Abstract
Purpose:
We investigated whether TBS differs by sex, race/ethnicity, body mass index (BMI), and other clinical variables.
Methods:
The VITamin D and OmegA-3 TriaL (VITAL) is determining effects of vitamin D3 and/or omega-3 fatty acid (FA) supplements in reducing risks of cancer and cardiovascular disease. In the VITAL: Effects on Bone Structure/Architecture ancillary study, effects of these interventions on bone will be investigated. Here we examine the associations of clinical risk factors with TBS assessments at baseline in the bone health subcohort, comprised of 672 participants (369 men and 303 women), mean (±SD) age 63.5±6.0 yrs; BMI ≤ 37 kg/m2, no bisphosphonates within 2 years or other bone active medications within 1 year.
Results:
TBS was greater in men than women (1.311 vs. 1.278; p<0.001) and lower with elevated BMIs (p<0.001), higher age (p=0.004), diabetes (p=0.008), SSRI use (p=0.044), and high alcohol intake (p=0.009). There was a trend for history of fragility fractures (p=0.072), and lower TBS. TBS did not vary when analyzed by race/ethnicity, smoking, history of falls, multivitamin or caffeine use.
Conclusions:
Lower TBS was associated with female sex, aging, BMI ≥25 kg/m2, SSRI use, alcohol use and presence of diabetes; there was a trend between lower TBS and history of fragility fractures. TBS may be useful clinically to assess structural changes that may be associated with fractures among patients who are overweight or obese, those on SSRIs or with diabetes. Ongoing follow-up studies will clarify the effects of supplemental vitamin D3 and/or FA’s on TBS and other bone health measures.
Keywords: Trabecular bone score, TBS, Osteoporosis, Fracture, SSRI
Mini Abstract:
We investigated the association of clinical variables with TBS at baseline in the bone health subcohort of the VITamin D and OmegA-3 TriaL (VITAL). Lower TBS was associated with female sex, aging, BMI ≥25 kg/m2, SSRI use, high alcohol intake, and presence of diabetes; there was a trend towards significance between lower TBS and history of fragility fractures.
Introduction:
Osteoporosis is the most common bone disease, and each year there are an estimated 2 million fractures in the U.S. and 200 million fractures worldwide [1,2]. Dual energy X-ray absorptiometry (DXA) is widely used for clinical care and research to measure bone mineral density (BMD). In the U.S., there are 53.6 million Americans who are at risk for fractures, including 10.2 million adults with osteoporosis according to BMD criteria and 43.4 million adults with osteopenia and low bone mass [2]. Since more than half of fragility fractures occur in individuals who have low bone mass, but are not osteoporotic by DXA, other factors must influence bone strength and fracture risk. These include clinical risk factors, disordered bone microarchitecture and/or bone remodeling, and accumulation of microfractures.
The Trabecular Bone Score (TBS) is a new, FDA-approved measure of bone texture that is generated from spinal BMD scans and is associated with bone microarchitecture [3]. Cross-sectional studies show that higher TBS is inversely associated with risk of osteoporotic fractures in postmenopausal women [4,5] and men, [6] regardless of whether the BMD T-score is in the osteoporotic or osteopenic range. Longitudinal studies also show that TBS predicts fracture risk in women [7,8] and men [9] and the results are additive to BMD in predicting fractures. The Fracture Risk Assessment Tool (FRAX®) score, which includes various clinical risk factors and femoral neck BMD, is widely used in adults with osteopenia to identify the absolute risk of fracture over 10 years. Recent studies show that FRAX score in combination with TBS can better predict fracture risk than FRAX alone [10]. A high TBS suggests strong microarchitecture, which should be resistant to fracture, whereas a low TBS indicates weaker, more fracture-prone bone.
New studies show that TBS increases in response to some osteoporosis therapies [11] and is lower in individuals with diabetes, chronic kidney disease, primary hyperparathyroidism, or history of glucocorticoid therapy [12–14]. For conditions like diabetes and obesity, the information provided by TBS is of particular interest as fracture risk is increased even though BMD values by DXA are higher than in non-diabetic and non-obese individuals, respectively [15,16]. As TBS is still a relatively new technology, determinants of TBS still need to be clarified and confirmed.
VITAL: Effects on Bone Structure and Architecture is a NIH-sponsored, ancillary study to the VITamin D and OmegA-3 Trial (VITAL) that is being conducted among a sub-cohort of participants at the Harvard Catalyst Clinical and Translational Science Center (CTSC) to test effects of supplemental vitamin D and omega-3 free fatty acid (FAs) on bone health imaging outcomes [17]. Vitamin D is widely used to promote skeletal health, but definitive data on benefits and risks of supplemental vitamin D have been inconsistent [18]. Results from the ongoing VITAL: Effects on Bone Structure and Architecture will clarify the relationship between vitamin D and bone health outcomes. The objective of this study was to determine in the large VITAL CTSC sub-cohort whether results of TBS at baseline vary by sex, race/ethnicity, body mass index (BMI), medication use and other clinical variables.
Materials and Methods:
Overview of study design
VITAL is a 2×2 factorial, double-blind, randomized, placebo-controlled trial investigating the effects of high-dose vitamin D3 (cholecalciferol, 2000 IU/d) and omega-3 fatty acid (1g/d; eicosapentaenoic acid and docosahexaenoic acid) supplementation on cancer and cardiovascular disease. The mean treatment period is 5 years. VITAL-Bone Health has been composed of two concurrent, ancillary studies, (1) VITAL: Effects on Fracture that has been determining the effects of supplemental vitamin D and/or omega-3 fatty acids on incident fractures in the large VITAL cohort (N=25,874 participants) and (2) VITAL: Effects on Bone Structure and Architecture that is being conducted among a sub-cohort of VITAL participants (N=771) using detailed, in-person phenotyping and bone assessments to determine the skeletal mechanisms through which these interventions may impact bone [17]. This study is included in the Clinical Trials.gov website (NCT01747447). These studies were approved by the Partners Human Research Committees, the Institutional Review Board of Brigham and Women’s Hospital (BWH).
Study population
As a primary prevention trial, VITAL participants (women aged ≥55 years and men ≥50 years) were enrolled from 50 states and had no prior history of cancer or cardiovascular disease. The parent VITAL trial recruited participants by sending screening questionnaires to a list of names and addresses assembled from commercially available U.S. mailing lists from professional organizations (e.g., licensed health professionals and business professionals), other organizations (e.g., AARP), and subscription lists of magazines for older adults and black individuals. Participants who had completed previous trials were also contacted for participation in VITAL and articles and advertisements were posted in newspapers and magazines. Safety exclusions included renal failure, hypercalcemia, hypo- or hyperparathyroidism, severe liver disease, sarcoidosis or other granulomatous disease, allergy to soy or fish, or other serious illness. Prior to enrollment, all participants signed a detailed informed consent form and were required to complete a 3-month, placebo run-in to demonstrate good pill-taking compliance (defined as taking ≥ 2/3 of the study pills). Participants were required to limit consumption of supplemental vitamin D to no more than 800 IU daily, consumption of supplemental calcium to no more than 1200 mg daily, and to abstain from the use of fish oil supplements during the run-in and randomization treatment periods. Study enrollment began in November 2011 and closed in March 2014 and included a final randomized cohort of 25,874 participants, including 5,107 African Americans/Blacks [19]. The intervention phase of VITAL ended on December 31, 2017, and the results of the parent trial will be reported in mid-to-late 2018.
