Abstract
Migraine is the third most common disease in the world (behind dental caries and tension-type headache) with an estimated global prevalence of 15%, yet its etiology remains poorly understood. Recent clinical trials have heralded the potential of therapeutic antibodies that block the actions of the neuropeptide calcitonin gene-related peptide (CGRP) or its receptor to prevent migraine. CGRP is believed to contribute to trigeminal nerve hypersensitivity and photosensitivity in migraine, but a direct role in pain associated with migraine has not been established. In this study, we report that peripherally administered CGRP can act in a light-independent manner to produce spontaneous pain in mice that is manifested as a facial grimace. As an objective validation of the orbital tightening action unit of the grimace response, we developed a squint assay using a video-based measurement of the eyelid fissure, which confirmed a significant squint response after CGRP injection, both in complete darkness and very bright light. These indicators of discomfort were completely blocked by preadministration of a monoclonal anti-CGRP blocking antibody. However, the nonsteroidal anti-inflammatory drug meloxicam failed to block the effect of CGRP. Interestingly, an apparent sex specific response to treatment was observed with the antimigraine drug sumatriptan partially blocking the CGRP response in male, but not female mice. These results demonstrate that CGRP can induce spontaneous pain, even in the absence of light, and that the squint response provides an objective biomarker for CGRP-induced pain that is translatable to humans.
Keywords: CGRP, migraine, grimace, non-evoked pain, squint assay, CGRP monoclonal antibody
1. Introduction
Migraine is a debilitating disorder characterized by recurrent severe headaches lasting 4–72 hours, with accompanying symptoms of abnormal sensory sensitivity, including photosensitivity [5; 9; 20; 51]. The etiology of migraine is not well understood, and there is an unmet need for better treatments. It is now recognized that actions of the neuropeptide calcitonin gene-related peptide (CGRP) are critical in migraine pathophysiology [17; 50; 56]. Clinically, elevated levels of CGRP have been reported in the cerebrospinal fluid [67], serum [19] and saliva [4] of migraineurs experiencing a headache and serum levels were normalized after treatment [18; 34]. Moreover, an injection of CGRP can trigger migraine-like headaches in migraineurs [26; 39]. Furthermore, CGRP receptor antagonists are effective in relieving acute migraine symptoms [10; 30; 49], and monoclonal antibodies targeting CGRP or its receptor have shown promise in clinical trials as preventative agents [13; 21; 59–61; 63].
CGRP is a potent vasodilator and a neuromodulator of nociceptive signaling and neurogenic inflammation [56]. CGRP and its receptor are present throughout the neuro-anatomical pain network, including the dorsal horn of the spinal cord and trigeminal nucleus caudalis, dorsal and trigeminal ganglia, periaqueductal grey, amygdala and thalamus [28; 33]. Multiple studies show that blocking CGRP actions by using receptor antagonists or CGRP null mice can prevent sensitization in inflammatory and neuropathic pain states [58; 69]. It is hypothesized that peripheral and central sensitization of the spinothalamic pathway by CGRP is a primary driver of the complex symptomatology experienced by migraineurs, including headache and photophobia [5; 56].
We have previously reported that mice avoid light after either central or peripheral administration of CGRP [35; 44; 53; 54]. This light-aversive behavior is attenuated by sumatriptan and a monoclonal CGRP antibody [44]. Light aversion was seen with wildtype mice exposed to bright light and with CGRP-sensitized transgenic mice exposed to dim light following intracerebroventricular injection of CGRP. Furthermore, the CGRP-sensitized mice showed mechanical allodynia in response to intrathecal CGRP, which is indicative of central sensitization [43]. Although CGRP has been associated with light aversion and nociceptive reflexes, no studies have shown spontaneous pain responses following CGRP injection in mice.
In this study, we characterized spontaneous pain in mice that receive peripheral CGRP using the mouse grimace scale (MGS) [38]. While this scale can reliably identify pain responses in mice, it relies on a subjective interpretation of facial features by trained scorers. Therefore, we also designed a squint assay that allowed us to objectively measure the eyelid fissure (distance between the eyelids). In both the grimace and squint assays, peripherally administered CGRP induced non-evoked, spontaneous pain.
2. Materials and Methods
2.1. Animals
Commercially available C57BL/6J (Jackson Labs, Bar Harbor, ME) and CD1 (Charles River Laboratories, Roanoke, IL) wildtype mice were used in this study. An equivalent number of male and female mice were used, between 10–14 weeks old. Mice were housed in groups of 5 on a 12-h light cycle with food and water ad libitum. All experiments were performed between 8 AM and 5 PM. For all experiments, investigators were blinded to genotype and/or drug treatment. Animal procedures were approved by the University of Iowa Animal Care and Use Committee and performed in accordance with the standards set by the National Institutes of Health.
2.2. Intraperitoneal drug administration
All injections were performed intraperitoneally (IP) with a 0.3mm × 13mm needle. Dulbecco PBS (Hyclone) was used as the diluent and vehicle. The amounts injected were as follows: 0.1 mg/kg rat α-CGRP (Sigma-Aldrich, St Louis, MO), 0.6 mg/kg sumatriptan succinate (APP Pharmaceuticals, Schaumburg, IL), 2 mg/kg meloxicam (Boehringer Ingelheim, Ridgefield, CT), and 30 mg/kg ALD405 (a monoclonal anti-CGRP antibody, Alder BioPharmaceuticals, Inc., Bothell, WA) or control antibodies (monoclonal antibody of the same isotype but with a different variable region targeting either digoxigenin or human PCSK9 protein). Sumatriptan was administered at the same time as CGRP or vehicle. Meloxicam was administered 30 minutes before CGRP or vehicle. The antibody and control antibody were administered 24 hours before baseline measurements. Animals were gently handled so no anesthetic agents were used during injections. All injections were performed by either AMF, LPS, BJR, or ASW. Following injection, each mouse was placed in a holding cage for 30 minutes to recover prior to testing, except for the free-moving experiment where earlier time points were assessed, as described below.
2.3. Free-moving MGS assay
Mice were acclimated to the testing room for one hour before the beginning of the experiment. Animals were then placed individually in a cubicle (15 × 15 × 15 cm high), with transparent Plexiglas walls and floor, and a ventilated transparent roof. Bedding from the animal’s home cage was placed in the cubicle in order to make the environment as stress-free as possible. Animals were left to acclimate to the cubicle for 5 to 10 minutes before pictures were taken with a digital camera at all time points (baseline, 5, 10, 15, 30, 60, 90 and/or 120 minutes after injection(s)). For all different time points, 4 pictures were taken at 20 second intervals to increase reliability. If the animal was grooming, actively sniffing or rearing, then the next available picture was used for scoring. Images were taken from multiple angles. Experimental designs and treatments for free-moving experiments are shown in the corresponding figures. For consistency in assessing grimace score, the same 2 individuals scored all the images in a blinded manner. Five facial parameters (action units) were scored: orbital tightening, nose bulge, cheek bulge, ear position, and whisker change. Each action unit was scored 0 (not present), 1 (moderately visible), or 2 (severe) on the basis of criteria described previously [38]. An initial grimace score of each photograph was calculated by averaging the scores of the 5 action units and a mean grimace score was obtained from the 4 images per time point. Scores from the two blinded individuals were averaged to give the final result. The accuracy and reliability of the scorers for those experiments are measured by correlation coefficients that range between 0.89 and 0.92 (details shown in Suppl. Fig. 1A).
2.4. Restrained MGS assay
Some assays required the use of a novel, gentle collar restraint for adequate observation of specific responses during video recording. This restraint was custom made from acrylic plastic. Mice were first acclimated to the restraint collar to decrease movements. For C57BL/6J, generally 3 acclimation sessions of 20 minutes each were sufficient, though occasionally up to 4 acclimation sessions were required prior to testing. For CD1, generally 4 acclimation sessions were sufficient though occasionally 5 sessions were required. Acclimation was evaluated by the mice laying still in the restraint for at least 10 minutes. An infrared-transparent dark acrylic chamber was placed over the mouse to eliminate extraneous light exposure during dark condition testing which was done in a dark room. For light condition testing the room light was turned on, the lid of the dark acrylic chamber was removed while an LED light array was simultaneously activated to deliver the bright light stimulus (Generay SpectroLED Essential 360 Daylight LED Light). Recording of grimace measurements in the light began 30 seconds post light activation. All experiments were recorded using three synchronized USB uEye ML video cameras (IDS Imaging Development Systems GmbH, Germany) capable of visible and infrared image recording.
