellent resource for pulling sharp electrodes (http://www.sutter.com/PDFs/pipette_cookbook.pdf). See the chapter on Intracellular Recording Electrodes for specific settings.
Once prepared, the aliquot of peptide can be stored at 4 °C and used for experiments over 1-2 days. During the injection, the peptide is typically diluted 5-10 times from the stock concentration. Thus, the final axonal concentration of AP2 pep will be 2-4 mM, a range that inhibits synaptic transmission and synaptic vesicle recycling at squid giant synapses (Morgan et al., 2000).
The best approach for impalement is to intersperse small microelectrode advances with short current pulses (1 ms) delivered through the “buzz” function on the amplifier. Upon entry of the microelectrode, a healthy lamprey giant axon will have a resting potential of −60 to −70 mV. The membrane potential may become slightly more hyperpolarized as the KCl in the electrode leaks out. However, if the membrane potential depolarizes, then the axon has not sealed properly and therefore is unlikely to be healthy enough to sustain the injection. Should this occur, discontinue and start over with a fresh piece of spinal cord.
The fluorescent dextran is used to estimate axonal concentration and diffusion of the peptide. To do so, compare the fluorescence intensity in the axon to a set of pre-prepared standard dilutions of the peptide solution. If the pipet clogs before the desired concentration is reached, try clearing the pipet by injecting a small pulse of current using the “buzz” or “clear” function on the amplifier. If this doesn’t work, then gently remove the microelectrode and re-impale with a new one, being careful not to damage the axon.
Some peptide solutions do not allow enough current to pass through the electrode to spike action potentials. If that occurs, gently remove the peptide-containing microelectrode and replace it with one containing 3M KCl for the stimulation.
The spinal cord can be stored in glut/para fixative at 4 °C for several days to weeks.
Osmium is extremely hazardous, and the vapors can fix mucous membranes of the eyes and nose and cause problems with breathing. Always work with osmium in a fume hood, and dispose of properly.
Uranyl acetate should be filtered at the time of use with a 0.22 μm syringe filter. UA is considered to be radioactive, but can be shielded by plastic, glass, or 6 inches of distance, and gloves. Store and dispose of in proper container.
Propylene oxide (PO) vapors are toxic, so work in the fume hood. Always use PO in glass or metal containers, as it can leech or dissolve plastics.
The giant axons should be visible, round, and uniformly stained within the ventromedial tract (Busch et al., 2014 shows a good example). If the cytoplasm is pulled away from the axolemma, this is a clear indication that the fixation was suboptimal, and the preparation should be discontinued for EM.
It is important to keep track of where you are sectioning relative to the injection site. This will allow you to estimate the peptide concentration at a particular location in the axon and match it with the observed phenotype.
The length of the lead citrate staining can be altered to achieve the desired tissue contrast. Also, the lead citrate will form large precipitates on the sections if exposed to oxygen. To prevent this, perform the lead citrate staining in a glass petri dish filled with pellets of NaOH (which absorb CO2) and sealed with parafilm. Do not breathe on the grids. Do not use lead citrate solution if it looks cloudy.
Synapses within the stimulated axon will exhibit a few clathrin-coated pits and vesicles, which are rarely if ever seen at unstimulated synapses. In addition, the vesicle cluster is slightly smaller and more dispersed, and the plasma membrane is somewhat evaginated (see Fig. 3C and compared to Fig. 4A). A single synapse may span 10 sections. It is useful to image all of the sections of a given synapse in the event that you want to perform 3D reconstructions from serial sections later on.
Do not perform image analysis on synapses within 25 μm of the injection site, because there can be local injection artifacts that could affect the results.