A sub-cohort of 1,054 VITAL participants from the New England region was enrolled for detailed, in-person phenotyping at the CTSC in Boston. Participants in the VITAL CTSC cohort were eligible for the VITAL: Effects on Structure and Architecture ancillary study if they did not have current use or prior history of bisphosphonates within the past two years or other osteoporosis medications including denosumab, human parathyroid hormone, calcitonin, raloxifene, tamoxifen, or systemic estrogens within the last year. Enrollment in the ancillary study (N = 771) exceeded the projected goal of 600 participants. Due to the 6- to 8-hour in-person visits, participants at baseline were generally healthier (less obese, hypertensive, diabetic, and smoked less) than the general U.S. population [20]. Of the 771 participants who had DXA scans, TBS was quantified among 672 participants who were within the weight-cut-offs (BMI ≤ 37 kg/m2) and had at least two suitable vertebrae available for TBS analysis (per Medimaps, Geneva, Switzerland). A total of 54 individuals with BMI >37 were excluded from the cohort and an additional 45 participants were excluded because they did not have vertebral images suitable for analysis.
Measurement of BMD
Baseline BMD of the spine (L1–L4), hip, and total body were measured by DXA (Discovery W, APEX Software Version 4.2, Hologic, Bedford, MA) in the Skeletal Health, Osteoporosis Center and Bone Density Unit at Brigham and Women’s Hospital. Reproducibility, reported as least significant change, for the spine BMD in the Bone Density Unit is 0.024 g/cm2.
Measurement of TBS:
TBS is an analytical, non-invasive measure using software incorporated into the DXA machine and generated from spinal BMD scans (Hologic Discovery, Bedford, MA) using the latest TBS iNsight software (version 2.1; Medimaps Group, Geneva, Switzerland) [8]. This technique determines vertebral texture by applying a quantitative algorithm to measure grey-level variation in 2-dimensional projection images to assess 3-dimensional textural characteristics. In vivo intra- and inter-machine precision values for TBS range from 1.1%−2.1% depending on the studies and the population [21].
Clinical Variables:
Risk factors that may affect bone health and body composition were assessed: age, sex, race/ethnicity, height, weight, BMI, smoking, alcohol use, physical activity (defined as total metabolic equivalent (MET) hours/week), osteoarthritis, baseline history of fragility fracture(s), history of falls, insulin-treated and non-insulin-treated diabetes, and SSRI-use.
Statistical Analysis:
Linear regression analysis was used to compare means of TBS across risk factor levels. Least-squares means adjusting for age, sex, Black race/ethnicity, and BMI are presented. Tests for interaction were performed across measures with ordinal levels. We ran a stepwise backward selection linear regression model including all the variables but forcing in age, sex, and BMI to mutually adjust for the clinical variables. Those with a p-value < 0.05 were retained in the model. All statistical analyses were performed using SAS (SAS Institute, Cary, NC). P values less than 0.05 were considered to be statistically significant.
Results:
Table 1. summarizes the mean TBS and baseline characteristics of the VITAL CTSC sub-cohort adjusted for age, sex/gender, and race/ethnicity. This study included 672 participants: 369 men (54.91%) and 303 women (45.09%) with a mean age of 63.5±6.0 years. Participants with a BMI > 37 kg/km2 (recommended BMI cut-off by the manufacturer) were excluded from the analysis. Mean TBS was greater in men than in women (1.311 vs. 1.278; p<0.001). TBS decreased with older age in the entire sub-cohort (p=0.004). This was not significant in men when the sexes were analyzed individually (Figure 1); however, the interaction with sex was not statistically significant (Appendix 1).
Table 1.
Baseline Characteristics of the VITAL CTSC, TBS Sub-cohort adjusted for age, sex/gender, Black race/ethnicity, and BMI
| Variable | Total (%) | TBS mean (95% CI) | P- value |
|---|---|---|---|
| Sex | N=672 | <0.001* | |
| Male | 369 (54.91%) | 1.311 (1.287 – 1.335) | |
| Female | 303 (45.09%) | 1.278 (1.253 – 1.302) | |
| Age | N=672 | 0.004* | |
| 50–54 | 50 (7.44%) | 1.321 (1.291 – 1.351) | |
| 55–59 | 149 (22.17%) | 1.307 (1.283 – 1.330) | |
| 60–64 | 218 (32.44%) | 1.285 (1.261 – 1.308) | |
| 65–69 | 168 (25.00%) | 1.287 (1.262 – 1.311) | |
| 70–74 | 58 (8.63%) | 1.267 (1.236 – 1.299) | |
| 75–79 | 23 (3.42%) | 1.266 (1.227 – 1.305) | |
| 80–84 | 6 (0.89%) | 1.328 (1.257 – 1.400) | |
| Race/Ethnicity | N=661 | 0.16 | |
| Non-Hispanic White | 549 (83.06%) | 1.303 (1.282 – 1.325) | |
| African American/Black | 58 (8.77%) | 1.288 (1.258 – 1.318) | |
| BMI | N=672 | <0.001* | |
| <18.5 | 4 (0.60%) | 1.320 (1.235 – 1.405) | |
| 18.5–24.9 | 187 (27.83%) | 1.355 (1.335 – 1.374) | |
| 25–29.9 | 310 (46.13%) | 1.324 (1.306 – 1.341) | |
| 30–34.9 | 148 (22.02%) | 1.277 (1.257 – 1.297) | |
| 35–37.0 | 23 (3.42%) | 1.197 (1.159 – 1.235) | |
|
History of fragility fracture at baseline |
N=655 | 0.072 | |
| No | 601 (91.76%) | 1.297 (1.273 – 1.320) | |
| Yes | 54 (8.24%) | 1.274 (1.242 – 1.307) | |
| Diabetes | N=664 | 0.008* | |
| No | 602 (90.66%) | 1.296 (1.273 – 1.320) | |
| Yes | 62 (9.34%) | 1.265 (1.235 – 1.295) | |
| Multivitamin use | N=634 | 0.87 | |
| No | 354 (55.84%) | 1.292 (1.267 – 1.316) | |
| Yes | 280 (44.16%) | 1.290 (1.265 – 1.316) | |
| Caffeine use | N=634 | 0.68 | |
| Never | 54 (8.52%) | 1.282 (1.250 – 1.314) | |
| >0- ⩽1 servings/day | 161 (25.39%) | 1.294 (1.269 – 1.320) | |
| >1- ⩽2.5 servings/day | 208 (32.81%) | 1.290 (1.263 – 1.317) | |
| >2.5- ⩽3.5 servings/day | 142 (22.40%) | 1.301 (1.272 – 1.330) | |
| >3.5 servings/day | 69 (10.88%) | 1.290 (1.259 – 1.322) | |
| Smoking status | N=668 | 0.28 | |
| Never | 342 (51.20%) | 1.300 (1.276 – 1.325) | |
| Past | 284 (42.51%) | 1.291 (1.266 – 1.316) | |
| Current | 42 (6.29%) | 1.285 (1.250 – 1.319) | |
| Alcohol use | N=634 | 0.009* | |
| Never / Rarely | 118 (18.61%) | 1.274 (1.247 – 1.301) | |
| 1–3/ month | 43 (6.78%) | 1.297 (1.262 – 1.331) | |
| 1/ week | 77 (12.15%) | 1.294 (1.265 – 1.323) | |
| 2–4/ week | 115 (18.14%) | 1.295 (1.267 – 1.324) | |
| 5–6/ week | 71 (11.20%) | 1.305 (1.274 – 1.336) | |
| Daily | 93 (14.67%) | 1.308 (1.278 – 1.338) | |
| 2–3/ day | 97 (15.30%) | 1.305 (1.275 – 1.335) | |
| 4–5/ day | 14 (2.21%) | 1.268 (1.215 – 1.321) | |
| 6+ /day | 6 (0.95%) | 1.199 (1.127 – 1.271) | |
| Total MET-hrs/week categories | N=669 | 0.19 | |
| 1st tertile (0–13.71) | 223 (33.33%) | 1.288 (1.264 – 1.313) | |
| 2nd tertile (13.72–32.48) | 223 (33.33%) | 1.297 (1.272 – 1.323) | |
| 3rd tertile (≥32.49) | 223 (33.33%) | 1.303 (1.278 – 1.329) | |
| SSRI use | N=662 | 0.044* | |
| No | 609 (91.99%) | 1.295 (1.272 – 1.319) | |
| Yes | 53 (8.01%) | 1.270 (1.236 – 1.304) | |
| Falls in the past year | N=638 | 0.35 | |
| None | 519 (81.35%) | 1.292 (1.267 – 1.316) | |
| One fall | 92 (14.42%) | 1.281 (1.251 – 1.310) | |
| Two falls | 15 (2.35%) | 1.322 (1.273 – 1.372) | |
| Three falls | 12 (1.88%) | 1.285 (1.231 – 1.339) |
p < 0.05
p-values for African-American/ Black and Non-Hispanic white are the comparison to all other race/ethnicity groups
Abbr: BMI, body mass index; MET, metabolic equivalent; SSRI, selective serotonin reuptake inhibitor
Figure 1.