Since an objective of these experiments was to test whether CGRP caused grimace even in the dark, we wanted to start with mice in the dark condition without them having experienced CGRP-induced pain in the light that may have persisted into the dark testing period. Mice were exposed to one pre-treatment run of the dark-light protocol (2 minutes in complete dark, 0 lux, followed by 2 minutes 27,000 lux, 5600k white light) to record baseline measurements. After a minimum 30 minutes rest period in the home cage, mice received treatment(s) IP in a blinded fashion. Following injection, each mouse was returned to a holding cage to recover for 30 minutes and then placed back in the gentle restraint and re-tested with the same light-dark protocol as described previously. Images of the mice were taken from the video recordings at baseline in the dark (BD), baseline in the light (BL), post-treatment in the dark (TxD) and post-treatment in the light (TxL). For each of those 4 conditions, images were obtained at 20, 40, 80, and 100 seconds of the video recordings, using a custom script in MATLAB. Experimental designs for restrained experiments are shown in the corresponding figures. The scores included orbital tightening, nose bulge, cheek bulge, and whisker change. Ear position was excluded due to interference by the collar. Each of the characteristic facial features of discomfort received a score from 0–2 as described above. Three trained individuals (out of a group of 5) scored the images of each experiment in a blinded manner. The accuracy and reliability of the scorers for those experiments are measured by a correlation coefficient that range between 0.82 and 0.95 with a mean interscorer correlation coefficient of 0.88 (Suppl. Fig. 1B). For comparison, Langford et al reported a value of 0.90 [38].
2.5. Squint assay
The squint assay was developed as an objective way to assess discomfort in mice. The images previously obtained for the restrained MGS assay were reused for this purpose. The restraint was equipped with a ruler (millimeter scale) affixed next to the head opening to properly scale each image for objective measurement. The images were analyzed using the measurement software (Infinity Analyze), where the maximum distance between inner surface of the eyelids, or palpebral fissure height, was measured for each image by a blinded investigator using digital calipers. After proper scaling, the investigator denoted the palprebral fissure height by marking a point at the center of each inner eyelid in the image and recorded the measurement equated to the pixel distance between the two points. For each time point the palpebral fissure heights of both eyes were measured and the mean distance was calculated.
2.7. Statistical analysis
All data are expressed as means ± S.E.M.
The effect of CGRP (compared to PBS) over time in free-moving setting was determined by a two-way ANOVA (factors: treatment and time) followed by Sidak’s multiple-comparison test comparing the CGRP and PBS groups at each time-point.
Effects of treatments in the free-moving assay were determined by a two-way repeated measure ANOVA with factors: treatment (5 treatment groups, corresponding to the different drug combination administered) and condition (3 levels, corresponding to baseline, treatments 1 and 2), followed by Dunnett’s multiple-comparison test to compare the effect of each treatment to their respective baseline. For comparison across treatment groups involving different animals, differences of changes from baseline were compared across treatment groups. Deltas (score at treatment time – score at baseline) were compared across treatment groups using a one-way ANOVA (with factor treatment) followed by Dunnett’s multiple comparison test.
Significance of experiments using the restraint and a light-dark paradigm were determined separately for results obtained in the dark and results obtained in the light, using a two-way repeated measure ANOVA with factors: treatment (PBS/CGRP) and condition (baseline/treatment) followed by Sidak’s multiple-comparison test to compare each treatment with its own baseline. For comparison across treatment groups involving different animals, observations were adjusted from baseline. Deltas (score at treatment time - score at baseline) were compared across treatment groups using an unpaired t-test when only two deltas were compared or a one-way ANOVA (treatment factor) followed by Dunnett’s multiple comparison test. Data were analyzed using GraphPad Prism software (RRID: SCR_002798). Significance was set at P < 0.05. For clarity, all statistical details (F and P values) can be found in Suppl. Table 1.
Principal components analysis is a procedure that converts a set of original correlated variables, such as the components in the grimace score, into a set of uncorrelated variables called the principal components. The first principal component represents the linear combination of the variables that explains most of the variation (that is, accounts for as much of the variability in the data as possible). The weights in the linear combination tell us which of the original variables contributes most to the principal component.
3. Results
3.1. CGRP induces facial signs of discomfort in the mouse grimace assay
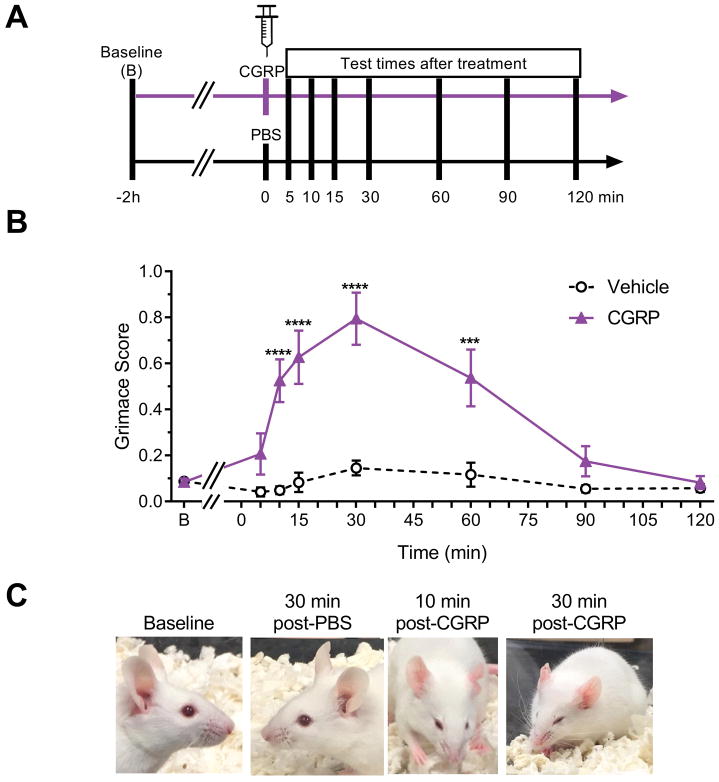
To establish whether CGRP could trigger a spontaneous pain response, the previously validated mouse grimace assay [38] was used in CD1 mice (Fig. 1A). Peripherally administered CGRP induced a significantly greater pain response than vehicle controls starting 10 minutes after injection, peaking at 30 minutes, and lasting for about 60 minutes (Fig. 1B). The vehicle (PBS) had no significant effect. Representative examples of the facial changes are shown in Fig. 1C. Ninety minutes after injection, pain responses were similar in both the CGRP and vehicle groups and back to baseline levels. Importantly, in addition to the grimace, we noticed that mice injected with CGRP showed piloerection, diarrhea (as described before [36]), and cutaneous vasodilation. The vasodilation is visible as redness of the ears (Fig. 1C). Analyzed individually, the different action units of the MGS all showed a significant effect of CGRP compared to vehicle controls (Suppl. Fig. 2A–E). For comparison with the subsequent restrained experiments where the ear position action unit had to be excluded, there was no difference in the pattern or statistical significance of the free-moving mouse data with or without this action unit (Suppl. Fig. 2F). There was no significant difference between males and females, although there was an apparent trend of a greater response in the female mice (Suppl. Fig. 3).
Figure 1. Peripheral CGRP induces spontaneous grimace in free-moving mice.
(A) Experimental design. (B) Mean grimace scores of CD1 mice measured at baseline (B) and after receiving vehicle (PBS, 10 ml/kg IP) or CGRP (0.1 mg/kg IP). (C) Representative pictures at baseline and post vehicle or CGRP injection as indicated. Average between 3 experiments. First experiment assessed baseline, 5, 10 and 15 minute time-points (n=10 per group). Second experiment assessed baseline, 15, 30, and 60 minute time-points (n=10 per group). Third experiment assessed baseline, 30, 90 and 120 minute time-points (n=10). Therefore n=10 to 30 per group in this figure. Experiments were scored by two blinded individuals, error bars indicate ± SEM. Two-way (with factors treatment and time) ANOVA P < 0.0001 for treatment factor, Sidak’s multiple comparison test, ***P < 0.001, ****P < 0.0001 when comparing CGRP to vehicle group at corresponding time point.
