Abstract
In Crohn’s disease, pathogenic Th17-cells express low levels of CD39 ectonucleotidase and are refractory to the immunosuppressive effects of unconjugated bilirubin (UCB), an endogenous ligand for aryl-hydrocarbon-receptor (AhR). This resistance to AhR ligation might be associated with alterations in responses to hypoxia. Limited exposure to hypoxia appears beneficial in acute tissue injury. However, in protracted inflammation, hypoxemia may paradoxically result in Th17-cell activation. We report here that in vitro exposure of Th17-cells from Crohn’s disease patients to hypoxia limits responsiveness to AhR stimulation by UCB, as reflected by lower CD39 levels. Blockade of hypoxia-inducible-factor-1alpha (HIF-1α) upregulates CD39 and favors Th17-cell regulatory responses. Resistance of Th17-cells to AhR signaling results, in part, from HIF-1α-dependent induction of ATP-binding cassette (ABC) transporters: multidrug-resistance-protein-1 (MDR1) and multidrug-resistance-associated-protein-4 (MRP4). Increased ABC transporters promote efflux of suppressive AhR ligands, such as UCB, from Th17-cells. Inhibition of MDR1, MRP4 and/or HIF-1α with ritonavir (RTV) reconstitutes AhR function in Th17-cells, enhancing therapeutic effects of UCB in dextran-sulfate-sodium-induced experimental colitis.
Deleterious effects of hypoxia on Th17-cells in Crohn’s disease can be ameliorated either by inhibiting HIF-1α or by suppressing ABC transporters to increase UCB availability as an AhR substrate. Targeting HIF-1α-ABC transporters could provide innovative therapeutic pathways for IBD.
Keywords: hypoxia, unconjugated bilirubin, ATP-binding cassette transporter, ectoenzyme, Th17-cells
1. Introduction
Severe hypoxia with tissue ischemia, during inflammatory insults, activates select transcriptional programs resulting in the upregulation of hypoxia-inducible-factor-1-alpha (HIF-1α) [1]. In the acute inflammatory setting, blockade of HIF-1α-mediated pathways is associated with over-production of pro-inflammatory mediators [2-4]. In the setting of chronic inflammation, however, HIF-1α can trigger differentiation of Th17-cells through the activation of RORγt and regulation of IL17 production [5]. This process also results in the inhibition of T-regulatory-1 (Tr1)-cells [6], a regulatory subset that becomes prominent during protracted inflammation [7]. These effects indicate putative differences in the kinetic impacts of decreased O2 levels and alterations of cell subsets during various inflammatory states.
We have previously shown that Th17-cells derived from patients with Crohn’s disease are refractory to the immunosuppressive effects of unconjugated bilirubin (UCB) [8], which otherwise serves as an immunoregulatory endogenous ligand for the aryl hydrocarbon receptor (AhR) [8-10]. AhR is a mediator of toxin/xenobiotic responses that also modulates adaptive immunity [11, 12]. Engagement of AhR through exogenous or endogenous ligands controls a variety of biological responses and results in the regulation of xenobiotic metabolizing enzymes, i.e. the cytochrome P4501A1 (CYP1A1) while promoting the transcription of a number of genes like the ATP-binding cassette (ABC) family of drug transporters and cytokines like IL10, IL17 and IL22 [13-15]. AhR expression per se has a key role in intestinal homeostasis as supported by studies in vivo in which exacerbation of dextran-sulfate-sodium (DSS)-induced colitis was noted in AhR−/− mice [16]. Recent investigations have further indicated that treatment with the AhR non-toxic agonist 2-(1’H-indole-3’-carbonyl)-thiazole-4-carboxylic acid methyl ester (ITE) ameliorates T-cell mediated colitis in humanized mice [17]. Further, administration of kynurenine, another AhR endogenous ligand derived from tryptophan metabolism, was associated with amelioration of DSS colitis and induction of IL10R expression on colonic epithelial cells [18]. We have also shown that treatment of mice with UCB contributes to recovery in DSS colitis through a mechanism mediated via AhR [8].
The immunomodulatory effects of AhR depend, in large part, on the upregulation of CD39, a nucleoside triphosphate diphosphohydrolase that catalyzes extracellular ATP and ADP into AMP, which is subsequently converted to adenosine via the ecto-5’-nucletidase CD73. CD39 is highly expressed on vascular endothelial cells and Tregs, where the catalytic activity contributes to suppressive function through the generation of immunosuppressive adenosine [19]. CD39 expression is altered in a proportion of patients with IBD; being regulated at the genetic level via single nucleotide polymorphisms (SNPs) in the non-coding regions of the gene that correlate with low CD39 levels and predisposition to IBD [20].
CD39 can also be induced on pathogenic Th17-cells where it imparts regulatory properties and contributes to the limitation of the effector potential [21, 22]. Defective CD39 expression and activity have previously been linked to IBD. Pathogenic Th17-cells, obtained from patients with Crohn’s disease, display defective expression of CD39, suggesting an inability of these cells to acquire regulatory properties with consequent permanence to an inflammatory state [21]. We have recently shown that Th17-cells obtained from Crohn’s patients fail to upregulate CD39 in response to UCB [8], suggesting selective defects in the response to AhR activation in these cells.
Here, we now report that in IBD, refractoriness of Th17-cells to AhR stimulation by UCB is dictated by decreased O2 levels that boost expression of ABC transporters. These molecular pumps increase the efflux of UCB out of cells, therefore limiting the availability of this ‘regulatory’ immunometabolite and ligand for AhR. Targeting hypoxia, in conjunction with AhR ligation, limits the deleterious effects of Th17-cell activation. Our data suggest that combined therapeutic approaches to target both pathways might have utility in IBD.
2. Materials and Methods
2.1. Subjects
Peripheral blood mononuclear cells (PBMC) and lamina propria mononuclear cells (LPMC) were isolated from 43 patients with Crohn’s disease (median Harvey-Bradshaw Index, 1, range 0 to 9), recruited from the Gastroenterology Division, Beth Israel Deaconess Medical Center (BIDMC), Boston, MA. Ten patients were studied during active disease, while the remaining were on clinical remission. At the time of study, 25 patients were on infliximab, 5 were on steroids and 7 were receiving 6-mercaptopurine. PBMC were also obtained from 25 healthy blood donors (Blood Donor Center at Children’s Hospital, Boston, MA). Human studies received IRB approval (2011P000202). Written consent was obtained from all study participants prior to inclusion in the study.
2.2. Mice
C57BL/6 wild type mice, originally purchased from Taconic, Hudson, NY were bred in our facility for 3-4 generations before being used and subjected to experimental colitis. 10-12-week old male and female were studied in accordance with standard institutional and welfare guidelines. Animal care and experiments were conducted under the guidelines of the National Institutes of Health for the care and use of laboratory animals. Protocols were approved by the Animal Care and Use Committee at BIDMC.
2.3. Cell isolation
2.3.1. Human:
PBMC were obtained by density gradient centrifugation on Ficoll-Paque (GE Healthcare Life Sciences, Pittsburgh, PA), as previously reported [21, 22]. LPMC were obtained from freshly biopsied colonic tissue in 9 patients with Crohn’s disease. In these patients, tissue was biopsied from both inflamed and non-inflamed areas (3-4 samples per bioptic areas). LPMC were then isolated as we previously described [8, 21] and results from the sites compared. PBMC and LPMC viability was checked by Trypan Blue exclusion and always exceeded 98%.
2.3.2. Mouse:
mononuclear cells were obtained from the spleen, mesenteric lymph nodes (MLN) and from colon intra-epithelial lymphocyte (IEL) and lamina propria (LP) fractions. IEL and LP were isolated as previously reported [8], following incubation in dissociation buffer (HBSS Ca2+ and Mg2+ free containing 2% FBS, 5 mM EDTA, 10 mM HEPES) and digestion buffer (HBSS with Ca2+ and Mg2+, 10% FBS, 0.5 mg/ml DNAse I [Sigma Aldrich, St Louis, MO] and 0.5 mg/ml collagenase type IV [Worthington Biochemical Corporation, Lakewood, NJ]), resuspension in 40% and layering onto 80% Percoll Plus (GE Healthcare Life Sciences).
2.4. In vitro cell conditioning and culture
Human PBMC and LPMC-derived CD4+ cells were purified according to the manufacturer’s instructions (Miltenyi Biotec, San Diego, CA). The purity of the sorted CD4 cells exceeded 92%. CD4 cells were cultured in RPMI 1640 medium, supplemented with 2 mM L-glutamine, 100 IU/ml penicillin, 100 mg/ml streptomycin, 1% non-essential amino acids and 10% FBS and exposed for 5 days to Th17 polarizing conditions consisting of IL6 (50 ng/ml), IL1β (10 ng/ml), TGFβ (3 ng/ml) [8] and with Dynabeads Human T activator CD3/CD28 for T-cell expansion (bead/cell ratio: 1/50) (Thermo Fisher Scientific, Cambridge, MA). All cytokines and neutralizing antibodies were from R&D Systems (Minneapolis, MN). On day 6, cells were treated with 20 μM UCB (Frontiers Scientific, Logan, UT) [8], the resuspension and addition of which was carried out limiting light exposure to the minimum. In parallel experiments, conditioned Th17-cells were placed in anaerobic chambers (VWR Scientific, Radnor, PA) and subjected to hypoxia (2% O2) in the absence or presence of HIF-1α blockade for the last 12 hours of culture.
To minimize the effects of re-oxygenation, at the end of the 12-hour incubation period, cells were processed immediately and either fixed and subsequently stained for flow cytometry markers or otherwise snap-frozen for qPCR analysis.