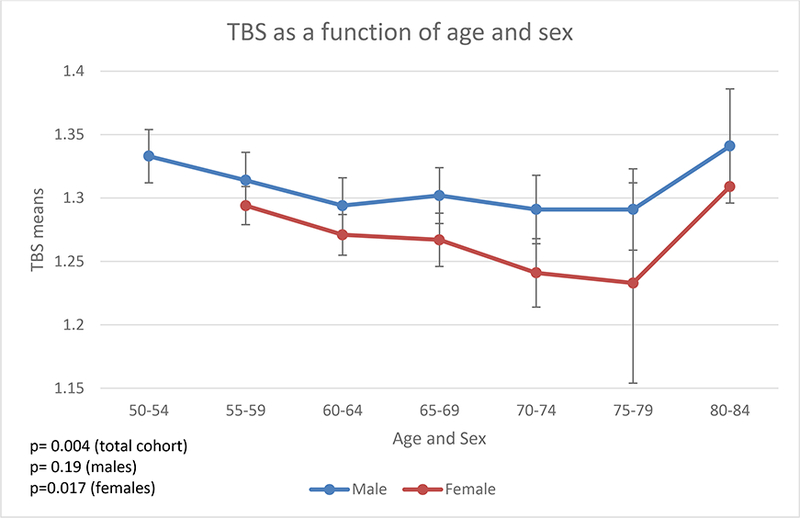
TBS as a function of age and sex
At baseline, the mean BMI in the entire cohort was 27.37 ± 3.92 kg/m2, and the mean BMI in the men and women were 27.81 ± 3.38 and 26.84 ± 4.43 kg/m2, respectively. The group with a BMI of 18.5–24.9 kg/m2 had the highest mean TBS (1.355); TBS was slightly lower when the BMI was <18.5 kg/m2 (1.320) but there were only 4 participants who had this extremely low BMI. Mean TBS progressively decreased with BMI’s ≥25 kg/m2 such that the lowest mean TBS (1.197) was seen in the group with a BMI of 35–37.0 kg/m2 (p<0.001).
Among the participants, 9.34 % had a history of diabetes at baseline, which was associated with a lower mean TBS when compared to participants without diabetes (p=0.008). An analysis of the association between use of SSRIs and TBS was significant (p=0.044) with lower TBS in those on an SSRI (1.295 vs. 1.270). History of fragility fracture showed a trend for lower baseline TBS (p=0.072). High alcohol intake was associated with lower TBS (p=0.009). There was no association with smoking, physical activity (MET scores), history of falls in the past year, or multivitamin or caffeine use.. Backward multiple regression analysis was conducted to examine the relationship between TBS and various predictors (Table 2). History of diabetes remained significant associated with TBS after mutual adjustment. In the overall cohort, TBS and spine BMD were highly correlated, but in participants with diabetes or on SSRIs, the correlations were not statistically significant (Table 3).
Table 2.
Stepwise regression analysis of baseline TBS adjusting for age, sex/ gender and race.
| Variable | β | Standard Error | P value |
|---|---|---|---|
| Intercept | 1.625 | 0.032 | <0.001* |
| Sex/ Gender (female vs. male) | −0.040 | 0.007 | <0.001* |
| Age | −0.013 | 0.005 | 0.018* |
| BMI | −0.008 | 0.001 | <0.001* |
| Diabetes | −0.034 | 0.013 | 0.008* |
| Alcohol use | 0.019 | 0.009 | 0.041* |
| SSRI use | −0.024 | 0.014 | 0.077 |
p < 0.05
Abbr: BMI, body mass index; TBS, trabecular bone score
Table 3.
Pearson Correlation between Spine BMD and TBS
| Pearson Correlation Coefficients |
P-value | |
|---|---|---|
| Overall Cohort (N=672) | 0.306 | <0.001* |
|
Overweight participants (N=110) |
0.264 | 0.005* |
|
Diabetic participants (N=62) |
0.163 | 0.21 |
| SSRI use (N=53) | 0.021 | 0.88 |
p < 0.05
Abbr: SSRI, selective serotonin reuptake inhibitor
Discussion:
In this study, we found several clinically important variables that were associated with either high or low TBS at baseline in the VITAL CTSC sub-cohort. Lower TBS was associated with female sex, older age, higher BMI, use of SSRIs, use of alcohol, and history of diabetes; there was a trend for lower TBS and history of fragility fracture (p=0.072). The findings from this study contrast with other studies, which showed that TBS, on average, was either lower in men than in women [6,9] or not statistically different [4,10]. Some have hypothesized that this could be due to men having a less “patterned” bone projection secondary to thicker trabeculae and/or wider vertebrae or different tissue composition leading to a less contrasted image [6,9]. Comparing TBS from different studies is complicated by differences in the software version used to generate TBS data, as a previous version was optimized for women of average body size and found to have limitations when used in men. In this study, we used TBS version 2.1, since the algorithm has since been improved to partially compensate for the effects of increased abdominal soft tissue that degrade image quality. Schacter et al. compared the TBS version 1 (TBS-v1) algorithm to the TBS version 2 (TBS-v2) algorithm [22] in a recently published analysis of the Manitoba cohort, and average TBS values were significantly lower in men than women using the TBS-v1 algorithm (p<0.001) and showed significant inverse correlations with BMI; however, with the TBS-v2 algorithm used herein, average values for men were slightly greater than in women (p<0.001) and there were no significant correlations with BMI.