REFERENCES:
- Augustine GJ, Morgan JR, Villalba-Galea CA, Jin S, Prasad K, and Lafer EM. 2006. Clathrin and synaptic vesicle endocytosis: studies at the squid giant synapse. Biochem Soc Trans 34:68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks SM, Busch DJ, Oliphint PA, Walsh RB, George JM, Lafer EM, Morgan JR. 2015. α-Synuclein interacts with Hsc70: A possible mechanism underlying the synaptic vesicle recycling defects in Parkinson’s Disease models Soc Neurosci Abst 36.13. Chicago, IL. [Google Scholar]
- Bourne J, Morgan JR, and Pieribone VA. 2006. Actin polymerization regulates clathrin coat maturation during early stages of synaptic vesicle recycling at lamprey synapses. J Comp Neurol 497:600–609. [DOI] [PubMed] [Google Scholar]
- Brodin L, Low P, and Shupliakov O. 2000. Sequential steps in clathrin-mediated synaptic vesicle endocytosis. Curr Opin Neurobiol 10:312–320. [DOI] [PubMed] [Google Scholar]
- Brodin L, and Shupliakov O. 2006. Giant reticulospinal synapse in lamprey: molecular links between active and periactive zones. Cell Tissue Res 326:301–310. [DOI] [PubMed] [Google Scholar]
- Busch DJ, Oliphint PA, Walsh RB, Banks SM, Woods WS, George JM, and Morgan JR. 2014. Acute increase of alpha-synuclein inhibits synaptic vesicle recycling evoked during intense stimulation. Mol Biol Cell. 25:3926–3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gad H, Ringstad N, Low P, Kjaerulff O, Gustafsson J, Wenk M, Di Paolo G, Nemoto Y, Crun J, Ellisman MH, De Camilli P, Shupliakov O, and Brodin L. 2000. Fission and uncoating of synaptic clathrin-coated vesicles are perturbed by disruption of interactions with the SH3 domain of endophilin. Neuron 27:301–312. [DOI] [PubMed] [Google Scholar]
- Heuser JE, and Reese TS. 1973. Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J Cell Biol 57:315–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JR, Di Paolo G, Werner H, Shchedrina VA, Pypaert M, Pieribone VA, and De Camilli P. 2004. A role for talin in presynaptic function. J Cell Biol 167:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JR, Jiang J, Oliphint PA, Jin S, Gimenez LE, Busch DJ, Foldes AE, Zhuo Y, Sousa R, and Lafer EM. 2013. A role for an Hsp70 nucleotide exchange factor in the regulation of synaptic vesicle endocytosis. J Neurosci 33:8009–8021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JR, Prasad K, Hao W, Augustine GJ, and Lafer EM. 2000. A conserved clathrin assembly motif essential for synaptic vesicle endocytosis. J Neurosci 20:8667–8676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JR, Prasad K, Jin S, Augustine GJ, and Lafer EM. 2001. Uncoating of clathrin-coated vesicles in presynaptic terminals: roles for Hsc70 and auxilin. Neuron 32:289–300. [DOI] [PubMed] [Google Scholar]
- Morgan JR, Prasad K, Jin S, Augustine GJ, and Lafer EM. 2003. Eps15 homology domain-NPF motif interactions regulate clathrin coat assembly during synaptic vesicle recycling. J Biol Chem 278:33583–33592. [DOI] [PubMed] [Google Scholar]
- Morgan JR, Zhao X, Womack M, Prasad K, Augustine GJ, and Lafer EM. 1999. A role for the clathrin assembly domain of AP180 in synaptic vesicle endocytosis. J Neurosci 19:10201–10212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang ZP, and Sudhof TC. 2010. Cell biology of Ca2+-triggered exocytosis. Curr Opin Cell Biol 22:496–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieribone VA, Shupliakov O, Brodin L, Hilfiker-Rothenfluh S, Czernik AJ, and Greengard P. 1995. Distinct pools of synaptic vesicles in neurotransmitter release. Nature. 375:493–497. [DOI] [PubMed] [Google Scholar]
- Ringstad N, Gad H, Low P, Di Paolo G, Brodin L, Shupliakov O, and De Camilli P. 1999. Endophilin/SH3p4 is required for the transition from early to late stages in clathrin-mediated synaptic vesicle endocytosis. Neuron 24:143–154. [DOI] [PubMed] [Google Scholar]
- Saheki Y, and De Camilli P. 2012. Synaptic vesicle endocytosis. Cold Spring Harb Perspect Biol 4:a005645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shupliakov O, Bloom O, Gustafsson JS, Kjaerulff O, Low P, Tomilin N, Pieribone VA, Greengard P, and Brodin L. 2002. Impaired recycling of synaptic vesicles after acute perturbation of the presynaptic actin cytoskeleton. Proc Natl Acad Sci U S A. 99:14476–14481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shupliakov O, Low P, Grabs D, Gad H, Chen H, David C, Takei K, De Camilli P, and Brodin L. 1997. Synaptic vesicle endocytosis impaired by disruption of dynamin-SH3 domain interactions. Science. 276:259–263. [DOI] [PubMed] [Google Scholar]
- von Kleist L, Stahlschmidt W, Bulut H, Gromova K, Puchkov D, Robertson MJ, MacGregor KA, Tomilin N, Pechstein A, Chau N, Chircop M, Sakoff J, von Kries JP, Saenger W, Krausslich HG, Shupliakov O, Robinson PJ, McCluskey A, and Haucke V. 2011. Role of the clathrin terminal domain in regulating coated pit dynamics revealed by small molecule inhibition. Cell. 146:471–484. [DOI] [PubMed] [Google Scholar]
- Zhuo Y, Cano KE, Wang L, Ilangovan U, Hinck AP, Sousa R, and Lafer EM. 2015. Nuclear Magnetic Resonance Structural Mapping Reveals Promiscuous Interactions between Clathrin-Box Motif Sequences and the N-Terminal Domain of the Clathrin Heavy Chain. Biochemistry. 54:2571–2580. [DOI] [PMC free article] [PubMed] [Google Scholar]