Abstract
Painful temporomandibular disorders (TMD) are both consequence and cause of change in multiple clinical, psychosocial, and biological factors. While longitudinal studies have identified antecedent biopsychosocial factors that increase risk for TMD onset and persistence, little is known about long-term change in those factors after TMD develops or remits. During a 7.6-year median follow-up period, we measured change in psychosocial characteristics, pain sensitivity, cardiovascular indicators of autonomic function, and clinical jaw function among 189 participants whose baseline chronic TMD status either persisted or remitted and 505 initially TMD-free participants, 83 of whom developed TMD. Among initially TMD-free participants who developed TMD, symptoms and pain sensitivity increased while psychological function worsened. In contrast, participants with chronic TMD at baseline tended to show improved TMD symptoms, improved jaw function, reduced somatic symptoms, and increased positive affect. In general, clinical and psychosocial variables more frequently changed in parallel with TMD status compared to pain sensitivity and autonomic measures. These findings demonstrate a complex pattern of considerable changes in biopsychosocial function associated with changes in TMD status. In particular, several biopsychosocial parameters improved among participants with chronic TMD despite pain persisting for years, suggesting considerable potential for ongoing coping and adaptation in response to persistent pain.
Introduction
Orofacial pain and altered masticatory function are the hallmarks of painful temporomandibular disorders (TMD), although patients often experience changes in multiple aspects of biological and psychosocial functioning [3;28;33]. Indeed, abundant evidence has identified numerous biopsychosocial factors that increase risk for TMD onset and persistence [1;12;18;19;22]. Previous findings from the OPPERA (Orofacial Pain: Prospective Evaluation and Risk Assessment) Study [47] demonstrate that clinical variables, such as comorbid conditions, non-painful orofacial symptoms and self-reported jaw function independently contribute to risk of developing first-onset TMD [26;41]. Likewise, psychological variables, including somatic symptoms, perceived stress, and negative mood increased risk for incident TMD [9;39]. Genetic analyses identified markers associated with increased TMD risk [40;50] as well as genes that interact with psychological phenotypes to heighten increased risk of developing TMD [48].
However, TMD status changes substantially over time, with onset of the condition representing only a single point along the highly variable course of TMD [8;12];[37]. For example, acute TMD becomes chronic in only a proportion of cases. Among patients seeking treatment for acute TMD, between 57 and 71% continued to report significant pain 6 months later [8;12]. Similarly, nearly half of people with TMD histories of varying duration at baseline reported no pain when re-assessed five years later, and another 14% of patients reported substantially improved pain [27]. Notably, in addition to improved TMD pain these groups showed reductions in sensitivity to palpation and in psychological distress. These findings suggest that the biopsychosocial characteristics that confer risk for development of TMD may also change over time as TMD status evolves.
To date, however, changes in clinical, psychological and biological functioning occurring after onset of TMD have been monitored for relatively short periods of time or in response to a particular treatment [8;12;22], with some exceptions [27]. Such studies typically monitor changes only in TMD cases, making it difficult to determine if observed changes in clinical, sensory, and psychological functioning exceed changes that would occur spontaneously in a reference population of TMD-free controls or in other groups whose TMD fluctuates across time [8;12;22;27]. Moreover, previous research has not included the broad range of clinical, psychosocial and biological measures that are available in our OPPERA cohort.
Therefore, the purpose of this study was to characterize long-term changes in TMD status in two groups of individuals: 1) those who were TMD-free at enrollment, and 2) those who had chronic TMD at enrollment. In particular, we aimed to investigate long-term trajectories of both TMD status and biopsychosocial functioning in people with and without TMD pain at baseline.
We hypothesized that: 1) biopsychosocial function would worsen in people who transitioned from being TMD-free to experiencing TMD and in those who experienced long-term persistence of TMD;and 2) biopsychosocial function would improve in people who transitioned from TMD to TMD-free status. Based on our previous findings [9;14;26;46], we anticipated that these patterns would be more apparent for clinical and psychosocial variables than for quantitative sensory testing and cardiovascular measures.
Methods
The data presented in this paper derive from two waves of the OPPERA study, a prospective cohort study designed to identify risk factors for TMD. At baseline, two groups of participants between the ages of 18 and 44 were recruited: 1) a group of 3,258 participants with no history of TMD, and 2) a group of 1088 individuals with chronic TMD. Previous publications describe methods and findings from other studies that are part of the OPPERA project [2;11;44;46]. Our description of the methodology and statistical analyses of this prospective cohort study follows STROBE guidelines [55]. All study procedures were approved by Institutional Review Boards at each study site, and all participants provided signed, informed consent to participate. In addition, a Certificate of Confidentiality (NIDCR-06-17) was obtained to further protect the privacy of research participants.