A recent study by Krueger et al. evaluating 90 women and 90 men with GE-Lunar iDXA by 3 different technologists showed no significant differences between sexes [4]. An analysis of the NHANES data showed that sex differences in TBS varied by age and race/ethnicity (p<0.001) [23]. In most of the nine demographic groups examined, TBS did not differ by sex (four subgroups) or was significantly higher in women (three subgroups). In our study using the updated TBS software (TBS-v2), men had a baseline mean TBS value of 1.311 (1.287–1.335) and women had a baseline mean TBS value of 1.278 (1.253–1.302; p<0.001) The sexual dimorphism of TBS, if confirmed, may result from differential effects of estrogens and androgens on bone. Of note, the average age of women in our study was older than men per study design and women on hormone therapy were excluded; >99% of women were postmenopausal. Further studies are needed to clarify how sex affects baseline TBS and fracture risk and to predict how this may affect the response to different osteoporosis therapies.
It is well known that aging is associated with a decrease in BMD and TBS in both men and women [21,24,25]. This decrease is seen regardless of which region of interest is assessed [25]. In one study of French women aged 45–85 years, a difference of 14.5% was observed, corresponding to a standard deviation of −2.25 SD between 45 and 85 years [25]. Shin and colleagues showed that aging is associated with a more degraded TBS than BMD [26], possibly due to age-dependent arthritic changes that may result in higher BMDs. In one study, BMD showed a weaker correlation with TBS in males (0.555) than females (0.655), and this correlation decreased with age in both sexes [24].
Selective serotonin reuptake inhibitors, or SSRIs, potently block the serotonin transporter both in the central nervous system and in the periphery, including on osteoblasts and osteocytes in bone [27]. It is possible that this leads to decreased differentiation of osteoclasts. Although these medications were developed for the treatment of major depression, they are widely used for many neuropsychiatric and other conditions. Several observational studies have shown an association between SSRIs with low BMD and a 70% increase in fracture and fall risk [28,29]. A recent systematic review and meta-analysis by Zhou et al. suggested that SSRI use is significantly associated with lower BMD of the lumbar spine but not of the total hip and femoral neck region [30]. They found that this bone loss is also greater in older people on SSRIs and in those who have been on the medication for longer amounts of time. To our knowledge, ours is the first study to report that SSRI use is associated with lower TBS including results adjusted for age, sex, and race (p=0.044). There was a significant interaction of SSRI use and sex/gender (p=0.025); the association between lower TBS and SSRI use was significant in men (p=0.013) but not women (p=0.80; Appendix 1). However, the sample size was small with only 23 men and 30 women using SSRIs. There was no association between SSRI use and increased risk of falls (data not shown). Since TBS is approved by the Food and Drug Administration (FDA) for clinical use in the U.S., this tool may advance assessment of those individuals on SSRIs who may be at increased fracture risk.
Diabetes confers a significantly increased risk of major osteoporotic fractures and hip fractures that is underestimated by FRAX. Like other recent reports, we demonstrated that participants with type 2 diabetes had a lower TBS than their non-diabetic counterparts [13,31]. Results of our study do not show a significant correlation between spine BMD and TBS in participants with diabetes (p=0.21). There was a trend for an interaction between diabetes and sex/gender (p=0.099) with diabetic men having significantly lower TBS (1.278 vs. 1.318; p=0.008); there was not a significant difference in TBS scores in women with and without diabetes (p=0.82; Appendix 1). Leslie et al. showed in the Manitoba cohort that type 2 diabetes was associated with higher BMD at all sites, but with lower lumbar spine TBS [13]. Abnormal trabecular microarchitecture may explain the paradox among diabetics of experiencing an increased risk of fractures at higher BMDs [31]. Kim et al. investigated lumbar spine TBS as an indicator for skeletal deterioration in diabetes in 1,229 men and 1,529 postmenopausal women in the Ansung cohort [32]. While BMD was higher in those with diabetes, TBS was negatively correlated with hemoglobin A1C, fasting plasma glucose, and fasting insulin in both genders, suggesting TBS may be useful as an indicator for identifying skeletal deterioration in diabetic patients with otherwise high BMDs [32]. Data on TBS in type 1 diabetes (T1DM) are sparse, but in a cross-sectional study, Neumann et al. showed a borderline reduction in mean TBS between diabetic patients and non-diabetic, gender-, age-, and BMI-matched controls (1.357 ± 0.129 vs. 1.389 ± 0.085, respectively, p = 0.075) [33]. T1DM patients with prevalent fractures (n=24) had a significantly lower TBS than T1DM patients without fractures (1.309 ± 0.125 versus 1.370 ± 0.127, p = 0.04).
TBS can enhance fracture prediction when used as an adjunct to FRAX and the output of FRAX can now be adjusted for TBS when this is entered through the FRAX website [34], which may be particularly helpful for both type 1 and type 2 diabetes patients. Reliable TBS cutoffs for fracture risk need to be determined and verified in larger studies in both patient populations as there may be inherent differences in the two diseases. We were not able to assess differences in type 1 vs type 2 diabetes in this study or evaluate the impact of insulin dependence or other hypoglycemic agents, given the small sample size.
High alcohol intake was also associated with lower TBS (p=0.009). According to the World Health Organization (WHO), three or more drinks per day is associated with an increased fracture risk. Other studies have found similar associations between alcohol use and low TBS [35].
Obesity was traditionally considered protective against osteoporosis and fracture, as BMI is positively correlated with BMD assessed by DXA [36,35]. However, recent data show an increased risk of falls and a higher risk of some fractures with obesity [36]. For example, in a sub-analysis of the Women’s Health Initiative Observational Cohort (WHI-OS), women with the highest BMI reported more falls, had more prevalent fractures, and lower measures of physical activity and function [37]. We found that higher BMI was associated with lower TBS, consistent with the results of others [35]. We also showed that BMD and TBS were strongly correlated among overweight participants. As there are concerns regarding 1) technical issues in performing textural analysis in obese subjects and 2) excessive soft tissue may give the false finding of a lower TBS, those with a BMI >37 kg/m2 were excluded from our analysis. When Leslie et al. excluded obese individuals with a BMI >30 kg/m2, this attenuated but did not eliminate the inverse correlation, suggesting that TBS may in fact capture alterations in bone structure in obese individuals that put them at greater risk of fracture [35]. Our data also support a lower TBS among adults with very low BMI’s <18.5 kg/m2 (1.320), however there were only 4 participants in this category. In a study examining TBS among adolescents with anorexia nervosa, >40% of subjects showed degraded architecture, which was correlated with BMI [38].