3.2. Effects of sumatriptan, meloxicam, and CGRP antibody treatments on CGRP-induced grimace
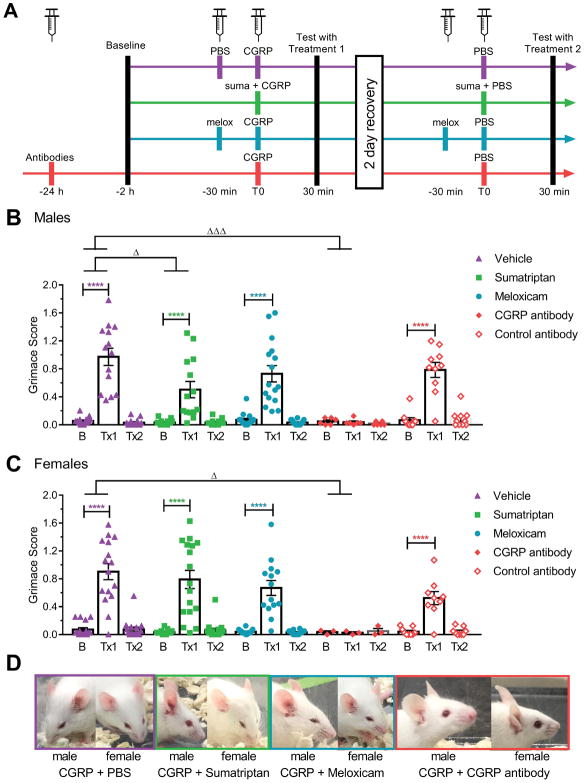
To help determine the nature of the pain elicited by peripheral CGRP, we examined the effect of three different treatments on the observed phenotype: sumatriptan (5-HT1B/D agonist considered as gold standard in migraine treatment), meloxicam (non-steroidal anti-inflammatory drug used to treat pain), and ALD405 (a CGRP-blocking monoclonal antibody) (Fig. 2A). In free-moving male CD1 mice, sumatriptan partially attenuated the effect of CGRP, as shown by a smaller difference (or delta) between treatment with CGRP + sumatriptan and its baseline (Tx1-baseline) compared to the delta between treatment with CGRP + vehicle and its baseline (Fig. 2B). This effect is only partial since the results obtained after Tx1 are still significantly different from baseline in the CGRP + sumatriptan group. In female mice, however, sumatriptan failed to significantly attenuate the effect of CGRP (Fig. 2C). Meloxicam and control antibody failed to attenuate the effect of CGRP in either males or females (Fig. 2B, C). As expected, the CGRP antibody ALD405 completely blocked the spontaneous pain induced by CGRP both in males (Fig. 2B) and in females (Fig. 2C). Additionally, the CGRP antibody was the only drug that was noted to be able to prevent piloerection, diarrhea and peripheral vasodilation (Fig. 2D).
Figure 2. Effect of treatments on CGRP-induced spontaneous grimace.
(A) Experimental design. (B, C) MGS scores of male (B) and female (C) CD1 mice at baseline (B) and after treatments 1 and 2 (Tx1 and Tx2). Mice were pretreated with isotype controls or CGRP monoclonal antibody ALD405 (30 mg/kg IP) 24 hours prior to testing, with meloxicam (2 mg/kg IP) 60 minutes prior to testing, or with vehicle (PBS, 10 ml/kg IP) or sumatriptan (0.6 mg/kg IP) 30 minutes prior to testing. For Tx1, all mice received CGRP (0.1 mg/kg IP) 30 minutes before testing. For Tx2, all mice received vehicle (PBS) 30 minutes before testing. The mean grimace scores were measured 30 minutes after the last injection. (D) Representative pictures of a male and a female taken during the assay 30 minutes after the last injection for the CGRP + PBS group (purple), CGRP + sumatriptan group (green), CGRP + meloxicam group (blue), CGRP + CGRP antibody (red). Average between 3 experiments scored by two blinded individuals (n=20 to 30 per group, ± SEM). Two-way repeated measures ANOVA P < 0.0001 for treatment factor, Dunnett’s multiple comparison test to compare treatment 1 to baseline, ****P < 0.0001. For comparisons across treatment groups involving different animals, differences of changes from baseline were compared across treatment groups. Deltas (score at treatment time – score at baseline) were compared across treatment groups using a one-way ANOVA (with factor treatment), followed by Dunnett’s multiple comparison test, ΔP < 0.05, ΔΔΔP < 0.001.
3.3. CGRP induces light-independent facial signs of discomfort
Given the subjective nature of the grimace scale, we wanted to develop a continuous and objective point-to-point measurement of the eyelid fissure. In addition, we wanted to be able to measure the eyelid fissure in the complete absence of light as well as under very bright light (~27,000 lux) in order to test whether the phenotype was simply a manifestation of the previously reported CGRP-induced light sensitivity. To achieve these dual goals, we created a gentle restraint collar (Fig. 3A) and an infrared-transparent dark acrylic chamber to monitor the mice at a known distance to the cameras in both complete dark and under very bright light (Fig. 3B). The first step was to validate the use of this restraint by detection of a CGRP-induced grimace response. One modification in the grimace scoring was exclusion of the ear position action unit since the collar prevented normal ear movement. At baseline, mice in the restraint collar had a higher grimace score than observed with free-moving mice (for example, compare baselines Fig. 1B and 3C). Nonetheless, CGRP still caused a significant grimace response in restrained CD1 mice in complete dark (Fig. 3C). In contrast, vehicle did not have a significant effect and the delta between baseline and CGRP treatment was significantly greater than the delta between baseline and vehicle treatment (Fig. 3C).
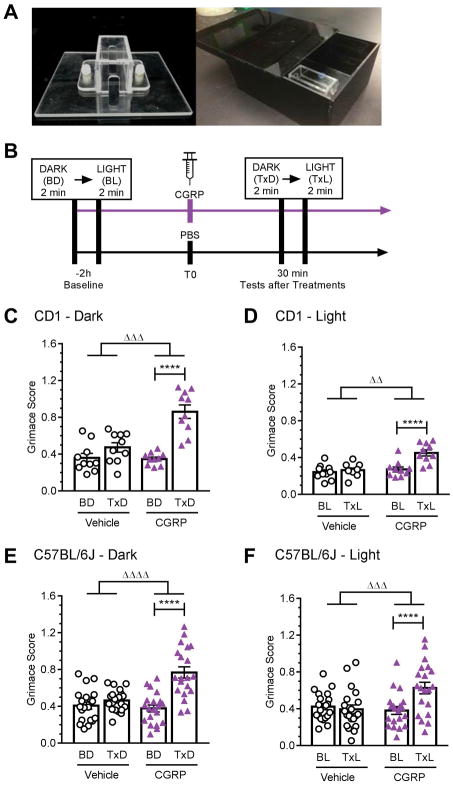
Figure 3. Peripheral CGRP induces grimace in restrained mice.
(A) Image of the restraint device and of the infrared-transparent dark acrylic chamber and lid allowing the dark-to-light transition. (B) Experimental design. (C–F) MGS scores were measured in CD1 mice in the dark (C) and in bright light (D), and in C57BL/6J mice in the dark (E) and bright light (F). Mice were restrained and recorded via camera during a two minute baseline dark condition (BD) followed by a two minute baseline light condition (BL) (cool white, 27,000 lux). After baseline conditions, mice were given an IP injection of either vehicle (PBS, 10 ml/kg IP) or CGRP (0.1 mg/kg). Thirty minutes post-injection mice were again restrained and recorded under a two minute treatment dark condition (TxD) followed by a two minute treatment light condition (TxL). Average of 2 experiments per strain, scored by 3 blinded individuals (n=10 per group for CD1 and n=20 per group for C57BL/6J, ± SEM). Two-way repeated measures ANOVA followed by Sidak’s multiple comparison test to compare baseline and treatment conditions, ****P < 0.001. To compare the effect of treatments, the deltas between the scores after treatment from baseline were compared by an unpaired t-test, ΔΔP < 0.01, ΔΔΔP < 0.001, ΔΔΔΔP < 0.0001.