Overall Study design
During the OPPERA-1 Study, between May 2006 and November 2008, we enrolled 3,258 adults who had no significant history of TMD. Participants were recruited using advertisements, emails and flyers distributed in communities surrounding the four U.S. study sites: Baltimore, MD; Buffalo, NY; Chapel Hill, NC; and Gainesville, FL. Details of the study settings and study participants are reported elsewhere [2]. In summary, screening procedures were used to identify potential participants who had all of the following characteristics: aged 18–44 years; English language fluency; intention to live in the area for at least two years; and absence of 13 specific health conditions. TMD-free individuals also reported fewer than five headaches/month in the three months before enrollment; no history of significant TMD symptoms (i.e. no orofacial pain in the month before enrollment and, prior to that period, no more than four days of orofacial pain per month, and examination findings confirming the absence of TMD); and no prior diagnosis or treatment for TMD. A major goal of the OPPERA Project was to identify risk factors for TMD onset [46]; therefore, every three months after enrollment, TMD-free participants completed quarterly health questionnaires to detect emergence of TMD symptoms. This initial observation period continued from first enrollment until May, 2011. Participants reporting symptoms during this period were invited to return for an in-clinic follow-up assessment at which they underwent the same clinical examination protocol to determine presence or absence of TMD. Therefore, for purposes of the present study, we have subdivided the initially TMD-Free participants into two groups: 1) those who remained TMD-free throughout the initial observation period (i.e. Controls), and 2) those who developed first onset TMD during the initial observation period (i.e. Incident TMD). It is important to emphasize that the Incident TMD group was TMD-free at the baseline assessment, but they developed TMD at a later time point. We have separated these groups, because our previous findings show that the Incident TMD group differed importantly from the Control group across a number of baseline variables [46;47].
In addition to enrolling TMD-free individuals, as part of the OPPERA-1 Case-Control Study, between May 2006 and May 2013 we recruited 1088 individuals with Chronic TMD based on modified Research Diagnostic Criteria for Temporomandibular Disorder [29;43]. Specifically, TMD cases met all three of the following criteria: 1) orofacial pain reported at least 15 days in the preceding month and at least five days per month in each of the five months preceding that; 2) during clinical examination, pain reported in the examiner-defined orofacial region for at least 5 days out of the prior 30 days; and 3) pain in response to palpation or jaw maneuver in at least three masticatory muscles or at least one temporomandibular joint [26;29].
As part of the OPPERA-2 Study (Protocol 12-050-E), we conducted a long-term follow-up of participants enrolled in OPPERA-1 described above. Thus, between September, 2014 and November 2016, we invited all active participants from OPPERA-1 to return for a follow-up clinic visit to ascertain their TMD status in OPPERA-2. This long-term follow-up visit also included collection of similar clinical, psychosocial and quantitative sensory measures as were collected during OPPERA-1, which are described in more detail below.
Composition of Study Groups
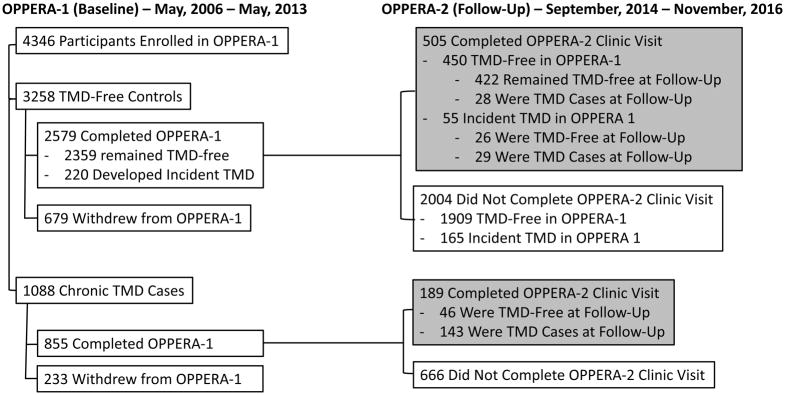
A flowchart of data collection is provided in Figure 1, which illustrates the case classification status for participants at baseline and follow-up. Combining the three possible classifications at baseline (Control, Incident TMD, Chronic TMD) with the two possible classifications at follow-up (Control, TMD) yielded the six study groups that were the focus of our analyses. Through April, 2017, a total of 694 participants completed the OPPERA-2 follow-up study. The OPPERA-1 status of these participants was: Controls (n=450), Incident TMD (n=55), and Chronic TMD (n=189). At Visit 2, 422 (89%) of the OPPERA-1 Controls remained TMD-free, while 28 (11%) Controls met criteria for TMD. Of the Incident TMD participants from OPPERA-1, 29 (54%) met TMD criteria while 26 (47%) were TMD-free at Visit 2. Finally, of the OPPERA-1 Chronic TMD cases, 143 (76%) continued to meet TMD criteria, while 46 (24%) were TMD-free at Visit 2.