Black individuals compared with whites have a lower risk for osteoporotic fractures, higher BMD and better bone quality, a finding that is consistent throughout life [39,40]. Higher skeletal mass does not completely explain their decreased fracture risk as they still have a lower risk of fracture at comparable bone densities [39]. An observed difference in bone microarchitecture in Black women has been hypothesized to be the explanation for this superior bone strength independent of density [41]. Using high resolution pQCT (HR-pQCT), Putnam et al. demonstrated that African American women had greater trabecular vBMD at the radius and higher cortical vBMD at the tibia [41]. In a cross-sectional analysis by Aloia et al. [42], of 518 postmenopausal African American women (mean age 66 years and BMI of 30.1), mean TBS was 1.300, which was significantly higher (2.5%) than the mean TBS of a database of Caucasian French women (p<0.0001). However, in the NHANES data, non-Hispanic Whites had higher TBS than non-Hispanic Blacks or Mexican Americans in all age groups [23]. Black men and women had lower TBS than did whites even with adjustment for age and tissue thickness [43]. These differences were present in nearly all age, BMI, and BMD groups. Difference in software calibration may have contributed to the discrepancy between these study results, and Jain [43] hypothesized that their study population is more reflective of the patients seen in clinical practice in which “ethnic differences are most relevant.” In our study, we found no difference in TBS between Caucasian and African-American participants. Although minority participation was high in the parent study with approximately 26% minority enrollment and an oversampling of Blacks [19], minority enrollment in this sub-cohort in the greater Boston area limited our ability to reach firm conclusions about the effect of race/ethnicity on TBS so these results need to be interpreted with caution. However, it is possible that TBS adjustments may be necessary for non-white ethnicities.
We acknowledge the limitations of this study. Although the data are from a large, clinical trial, these analyses are of the baseline data only. Although we showed an effect of diabetes on baseline TBS, the number of individuals in the cohort on insulin (9 subjects) was too small to draw any additional conclusions about the impact of insulin therapy. In addition, because VITAL enrolled older adults, these results are not generalizable to younger men and women.
In conclusion, our study investigated the associations of clinical risk factors for osteoporosis with TBS in the VITAL CTSC sub-cohort at baseline. Lower TBS was associated with female sex, BMI >25 kg/m2, high alcohol intake, and older age. A lower TBS was also associated with the use of SSRIs and presence of diabetes, and there was a trend for lower TBS with history of fragility fractures. Lower TBS in these settings, irrespective of BMD, may potentially predict the risk of skeletal fragility associated with these clinical conditions or therapy. Further studies are needed to confirm the relationships between these clinical factors, TBS and fracture risk. As there were significant differences in TBS between men and women, it is unclear whether the same TBS cut-offs may have different predictive fracture risk between the two sexes. Ongoing follow-up studies at 2 years post-randomization will clarify the effects of high doses of supplemental vitamin D and/or omega-3 fatty acids on TBS and other bone health measures.
Acknowledgments:
The ancillary studies VITAL: Effects on Fractures and VITAL: Effects on Structure and Architecture are supported by the grants R01AR060574 and R01AR59775, respectively, from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The parent trial is supported by grant U01CA138962. Members of the VITAL Data and Safety Monitoring Board include Lawrence S. Cohen, Theodore Colton, Mark A. Espeland, I. Craig Henderson, Alice H. Lichtenstein, Rebecca A. Silliman, and Nanette K. Wenger (chair), and Josephine Boyington, Cindy D. Davis, Rebecca B. Costello, Gabriela Riscuta, Harold Seifried, Lawrence Fine, and Peter Greenwald (ex-officio members). We would also like to acknowledge Vadim Bubes and Gregory Kotler, the data analysts for this project. We would also like to acknowledge the contribution of the Steve Cobb Junior Faculty Education Fund. In addition, we would like to acknowledge Andrea Alvarez, a summer research student from Winsor High School, for her contributions to this research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was conducted with support from Harvard Catalyst — the Harvard Clinical and Translational Science Center (NCRR and NCATS, NIH Award UL1 TR001102) and financial contributions from Harvard University and its affiliated academic healthcare centers.
Grants:
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Numbers R01AR59775 (LeBoff, M.S., PI) and R01AR060574 (LeBoff, M.S., PI), respectively. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The parent VITAL trial is supported by grant U01CA138962 (Manson, J. and Buring, J, PIs). Support for this research was also provided from the Harvard Catalyst Clinical and Translational Science Center (CTSC) (NIH Award UL1 TR001102) and the Steve Cobb Junior Faculty and Fellow Education Fund. Pharmavite LLC of Northridge, California (vitamin D) and Pronova BioPharma (BASF) of Norway (Omacor® fish oil) donated the study agents, matching placebos, and packaging in the form of calendar packs.
Appendix 1: Baseline characteristics of the VITAL CTSC TBS Sub-cohort, according to sex and adjusted for age, Black race/ethnicity and BMI
| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | N (%) | TBS Least Square Mean (%95CLI) |
P value for trend |
N (%) | TBS Least Square Mean (%95CLI) |
P value for trend |
P value for Interaction |
|
| Age (years) | N= 369 | 0.19 | N= 303 | 0.017* | 0.15 | |||
| 50–54 | 50 (13.55%) | 1.333 (1.291 – 1.376) | ||||||
| 55–59 | 66 (17.89%) | 1.314 (1.270 – 1.357) | 83 (27.39%) | 1.294 (1.264 – 1.325) | ||||
| 60–64 | 116 (31.44%) | 1.294 (1.252 – 1.336) | 102 (33.66%) | 1.271 (1.242 – 1.301) | ||||
| 65–69 | 90 (24.39%) | 1.302 (1.259 – 1.345) | 78 (25.74%) | 1.267 (1.235 – 1.299) | ||||
| 70–74 | 29 (7.86%) | 1.291 (1.239 – 1.344) | 29 (9.57%) | 1.241 (1.201 – 1.282) | ||||
| 75–79 | 13 (3.52%) | 1.291 (1.229 – 1.353) | 10 (3.30%) | 1.233 (1.180 – 1.285) | ||||
| 80–84 | 5 (1.36%) | 1.341 (1.253 – 1.429) | 1 (0.33%) | 1.309 (1.152 – 1.465) | ||||
| Race/Ethnicity | N= 364 | 0.62 | N= 297 | 0.16 | 0.86 | |||
| Non-Hispanic White |
294 (80.77%) | 1.310 (1.270 – 1.349) | 255 (85.86%) | 1.294 (1.260 – 1.328) | ||||
| African American/Black |
42 (11.54%) | 1.308 (1.261 – 1.356) | 16 (5.39%) | 1.247 (1.197 – 1.298) | ||||
| BMI (kg/m2) | N= 369 | <0.001* | N= 303 | <0.001* | 0.008* | |||
| <18.5 | 1 (0.27%) | 1.351 (1.173 – 1.528) | 3 (0.99%) | 1.280 (1.185 – 1.376) | ||||
| 18.5–24.9 | 74 (20.05%) | 1.389 (1.361 – 1.417) | 113 (37.29%) | 1.310 (1.274 – 1.345) | ||||
| 25–29.9 | 206 (55.83%) | 1.349 (1.329 – 1.370) | 104 (34.32%) | 1.281 (1.246 – 1.317) | ||||
| 30–34.9 | 76 (20.60%) | 1.299 (1.273 – 1.326) | 72 (23.76%) | 1.240 (1.203 – 1.276) | ||||
| 35–37.0 | 12 (3.25%) | 1.159 (1.106 – 1.212) | 11 (3.63%) | 1.236 (1.180 – 1.293) | ||||
|
History of fragility fracture at baseline |
N= 360 | 0.077 | N= 295 | 0.43 | 0.53 | |||
| No | 341 (94.72%) | 1.312 (1.271 – 1.353) | 260 (88.14%) | 1.272 (1.234 – 1.310) | ||||
| Yes | 19 (5.28%) | 1.273 (1.215 – 1.331) | 35 (11.86%) | 1.260 (1.213 – 1.307) | ||||
| Diabetes | N= 365 | 0.008* | N= 299 | 0.82 | 0.099 | |||
| No | 322 (88.22%) | 1.318 (1.276 – 1.360) | 280 (93.65%) | 1.260 (1.223 – 1.298) | ||||
| Yes | 43 (11.78%) | 1.278 (1.231 – 1.324) | 19 (6.35%) | 1.256 (1.206 – 1.306) | ||||
|
Multivitamin use |
N= 342 | 0.80 | N= 292 | 0.77 | 0.63 | |||
| No | 177 (51.75%) | 1.310 (1.266 – 1.354) | 177 (60.62%) | 1.269 (1.229 – 1.309) | ||||
| Yes | 165 (48.25%) | 1.308 (1.265 – 1.350) | 115 (39.38%) | 1.272 (1.230 – 1.314) | ||||
| Caffeine use | N= 343 | 0.30 | N= 291 | 0.93 | 0.26 | |||
| Never | 31 (9.04%) | 1.289 (1.240 – 1.338) | 23 (7.90%) | 1.277 (1.225 – 1.329) | ||||
| >0- ⩽1 servings/day |
103 (30.03%) | 1.316 (1.272 – 1.360) | 58 (19.93%) | 1.273 (1.229 – 1.317) | ||||
| >1- ⩽2.5 servings/day |
109 (31.78%) | 1.316 (1.270 – 1.362) | 99 (34.02%) | 1.265 (1.221 – 1.308) | ||||
| >2.5- ⩽3.5 servings/day |
58 (16.91%) | 1.334 (1.284 – 1.384) | 84 (28.87%) | 1.268 (1.223 – 1.314) | ||||
| >3.5 servings/day |
42 (12.24%) | 1.322 (1.270 – 1.374) | 27 (9.28%) | 1.261 (1.213 – 1.309) | ||||
| Smoking status | N= 366 | 0.74 | N= 302 | 0.092 | 0.12 | |||
| Never | 181 (49.45%) | 1.310 (1.268 – 1.353) | 161 (53.31%) | 1.278 (1.240 – 1.316) | ||||
| Past | 161 (43.99%) | 1.305 (1.262 – 1.348) | 123 (40.73%) | 1.266 (1.226 – 1.306) | ||||
| Current | 24 (6.56%) | 1.319 (1.264 – 1.375) | 18 (5.96%) | 1.237 (1.186 – 1.289) | ||||
| Alcohol use | N= 343 | 0.018* | N= 291 | 0.24 | 0.28 | |||
| Never / Rarely | 62 (18.08%) | 1.293 (1.249 – 1.337) | 56 (19.24%) | 1.252 (1.207 – 1.297) | ||||
| 1–3/ month | 13 (3.79%) | 1.336 (1.272 – 1.399) | 30 (10.31%) | 1.261 (1.213 – 1.308) | ||||
| 1/ week | 34 (9.91%) | 1.306 (1.255 – 1.357) | 43 (14.78%) | 1.281 (1.236 – 1.326) | ||||
| 2–4/ week | 64 (18.66%) | 1.320 (1.273 – 1.367) | 51 (17.53%) | 1.269 (1.225 – 1.313) | ||||
| 5–6/ week | 40 (11.66%) | 1.340 (1.290 – 1.390) | 31 (10.65%) | 1.268 (1.217 – 1.318) | ||||
| 1 / day | 53 (15.45%) | 1.333 (1.285 – 1.382) | 40 (13.75%) | 1.279 (1.230 – 1.328) | ||||
| 2–3/ day | 61 (17.78%) | 1.314 (1.267 – 1.362) | 36 (12.37%) | 1.301 (1.252 – 1.351) | ||||
| 4–5/ day | 11 (3.21%) | 1.285 (1.218 – 1.353) | 3 (1.03%) | 1.245 (1.126 – 1.363) | ||||
| 6+ /day | 5 (1.46%) | 1.210 (1.122 – 1.299) | 1 (0.34%) | 1.223 (1.062 – 1.385) | ||||
|
Total MET- hrs/week categories |
N= 368 | 0.10 | N= 301 | 0.35 | 0.54 | |||
| 1st tertile (0– 13.71) |
113 (30.71%) | 1.301 (1.259 – 1.343) | 110 (36.54%) | 1.265 (1.226 – 1.304) | ||||
| 2nd tertile (13.72– 32.48) |
129 (35.05%) | 1.308 (1.265 – 1.352) | 94 (31.23%) | 1.281 (1.240 – 1.322) | ||||
| 3rd tertile (⩾32.49) |
126 (34.24%) | 1.326 (1.282 – 1.369) | 97 (32.23%) | 1.277 (1.236 – 1.319) | ||||
| SSRI use | N= 362 | 0.013* | N= 300 | 0.80 | 0.025* | |||
| No | 339 (93.65%) | 1.310 (1.269 – 1.351) | 270 (90.00%) | 1.271 (1.233 – 1.309) | ||||
| Yes | 23 (6.35%) | 1.260 (1.204 – 1.316) | 30 (10.00%) | 1.267 (1.218 – 1.316) | ||||
|
Falls in the past year |
N= 345 | 0.49 | N= 293 | 0.20 | ||||
| None | 293 (84.93%) | 1.311 (1.268 – 1.353) | 226 (77.13%) | 1.267 (1.228 – 1.307) | ||||
| One fall | 42 (12.17%) | 1.287 (1.238 – 1.337) | 50 (17.06%) | 1.265 (1.219 – 1.310) | ||||
| Two falls | 6 (1.74%) | 1.306 (1.222 – 1.390) | 9 (3.07%) | 1.324 (1.260 – 1.389) | ||||
| Three falls | 4 (1.16%) | 1.290 (1.191 – 1.389) | 8 (2.73%) | 1.259 (1.191 – 1.328) | ||||
Footnotes
Disclosure Statement:
Anna L. Goldman: No disclosures.
Catherine M. Donlon: No disclosures.
Nancy R. Cook: No disclosures.
JoAnn E. Manson: No disclosures.
Julie E. Buring: No disclosures.
Trisha Copeland: No disclosures.
Cindy Y. Yu: No disclosures.
Meryl S. LeBoff: No disclosures.