2.5. HIF-1α blockade
HIF-1α blockade was achieved upon treatment of differentiated Th17-cells with HIF-1α-specific siRNAs (Thermo Fisher Scientific). Cells were resuspended in Opti-MEM medium (Thermo Fisher Scientific) and seeded at 2.5-3×105/well of a 96-well plate. HIF-1α-specific siRNAs, used at a final concentration of 1 pmol/well, were added to the cells for the last 24 hours of culture. A negative control siRNA (Thermo Fisher Scientific) served as control. In additional experiments, differentiated Th17-cells were treated with ritonavir (RTV, Sigma Aldrich), a HIF-1α non-specific inhibitor that also antagonizes MDR1 and MRP4. RTV was added at 5 μM [23] for 24 hours before Th17-cells were exposed to UCB.
2.6. Induction and clinical assessment of experimental colitis
C57BL/6 wild type mice were treated with 3% DSS in standard drinking water, provided at libitum for 6 days. Mice were concomitantly injected with UCB at 20 μmol/kg/day intraperitoneally alone or in combination with RTV at 10 mg/kg/day intraperitoneally [24]. Mice were sacrificed either on day 7 at the end of DSS treatment (n=24) or on day 10 after recovery (n=24). UCB resuspension was carried out as described before [8]; RTV was administered after resuspension in 10% ethanol. UCB and/or RTV administration was continued at the same dose and frequency after DSS treatment was replaced with normal water for 4 days. Body weight score was calculated on the basis of body weight loss (‘0’: 0-1%; ‘1’: >1-5%; ‘2’: >5-10%; ‘3’: >10-15%; ‘4’: >15-20%) and assessed on a daily basis. On day 10 mice were sacrificed, colon dissected and length measured from the ileocecal junction to the anal verge.
2.7. Histological evaluation of colitis
Colonic tissue was removed and assessed for histological evaluation upon freezing into Optimum Cutting Temperature (OCT) compound. 6 μM tissue sections were stained with hematoxylin and eosin and then evaluated for the presence of colitis, as reported elsewhere [20, 25]. Histology score was calculated as indicated before [8].
2.8. Flow cytometry staining
2.8.1. Human:
Flow cytometry was performed following incubation with PE, APC, Pacific Blue and PE-Cy7-conjugated anti-human antibodies to CD4 (clone # OKT4), CCR6 (clone # G034E3), CXCR3 (clone # G025H7), CD39 (clone # A1), CD49b (clone # P1E6-C5) and LAG3 (clone # 11C3C65), all from Biolegend, San Diego, CA. Frequency and MFI of FOXP3 and RORC were assessed by intracellular staining, following cell fixation and permeabilization with Cytofix/Cytoperm (BD Biosciences, San José, CA) and incubation with PE and APC-conjugated antibodies to human FOXP3 (clone # PCH101) and RORC (clone # AFKJS-9), all from eBioscience, San Diego, CA. Frequency of cytokine producing cells was determined after exposure to cell stimulation cocktail plus protein transport inhibitors (eBioscience) at 2 μl/ml according to the manufacturer’s instructions for 5 hours. Staining was carried out using Alexa-Fluor 488, PE, APC and PerCP-Cy5.5 anti-human antibodies to IL17A (clone # BL168), IL10 (clone # JES3-9D7) and IFNγ (clone # 4S.B3), all from Biolegend. Flow cytometry was performed as reported before [21]. Cells were acquired on a BD LSRII (BD Biosciences) and analyzed using FlowJo 2 software (version 10, TreeStar, Ashland, OR). Positively stained cell populations were gated based on unstained, single stained and isotype stained controls (BD Biosciences). Fluorescence compensation was adjusted based on fluorescence-minus-one method.
2.8.2. Mouse:
Lymphomononuclear cells from spleen, MLN, IEL and LP were tested for CD4, IL17, IL10, FOXP3 and CD39 positivity using PE-Cy5, PE-Cy7, PE, APC and APC-Cy7 antibodies to mouse CD4 (clone # GK1.5), IL17A (clone # TC11-18H101), IL10 (clone JES5-16E3), CD39 (clone # A1) from Biolegend and for FOXP3 (clone # FJK-16s) from eBioscience. Flow cytometry analysis was carried out as indicated above.
2.9. Suppression assay
Th17-cell ability to suppress autologous CD4+CD25− cell effector function was assessed in co-culture experiments, in which untreated, UCB or UCB and RTV treated Th17-cells were added at a ratio of 1/8 to CD4+CD25− targets [21, 26, 27]. Th17-cells were used after sorting of CCR6+CXCR3− cells from CD4 cells conditioned in the presence of Th17 polarizing conditions [8]. Cultures of CD4+CD25− cells without Th17-cells were performed in parallel under identical conditions. Responder cells were activated using IL2 (30 IU/ml) and Dynabeads Human T activator CD3/CD28 (bead/cell ratio: 1:2). Responder cell IL17 and IFNγ production in the absence or presence of Th17-cells was tested by intracellular cytokine staining after 3-4 days co-culture.
2.10. MDR1 and MRP4 activity
MDR1 and MRP4 activity of human Th17-cells was assessed by rhodamine (EMD Millipore, Billerica, MA) and Calcein AM (BD Pharmingen) staining respectively.
When determining MDR1 activity, cells were incubated with rhodamine (1/100 dilution) for 1 hour on ice. Following centrifugation, cells were resuspended in cold efflux buffer consisting of RPMI 1640, 30% BSA and gentamycin solution.
When determining MRP4 activity, cells were incubated with Calcein AM dye (1μM) in the dark for 30 minutes at room temperature, washed and resuspended in PBS containing 1% FBS.
For both assays, Th17-cells were analyzed by flow cytometry. As per manufacturer’s recommendations, MDR1 and MRP4 drug transporter activity was measured on the basis of changes in rhodamine and Calcein AM fluorescence before and after exposure to UCB, alone or in combination with RTV.
High Th17-cell MDR1 and MRP4 fluorescence was indicative of low activity (i.e. when transporters are poorly active, the cell retains the dye) while decreases in MDR1 and MRP4 fluorescence signified increases in drug transporter activity.
2.11. Immunoblotting
Immunoblot analysis was carried out as previously described [21] using 3-5×105 cells. Rabbit anti-human HIF-1α (cat. # 3716S, Cell Signaling, Danvers, MA) and mouse anti-human AhR (cat. # ab2770, Abcam, Cambridge, MA) or aryl hydrocarbon receptor nuclear translocator (ARNT, clone # 775146, R&D Systems) primary antibodies were applied at 1/2,000, 1/5,000 and 2 μg/ml respectively. HRP-labeled goat anti-mouse (for AhR and ARNT, Thermo Fisher Scientific) and mouse anti-rabbit (for HIF-1α, Thermo Fisher Scientific) secondary antibodies (Thermo Fisher Scientific) were used at 1/50,000. Bands were visualized using Super Signal West Femto Sensitivity Substrate (Thermo Fisher Scientific) according to the manufacturer’s instructions. For immunoblot normalization, the same cell lysates were also tested for β-actin (cat. # ab8226, Abcam) antibodies at 1/10,000 and, subsequently, with an HRP-labeled goat anti-mouse antibody at 1/20,000 (Thermo Fisher Scientific). Band density was measured by Image J software.
2.12. qPCR
Expression of human ENTPD1, FOXP3, IL10, CYP1A1, ABCB1 and ABCC4 mRNA expression was determined by qPCR. Total RNA was extracted from 3-5×105 cells using TRIzol reagent (Thermo Fisher Scientific) and mRNA was reverse transcribed using iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA) according to the manufacturer’s instructions. ENTPD1, IL10 and ABCB1 primer sequences were as previously reported [8, 28]. FOXP3, CYP1A1 and ABCC4 primer sequences were as follows.
FOXP3
Forward 5’ GAAACAGCACATTCCCAGAGTTC 3’
Reverse 5’ GCACTTGTGCAGACTCAGGTTG 3’
CYP1A1
Forward 5’ ATTATCTTTGGCATGGGCAAGCGG 3’
Reverse 5’ CAGCTGCATTTGGAAGTGCTCACA 3’
ABCC4
Forward 5’ GCCAAGATGTTGCCTATGTGCTT 3’
Reverse 5’ CTTGCTATGCCAAAAAGAACGGT 3’
PCR amplification conditions were as reported previously [21, 29]. Samples were run on a StepOne Plus (Applied Biosystems, Foster City, CA) and results analyzed by matched software and expressed as relative quantification. Relative gene expression was determined after normalization to human β-actin.
2.13. Chromatin immunoprecipitation
Chromatin was extracted from Th17-cells differentiated from healthy blood donors (4×106/reaction) according to the manufacturer’s instructions (Pierce Agarose ChIP Kit, Thermo Scientific). Rabbit anti-human HIF-1α (clone # D2U3T, Cell Signaling) antibody and rabbit IgG (Thermo Scientific) as negative control were used to immunoprecipitate chromatin. Immunoprecipitated DNA was analyzed by qPCR using the following sets of primers.
ABCB1
Forward 5’ GCAACGGAAGCCAGAACATT 3’
Reverse 5’ AGGCTTCCTGTGGCAAAGAG 3’
ABCC4
Forward 5’ AAGCGGCTGCTTCACAGG 3’
Reverse 5’ CTGGACCTCAAGCAGGGATG 3’
Data are presented as fold enrichment compared to IgG control.
2.14. Thin layer chromatography
Thin layer chromatography (TLC) was performed as previously described [21]. Untreated or UCB treated 3×105 Th17-cells under normoxic or hypoxic conditions were incubated with 2 mCi/ml [C14] ADP (GE Healthcare Life Sciences) in 10 mM Ca2+ and 5 mM Mg2+. [C14] labeled ADP, AMP and adenosine served as standards. TLC band density was examined using Image J software.
2.15. Statistics
Normality of variable distribution was assessed by Kolmogorov-Smirnov goodness-of-fit test. Comparisons were performed using parametric (paired or unpaired Student’s t test) or non-parametric (Wilcoxon signed-rank or Mann-Whitney test) tests according to data distribution. One-way ANOVA or Kruskal-Wallis tests, followed by Tukey’s or Dunn’s multiple comparison tests, were used when comparing more than two sets of data. For all comparisons, P<0.05 was considered significant. P values less than 0.1 were considered to indicate a trend to significance.