Figure 1.
Flow Diagram of Participant Matriculation through the Protocol.
Detailed Study Procedures
The primary purpose of this prospective follow-up study was to characterize long-term changes in biopsychosocial functioning associated with changes in TMD status. Thus, we ascertained TMD status and collected clinical, psychosocial and QST data at Visit 1 (OPPERA-1) and Visit 2 (OPPERA-2). These measures are described briefly below and in greater detail in previous publications from the OPPERA Project [9;14;26;41;45].
Clinical Examination
The clinical examination protocol in OPPERA-2 was adapted from the Diagnostic Criteria for TMD [42]. TMD cases fulfilled all three of the following criteria: 1) history of pain in the orofacial region for ≥5 days in the prior 30 days as assessed at the time of the examination; 2) reproduction of similar pain symptoms during examination, as determined from palpation and/or jaw maneuver that evoked pain cited as “familiar” at one or more of six muscle groups (myalgia) or either temporomandibular joint (arthralgia); and 3) a positive response to questions as to whether evoked pain was modified by one or more of four types of jaw function (chewing, jaw opening, jaw habits, and behaviors such as yawning). The case classification for OPPERA-1 examinations used criteria from the Research Diagnostic Criteria for TMD [7], which, at the time, was the benchmark for classifying TMD. It likewise classified myalgia and arthralgia based on positive responses to pain history and examiner-evoked pain [29]. Summary variables from examinations were calculated to describe characteristics of the study sample. Examiners were trained in the diagnostic criteria and examination procedures prior to study initiation and they had excellent inter-examiner reliability when compared to the study’s reference examiner [44]. In addition, to assess global sensitivity to digital palpation, the examiner assessed pain from palpation at seven body sites outside of the orofacial region (middle part of upper belly of trapezius, supraspinatus, second rib, lateral epicondyle, medial gluteus, greater trochanter, and medial knee).
Clinical Questionnaires
The following questionnaires were administered at both visits to assess health status and clinical information. Participants rated their facial pain and the extent to which pain interfered in activities using the Graded Chronic Pain Scale (GCPS), which yields overall measures of pain (Characteristic Pain Intensity) and pain-related activity interference [56;57]. Because the GPCS was used to characterize the severity of clinical TMD pain in the sample, data are available only for those individuals reporting TMD at both time points. A summary variable reflecting how many of six non-specific orofacial symptoms were endorsed (i.e. stiffness, cramping, fatigue, pressure, soreness, and ache) [26;29] was derived from an OPPERA-specific measure.
The Oral Behaviors Checklist assesses the past month frequency of 21 jaw activities, such as clenching the teeth or bracing the jaw [24;25]. An overall measure was obtained by summing the responses to each of the 21 items. The Jaw Functional Limitation Scale (JFLS) was used to measure limitations in jaw function overall and across three domains: mastication, vertical jaw mobility, and verbal and emotional expression [30;31].
Health Status Questionnaires
The following health-related measures were administered at both visits. The Pittsburgh Sleep Quality Index (PSQI) is the most commonly used instrument to assess sleep quality. Respondents are asked about their previous month’s sleep across multiple domains; a global score reflects overall sleep quality, with higher scores reflecting poorer sleep [4]. The Medical Outcomes Study Short Form 12 Item Version (SF-12) is a brief measure of health-related quality of life, which yields two scale scores, a Mental Health Composite Score (MCS) and a Physical Health Composite Score (PCS) [58].