Clinical Trial Registration Number: NCT01747447
References:
- 1.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A (2007) Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res 22 (3):465–475. doi: 10.1359/jbmr.061113 [DOI] [PubMed] [Google Scholar]
- 2.Wright NC, Looker AC, Saag KG, Curtis JR, Delzell ES, Randall S, Dawson-Hughes B (2014) The recent prevalence of osteoporosis and low bone mass in the United States based on 24 bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res 29 (11):2520–2526. doi: 10.1002/jbmr.2269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hans D, Barthe N, Boutroy S, Pothuaud L, Winzenrieth R, Krieg MA (2011) Correlations between trabecular bone score, measured using anteroposterior dual-energy X-ray absorptiometry acquisition, and 3-dimensional parameters of bone microarchitecture: an experimental study on human cadaver vertebrae. J Clin Densitom 14 (3):302–312. doi: 10.1016/j.jocd.2011.05.005 [DOI] [PubMed] [Google Scholar]
- 4.Krueger D, Fidler E, Libber J, Aubry-Rozier B, Hans D, Binkley N (2013) Spine Trabecular Bone Score Subsequent to Bone Mineral Density Improves Fracture Discrimination in Women. J Clin Densitom. doi: 10.1016/j.jocd.2013.05.001 [DOI] [PubMed] [Google Scholar]
- 5.Rabier B, Heraud A, Grand-Lenoir C, Winzenrieth R, Hans D (2010) A multicentre, retrospective case-control study assessing the role of trabecular bone score (TBS) in menopausal Caucasian women with low areal bone mineral density (BMDa): Analysing the odds of vertebral fracture. Bone 46 (1):176–181. doi: 10.1016/j.bone.2009.06.032 [DOI] [PubMed] [Google Scholar]
- 6.Leib E, Winzenrieth R, Aubry-Rozier B, Hans D (2014) Vertebral microarchitecture and fragility fracture in men: a TBS study. Bone 62:51–55. doi: 10.1016/j.bone.2013.12.015 [DOI] [PubMed] [Google Scholar]
- 7.Boutroy S, Hans D, Sornay-Rendu E, Vilayphiou N, Winzenrieth R, Chapurlat R (2013) Trabecular bone score improves fracture risk prediction in non-osteoporotic women: the OFELY study. Osteoporos Int 24 (1):77–85. doi: 10.1007/s00198-012-2188-2 [DOI] [PubMed] [Google Scholar]
- 8.Hans D, Goertzen AL, Krieg MA, Leslie WD (2011) Bone microarchitecture assessed by TBS predicts osteoporotic fractures independent of bone density: the Manitoba study. J Bone Miner Res 26 (11):2762–2769. doi: 10.1002/jbmr.499 [DOI] [PubMed] [Google Scholar]
- 9.Leslie WD, Aubry-Rozier B, Lix LM, Morin SN, Majumdar SR, Hans D (2014) Spine bone texture assessed by trabecular bone score (TBS) predicts osteoporotic fractures in men: the Manitoba Bone Density Program. Bone 67:10–14. doi: 10.1016/j.bone.2014.06.034 [DOI] [PubMed] [Google Scholar]
- 10.McCloskey EV, Oden A, Harvey NC, Leslie WD, Hans D, Johansson H, Barkmann R, Boutroy S, Brown J, Chapurlat R, Elders PJ, Fujita Y, Gluer CC, Goltzman D, Iki M, Karlsson M, Kindmark A, Kotowicz M, Kurumatani N, Kwok T, Lamy O, Leung J, Lippuner K, Ljunggren O, Lorentzon M, Mellstrom D, Merlijn T, Oei L, Ohlsson C, Pasco JA, Rivadeneira F, Rosengren B, Sornay-Rendu E, Szulc P, Tamaki J, Kanis JA (2016) A Meta-Analysis of Trabecular Bone Score in Fracture Risk Prediction and Its Relationship to FRAX. J Bone Miner Res 31 (5):940–948. doi: 10.1002/jbmr.2734 [DOI] [PubMed] [Google Scholar]
- 11.Krieg MA, Aubry-Rozier B, Hans D, Leslie WD (2013) Effects of anti-resorptive agents on trabecular bone score (TBS) in older women. Osteoporos Int 24 (3):1073–1078. doi: 10.1007/s00198-012-2155-y [DOI] [PubMed] [Google Scholar]
- 12.Paggiosi MA, Peel NF, Eastell R (2015) The impact of glucocorticoid therapy on trabecular bone score in older women. Osteoporos Int 26 (6):1773–1780. doi: 10.1007/s00198-015-3078-1 [DOI] [PubMed] [Google Scholar]
- 13.Leslie WD, Aubry-Rozier B, Lamy O, Hans D, Manitoba Bone Density P (2013) TBS (trabecular bone score) and diabetes-related fracture risk. J Clin Endocrinol Metab 98 (2):602–609. doi: 10.1210/jc.2012-3118 [DOI] [PubMed] [Google Scholar]
- 14.Romagnoli E, Cipriani C, Nofroni I, Castro C, Angelozzi M, Scarpiello A, Pepe J, Diacinti D, Piemonte S, Carnevale V, Minisola S (2013) “Trabecular Bone Score” (TBS): an indirect measure of bone micro-architecture in postmenopausal patients with primary hyperparathyroidism. Bone 53 (1):154–159. doi: 10.1016/j.bone.2012.11.04125 [DOI] [PubMed] [Google Scholar]
- 15.de II, van der Klift M, de Laet CE, van Daele PL, Hofman A, Pols HA(2005) Bone mineral density and fracture risk in type-2 diabetes mellitus: the Rotterdam Study. Osteoporos Int 16 (12):1713–1720. doi: 10.1007/s00198-005-1909-1 [DOI] [PubMed] [Google Scholar]
- 16.Felson DT, Zhang Y, Hannan MT, Anderson JJ (1993) Effects of weight and body mass index on bone mineral density in men and women: the Framingham study. J Bone Miner Res 8 (5):567–573. doi: 10.1002/jbmr.5650080507 [DOI] [PubMed] [Google Scholar]
- 17.LeBoff MS, Yue AY, Copeland T, Cook NR, Buring JE, Manson JE (2015) VITAL-Bone Health: Rationale and design of two ancillary studies evaluating the effects of vitamin D and/or omega-3 fatty acid supplements on incident fractures and bone health outcomes in the VITamin D and OmegA-3 TriaL (VITAL). Contemp Clin Trials 41C:259–268. doi: 10.1016/j.cct.2015.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weaver CM, Alexander DD, Boushey CJ, Dawson-Hughes B, Lappe JM, LeBoff MS, Liu S, Looker AC, Wallace TC, Wang DD (2016) Calcium plus vitamin D supplementation and risk of fractures: an updated meta-analysis from the National Osteoporosis Foundation. Osteoporos Int 27 (1):367–376. doi: 10.1007/s00198-015-3386-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manson JE, Bassuk SS, Lee IM, Cook NR, Albert MA, Gordon D, Zaharris E, Macfadyen JG, Danielson E, Lin J, Zhang SM, Buring JE (2011) The VITamin D and OmegA-3 TriaL (VITAL): Rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemp Clin Trials 33(1):159–171. doi:S1551-7144(11)00245-X [pii] 10.1016/j.cct.2011.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Donlon CM, LeBoff MS, Chou SH, Cook NR, Copeland T, Buring JE, Bubes V, Kotler G, Manson JE (2018) Baseline characteristics of participants in the VITamin D and OmegA-3 TriaL (VITAL): Effects on Bone Structure and Architecture. Contemp Clin Trials 67:56–67. doi: 10.1016/j.cct.2018.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silva BC, Leslie WD, Resch H, Lamy O, Lesnyak O, Binkley N, McCloskey EV, Kanis JA, Bilezikian JP (2014) Trabecular bone score: a noninvasive analytical method based upon the DXA image. J Bone Miner Res 29 (3):518–530. doi: 10.1002/jbmr.2176 [DOI] [PubMed] [Google Scholar]