Statistical analysis was performed using SPSS version 22.
3. Results
3.1. Hypoxia in Crohn’s disease renders Th17-cells refractory to immunoregulation
We have previously shown that Th17-cells from Crohn’s disease patients are refractory to the immunomodulatory properties of UCB [8]. In this setting, Th17-cell impaired responsiveness can be reflected by lack of CD39 and FOXP3 upregulation upon UCB exposure [8]. To further understand the molecular mechanisms regulating UCB-triggered immunomodulation of Th17-cells, we explored the role of HIF-1α, which is upregulated during inflammatory conditions and controls AhR [6]. Recent work proposes that HIF-1α modulates AhR expression in Tr1-cells and interferes with differentiation and metabolic programming [6].
Th17-cells were derived from PBMC of healthy individuals and Crohn’s disease patients upon conditioning of CD4+ T-cells in the presence of IL6, IL1β and TGFβ. The average proportion of IL17 producing cells that also expressed CCR6+, CXCR3− and RORC+ ranged from 12 to 14%, similar to what we previously reported [8]. Following polarization, cells were subjected to hypoxic conditions (2% O2) and then exposed to UCB for the last six hours of culture. Levels of both AhR and ARNT, the heterodimeric partner of HIF-1α that is shared with AhR, remained low in Crohn’s disease both under normoxic and hypoxic conditions (Fig. 1A). In contrast, HIF-1α levels were higher in Th17-cells differentiated from patients than in those obtained from healthy subjects both at baseline and after hypoxia treatment (Fig. 1B). Indeed, HIF-1α underwent upregulation following Th17-cell exposure to hypoxic environment, both in heathy subjects and Crohn’s patients (Fig. 1B).
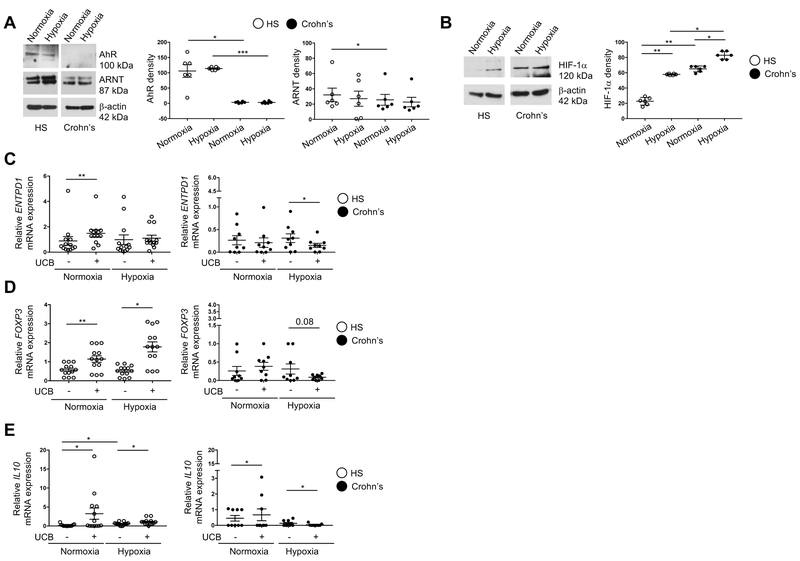
Fig. 1. Hypoxia alters Th17-cell response to AhR ligation in Crohn’s disease.
(A) Representative Western Blot analysis showing Th17-cell expression of AhR and ARNT under normoxic and hypoxic (2% O2) conditions in one representative healthy subject (HS) and one patient with Crohn’s disease. Mean ± SEM AhR and ARNT density in Th17-cells (HS, n=6; Crohn’s patients, n=6). (B) Representative Western Blot analysis showing HIF-1α expression by Th17-cells of one HS and one Crohn’s patient. Mean ± SEM HIF-1α density in Th17-cells from the same HS and Crohn’s patients. Mean ± SEM of relative ENTPD1 (C), FOXP3 (D) and IL10 (E) mRNA expression by Th17-cells from HS (n=13) and Crohn’s patients (n=9).
Comparisons were made using one-way ANOVA or Kruskal-Wallis test, followed by Tukey’s or Dunn’s multiple comparison tests. *P≤0.05;**P≤0.01.
We previously showed that UCB via AhR has immunoregulatory properties by upregulating CD39 and FOXP3 expression [8]. We next tested the expression of these two molecules under normoxic and hypoxic conditions. Hypoxia impaired the response of Crohn’s-derived Th17-cells to UCB by downregulating both ENTPD1 (means of data indicating 2.1-fold decrease) and FOXP3 (3-fold decrease) mRNA expression (Fig.1, C-D). Such effects were not observed in healthy control samples, where expression of CD39 remained stable and FOXP3 levels were increased (3.4-fold) following UCB stimulation (Fig. 1, C-D). Downregulation of CD39 (2.8-fold) and FOXP3 (2.2-fold) expression upon stimulation of Th17-cells with UCB, in the presence of hypoxic conditions, was also observed at the protein level in Crohn’s patients but again not in healthy control samples (Supplementary Fig. 1, A-B).
Exposure of Crohn’s-derived Th17-cells to hypoxic conditions also impacted IL10 mRNA levels (4-fold decrease) (Fig. 1E) as well as the proportion of IL10 producing lymphocytes (5.6-fold decrease) within the Th17-cell subset (Supplementary Fig. 1C). No differences in Th17 response to hypoxia were noted between untreated (n=3) and treated (n=13) patients and between patients with mild or moderate disease activity (n=3) and those in remission (n=13).
No changes in the ectoenzymatic activity of CD39 and CD73 - the ectoenzyme that works in tandem with CD39 - were observed in untreated and UCB-treated Th17-cells under hypoxic conditions both in healthy subjects and Crohn’s disease patients (Supplementary Fig. 2, A-B).
Overall, these data indicate that in Crohn’s disease hypoxia via HIF-1α dampens the response of Th17-cells to AhR activation brought about by UCB.
3.2. Blockade of HIF-1α restores Th17 responsiveness to UCB in Crohn’s disease
To further dissect the role of hypoxia and HIF-1α in the control of AhR-mediated UCB effects, we subjected Th17-cells from healthy individuals and Crohn’s disease patients to HIF-1α siRNA treatment and then tested their response following exposure to UCB under normoxic and hypoxic conditions.
In cells from normal individuals, treatment of Th17-cells with HIF-1α-specific siRNA resulted in increased levels of CD39 compared to untreated or UCB-treated Th17-cells under normoxic and hypoxic conditions (Fig. 2, A-B).
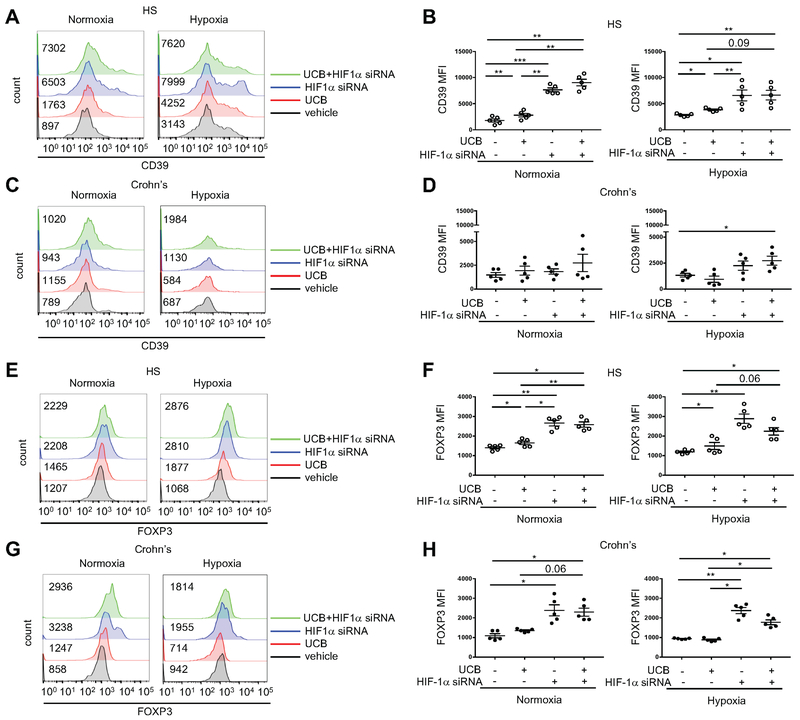
Fig. 2. Blockade of hypoxia restores Th17-cell ability to respond to AhR ligation.
Representative histograms of (A and C) CD39 and (E and G) FOXP3 mean fluorescence intensity (MFI) of Th17-cells differentiated from peripheral blood CD4+ cells of one HS and one patient with Crohn’s disease. Cells were treated with either UCB, HIF-1α siRNA or UCB plus HIF-1α siRNA, under normoxic or hypoxic conditions. Untreated (vehicle) controls are also shown. For the flow cytometry analysis, cells were initially gated on CD4+IL17+ lymphocytes; then CD39 and FOXP3 MFI was measured. Numerical values of CD39 and FOXP3 MFI in untreated, UCB or UCB plus HIF-1α siRNA-treated Th17-cells are indicated within the histogram plots. Mean ± SEM (B and D) CD39 and (F and H) FOXP3 MFI of Th17-cells (HS, n=5; Crohn’s patients, n=5).
Comparisons were made using one-way ANOVA, followed by Tukey’s multiple comparison test. *P≤0.05;**P≤0.01;***P≤0.001.
No significant changes in CD39 MFI levels were found upon treatment of Crohn’s-derived Th17-cells with HIF-1αs-specific siRNA under normoxia and hypoxic conditions (Fig. 2, C-D).