CGRP-induced grimace was also observed under very bright light conditions (Fig. 3D). Surprisingly, the grimace scores were higher in the dark than in bright light for the CD1 mice (comparison of the delta between baseline and CGRP treatments in dark versus light; p = 0.0002). Subsequent analysis of all four action units in the absence of CGRP showed that the transition to light caused a significant decrease in orbital tightening and nose bulge at baseline and with vehicle treatment (Suppl. Fig. 4). This could explain the reduced grimace response in bright light. In addition, as seen in the dark, vehicle did not cause a significant response and the delta between baseline and CGRP treatment was significantly greater than with vehicle (Fig. 3D).
Since we have previously tested C57BL/6J mice for CGRP-induced migraine-like symptoms, we then asked if CGRP also causes a grimace response in this strain of mice. C57BL/6J mice showed comparable grimace responses following CGRP administration both in the dark (Fig. 3E) and light (Fig. 3F). Unlike the CD1 mice, there was no significant difference between the CGRP-induced grimace in light and dark. As with the CD1 mice, vehicle did not cause a significant response and the delta between baseline and CGRP treatment was significantly greater than the delta between baseline and vehicle treatment in both the light and dark (Fig. 3E, F). As with the free-moving mice, for both strains of mice and in the light and dark, all the MGS action units showed a significant effect of CGRP compared to vehicle (data not shown). Collectively, these results indicate that CGRP induces spontaneous pain in mice through a light independent pathway and validate the use of the restraint collar.
3.4. CGRP antibody completely blocks the facial signs of discomfort induced by CGRP in restrained mice
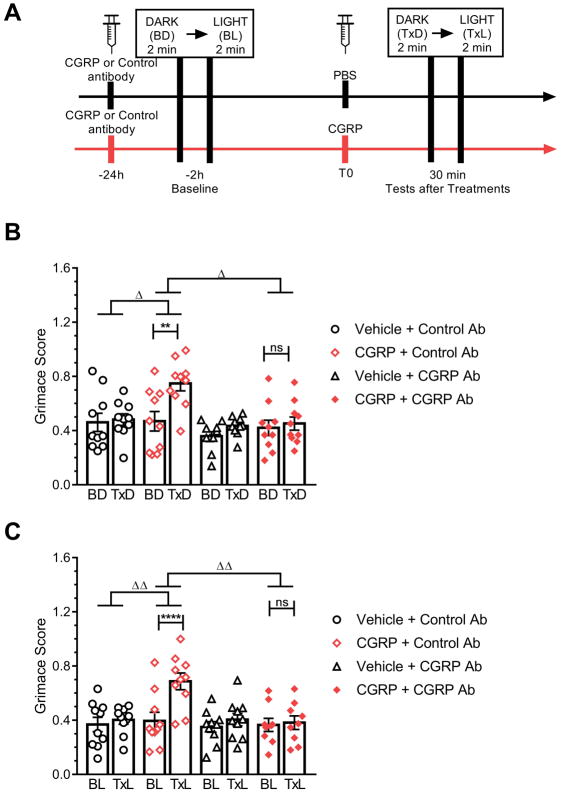
The efficacy of the CGRP antibody ALD405 was also assessed in both the dark and light using the restrained C57BL/6J mice (Fig. 4A). The CGRP antibody ALD405, when co-administered with CGRP, completely prevented the effect of CGRP in both the dark (Fig. 4B) and light (Fig. 4C). The control antibody, when co-administered with CGRP, did not have any effect, as CGRP significantly increased the grimace score in this group compared to their baseline levels in both the dark and light (Fig. 4B, C). Together, these results show that the effect of CGRP and its attenuation by CGRP antibody can be visualized in restrained animals and is light independent.
Figure 4. CGRP-induced grimace is blocked by a CGRP antibody in restrained mice.
(A) Experimental design. (B, C) C57BL/6J mice in dark (B) and in bright light (C). Mice were pretreated by IP injection with isotype control or CGRP monoclonal antibody (30 mg/kg). 24 hours later, mice were restrained and recorded via camera during a two minute baseline dark condition (BD) followed by a two minute light treatment condition (BL) (cool white, 27,000 lux). After baseline conditions, mice were given an IP injection of either vehicle (PBS, 10 ml/kg IP) or CGRP (0.1 mg/kg). Thirty minute post-injection mice were again restrained and recorded under a two minute dark condition (TxD) followed by a two minute light condition (TxL). Average of 2 experiments per treatment, scored by 3 blinded individuals (n=10 per group, ± SEM). Two-way repeated measures ANOVA followed by Sidak’s multiple comparison test to compare baseline and treatment conditions, **P < 0.01, ****P < 0.001, not significant (ns). To compare the effect of treatments, the deltas between the scores after treatment from baseline were compared with a one-way ANOVA followed by Dunnett’s multiple comparison test, ΔP < 0.05, ΔΔP < 0.01.
3.5. Detection of the CGRP pain response by an objective squint assay
Even though the grimace scale is now fairly well accepted as a measure of spontaneous pain, its main criticisms are the subjectivity of the assay, the time-consuming scoring, and the use of a non-continuous scale. We applied a principal component analysis to the four components of our grimace score. The first principal component (see methods), which put most of its weight on the orbital tightening, explained 77.1% of the total variation. This suggests that the squint or orbital tightening carries the largest weight among the four components that are averaged into the grimace score. We therefore reanalyzed pictures used for the restrained MGS to measure the eyelid fissure as an assessment of orbital tightening. The point to point measurements of eyelid fissure and grimace score had a Pearson correlation of −0.69. The squint assay has a markedly smaller coefficient of variation of 18.1 in dark and 15.6 in light than the grimace values of 46.8 in dark and 49.8 in light.
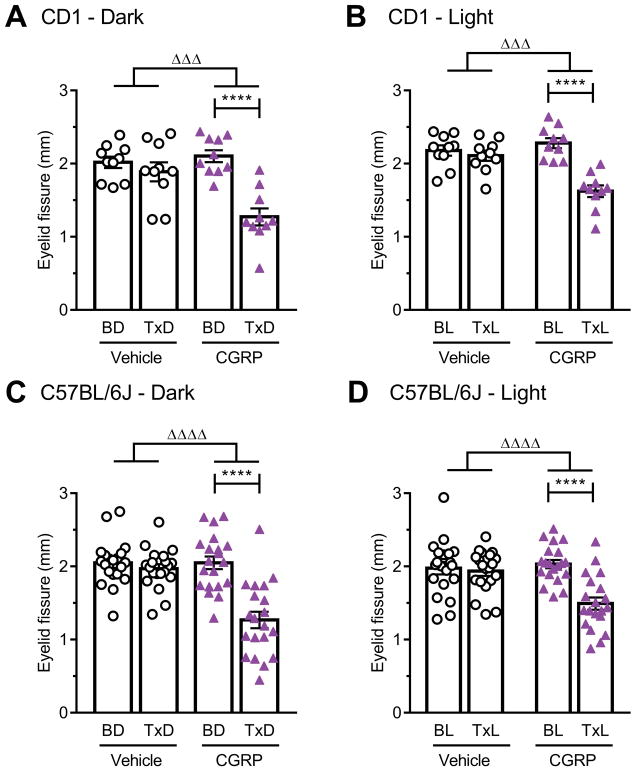
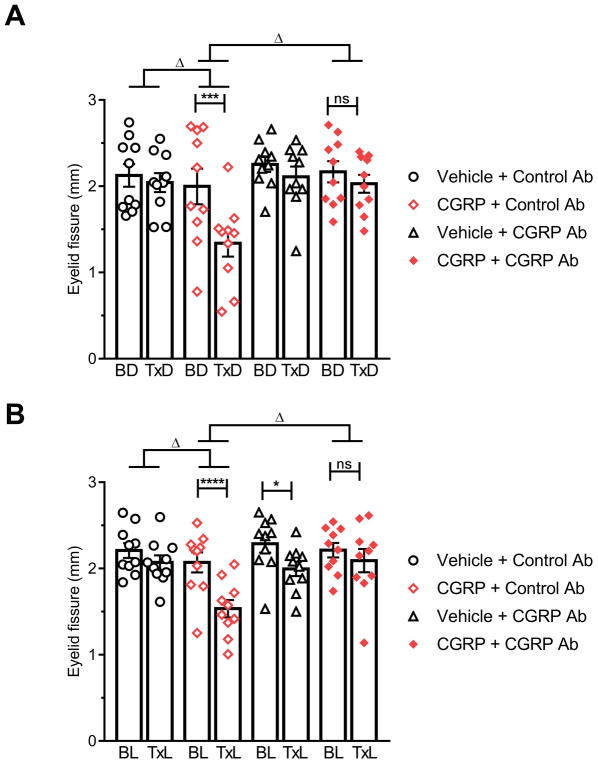
Using this squint assay, CGRP was found to significantly decrease the eyelid fissure compared to baseline in both the dark and light for CD1 mice (Fig. 5A, B) and C57BL/6J mice (Fig. 5C, D). The vehicle had no effect and the changes between baseline and CGRP treatment were also significantly different from vehicle (Fig. 5). Similar to the effect of light on the grimace orbital tightening action unit, we also observed that light caused CD1 mice to open their eyes, independent of CGRP, at baseline and with vehicle treatment (Suppl. Fig. 4). Also, as with the grimace assay, there was no difference in eyelid fissures between the dark and light states in untreated C57BL/6J mice. Analysis of individual eyes of both strains revealed that most mice had one eye more open than the other at any given time both in the light and dark, pre-and post-treatment (Suppl. Fig. 5). This laterality was apparently not greater after CGRP injection compared to baseline or vehicle. There was no difference between males and females (data not shown). Finally, the CGRP antibody completely blocked CGRP-induced squint in C57BL/6J mice in the dark (Fig. 6A) and light (Fig. 6B), while the control antibody had no significant effect.