Measures of Psychological Function
The following measures were used to assess psychological functioning, including somatic symptoms, emotional functioning, pain coping, and perceived stress [9;10]. The Profile of Mood States-Bipolar (POMS-Bi) assesses the overall mood state over the past week and yields global indices of Positive Affect and Negative Affect [21]. The 10-item Perceived Stress Scale (PSS) assesses individual’s perceptions of recent situations as stressful and their perceived ability to cope with these situations [5]. The Symptom Checklist 90-Revised (SCL90R) evaluates a broad range of psychological symptoms, and our analysis included the Somatization and Depression subscales, which assess nonspecific physical symptoms and general depression-related symptoms, respectively [6]. The Trait Anxiety Inventory includes 20 items that assess trait anxiety by asking respondents to respond based on how they “generally feel.” [51]. The Pennebaker Inventory of Limbic Languidness (PILL) assesses the frequency of 54 common physical symptoms and sensations, yielding a total score that reflects somatic awareness or the general tendency to endorse physical symptoms [34]. The Coping Strategies Questionnaire-Revised (CSQ-R) assesses the extent to which individuals employ different cognitive approaches to cope with pain experiences, and it yields several subscales, each reflecting a different coping strategy [36].
Quantitative Sensory Testing (QST)
The following QST measures were obtained at both time points, and more detailed methods have been previously reported [14;15]. Pressure Pain Thresholds (PPTs) were assessed using a pressure algometer (Somedic, Horby, Sweden) bilaterally at five anatomical locations (temporalis muscles, masseter muscles, TMJs, trapezius muscles, and the lateral epicondyles). Pressure using a 1cm2 tip was increased at the rate of 30 kPa/s until either the participant indicated first pain sensation or until 600 kPa was applied. Measurements were repeated at each location either until two values were obtained within 20kPa of one another or until five trials were administered. The two closest values were then averaged to obtain the PPT value for each site. Mechanical Cutaneous Pain was assessed using a set of locally-constructed weighted probes with a flat contact area of 0.2 mm diameter applied to the dorsum of digits 2–4. Measures included mechanical pain threshold, 0–100 ratings of pain intensity in response to a 512 mN stimulus applied first singly and then once per second for 10 trials, and finally a single 0–100 rating of pain after-sensation 15-sec after the 10-trial series. Heat Pain measures were obtained using a commercially available thermal stimulator (Pathway; MEDOC; Ramat Yishai, Israel). Stimuli were applied on the ventral forearm. Heat pain tolerance was determined using a contact thermode (2.56cm2) whose temperature increased from 32°C at a rate of 0.5°C/s until the subject pushed a button indicating s/he could no longer tolerate the pain, with an uninformed ceiling temperature of 51°C. The average of 4 trials was computed. Following tolerance testing, subjects rated the pain intensity (0–100) evoked by ten 48°C thermal stimuli delivered at 2.5 sec inter-stimulus intervals with a CHEPS thermode (5.73 cm2) from a baseline temperature of 38°C. Based on our previous findings, measures used in this analysis include the mean pain rating across the 10 trials, and the pain after-sensation rated 15-sec after the last trial [14].
Cardiovascular Measures
We assessed the following cardiovascular variables at rest, as previously described [14;23]. Systolic blood pressure and heart rate were assessed using an automated blood pressure monitor (Datascope Accutorr). The protocols for collection of these measures differed slightly at baseline and follow-up. At baseline, we collected these measures thrice, once every 2.5 minutes during a 20-minute rest (i.e. at minutes 15, 17.5 and 20). At follow-up, we collected the measures thrice, once every three minutes during a 15-minute rest (i.e. at minutes 9, 12, and 15). The average of the three measures was computed for SBP and HR.
Control Variables
Demographic characteristics of sex, age, race and ethnicity were self-reported in screening interviews at the time of enrollment. Questions about race and ethnicity were combined to form three groups: non-Hispanic White, African-American, and other or unstated racial-ethnic groups. Study site was a categorical variable denoting the study site at which participants were enrolled: FL, MD, NC, or NY. All analyses controlled for age, sex, race/ethnicity, and study site.
Data analysis
Descriptive statistics were computed for each variable at baseline and follow-up time points. These data are included in Appendix Tables 1–3. Next, change scores were computed for each variable by subtracting the baseline value from the value obtained at follow-up. These change scores were used as the dependent measures in separate general linear models in which study group (six categories) was the main explanatory variable and covariates were demographic variables (sex, race/ethnicity, age) and study site. We considered also adjusting for baseline values when analyzing change scores; however, we decided to not take this approach for several reasons. First, there is no consensus in the literature regarding the appropriateness of adjusting for baseline values when examining change scores [13;53]. Second, adjustment for baseline values is most often recommended in the context of intervention studies, in which group assignments are randomly determined and the people from each group are considered to have been drawn from the same population and therefore have equivalent expected values [20;54]; neither of these conditions was present in this observational study. Finally, because this was intended to be primarily a descriptive study, adjustment for baseline may distort the change scores and confuse interpretation of results.