- 22.Schacter GI, Leslie WD, Majumdar SR, Morin SN, Lix LM, Hans D (2017) Clinical performance of an updated trabecular bone score (TBS) algorithm in men and women: the Manitoba BMD cohort. Osteoporos Int 28 (11):3199–3203. doi: 10.1007/s00198-017-4166-1 [DOI] [PubMed] [Google Scholar]
- 23.Looker AC, Sarafrazi Isfahani N, Fan B, Shepherd JA (2016) Trabecular bone scores and lumbar spine bone mineral density of US adults: comparison of relationships with demographic and body size variables. Osteoporos Int 27 (8):2467–2475. doi: 10.1007/s00198-016-3550-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bazzocchi A, Ponti F, Diano D, Amadori M, Albisinni U, Battista G, Guglielmi G (2015) Trabecular bone score in healthy ageing. The British Journal of Radiology 88 (1052):20140865. doi: 10.1259/bjr.20140865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dufour R, Winzenrieth R, Heraud A, Hans D, Mehsen N (2013) Generation and validation of a normative, age-specific reference curve for lumbar spine trabecular bone score (TBS) in French women. Osteoporos Int. doi: 10.1007/s00198-013-2384-8 [DOI] [PubMed] [Google Scholar]
- 26.Shin YH, Gong HS, Lee KJ, Baek GH (2017) Older Age and Higher Body Mass Index Are Associated With a More Degraded Trabecular Bone Score Compared to Bone Mineral Density. J Clin Densitom. doi: 10.1016/j.jocd.2017.06.006 [DOI] [PubMed] [Google Scholar]
- 27.Haney EM, Chan BK, Diem SJ, Ensrud KE, Cauley JA, Barrett-Connor E, Orwoll E, Bliziotes MM (2007) Association of low bone mineral density with selective serotonin reuptake 26 inhibitor use by older men. Arch Intern Med 167 (12):1246–1251. doi: 10.1001/archinte.167.12.1246 [DOI] [PubMed] [Google Scholar]
- 28.Williams LJ, Henry MJ, Berk M, Dodd S, Jacka FN, Kotowicz MA, Nicholson GC, Pasco JA (2008) Selective serotonin reuptake inhibitor use and bone mineral density in women with a history of depression. International clinical psychopharmacology 23 (2):84–87. doi: 10.1097/YIC.0b013e3282f2b3bb [DOI] [PubMed] [Google Scholar]
- 29.Wang CY, Fu SH, Wang CL, Chen PJ, Wu FL, Hsiao FY (2016) Serotonergic antidepressant use and the risk of fracture: a population-based nested case-control study. Osteoporos Int 27 (1):57–63. doi: 10.1007/s00198-015-3213-z [DOI] [PubMed] [Google Scholar]
- 30.Zhou C, Fang L, Chen Y, Zhong J, Wang H, Xie P (2018) Effect of selective serotonin reuptake inhibitors on bone mineral density: a systematic review and meta-analysis. Osteoporos Int. doi: 10.1007/s00198-018-4413-0 [DOI] [PubMed] [Google Scholar]
- 31.Dhaliwal R, Cibula D, Ghosh C, Weinstock RS, Moses AM (2014) Bone quality assessment in type 2 diabetes mellitus. Osteoporos Int 25 (7):1969–1973. doi: 10.1007/s00198-014-2704-7 [DOI] [PubMed] [Google Scholar]
- 32.Kim JH, Choi HJ, Ku EJ, Kim KM, Kim SW, Cho NH, Shin CS (2015) Trabecular bone score as an indicator for skeletal deterioration in diabetes. J Clin Endocrinol Metab 100 (2):475–482. doi: 10.1210/jc.2014-2047 [DOI] [PubMed] [Google Scholar]
- 33.Neumann T, Lodes S, Kastner B, Lehmann T, Hans D, Lamy O, Muller UA, Wolf G, Samann A (2016) Trabecular bone score in type 1 diabetes--a cross-sectional study. Osteoporos Int 27 (1):127–133. doi: 10.1007/s00198-015-3222-y [DOI] [PubMed] [Google Scholar]
- 34.Silva BC, Broy SB, Boutroy S, Schousboe JT, Shepherd JA, Leslie WD (2015) Fracture Risk Prediction by Non-BMD DXA Measures: the 2015 ISCD Official Positions Part 2: Trabecular Bone Score. J Clin Densitom 18 (3):309–330. doi: 10.1016/j.jocd.2015.06.008 [DOI] [PubMed] [Google Scholar]
- 35.Leslie WD, Krieg MA, Hans D (2013) Clinical factors associated with trabecular bone score. J Clin Densitom 16 (3):374–379. doi: 10.1016/j.jocd.2013.01.006 [DOI] [PubMed] [Google Scholar]
- 36.Poiana C, Carsote M, Radoi V, Mihai A, Capatina C (2015) Prevalent osteoporotic fractures in 622 obese and non-obese menopausal women. Journal of medicine and life 8 (4):462–466 [PMC free article] [PubMed] [Google Scholar]
- 37.Beck TJ, Petit MA, Wu G, LeBoff MS, Cauley JA, Chen Z (2009) Does obesity really make the femur stronger? BMD, geometry, and fracture incidence in the women’s health initiative-observational study. J Bone Miner Res 24 (8):1369–1379. doi: 10.1359/jbmr.090307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Donaldson AA, Feldman HA, O’Donnell JM, Gopalakrishnan G, Gordon CM (2015) Spinal Bone Texture Assessed by Trabecular Bone Score in Adolescent Girls With Anorexia Nervosa. J Clin Endocrinol Metab 100 (9):3436–3442. doi: 10.1210/jc.2015-2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cauley JA, Lui L, Ensrud KE, Zmuda JM, Stone KL, Hochberg MC, Cummings SR (2005) Bone mineral density and the risk of incident nonspinal fractures in black and white women. JAMA 293 (17):2102–2108. doi: 10.1001/jama.293.17.2102 [DOI] [PubMed] [Google Scholar]
- 40.Luckey MM, Meier DE, Mandeli JP, DaCosta MC, Hubbard ML, Goldsmith SJ (1989) Radial and vertebral bone density in white and black women: evidence for racial differences in premenopausal bone homeostasis. J Clin Endocrinol Metab 69 (4):762–770. doi: 10.1210/jcem-69-4-762 [DOI] [PubMed] [Google Scholar]
- 41.Putman MS, Yu EW, Lee H, Neer RM, Schindler E, Taylor AP, Cheston E, Bouxsein ML, Finkelstein JS (2013) Differences in skeletal microarchitecture and strength in African-American and white women. J Bone Miner Res 28 (10):2177–2185. doi: 10.1002/jbmr.1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aloia JF, Mikhail M, Usera G, Dhaliwal R, Islam S (2015) Trabecular bone score (TBS) in postmenopausal African American women. Osteoporos Int 26 (3):1155–1161. doi: 10.1007/s00198-014-2928-6 27 [DOI] [PubMed] [Google Scholar]
- 43.Jain RK, Vokes TJ (2017) African Americans have lower TBS than whites among densitometry patients at a Chicago academic center. Osteoporos Int 28 (3):917–923. doi: 10.1007/s00198-016-3796-z [DOI] [PubMed] [Google Scholar]