Addition of UCB in conjunction with siRNA treatment did not further increase CD39 levels in Th17-cells from healthy individuals (Fig. 2, A-B); however, under hypoxia conditions, an increase in CD39 in Th17-cells derived from Crohn’s disease patients over basal unstimulated cells was observed (Fig. 2, C-D). Exposure of Th17-cells to HIF-1α-specific siRNA, alone or in combination with UCB, resulted in increased FOXP3 levels in Th17-cells from healthy individuals (Fig. 2, E-F) and Crohn’s disease patients (Fig. 2, G-H) under both normoxic and hypoxic conditions.
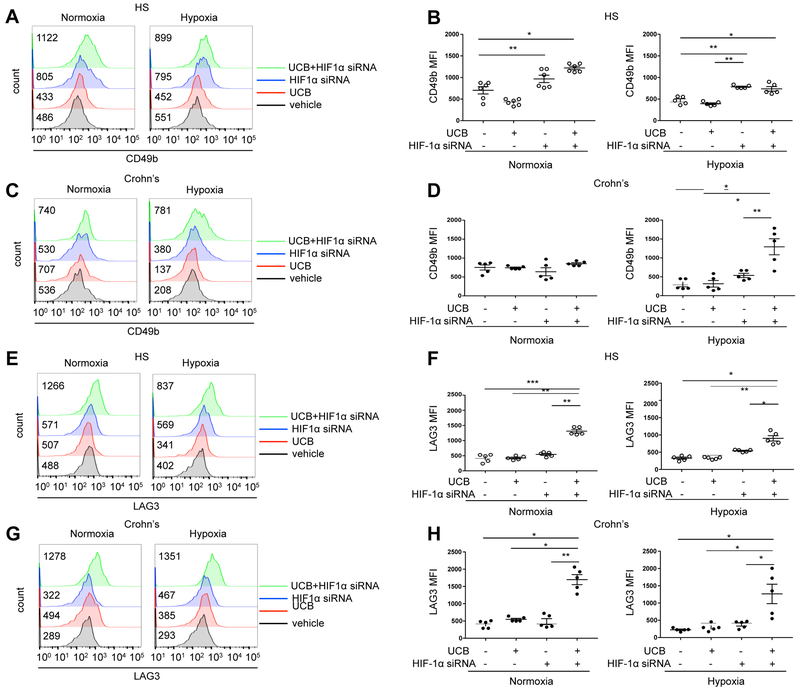
Since hypoxia dampens levels of IL10 expression in Crohn’s disease-derived Th17-cells (Fig. 1E and Supplementary Fig. 1C) and because previous work demonstrated inhibitory effects of HIF-1α over Tr1 differentiation [6], we next tested whether blockade of HIF-1α impacted expression of CD49b and LAG3, the identifying markers of IL10 producing Tr1-cells [30]. We found that exposure of Th17-cells, in which HIF-1α was blocked, resulted in significantly increased expression of CD49b in Th17-cells from healthy individuals but not in cells from Crohn’s patients both under normoxic and hypoxic conditions (Fig. 3, A-D). Addition of UCB to HIF-1α siRNA treatment did not cause further increases in CD49b levels in cells from healthy individuals (Fig. 3, A-B), but boosted expression of CD49b in Crohn’s-derived Th17-cells under hypoxia (Fig. 3, C-D). Treatment of Th17-cells with HIF-1α siRNA did not significantly change the expression of LAG3 under normoxic or hypoxic conditions in cells from healthy (Fig. 3, E-F) or Crohn’s disease patients (Fig. 3, G-H). Addition of UCB to HIF-1α siRNA treatment, however, resulted in significantly higher levels of LAG3 expression in cells from both health and Crohn’s patients and under both normoxic and hypoxic conditions (Fig. 3, E-H).
Fig. 3. Blockade of hypoxia boosts expression of Trl-cell markers by Th17-cells in Crohn’s disease samples.
Representative histograms of (A and C) CD49b and (E and G) LAG3 MFI in Th17-cells of one HS and one patient with Crohn’s disease are shown. Cells were treated either with UCB, HIF-1 siRNA or UCB plus HIF-1 siRNA, under normoxic or hypoxic conditions. Untreated (vehicle) controls are also shown. For the flow cytometry analysis, cells were initially gated on CD4+IL17+ lymphocytes; then CD49b and LAG3 MFI was measured. Numerical values of CD49b and LAG3 MFI in untreated, UCB or UCB plus HIF-1α siRNA-treated Th17-cells are indicated within the histogram plots. Mean ± SEM (B and D) CD49b and (F and H) LAG3 MFI of untreated, UCB, HIF-1α siRNA or UCB plus HIF-1α siRNA-treated Th17-cells under normoxic or hypoxic conditions (HS, n=5; Crohn’s patients, n=5).
Comparisons were made using one-way ANOVA, followed by Tukey’s multiple comparison test. *P≤0.05;**P≤0.01;***P≤0.001.
Fold changes in Th17-cell MFI for CD39, FOXP3, CD49b and LAG3 are summarized in Supplementary Table 1.
No differences in the response to HIF-1α blockade - alone or in combination with UCB - were noted between untreated (n=2) and treated patients (n=6).
Collectively, these data show that blockade of HIF-1α enhances the immunomodulatory properties of UCB, that in turn increases levels of Treg and Tr1 markers also in Th17-cells derived from patients with Crohn’s disease.
3.3. Hypoxia impacts on AhR downstream signaling
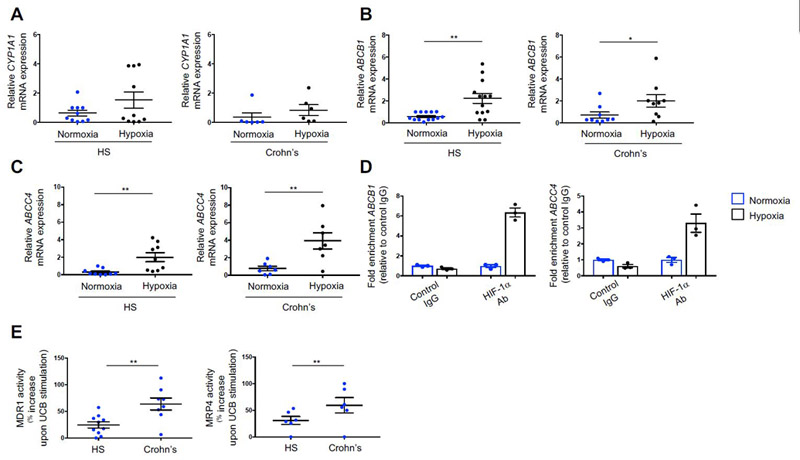
Because our data show that hypoxia does not directly impact levels of AhR and ARNT in Th17-cells derived from both healthy individuals and Crohn’s disease patients, we next investigated the effects of hypoxia on expression of molecules that are directly regulated by either AhR or ARNT, namely CYP1A1 and the ABC transporters multidrug-resistance-associated-protein-4 (MRP4/ABCC4) and the multidrug-resistance-protein-1 (MDR1/ABCB1). Levels of CYP1A1 and ABCC4, which are regulated by AhR [31], and ABCB1, which is modulated by ARNT [32] were measured in Th17-cells under normoxic and hypoxic conditions.
We found that hypoxia while not significantly impacting the expression of CYP1A1 (Fig. 4A), increased the levels of ABCB1 and ABCC4 in both healthy and Crohn’s disease-derived Th17-cells (Fig. 4, B-C), implicating a direct interaction between HIF-1α and ABCB1 and ABCC4 in human Th17-cells. To further dissect and study this link, we performed sequence analysis of the ABCB1 and ABCC4 promoter region and identified putative binding sites for HIF-1α (Supplementary Fig. 3, A-B). Chromatin immunoprecipitation analysis showed that upon Th17-cell exposure to hypoxia, there is enrichment in ABCB1 and ABCC4 expression upon binding of HIF-1α to the promoter region of the two genes (Fig. 4D).
Fig. 4. Hypoxia boosts the expression of AhR/ARNT-regulated ABC transporters.
Mean ± SEM (A) CYP1A1, (B) ABCB1 and (C) ABCC4 mRNA expression by Th17-cells from HS (n=10 for A; n=13 for B and n=9 for C) and patients with Crohn’s disease (n=6 for A; n=9 for B and n=7 for C). (D) Chromatin immunoprecipitation analysis showing enrichment of ABCB1 and ABCC4 mRNA expression in the promoter region bound by HIF-1α antibody, as compared to IgG negative control. Mean ± SEM ABCB1 and ABCC4 mRNA levels from three independent experiments performed using Th17-cells obtained from one healthy blood donor. (E) Mean ± SEM MDR1 and MRP4 activity, measured as percentage increase upon UCB stimulation in HS and Crohn’s disease patients (HS, n=10 for MDR1 and n=6 for MRP4; Crohn’s patients: n=8 for MDR1 and n=6 for MRP4). Comparisons were made using Wilcoxon signed-rank test (A-B) and paired t test (C, E). *P≤0.05;**P≤0.01.
Collectively, these data show that despite not having a direct impact on AhR and ARNT expression in human Th17-cells, hypoxia per se modulates the AhR/ARNT downstream signaling through upregulation of ABCB1 and ABCC4 mRNA levels.
ABCB1 and ABCC4 encode for the MDR1 and MRP4 ABC transporters, which pump drugs and metabolites out of cells and have been previously shown to be highly expressed on the intestinal epithelium (MDR1) and on lymphocytes (MDR1 and MRP4) [33, 34]. To investigate whether MDR1 and MRP4 have a role in favoring UCB efflux out of cells, we measured the activity of these two transporters in healthy control and Crohn’s-derived Th17-cells before and after exposure to UCB. We found that Th17-cells from Crohn’s disease patients display higher MDR1 and MRP4 activity upon stimulation with UCB (Fig. 4E).
Expression of MDR1 at baseline was lower in healthy control than in Crohn’s-derived Th17-cells (Supplementary Fig. 4A); there were no differences in the baseline MRP4 MFI data between healthy control and Crohn’s-derived Th17-cells (Supplementary Fig. 4B). Exposure of Th17-cells to UCB, activated MRP4 alone, in healthy subjects, and both MDR1 and MRP4 transporters, in Crohn’s disease (Supplementary Fig. 4, A-B). These data indicate that hypoxia increases levels of MDR1 and MRP4, the activity of which is further heightened in Crohn’s-derived Th17-cells that leads to heightened UCB efflux from the cell.