Figure 5. Peripheral CGRP induces squint in restrained mice.
Scored images obtained during the restrained MGS experiment (Fig. 3C, D, E, F) were used to measure eyelid fissure. (A) CD1 in dark, (B) CD1 in light, (C) C57BL/6J in dark and (D) C57BL/6J in light. Average of 2 experiments per strain, scored by 1 blinded individual (n=10 per group for CD1 and n=20 per group for C57BL/6J, ± SEM). Two-way repeated measures ANOVA followed by Sidak’s multiple comparison test to compare baseline and treatment conditions, ****P < 0.001. To compare the effect of treatments, the deltas between the scores after treatment from baseline were compared with an unpaired t-test, ΔΔΔP < 0.001, ΔΔΔΔP < 0.0001.
Figure 6. CGRP-induced squint is blocked by a CGRP antibody.
(A) C57BL/6J in dark, (B) C57BL/6J in light. Scored images obtained during the restrained MGS experiments (Fig. 4A, B) were used to measure eyelid fissure. Average of 2 experiments scored by 1 blinded individual (n=10 per group, ± SEM). Two-way repeated measures ANOVA followed by Sidak’s multiple comparison test to compare baseline and treatment conditions, *P < 0.05, ***P < 0.001, ****P < 0.001, not significant (ns). To compare the effect of treatments, the deltas between the scores after treatment from baseline were compared with a one-way ANOVA followed by Dunnett’s multiple comparison test, ΔP < 0.05.
4. Discussion
In this report, we describe for the first time a spontaneous pain phenotype induced by CGRP in mice. This observation fits with human studies in which injection of CGRP caused headache pain [23; 39] and CGRP receptor antagonists reduced headache intensity [29; 49]. The time course of CGRP-induced pain in wildtype mice in this study matches the initial mild headache reported by both migraineurs and non-migraine subjects. Whether a prolonged or biphasic pain phenotype will be seen in sensitized mouse models similar to that seen in migraineurs remains to be tested. While migraine attacks can be evoked by specific triggers, the pain is spontaneous and therefore has been difficult to assess in pre-clinical studies. Of importance, we find that CGRP-induced spontaneous pain is light-independent, which was relevant since CGRP induces light-aversion in mice [44]. The results demonstrate that migraine-related light-aversion and spontaneous pain are independent.
There have been only three other direct assessments of spontaneous pain in rodent models of migraine. The first was with a transgenic model of familial hemiplegic migraine 1 (FHM1), which showed an increased grimace compared to littermates [38], along with increased facial grooming and eye-blinking rates [8]. Consistent with our findings, the FHM1 study also reported that restrained mice had higher baseline grimace scores compared to free-moving mice [38]. The second report was repeated cortical spreading depression events, which yielded an increased grimace compared to sham mice [37]. The latest report was that repeated nitroglycerin administrations in rats increased facial pain expressions [27]. Thus, there are now four migraine-related triggers that cause grimace responses in rodents: CGRP, FHM1 mutation, cortical spreading depression and nitroglycerin.
Other studies have indirectly measured spontaneous pain by assessing non-evoked behaviors during a migraine-like episode. Application of inflammatory soup onto the dura increased resting and freezing, decreased motility/exploratory behavior, increased facial grooming, altered conditioned place preference, and decreased food intake [12; 31; 42; 45; 64]. Likewise, TrpA1 agonists on the dura decreased vertical rearing [14]. Taken together, these results support the use of preclinical assays to measure migraine-like pain.
Our finding that CGRP can cause spontaneous pain builds on decades of studies linking CGRP and nociception. CGRP is widely expressed in nociceptive sensory neurons and has been pharmacologically tied to thermal and mechanical evoked nociceptive responses [33]. For example, intrathecal injection of CGRP increased sensitivity to mechanical stimuli and this tactile allodynia was enhanced in a migraine mouse model genetically sensitized to CGRP [43]. Where and how might CGRP be acting in the periphery to cause pain? We have previously proposed that CGRP can act in the meninges and trigeminal ganglia as a neuromodulator of peripheral sensitization [57]. In the meninges, CGRP could alter the microenvironment to sensitize trigeminal nociceptors to mechanical stimulation, for example from vascular pulsations. In the ganglia, CGRP could initiate paracrine, and possibly autocrine, actions to sensitize nociceptors, for example by ATP-gated ion channels. In both cases, CGRP would be acting as a neuromodulator of excitatory stimuli that trigger pain sensations in the CNS. The CGRP monoclonal antibody would prevent this peripheral sensitization by sequestering CGRP.
In this study, we found that the anti-migraine drug sumatriptan, at a dose sufficient to inhibit CGRP-induced light aversion [44], could partially attenuate the CGRP-induced pain response. Unexpectedly, sumatriptan was effective, albeit partially, only in males, not in females. The reason for this gender difference is not known, but one possibility is that females had a stronger CGRP response than males and hence could not be rescued by sumatriptan. This possibility is supported by recent studies showing more robust or more rapidly developed phenotypes in female than male rodents in response to migraine-like triggers [31; 52; 64]. We have also noted that female mice show a trend of greater CGRP-induced light aversion compared to males [44; 54]. However, while these examples suggest that females could be more sensitive to CGRP than males, a caveat is that we observed similar male and female grimace and squint responses. It is possible though that the CGRP dose used in the present study had a ceiling effect that masked gender differences. Furthermore, until recently, pre-clinical studies of migraine treatments were mainly assessed only in male rodents [3; 15; 45]. More recent studies include female rodents, but often fail to analyze or report sexual dimorphisms [7; 44; 52]. Interestingly, there are sexually dimorphic pronociceptive effects of sumatriptan [2]. More studies, including a sumatriptan dose response, are needed. To our knowledge, no clinical study has reported gender differences for sumatriptan efficacy [25; 32].
In addition to sumatriptan, two other potential therapeutics were tested. A nonsteroidal anti-inflammatory agent, meloxicam, failed to block CGRP-induced grimace at the suggested dose to relieve post-surgical pain in mice [6; 65], although other studies have reported only partial or no relief [40; 46; 55]. As expected, spontaneous pain was completely prevented by a CGRP antibody. This is consistent with its efficacy in preventing CGRP-induced light aversion [44]. In addition, we observed that the CGRP antibody seemed to block piloerection, diarrhea and peripheral vasodilation (based on redness of the ears). The prevention of diarrhea is in agreement with our previous study [36]. This efficacy both in males and females in two different strains of mice is consistent with the promising results of clinical trials using CGRP and CGRP receptor antibodies to prevent migraine [13; 21; 59–61; 63].