3.4. Pharmacological blockade of MDR1 and MRP4 boosts immunosuppressive properties of UCB on Th17-cells in Crohn’s disease
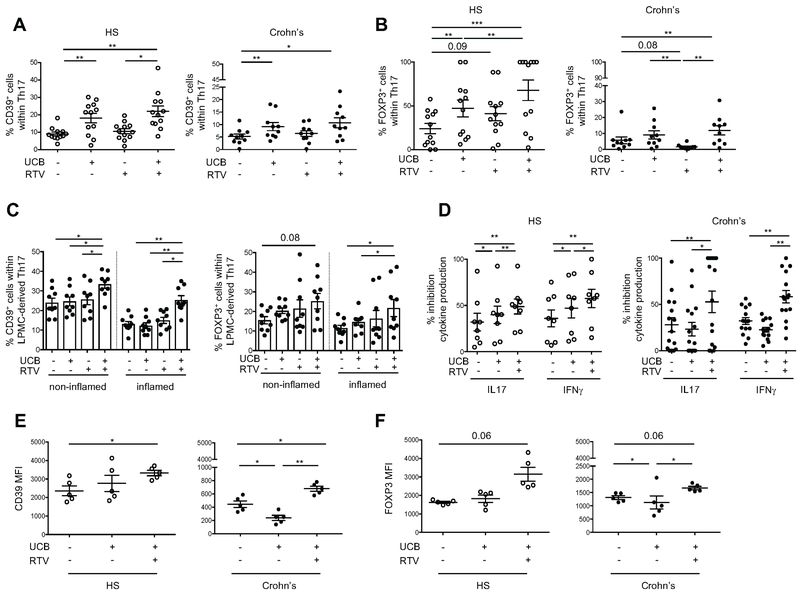
Given the heightened levels of HIF-1α in Th17-cells from patients with Crohn’s disease (Fig. 1B), the role of HIF-1α in promoting ABCB1 and ABCC4 expression (Fig. 4, B-C), and the increased MDR1 and MRP4 activity displayed by Crohn’s Th17-cells upon stimulation with UCB (Fig. 4E), we next tested the effect of pharmacological blockade of MDR1 and MRP4 on the Th17-cell response to UCB in vitro. To this end, we used RTV, an anti-retroviral drug that in addition to antagonizing HIF-1α [35] (Supplementary Fig. 5A) can also inhibit MDR1 [36] and MRP4 [37] (Supplementary Fig. 5, B-C), and tested its effects on UCB immunoregulatory properties in Th17-cells derived from the peripheral blood of both healthy individuals and patients with Crohn’s disease. Compared to stimulation with UCB only, treatment with UCB plus RTV resulted in a high proportion of peripheral blood-derived Th17-cells being positive for CD39 in both healthy controls and in patients with Crohn’s disease (Fig. 5A and Supplementary Fig. 6A). Further, this combination increased the frequency of Th17-cells expressing FOXP3 from both healthy volunteers and Crohn’s patients (Fig. 5B and Supplementary Fig. 6B). No differences were observed in the response of Th17-cells to the combination of RTV plus UCB comparing untreated (n=3) and treated (n=10) patients and between patients with mild disease activity (n=3) and patients in remission (n=10).
Fig. 5. Pharmacological inhibition of MDR1/MRP4 boosts UCB effects in human Th17-cells.
Mean ± SEM frequency of CD39+ (A) and FOXP3+ (B) lymphocytes within UCB, RTV or UCB plus RTV-treated Th17-cells (gated as CD4+IL17+ lymphocytes) from n=12 HS and n=10 Crohn’s disease patients. Untreated (vehicle) controls are also shown. (C) Mean ± SEM frequency of CD39+ and FOXP3+ lymphocytes within LPMC-derived Th17-cells isolated from non-inflamed and inflamed bioptic colonic areas (n=9 Crohn’s). Cells were exposed to the same treatments as in (A) and (B). (D) Mean ± SEM % inhibition of IL17 and IFNγ production by CD4+CD25− responders in the presence of untreated, UCB, RTV or UCB plus RTV-treated Th17-cells (n=8 HS and n=15 Crohn’s); (E) Mean ± SEM CD39 and (F) FOXP3 MFI of untreated and UCB, RTV or UCB plus RTV-treated Th17-cells under normoxic or hypoxic conditions (n=5 HS and n=5 Crohn’s).
Comparisons were made using one-way ANOVA or Kruskal-Wallis test, followed by Tukey’s or Dunn’s multiple comparison tests. *P≤0.05;**P≤0.01;***P≤0.001.
Notably, when analyzing Th17-cells derived from the lamina propria of Crohn’s disease patients, the UCB plus RTV treatment resulted in increases in the proportion of CD39+ and FOXP3+ lymphocytes (Fig. 5C). This effect was noted in Th17-cells that were obtained from both inflamed and non-inflamed (used as intra-individual control) colonic areas of the same patient (Fig. 5C).
Analysis of suppressive function showed that exposure to UCB plus RTV boosted Th17-cell ability to suppress IL17 and IFNγ production by responder CD4+CD25− cells from both healthy subjects and Crohn’s disease patients (Fig. 5D).
We then determined whether treatment with UCB plus RTV could be beneficial in modulating the response of Th17-cells to hypoxia. Stimulation with both compounds resulted in augmented CD39 expression levels, in healthy subjects and Crohn’s disease patients (Fig. 5E); in both groups, there was also a trend to increase in FOXP3 levels (Fig. 5F). Overall the data presented here show that RTV boosts the immunosuppressive effects of UCB, as seen in Crohn’s disease and restores Th17-cell responsiveness to UCB under hypoxic conditions.
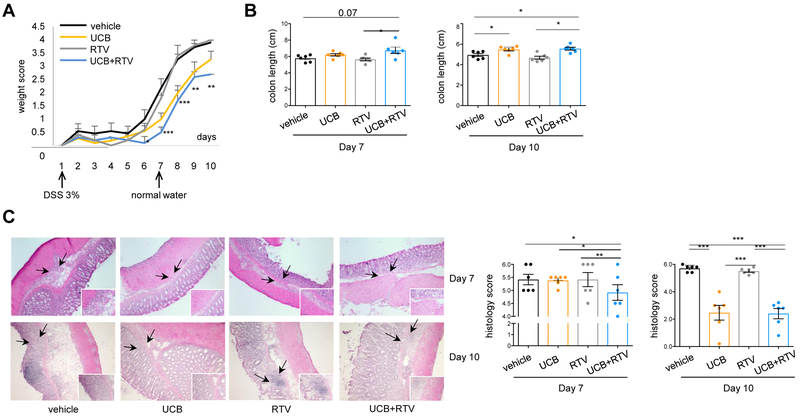
3.5. Pharmacological blockade of MDR1 and MRP4 enhances UCB immunosuppressive properties in experimental colitis
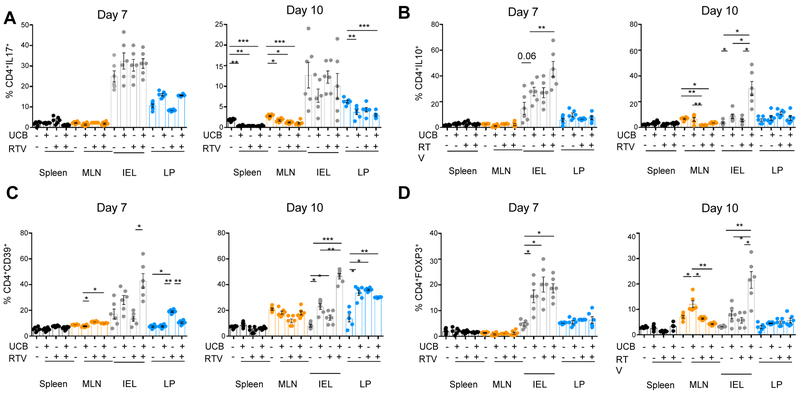
The effect of MDR1 and MRP4 blockade by RTV on UCB immunoregulatory properties was also tested in vivo in DSS-induced colitis in mice. RTV was administered alone or in combination with UCB to WT mice during the ‘induction’ and the ‘recovery’ phase of colitis, when DSS was replaced with standard drinking water. As depicted in Fig. 6A, the combination of UCB and RTV significantly decreased the body conditioning score from day 6 during DSS treatment and throughout recovery, when compared to vehicle or treatment with UCB or RTV only (Fig. 6A). On day 7, at the end of DSS treatment, mice subjected to combined treatment with RTV and UCB had the highest colon length (Fig. 6B), the lowest histology score (Fig. 6C) and the highest frequency of CD4+IL10+ (Fig. 7A) or CD4+CD39+ cells (Fig. 7B) amongst intra-epithelial lymphocytes (IEL). At day 10, mice treated with UCB and RTV displayed colon length and histology score similar to mice treated with UCB only (Fig. 6B-C). Combined treatment was associated with the lowest frequency of CD4+IL17+ cells in the spleen, MLN and lamina propria (Fig. 7A); and with the highest proportions of CD4+IL10+ (Fig. 7B), CD4+CD39+ (Fig. 7C) and CD4+FOXP3+ lymphocytes amongst IEL (Fig. 7D).
Fig. 6. Pharmacological inhibition of MDR1/MRP4 enhances immunoregulatory effects of UCB in DSS colitis.
WT mice (n=24) were treated with 3% DSS for 6 days and sacrificed on day 7. In additional n=24 mice, DSS treatment was replaced with normal water for additional 4 days. For the whole duration of experiment, mice were administered vehicle (n=12), UCB (n=12), RTV (n=12), or UCB plus RTV (n=12). (A) Mean ± SEM weight score in vehicle, UCB, RTV and UCB plus RTV-treated WT mice. (B) Mean ± SEM colon length (cm) on days 7 and 10. (C) Representative hematoxylin & eosin staining of colon sections. Mean ± SEM histology score on days 7 and 10 is also shown. Comparisons were made using one-way ANOVA, followed by Tukey’s multiple comparison test. *P≤0.05;**P≤0.01;***P≤0.001.
Fig. 7. Immunological effects of combined treatment with UCB and MDR1/MRP4 inhibitors in DSS colitis.
WT mice (n=24) were treated with 3% DSS for 6 days and sacrificed on day 7. In additional n=24 mice, DSS treatment was replaced with normal water for additional 4 days. For the whole duration of experiment, mice were administered vehicle (n=12), UCB (n=12), RTV (n=12), or UCB plus RTV (n=12). Mean ± SEM frequency of (A) CD4+IL17+, (B) CD4+IL10+, (C) CD4+CD39+ and (D) CD4+FOXP3+cells among spleen, MLN, IEL, LP-derived mononuclear cells obtained from mice on day 7 or 10.
Comparisons were made using one-way ANOVA or Kruskal-Wallis test, followed by Tukey’s or Dunn’s multiple comparison tests. *P≤0.05;**P≤0.01;***P≤0.001.
Collectively, these data indicate that administration of RTV in association with UCB enhances the salutary effects of UCB on colitis, including the induction phase.
4. Discussion
Our results show that hypoxia impairs Th17 regulatory responses to AhR ligation in IBD. This effect can be directly linked to enhanced UCB efflux secondary to high ABC transporter levels and xenobiotic pump activity thereby removing UCB from cells. Notably, pharmacological inhibition of MDR1 and MRP4, two of the ABC transporters expressed by Th17-cells and directly controlled by AhR/ARNT signaling, boosts the salutary properties of UCB in vivo. This intervention also abrogates Th17-cell resistance to AhR-mediated immunomodulation and allows immunoregulation.
Further, these data substantiate a complex role for hypoxia in the control of T-cell immunity. Previous reports show that severe and sudden hypoxia, such as that occurring during acute inflammation [2-4] activates transcriptional programs culminating with development of Tregs through the induction of FOXP3 by HIF-1α [1]. Moreover, in the context of hepatic ischemia reperfusion, hypoxia induces CD39 through the activation of the transcription factor Sp, whereas the linked ectonucleotidase CD73 is boosted through the induction of HIF-1α [38-40].
In the context of ongoing/protracted inflammation, heightened levels of HIF-1α that possibly result from post-transcriptional modifications including neddylation [41], have opposing effects on Th17-cells. These limiting effects leads to reduced responsiveness to AhR, without directly impacting AhR expression, as previously shown in mouse Tr1-cells [6]. This suggests in part a differential role for hypoxia and HIF-1α in acute vs. chronic states and in different cell subsets, like Th17, Tregs and Tr1-cells. The dualistic effects of hypoxia as either an immunomodulator in physiological immunological niches or a promoter of tissue dysfunction and disease development has recently been proposed as summarized in a review by Taylor & Colgan [42].
Blockade of HIF-1α with siRNAs effectively restored the Th17-cell response to AhR ligation. We demonstrated previously that UCB imparts regulatory properties to Th17-cells by upregulating CD39 and FOXP3 [8]. These properties, deeply impaired in the context of TBD inflammation likely as result of increased HIF-1α levels, are reacquired upon transcriptional control of HIF-1α under normoxic and hypoxic conditions.
In the context of chronic inflammatory states, as in longstanding autoimmune disease, hypoxia modulates T-cell metabolism by promoting glycolysis through the upregulation of Glut1 and glycolytic enzymes [43]. The shift towards aerobic glycolytic metabolism is accompanied by RORγt induction and proteosomal degradation of Foxp3 [5]. It is plausible that blockade of hypoxia/HIF-1α might, at least in part, reverse these metabolic changes. Such salutary effects could be further enhanced in the presence of AhR ligands - e.g. UCB in the experiments noted here - to further promote immunoregulation.
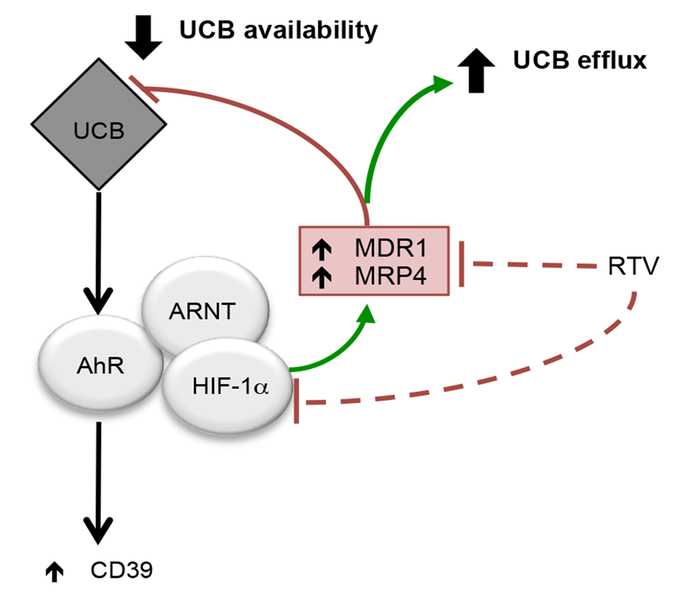
Notably, our results propose a novel mechanism through which hypoxia modulates refractoriness of Th17-cells to AhR signaling/ligation (Fig. 8). This involves a direct interaction between HIF-1α with ABCB1 and ABCC4, which encode for MDR1 and MRP4 ABC transporters. This has been confirmed by chromatin immunoprecipitation that identified direct binding of HIF-1α to the promoters of MDRl and MRP4. Induction of MDR1 by hypoxia was previously reported for epithelial cells, as well as in primary human microvascular endothelial cells [15].
Fig. 8. Hypoxia inhibits Th17-cell response to AhR ligation, by favoring efflux of immunomodulatory metabolites.
UCB boosts the immunoregulatory properties of Th17-cells by upregulating the CD39 ectoenzyme [8]. This mechanism is mediated by AhR, for which UCB serves as an endogenous ligand. In IBD, inflammation induced hypoxia and consequent upregulation of HIF-1α, boosts ABC transporter levels with consequent increase in the efflux of immunometabolites like UCB out of cells. This might reduce UCB or other substrate availability to AhR resulting in defective response by Th17-cells to AhR ligation.
Of note, the enzymatic activity of both MDR1 and MRP4 is elevated in TBD patient-derived Th17-cells, which also display heightened expression of MDR1, as previously reported [44]. Due to the increase in MDR1 and MRP4 function, Th17-cells from IBD patients can rapidly pump xenobiotics and endogenous metabolites like UCB, out of cells, significantly decreasing the time for AhR ligand interactions. Previous work had shown that ABC transporters, including MDR1, mediate UCB transport and efflux [45, 46].
As a consequence of increased efflux, as in the IBD setting, UCB might fail to both impart the typical Th17-cell regulatory properties and also perturb transition to a less inflammatory state. Based on our data, hypoxia-induced HIF-1α largely impacts AhR/ARNT signaling by diminishing the availability of AhR ligands.
We have recently shown that Crohn’s patients display circulating and lamina propria-derived Th17-cells that express low AhR levels [8]. We can therefore postulate multiple AhR-related defects in Crohn’s disease, including impaired expression and altered modulation by hypoxia and ABC transporters, the latter resulting in reduced substrate availability.
RTV, a non-specific inhibitor of MDR1 and MRP4, has been also shown to have antagonistic effects on HIF-1α [35] (Supplementary Fig. 5). This drug significantly boosted the immunosuppressive properties of UCB in DSS-induced colitis where, compared to UCB only, the clinical course was also improved in the induction phase of the injury. Further, combination treatment with UCB plus RTV resulted in increases in the expression of immunoregulatory molecules by CD4 cells, with IL10 and CD39 levels already heightened from the induction phase.
Notably, in human IBD-derived Th17-cells the combinational treatment resulted in restoration of the ability of these cells to respond to AhR stimulation, this being particularly evident when analyzing the phenotype of lamina propria-derived Th17-cells. In previous work, we showed that IBD-derived Th17-cells were found to be particularly refractory to the beneficial properties of UCB [8]. In the current study, the combination of UCB and RTV conferred Th17-cell immunoregulatory properties under both normoxic and hypoxic conditions, mimicking the effect of HIF-1α blockade in the latter setting. UCB and RTV-treated Th17-cells exert increased regulatory control over effector cell function, also in the experimentation when these cells were obtained from Crohn’s disease patients. Whether the phenotypic and functional immunoregulatory properties acquired by Th17-cells following UCB and RTV treatment are maintained over time remains to be addressed. This scenario also implicates the acquisition of a ‘suppressor-like’ phenotype by Th17-cells, as we have reported previously [21].
Our data also highlight that in the context of IBD, hypoxia decreases the expression of IL10 in Th17-cells. IL10 is one of the regulatory features UCB imparts on pathogenic Th17-cells [8]. Notably, control of HIF-1α using siRNA silencing reverses these inhibitory effects, by boosting the levels of both CD49b and LAG3, two markers that phenotypically identify Tr1-cells in mouse and humans [30]. Hypoxia and HIF-1α were previously shown to interfere with Tr1 differentiation by impacting AhR expression levels [6]. Our data here propose that hypoxia alters the Th17 response to AhR ligation protracting Th17 inflammatory status, whereas inhibition of hypoxia favors the acquisition of Tr1 properties upon UCB treatment. Th17/Tr1 plasticity was reported in previous studies and referred to as ‘Th17 transdifferentiation’ [47, 48]. It is likely that in this experimental model Th17-cells do not fully transdifferentiate into Tr1-cells. Despite an increase in CD49b and LAG3 levels, these cells also express high levels of FOXP3 that normally signify Tregs in the clinical setting. In the presence of HIF-1α blockade UCB exerts more effective control over Th17-cell effector function and confer on Th17-cells the concomitant ability to act as regulatory cells.