The major tool of this study was the grimace scale. Development of the grimace scale by Mogil and colleagues was a major advance that allowed scientists to assess spontaneous pain in diverse animal models [1; 11; 24; 38; 47; 62; 66]. It represents a translatable non-evoked assay that can stratify the severity and evaluate the efficacy of treatments, but there are caveats, including its use as an indicator of pain. For example, grimace may not always equate to pain and is generally more indicative of acute rather than chronic pain [38; 47]. An inherent weakness of the assay is that it is subjective, its reliability depends on the level of training of the scorer, and the scoring is non-continuous, which decreases its sensitivity. Likewise, some of the action units might influence one another. For example, it’s likely that a nose bulge will in turn induce a cheek bulge. If both action units are scored independently when they are dependent variables, it could bias the outcome of the grimace score. Finally, the MGS is time-consuming and requires a minimum of two blinded scorers per experiment. In light of those shortcomings in speed and objectivity, automation of pain analysis is a logical step forward. Recently, Tuttle and colleagues have begun to address these factors with the use of a convolutional neural network to attribute binary pain/no-pain assessment to facial images of mice [66].
We sought to develop a simplified indicator of grimace. Towards this goal, we found that the principal component driving the final grimace score is the orbital tightening action unit. As such, we reasoned that measuring the squint (eyelid fissure) on a continuous scale could provide a more sensitive and objective way to assess spontaneous pain. Using restrained animals, we were able to quantify this squint and as a proof of principle, we found that the results obtained by this method are similar to the MGS. Importantly, the squint assay has a smaller coefficient of variation, which is likely due to the objectivity of the assay. Thus, another advantage of the squint assay is that it should be powered to require fewer animals compared to the grimace assay. However, the squint assay does not completely replace the grimace assay as there is a Pearson correlation of −0.69 between the assays, and a stimulus could trigger eye closure or opening without affecting other indicators of pain. For example, a light-triggered eye-opening apparently reduced the grimace score of CD1 mice in the light. Moreover, this test needs to be validated with other stimuli and developed in free moving animals, since the assay with restrained mice has a diminished dynamic range, due to an increased MGS score at baseline. Future studies will allow improvement of this assay with automation to capture eye measurements in freely moving animals that could be translatable to humans.
In conclusion, we can now add spontaneous pain to the list of migraine symptoms caused by CGRP. Combined with CGRP roles in light aversive behavior and tactile allodynia, it is increasingly clear that CGRP directly contributes to multiple migraine symptoms in preclinical models. The apparent gender difference revealed by sumatriptan treatments further emphasizes the necessity of studies in both male and female mice. Finally, development of an objective and continuous scale measuring the squint response will facilitate further studies on non-evoked pain in mouse models.
Supplementary Material
Acknowledgments
Acknowledgments and conflict of interest statement
The authors thank members of the Russo lab for interesting discussions. This work was supported by grants to AFR from the NIH (NS075599), Veterans Affairs Medical Center (1I01RX002101), and Department of Defense USAMRAA (W81XWH-16-1-0071 and W81XWH-16-1-0211). AFR is a consultant to Alder BioPharmaceuticals, Inc. and Pharmnovo, and has served as a consultant to Eli Lilly and Amgen/Novartis. This work was also supported by a grant to LPS from Veterans Affairs Medical Center (IK2 RX-002010-01). LFGM and MCML are both employees at Alder BioPharmaceuticals, Inc.
References
- 1.Akintola T, Raver C, Studlack P, Uddin O, Masri R, Keller A. The grimace scale reliably assesses chronic pain in a rodent model of trigeminal neuropathic pain. Neurobiol Pain. 2017;2:13–17. doi: 10.1016/j.ynpai.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Araldi D, Ferrari LF, Green P, Levine JD. Marked sexual dimorphism in 5-HT1 receptors mediating pronociceptive effects of sumatriptan. Neuroscience. 2017;344:394–405. doi: 10.1016/j.neuroscience.2016.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bates EA, Nikai T, Brennan KC, Fu YH, Charles AC, Basbaum AI, Ptacek LJ, Ahn AH. Sumatriptan alleviates nitroglycerin-induced mechanical and thermal allodynia in mice. Cephalalgia. 2010;30(2):170–178. doi: 10.1111/j.1468-2982.2009.01864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellamy JL, Cady RK, Durham PL. Salivary levels of CGRP and VIP in rhinosinusitis and migraine patients. Headache. 2006;46(1):24–33. doi: 10.1111/j.1526-4610.2006.00294.x. [DOI] [PubMed] [Google Scholar]
- 5.Burstein R, Noseda R, Borsook D. Migraine: multiple processes, complex pathophysiology. J Neurosci. 2015;35(17):6619–6629. doi: 10.1523/JNEUROSCI.0373-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carpenter JW. Exotic Animal Formulary. 4. St Louis, MO: Elsevier Health Sciences; 2012. [Google Scholar]
- 7.Chan KY, Labastida-Ramirez A, Ramirez-Rosas MB, Labruijere S, Garrelds IM, Danser AH, van den Maagdenberg AM, Maassen Van Den Brink A. Trigeminovascular calcitonin gene-related peptide function in Cacna1a R192Q-mutated knock-in mice. J Cereb Blood Flow Metab. 2017 doi: 10.1177/0271678X17725673. 271678X17725673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chanda ML, Tuttle AH, Baran I, Atlin C, Guindi D, Hathaway G, Israelian N, Levenstadt J, Low D, Macrae L, O’Shea L, Silver A, Zendegui E, Mariette Lenselink A, Spijker S, Ferrari MD, van den Maagdenberg AM, Mogil JS. Behavioral evidence for photophobia and stress-related ipsilateral head pain in transgenic Cacna1a mutant mice. Pain. 2013;154(8):1254–1262. doi: 10.1016/j.pain.2013.03.038. [DOI] [PubMed] [Google Scholar]
- 9.Charles A. The evolution of a migraine attack - a review of recent evidence. Headache. 2013;53(2):413–419. doi: 10.1111/head.12026. [DOI] [PubMed] [Google Scholar]
- 10.Connor KM, Shapiro RE, Diener HC, Lucas S, Kost J, Fan X, Fei K, Assaid C, Lines C, Ho TW. Randomized, controlled trial of telcagepant for the acute treatment of migraine. Neurology. 2009;73(12):970–977. doi: 10.1212/WNL.0b013e3181b87942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dalla Costa E, Minero M, Lebelt D, Stucke D, Canali E, Leach MC. Development of the Horse Grimace Scale (HGS) as a pain assessment tool in horses undergoing routine castration. PLoS One. 2014;9(3):e92281. doi: 10.1371/journal.pone.0092281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Felice M, Eyde N, Dodick D, Dussor GO, Ossipov MH, Fields HL, Porreca F. Capturing the aversive state of cephalic pain preclinically. Ann Neurol. 2013;74(2):257–265. doi: 10.1002/ana.23922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dodick DW, Ashina M, Brandes JL, Kudrow D, Lanteri-Minet M, Osipova V, Palmer K, Picard H, Mikol DD, Lenz RA. ARISE: A Phase 3 randomized trial of erenumab for episodic migraine. Cephalalgia. 2018 doi: 10.1177/0333102418759786. 333102418759786. [DOI] [PubMed] [Google Scholar]
- 14.Edelmayer RM, Le LN, Yan J, Wei X, Nassini R, Materazzi S, Preti D, Appendino G, Geppetti P, Dodick DW, Vanderah TW, Porreca F, Dussor G. Activation of TRPA1 on dural afferents: a potential mechanism of headache pain. Pain. 2012;153(9):1949–1958. doi: 10.1016/j.pain.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edelmayer RM, Vanderah TW, Majuta L, Zhang ET, Fioravanti B, De Felice M, Chichorro JG, Ossipov MH, King T, Lai J, Kori SH, Nelsen AC, Cannon KE, Heinricher MM, Porreca F. Medullary pain facilitating neurons mediate allodynia in headache-related pain. Ann Neurol. 2009;65(2):184–193. doi: 10.1002/ana.21537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edvinsson L. The Journey to Establish CGRP as a Migraine Target: A Retrospective View. Headache. 2015;55(9):1249–1255. doi: 10.1111/head.12656. [DOI] [PubMed] [Google Scholar]
- 17.Edvinsson L, Warfvinge K. Recognizing the role of CGRP and CGRP receptors in migraine and its treatment. Cephalalgia. 2017 doi: 10.1177/0333102417736900. 333102417736900. [DOI] [PubMed] [Google Scholar]
- 18.Goadsby PJ, Edvinsson L. The trigeminovascular system and migraine: studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann Neurol. 1993;33(1):48–56. doi: 10.1002/ana.410330109. [DOI] [PubMed] [Google Scholar]
- 19.Goadsby PJ, Edvinsson L, Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol. 1990;28(2):183–187. doi: 10.1002/ana.410280213. [DOI] [PubMed] [Google Scholar]
- 20.Goadsby PJ, Holland PR, Martins-Oliveira M, Hoffmann J, Schankin C, Akerman S. Pathophysiology of Migraine: A Disorder of Sensory Processing. Physiol Rev. 2017;97(2):553–622. doi: 10.1152/physrev.00034.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goadsby PJ, Reuter U, Hallstrom Y, Broessner G, Bonner JH, Zhang F, Sapra S, Picard H, Mikol DD, Lenz RA. A Controlled Trial of Erenumab for Episodic Migraine. N Engl J Med. 2017;377(22):2123–2132. doi: 10.1056/NEJMoa1705848. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez C, Zegpi C, Noriega V, Prieto JC, Miranda HF. Synergism between dexketoprofen and meloxicam in an orofacial formalin test was not modified by opioid antagonists. Pharmacol Rep. 2011;63(2):433–440. doi: 10.1016/s1734-1140(11)70509-6. [DOI] [PubMed] [Google Scholar]
- 23.Guo S, Vollesen AL, Olesen J, Ashina M. Premonitory and nonheadache symptoms induced by CGRP and PACAP38 in patients with migraine. Pain. 2016;157(12):2773–2781. doi: 10.1097/j.pain.0000000000000702. [DOI] [PubMed] [Google Scholar]
- 24.Hampshire V, Robertson S. Using the facial grimace scale to evaluate rabbit wellness in post-procedural monitoring. Lab Anim (NY) 2015;44(7):259–260. doi: 10.1038/laban.806. [DOI] [PubMed] [Google Scholar]
- 25.Hansen JM, Goadsby PJ, Charles A. Reduced efficacy of sumatriptan in migraine with aura vs without aura. Neurology. 2015;84(18):1880–1885. doi: 10.1212/WNL.0000000000001535. [DOI] [PubMed] [Google Scholar]
- 26.Hansen JM, Hauge AW, Olesen J, Ashina M. Calcitonin gene-related peptide triggers migraine-like attacks in patients with migraine with aura. Cephalalgia. 2010;30(10):1179–1186. doi: 10.1177/0333102410368444. [DOI] [PubMed] [Google Scholar]
- 27.Harris HM, Carpenter JM, Black JR, Smitherman TA, Sufka KJ. The effects of repeated nitroglycerin administrations in rats; modeling migraine-related endpoints and chronification. J Neurosci Methods. 2017;284:63–70. doi: 10.1016/j.jneumeth.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 28.Hay DL, Walker CS. CGRP and its receptors. Headache. 2017;57(4):625–636. doi: 10.1111/head.13064. [DOI] [PubMed] [Google Scholar]
- 29.Ho TW, Ferrari MD, Dodick DW, Galet V, Kost J, Fan X, Leibensperger H, Froman S, Assaid C, Lines C, Koppen H, Winner PK. Efficacy and tolerability of MK-0974 (telcagepant), a new oral antagonist of calcitonin gene-related peptide receptor, compared with zolmitriptan for acute migraine: a randomised, placebo-controlled, parallel-treatment trial. Lancet. 2008;372(9656):2115–2123. doi: 10.1016/S0140-6736(08)61626-8. [DOI] [PubMed] [Google Scholar]
- 30.Ho TW, Mannix LK, Fan X, Assaid C, Furtek C, Jones CJ, Lines CR, Rapoport AM group MKPs. Randomized controlled trial of an oral CGRP receptor antagonist, MK-0974, in acute treatment of migraine. Neurology. 2008;70(16):1304–1312. doi: 10.1212/01.WNL.0000286940.29755.61. [DOI] [PubMed] [Google Scholar]
- 31.Huang DY, Ren L, Qiu CS, Liu P, Peterson J, Yanagawa YC, Cao YQ. Characterization of a mouse model of headache. Pain. 2016;157(8):1744–1760. doi: 10.1097/j.pain.0000000000000578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iversen HK, Olesen J. Headache induced by a nitric oxide donor (nitroglycerin) responds to sumatriptan. A human model for development of migraine drugs. Cephalalgia. 1996;16(6):412–418. doi: 10.1046/j.1468-2982.1996.1606412.x. [DOI] [PubMed] [Google Scholar]
- 33.Iyengar S, Ossipov MH, Johnson KW. The role of calcitonin gene-related peptide in peripheral and central pain mechanisms including migraine. Pain. 2017;158(4):543–559. doi: 10.1097/j.pain.0000000000000831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Juhasz G, Zsombok T, Jakab B, Nemeth J, Szolcsanyi J, Bagdy G. Sumatriptan causes parallel decrease in plasma calcitonin gene-related peptide (CGRP) concentration and migraine headache during nitroglycerin induced migraine attack. Cephalalgia. 2005;25(3):179–183. doi: 10.1111/j.1468-2982.2005.00836.x. [DOI] [PubMed] [Google Scholar]
- 35.Kaiser EA, Kuburas A, Recober A, Russo AF. Modulation of CGRP-induced light aversion in wild-type mice by a 5-HT(1B/D) agonist. J Neurosci. 2012;32(44):15439–15449. doi: 10.1523/JNEUROSCI.3265-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaiser EA, Rea BJ, Kuburas A, Kovacevich BR, Garcia-Martinez LF, Recober A, Russo AF. Anti-CGRP antibodies block CGRP-induced diarrhea in mice. Neuropeptides. 2017;64:95–99. doi: 10.1016/j.npep.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karatas H, Erdener SE, Gursoy-Ozdemir Y, Lule S, Eren-Kocak E, Sen ZD, Dalkara T. Spreading depression triggers headache by activating neuronal Panx1 channels. Science. 2013;339(6123):1092–1095. doi: 10.1126/science.1231897. [DOI] [PubMed] [Google Scholar]
- 38.Langford DJ, Bailey AL, Chanda ML, Clarke SE, Drummond TE, Echols S, Glick S, Ingrao J, Klassen-Ross T, Lacroix-Fralish ML, Matsumiya L, Sorge RE, Sotocinal SG, Tabaka JM, Wong D, van den Maagdenberg AM, Ferrari MD, Craig KD, Mogil JS. Coding of facial expressions of pain in the laboratory mouse. Nat Methods. 2010;7(6):447–449. doi: 10.1038/nmeth.1455. [DOI] [PubMed] [Google Scholar]
- 39.Lassen LH, Haderslev PA, Jacobsen VB, Iversen HK, Sperling B, Olesen J. CGRP may play a causative role in migraine. Cephalalgia. 2002;22(1):54–61. doi: 10.1046/j.1468-2982.2002.00310.x. [DOI] [PubMed] [Google Scholar]