In conclusion, our investigations identify a novel mechanism of Th17 effector cell biology in human IBD. Based on this model, chronic inflammation and resulting hypoxia alter the sensitivity of Th17-cells to AhR ligation, by promoting MDR1 and MRP4. This has the effect of diminishing the availability of AhR ligands in the pathologic state, such as in Crohn’s disease. This impact is compounded by already elevated MDR1 and MRP4 activity in these circumstances in Th17-cells. Pharmacologic manipulation of these pro-inflammatory pathways may restore responsiveness to UCB and potentially other natural AhR ligands. This approach could eventually re-establish immune homeostasis by increasing immunoregulatory properties to Th17-cells and thereby limit the pathogenic potential of these immune cells in disease states such as Crohn’s disease.
Supplementary Material
Mean ± SEM (A) CD39 and (B) FOXP3 MFI of Th17-cells obtained from HS (n=5) and patients with Crohn’s disease (n=5). (C) Representative flow cytometry plots showing the frequency of IL10+ lymphocytes within CD4+IL17+ cells from one HS and one patient with Crohn’s. Mean ± SEM frequency of IL10+ cells within the CD4+IL17+ subset (HS n=6; Crohn’s patients, n=10).
Comparisons were made using one-way ANOVA or Kruskal-Wallis test, followed by Tukey’s or Dunn’s multiple comparison tests. *P≤0.05;**P≤0.01;***P≤0.001.
Putative HIF-1α binding sites (in blue) within the (A) ABCB1 and the (B) ABCC4 promoter regions.
Levels of MDR1 and MRP4 were determined by flow cytometry using rhodamine or Calcein AM staining respectively. Mean ± SEM (A) MDR1 and (B) MRP4 MFI of untreated and UCB-treated Th17-cells from healthy subjects (HS, n=10 for MDR1 and n=6 for MRP4 determination) and Crohn’s disease patients (n=8 for MDR1 and n=6 for MRP4 determination).
Comparisons were made using one-way ANOVA, followed by Tukey’s multiple comparison test. *P≤0.05;**P≤0.01.
Th17-cells were obtained from the peripheral blood of healthy subjects (HS) and then exposed to 5 μM RTV for the last 24 hours of culture. (A) Mean ± SEM HIF-1α mRNA levels in untreated and RTV-treated Th17-cells (HS, n=9). Representative histograms showing (B) MDR1 and (C) MRP4 MFI of untreated, UCB or UCB plus RTV-treated Th17-cells. Numerical values of MDR1 and MRP4 MFI in untreated, UCB or UCB plus RTV-treated Th17-cells are indicated within the histogram plots. Mean ± SEM MDR1 and MRP4 MFI from 5 HS are also shown.
Comparisons were made using Wilcoxon signed-rank test (A) and one-way ANOVA, followed by Tukey’s multiple comparison test (B-C). *P≤0.05;**P≤0.01.
Th17-cells were differentiated from peripheral blood-derived CD4+ cells and then exposed to vehicle, UCB, RTV or the combination of UCB and RTV. Frequencies of CD39+ and FOXP3+ lymphocytes within the CD4+IL17+ subset were determined by flow cytometry. Flow cytometry plots of CD4 (X axis) and (A) CD39 or FOXP3 (B) (Y axis) fluorescence intensity in one HS (representative of 12) and one patient with Crohn’s disease (representative of 10) are shown.
(A) ADPase ectoenzymatic activity of untreated and UCB-treated Th17-cells under normoxic or hypoxic conditions was determined by TLC upon cell incubation with 14C-labeled ADP. A representative of 4 independent experiments is shown. (B) Mean ± SEM ADP/AMP ratio of untreated and UCB-treated Th17-cells under normoxic or hypoxic conditions (HS n=4; Crohn’s patients, n=4).
Highlights.
Hypoxia curbs Th17-cell response to unconjugated bilirubin (UCB) in Crohn’s disease
Blockade of HIF-1α upregulates CD39 and reconstitutes Th17-cell response to UCB
Th17 resistance to UCB results from HIF-1α-induced upregulation of ABC transporters
Inhibition of HIF-1α/ABC transporters ameliorates UCB properties in vivo
Inhibition of HIF-1α/ABC transporters restores Th17 response to UCB in Crohn’s disease
Acknowledgments
Funding
This work has been supported by the National Institute of Health (R01 DK108894 to M.S.L.; P01 HL107152 and R21 CA164970 to S.C.R.; ES02530, AI126880 and AI093903 to F.J.Q.); Pfizer research support to S.C.R.; the Helmsley Charitable Trust (grant 281574.5069091.0010 to S.C.R. and A.C.M.); and by the Department of Defense Award W81XWH-16-0464 (to L.E.O and S.C.R.).
Abbreviations:
- (UCB)
unconjugated bilirubin
- (AhR)
aryl hydrocarbon receptor
- (ARNT)
aryl hydrocarbon receptor nuclear translocator
- (HIF-1α)
hypoxia inducible factor 1 alpha
- (ABC)
ATP binding cassette
- (MDR1)
multidrug resistance protein 1
- (MRP4)
multidrug resistance associated protein 4
- (RTV)
ritonavir
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interest statement: The Authors declare that no competing interests exist
References
- [1].Clambey ET, McNamee EN, Westrich JA, Glover LE, Campbell EL, Jedlicka P et al. Hypoxia-inducible factor-1 alpha-dependent induction of FoxP3 drives regulatory T-cell abundance and function during inflammatory hypoxia of the mucosa. Proc Natl Acad Sci U S A, 2012;109:E2784–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Caldwell CC, Kojima H, Lukashev D, Armstrong J, Farber M, Apasov SG et al. Differential effects of physiologically relevant hypoxic conditions on T lymphocyte development and effector functions. J Immunol, 2001;167:6140–9. [DOI] [PubMed] [Google Scholar]
- [3].Thiel M, Caldwell CC, Kreth S, Kuboki S, Chen P, Smith P et al. Targeted deletion of HIF-1alpha gene in T cells prevents their inhibition in hypoxic inflamed tissues and improves septic mice survival. PLoS One, 2007;2:e853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lukashev D, Klebanov B, Kojima H, Grinberg A, Ohta A, Berenfeld L et al. Cutting edge: hypoxia-inducible factor 1alpha and its activation-inducible short isoform I.1 negatively regulate functions of CD4+ and CD8+ T lymphocytes. J Immunol, 2006;177:4962–5. [DOI] [PubMed] [Google Scholar]
- [5].Dang EV, Barbi J, Yang HY, Jinasena D, Yu H, Zheng Y et al. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell, 2011;146:772–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Mascanfroni ID, Takenaka MC, Yeste A, Patel B, Wu Y, Kenison JE et al. Metabolic control of type 1 regulatory T cell differentiation by AHR and HIF1-alpha. Nat Med, 2015;21:638–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Roncarolo MG, Gregori S, Bacchetta R, Battaglia M. Tr1 cells and the counter-regulation of immunity: natural mechanisms and therapeutic applications. Curr Top Microbiol Immunol, 2014;380:39–68. [DOI] [PubMed] [Google Scholar]
- [8].Longhi MS, Vuerich M, Kalbasi A, Kenison JE, Yeste A, Csizmadia E et al. Bilirubin suppresses Th17 immunity in colitis by upregulating CD39. JCI Insight, 2017;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Phelan D, Winter GM, Rogers WJ, Lam JC, Denison MS. Activation of the Ah receptor signal transduction pathway by bilirubin and biliverdin. Arch Biochem Biophys, 1998;357:155–63. [DOI] [PubMed] [Google Scholar]
- [10].Gutierrez-Vazquez C, Quintana FJ. Regulation of the Immune Response by the Aryl Hydrocarbon Receptor. Immunity, 2018;48:19–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E et al. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature, 2008;453:65–71. [DOI] [PubMed] [Google Scholar]
- [12].Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature, 2008;453:106–9. [DOI] [PubMed] [Google Scholar]
- [13].Omiecinski CJ, Vanden Heuvel JP, Perdew GH, Peters JM. Xenobiotic metabolism, disposition, and regulation by receptors: from biochemical phenomenon to predictors of major toxicities. Toxicol Sci, 2011;120 Suppl 1:S49–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Fletcher JI, Haber M, Henderson MJ, Norris MD. ABC transporters in cancer: more than just drug efflux pumps. Nat Rev Cancer, 2010;10:147–56. [DOI] [PubMed] [Google Scholar]
- [15].Comerford KM, Wallace TJ, Karhausen J, Louis NA, Montalto MC, Colgan SP. Hypoxia-inducible factor-1-dependent regulation of the multidrug resistance (MDR1) gene. Cancer Res, 2002;62:3387–94. [PubMed] [Google Scholar]
- [16].Arsenescu R, Arsenescu V, Zhong J, Nasser M, Melinte R, Dingle RW et al. Role of the xenobiotic receptor in inflammatory bowel disease. Inflamm Bowel Dis, 2011;17:1149–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Goettel JA, Gandhi R, Kenison JE, Yeste A, Murugaiyan G, Sambanthamoorthy S et al. AHR Activation Is Protective against Colitis Driven by T Cells in Humanized Mice. Cell Rep, 2016;17:1318–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lanis JM, Alexeev EE, Curtis VF, Kitzenberg DA, Kao DJ, Battista KD et al. Tryptophan metabolite activation of the aryl hydrocarbon receptor regulates IL-10 receptor expression on intestinal epithelia. Mucosal Immunol, 2017;10:1133–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med, 2007;204:1257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Friedman DJ, Kunzli BM, YI AR, Sevigny J, Berberat PO, Enjyoji K et al. From the Cover: CD39 deletion exacerbates experimental murine colitis and human polymorphisms increase susceptibility to inflammatory bowel disease. Proc Natl Acad Sci U S A, 2009;106:16788–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Longhi MS, Moss A, Bai A, Wu Y, Huang H, Cheifetz A et al. Characterization of human CD39+ Th17 cells with suppressor activity and modulation in inflammatory bowel disease. PLoS One, 2014;9:e87956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Liberal R, Grant CR, Ma Y, Csizmadia E, Jiang ZG, Heneghan MA et al. CD39 mediated regulation of Th17-cell effector function is impaired in juvenile autoimmune liver disease. J Autoimmun, 2016;72:102–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Delmonte OM, Bertolotto G, Ricotti E, Tovo PA. Immunomodulatory effects of two HIV protease inhibitors, Saquinavir and Ritonavir, on lymphocytes from healthy seronegative individuals. Immunol Lett, 2007;111:111–5. [DOI] [PubMed] [Google Scholar]