- 40.Leach MC, Klaus K, Miller AL, Scotto di Perrotolo M, Sotocinal SG, Flecknell PA. The assessment of post-vasectomy pain in mice using behaviour and the Mouse Grimace Scale. PLoS One. 2012;7(4):e35656. doi: 10.1371/journal.pone.0035656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li D, Ren Y, Xu X, Zou X, Fang L, Lin Q. Sensitization of primary afferent nociceptors induced by intradermal capsaicin involves the peripheral release of calcitonin gene-related Peptide driven by dorsal root reflexes. J Pain. 2008;9(12):1155–1168. doi: 10.1016/j.jpain.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malick A, Jakubowski M, Elmquist JK, Saper CB, Burstein R. A neurohistochemical blueprint for pain-induced loss of appetite. Proc Natl Acad Sci U S A. 2001;98(17):9930–9935. doi: 10.1073/pnas.171616898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marquez de Prado B, Hammond DL, Russo AF. Genetic enhancement of calcitonin gene-related Peptide-induced central sensitization to mechanical stimuli in mice. J Pain. 2009;10(9):992–1000. doi: 10.1016/j.jpain.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mason BN, Kaiser EA, Kuburas A, Loomis MM, Latham JA, Garcia-Martinez LF, Russo AF. Induction of Migraine-Like Photophobic Behavior in Mice by Both Peripheral and Central CGRP Mechanisms. J Neurosci. 2017;37(1):204–216. doi: 10.1523/JNEUROSCI.2967-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Melo-Carrillo A, Lopez-Avila A. A chronic animal model of migraine, induced by repeated meningeal nociception, characterized by a behavioral and pharmacological approach. Cephalalgia. 2013;33(13):1096–1105. doi: 10.1177/0333102413486320. [DOI] [PubMed] [Google Scholar]
- 46.Miller AL, Kitson GL, Skalkoyannis B, Flecknell PA, Leach MC. Using the mouse grimace scale and behaviour to assess pain in CBA mice following vasectomy. Appl Anim Behav Sci. 2016;181:160–165. doi: 10.1016/j.applanim.2016.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miller AL, Leach MC. The Mouse Grimace Scale: A Clinically Useful Tool? PLoS One. 2015;10(9):e0136000. doi: 10.1371/journal.pone.0136000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mogil JS, Miermeister F, Seifert F, Strasburg K, Zimmermann K, Reinold H, Austin JS, Bernardini N, Chesler EJ, Hofmann HA, Hordo C, Messlinger K, Nemmani KV, Rankin AL, Ritchie J, Siegling A, Smith SB, Sotocinal S, Vater A, Lehto SG, Klussmann S, Quirion R, Michaelis M, Devor M, Reeh PW. Variable sensitivity to noxious heat is mediated by differential expression of the CGRP gene. Proc Natl Acad Sci U S A. 2005;102(36):12938–12943. doi: 10.1073/pnas.0503264102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Olesen J, Diener HC, Husstedt IW, Goadsby PJ, Hall D, Meier U, Pollentier S, Lesko LM Group BBCPoCS. Calcitonin gene-related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N Engl J Med. 2004;350(11):1104–1110. doi: 10.1056/NEJMoa030505. [DOI] [PubMed] [Google Scholar]
- 50.Ong JJY, Wei DY, Goadsby PJ. Recent Advances in Pharmacotherapy for Migraine Prevention: From Pathophysiology to New Drugs. Drugs. 2018 doi: 10.1007/s40265-018-0865-y. [DOI] [PubMed] [Google Scholar]
- 51.Pietrobon D, Moskowitz MA. Pathophysiology of migraine. Annu Rev Physiol. 2013;75:365–391. doi: 10.1146/annurev-physiol-030212-183717. [DOI] [PubMed] [Google Scholar]
- 52.Pradhan AA, Smith ML, McGuire B, Tarash I, Evans CJ, Charles A. Characterization of a novel model of chronic migraine. Pain. 2014;155(2):269–274. doi: 10.1016/j.pain.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Recober A, Kaiser EA, Kuburas A, Russo AF. Induction of multiple photophobic behaviors in a transgenic mouse sensitized to CGRP. Neuropharmacology. 2010;58(1):156–165. doi: 10.1016/j.neuropharm.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Recober A, Kuburas A, Zhang Z, Wemmie JA, Anderson MG, Russo AF. Role of calcitonin gene-related peptide in light-aversive behavior: implications for migraine. J Neurosci. 2009;29(27):8798–8804. doi: 10.1523/JNEUROSCI.1727-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roughan JV, Bertrand HG, Isles HM. Meloxicam prevents COX-2-mediated post-surgical inflammation but not pain following laparotomy in mice. Eur J Pain. 2016;20(2):231–240. doi: 10.1002/ejp.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Russo AF. Calcitonin gene-related peptide (CGRP): a new target for migraine. Annu Rev Pharmacol Toxicol. 2015;55:533–552. doi: 10.1146/annurev-pharmtox-010814-124701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Russo AF. CGRP as a neuropeptide in migraine: lessons from mice. Br J Clin Pharmacol. 2015;80(3):403–414. doi: 10.1111/bcp.12686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Salmon AM, Damaj MI, Marubio LM, Epping-Jordan MP, Merlo-Pich E, Changeux JP. Altered neuroadaptation in opiate dependence and neurogenic inflammatory nociception in alpha CGRP-deficient mice. Nat Neurosci. 2001;4(4):357–358. doi: 10.1038/86001. [DOI] [PubMed] [Google Scholar]
- 59.Saper J, Lipton RB, Kudrow D, Hirman J, Dodick D, Silberstein S, Chakhava G, Smith J, Biondi D. A phase 3, randomized, doubleblind, placebo-controlled study to evaluate the efficacy and safety of eptinezumab in frequent episodic migraine prevention: primary results of the PROMISE 1 (prevention of migraine via intravenous eptinezumab safety and efficacy 1) trial. Cephalalgia. 2017;37(Suppl. 1):337. (abstr) [Google Scholar]
- 60.Silberstein SD, Dodick DW, Bigal ME, Yeung PP, Goadsby PJ, Blankenbiller T, Grozinski-Wolff M, Yang R, Ma Y, Aycardi E. Fremanezumab for the Preventive Treatment of Chronic Migraine. N Engl J Med. 2017;377(22):2113–2122. doi: 10.1056/NEJMoa1709038. [DOI] [PubMed] [Google Scholar]
- 61.Skljarevski V, Oakes TM, Zhang Q, Ferguson MB, Martinez J, Camporeale A, Johnson KW, Shan Q, Carter J, Schacht A, Goadsby PJ, Dodick DW. Effect of Different Doses of Galcanezumab vs Placebo for Episodic Migraine Prevention: A Randomized Clinical Trial. JAMA Neurol. 2018;75(2):187–193. doi: 10.1001/jamaneurol.2017.3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sotocinal SG, Sorge RE, Zaloum A, Tuttle AH, Martin LJ, Wieskopf JS, Mapplebeck JC, Wei P, Zhan S, Zhang S, McDougall JJ, King OD, Mogil JS. The Rat Grimace Scale: a partially automated method for quantifying pain in the laboratory rat via facial expressions. Mol Pain. 2011;7:55. doi: 10.1186/1744-8069-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stauffer VL, Sides R, Camporeale A, Skljarevski V, Ahl J, Aurora SK. A Phase 3, Long-Term, Open-Label Safety Study of Self-Administered Galcanezumab Injections in Patients with Migraine. Cephalalgia. 2017;37(Suppl. 1):330. (abstr) [Google Scholar]
- 64.Stucky NL, Gregory E, Winter MK, He YY, Hamilton ES, McCarson KE, Berman NE. Sex differences in behavior and expression of CGRP-related genes in a rodent model of chronic migraine. Headache. 2011;51(5):674–692. doi: 10.1111/j.1526-4610.2011.01882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tubbs JT, Kissling GE, Travlos GS, Goulding DR, Clark JA, King-Herbert AP, Blankenship-Paris TL. Effects of buprenorphine, meloxicam, and flunixin meglumine as postoperative analgesia in mice. J Am Assoc Lab Anim Sci. 2011;50(2):185–191. [PMC free article] [PubMed] [Google Scholar]
- 66.Tuttle AH, Molinaro MJ, Jethwa JF, Sotocinal SG, Prieto JC, Styner MA, Mogil JS, Zylka MJ. A deep neural network to assess spontaneous pain from mouse facial expressions. Mol Pain. 2018;14 doi: 10.1177/1744806918763658. 1744806918763658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van Dongen RM, Zielman R, Noga M, Dekkers OM, Hankemeier T, van den Maagdenberg AM, Terwindt GM, Ferrari MD. Migraine biomarkers in cerebrospinal fluid: A systematic review and meta-analysis. Cephalalgia. 2017;37(1):49–63. doi: 10.1177/0333102415625614. [DOI] [PubMed] [Google Scholar]
- 68.Yin K, Deuis JR, Lewis RJ, Vetter I. Transcriptomic and behavioural characterisation of a mouse model of burn pain identify the cholecystokinin 2 receptor as an analgesic target. Mol Pain. 2016:12. doi: 10.1177/1744806916665366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yu LC, Hansson P, Lundeberg T. The calcitonin gene-related peptide antagonist CGRP8-37 increases the latency to withdrawal responses in rats. Brain Res. 1994;653(1–2):223–230. doi: 10.1016/0006-8993(94)90393-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.