- [24].Hruz PW, Yan Q, Struthers H, Jay PY. HIV protease inhibitors that block GLUT4 precipitate acute, decompensated heart failure in a mouse model of dilated cardiomyopathy. FASEB J, 2008;22:2161–7. [DOI] [PubMed] [Google Scholar]
- [25].Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc, 2007;2:541–6. [DOI] [PubMed] [Google Scholar]
- [26].Longhi MS, Ma Y, Grant CR, Samyn M, Gordon P, Mieli-Vergani G et al. T-regs in autoimmune hepatitis-systemic lupus erythematosus/mixed connective tissue disease overlap syndrome are functionally defective and display a Th1 cytokine profile. J Autoimmun, 2013;41:146–51. [DOI] [PubMed] [Google Scholar]
- [27].Holder BS, Grant CR, Liberal R, Ma Y, Heneghan MA, Mieli-Vergani G et al. Retinoic acid stabilizes antigen-specific regulatory T-cell function in autoimmune hepatitis type 2. J Autoimmun, 2014;53:26–32. [DOI] [PubMed] [Google Scholar]
- [28].Tomiyasu H, Watanabe M, Sugita K, Goto-Koshino Y, Fujino Y, Ohno K et al. Regulations of ABCB1 and ABCG2 expression through MAPK pathways in acute lymphoblastic leukemia cell lines. Anticancer Res, 2013;33:5317–23. [PubMed] [Google Scholar]
- [29].Grant CR, Liberal R, Holder BS, Cardone J, Ma Y, Robson SC et al. Dysfunctional CD39(POS) regulatory T cells and aberrant control of T-helper type 17 cells in autoimmune hepatitis. Hepatology, 2014;59:1007–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gagliani N, Magnani CF, Huber S, Gianolini ME, Pala M, Licona-Limon P et al. Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nat Med, 2013;19:739–46. [DOI] [PubMed] [Google Scholar]
- [31].Xu S, Weerachayaphorn J, Cai SY, Soroka CJ, Boyer JL. Aryl hydrocarbon receptor and NF-E2-related factor 2 are key regulators of human MRP4 expression. Am J Physiol Gastrointest Liver Physiol, 2010;299:G126–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Chan YY, Kalpana S, Chang WC, Chang WC, Chen BK. Expression of aryl hydrocarbon receptor nuclear translocator enhances cisplatin resistance by upregulating MDR1 expression in cancer cells. Mol Pharmacol, 2013;84:591–602. [DOI] [PubMed] [Google Scholar]
- [33].Thiebaut F, Tsuruo T, Hamada H, Gottesman MM, Pastan I, Willingham MC. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci U S A, 1987;84:7735–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Turriziani O, Gianotti N, Falasca F, Boni A, Vestri AR, Zoccoli A et al. Expression levels of MDR1, MRP1, MRP4, and MRP5 in peripheral blood mononuclear cells from HIV infected patients failing antiretroviral therapy. J Med Virol, 2008;80:766–71. [DOI] [PubMed] [Google Scholar]
- [35].Vadlapatla RK, Vadlapudi AD, Pal D, Mukherji M, Mitra AK. Ritonavir inhibits HIF-1alpha-mediated VEGF expression in retinal pigment epithelial cells in vitro. Eye (Lond), 2014;28:93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Bierman WF, Scheffer GL, Schoonderwoerd A, Jansen G, van Agtmael MA, Danner SA et al. Protease inhibitors atazanavir, lopinavir and ritonavir are potent blockers, but poor substrates, of ABC transporters in a broad panel of ABC transporter-overexpressing cell lines. J Antimicrob Chemother, 2010;65:1672–80. [DOI] [PubMed] [Google Scholar]
- [37].Fukuda Y, Takenaka K, Sparreboom A, Cheepala SB, Wu CP, Ekins S et al. Human immunodeficiency virus protease inhibitors interact with ATP binding cassette transporter 4/multidrug resistance protein 4: a basis for unanticipated enhanced cytotoxicity. Mol Pharmacol, 2013;84:361–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hart ML, Gorzolla IC, Schittenhelm J, Robson SC, Eltzschig HK. SP1-dependent induction of CD39 facilitates hepatic ischemic preconditioning. J Immunol, 2010;184:4017–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Eltzschig HK, Kohler D, Eckle T, Kong T, Robson SC, Colgan SP. Central role of Sp1-regulated CD39 in hypoxia/ischemia protection. Blood, 2009;113:224–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Haschemi A, Wagner O, Marculescu R, Wegiel B, Robson SC, Gagliani N et al. Cross-regulation of carbon monoxide and the adenosine A2a receptor in macrophages. J Immunol, 2007;178:5921–9. [DOI] [PubMed] [Google Scholar]
- [41].Curtis VF, Ehrentraut SF, Campbell EL, Glover LE, Bayless A, Kelly CJ et al. Stabilization of HIF through inhibition of Cullin-2 neddylation is protective in mucosal inflammatory responses. FASEB J, 2015;29:208–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Taylor CT, Colgan SP. Regulation of immunity and inflammation by hypoxia in immunological niches. Nat Rev Immunol, 2017;17:774–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Shi LZ, Wang R, Huang G, Vogel P, Neale G, Green DR et al. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med, 2011;208:1367–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Ramesh R, Kozhaya L, McKevitt K, Djuretic IM, Carlson TJ, Quintero MA et al. Pro-inflammatory human Th17 cells selectively express P-glycoprotein and are refractory to glucocorticoids. J Exp Med, 2014;211:89–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Watchko JF, Daood MJ, Hansen TW. Brain bilirubin content is increased in P-glycoprotein-deficient transgenic null mutant mice. Pediatr Res, 1998;44:763–6. [DOI] [PubMed] [Google Scholar]
- [46].Bellarosa C, Bortolussi G, Tiribelli C. The role of ABC transporters in protecting cells from bilirubin toxicity. Curr Pharm Des, 2009;15:2884–92. [DOI] [PubMed] [Google Scholar]
- [47].Esplugues E, Huber S, Gagliani N, Hauser AE, Town T, Wan YY et al. Control of TH17 cells occurs in the small intestine. Nature, 2011;475:514–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Gagliani N, Amezcua Vesely MC, Iseppon A, Brockmann L, Xu H, Palm NW et al. Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature, 2015;523:221–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mean ± SEM (A) CD39 and (B) FOXP3 MFI of Th17-cells obtained from HS (n=5) and patients with Crohn’s disease (n=5). (C) Representative flow cytometry plots showing the frequency of IL10+ lymphocytes within CD4+IL17+ cells from one HS and one patient with Crohn’s. Mean ± SEM frequency of IL10+ cells within the CD4+IL17+ subset (HS n=6; Crohn’s patients, n=10).
Comparisons were made using one-way ANOVA or Kruskal-Wallis test, followed by Tukey’s or Dunn’s multiple comparison tests. *P≤0.05;**P≤0.01;***P≤0.001.
Putative HIF-1α binding sites (in blue) within the (A) ABCB1 and the (B) ABCC4 promoter regions.
Levels of MDR1 and MRP4 were determined by flow cytometry using rhodamine or Calcein AM staining respectively. Mean ± SEM (A) MDR1 and (B) MRP4 MFI of untreated and UCB-treated Th17-cells from healthy subjects (HS, n=10 for MDR1 and n=6 for MRP4 determination) and Crohn’s disease patients (n=8 for MDR1 and n=6 for MRP4 determination).
Comparisons were made using one-way ANOVA, followed by Tukey’s multiple comparison test. *P≤0.05;**P≤0.01.
Th17-cells were obtained from the peripheral blood of healthy subjects (HS) and then exposed to 5 μM RTV for the last 24 hours of culture. (A) Mean ± SEM HIF-1α mRNA levels in untreated and RTV-treated Th17-cells (HS, n=9). Representative histograms showing (B) MDR1 and (C) MRP4 MFI of untreated, UCB or UCB plus RTV-treated Th17-cells. Numerical values of MDR1 and MRP4 MFI in untreated, UCB or UCB plus RTV-treated Th17-cells are indicated within the histogram plots. Mean ± SEM MDR1 and MRP4 MFI from 5 HS are also shown.
Comparisons were made using Wilcoxon signed-rank test (A) and one-way ANOVA, followed by Tukey’s multiple comparison test (B-C). *P≤0.05;**P≤0.01.
Th17-cells were differentiated from peripheral blood-derived CD4+ cells and then exposed to vehicle, UCB, RTV or the combination of UCB and RTV. Frequencies of CD39+ and FOXP3+ lymphocytes within the CD4+IL17+ subset were determined by flow cytometry. Flow cytometry plots of CD4 (X axis) and (A) CD39 or FOXP3 (B) (Y axis) fluorescence intensity in one HS (representative of 12) and one patient with Crohn’s disease (representative of 10) are shown.
(A) ADPase ectoenzymatic activity of untreated and UCB-treated Th17-cells under normoxic or hypoxic conditions was determined by TLC upon cell incubation with 14C-labeled ADP. A representative of 4 independent experiments is shown. (B) Mean ± SEM ADP/AMP ratio of untreated and UCB-treated Th17-cells under normoxic or hypoxic conditions (HS n=4; Crohn’s patients, n=4).