The null hypothesis of no change between study visits was tested using least-squares means estimated for each of the six study groups. We then compared the magnitude of change between the reference group of controls who remained TMD-free at both time-points with each of the remaining five study groups, and we tested for group differences using Wald’s test applied to the least squares means.
Results
The demographic characteristics of the sample at enrollment are presented in Table 1. Chronic TMD cases were more likely to be females and non-Hispanic white compared to Controls, and Chronic TMD cases were slightly younger. Also, due to differences in enrollment rates, the four sites recruited different numbers of both Controls and TMD cases. Thus, all analyses controlled for demographic variables and study site.
Table 1.
Demographic Variables across Groups
| Baseline Case Classification | Non-TMD (n=450) | Incident-TMD (n=55) | Chronic-TMD (n=189) | |||
|---|---|---|---|---|---|---|
| Follow-Up Case Classification | Non-TMD (n=422) | TMD (n=28) | Non-TMD (n=29) | TMD (n=26) | Non-TMD (n=143) | TMD (n=46) |
| Age (years) Baseline | 38 (8.4) | 36.5 (8.8) | 40.4 (9.0) | 36.4 (8.4) | 36.4 (7.8) | 35.5 (8.3) |
| Sex (% F) | 62.1 | 60.7 | 76.9 | 65.5 | 80.4 | 84.6 |
| Race (% NHW) | 59.5 | 42.9 | 42.3 | 62.1 | 87.0 | 76.9 |
| Study Site | ||||||
| North Carolina | 39.6 | 50.0 | 11.5 | 27.6 | 41.3 | 39.9 |
| New York | 32.9 | 14.3 | 61.5 | 31.0 | 32.6 | 37.8 |
| Florida | 14.7 | 17.9 | 15.4 | 31.0 | 26.1 | 21.7 |
| Maryland | 12.8 | 17.9 | 11.5 | 10.3 | 0.0 | 0.7 |
NHW=non-Hispanic white
Comparability of Participants Retained in the Study versus Lost to Follow-Up
The follow-up rate was similar for Controls (19.6%) and TMD Cases (22.1%). Follow-up rates were slightly lower for males (18%) than females (21.4%) and were lower for Asian (8.1%) and Hispanic (11.5%) than non-Hispanic white (21%) and African American (26.6%) subjects. Subjects who returned for a follow-up visit were older (Mean Age = 30.5 years when enrolled in OPPERA-1) than those lost to follow-up (Mean Age = 25.6 when enrolled in OPPERA-1). Fewer participants returned for follow-up at Maryland (6.5%) and Florida (10.8%) compared to North Carolina (23.3%) and New York (22.6%). This low rate of follow-up is likely due to the fact that at the time of enrollment, the follow-up study had not been conceived; therefore, participants were not selected for their availability for long-term follow-up. This, combined with the extended follow-up period contributed to high attrition. The median time between Visits 1 and 2 was 7.6 years overall and was significantly shorter, by protocol, for participants whose OPPERA-1 status was Chronic TMD (5.0 years) compared to Control (8.0 years) or Incident TMD (7.9 Years). Individuals returning for follow-up differed significantly from those lost to follow-up on many study-relevant variables (see Appendix Table 1). Specifically, those retained in the study were older and more likely to be female and non-Hispanic white compared to those lost to follow-up. In addition, among Controls, those who were seen at follow-up reported lower perceived stress, higher positive affect, and lower pain catastrophizing. Also, retained Controls had lower pressure pain threshold at the temporalis and reported lower mechanical aftersensation. However, these differences in psychological and QST measures were quite small in magnitudes. a higher number of body sites painful to palpation, had lower pressure pain thresholds at masseter and temporalis muscles, and reported less pain catastrophizing.
Changes from Baseline to Follow-up
Graded Chronic Pain Scale
GCPS data are intrinsically available only for the Chronic TMD-to-TMD group. These data show that Characteristic Pain Intensity decreased significantly from baseline (Mean=55.1, SD=7.6) to follow-up (Mean=45.2, SD=9.3, p < 0.001). Overall Pain Interference declined slightly but not significantly from baseline (Mean=23.5, SD=2.9) to follow-up (Mean=20.7, SD=3.5, p > 0.2).
Clinical-Health Variables
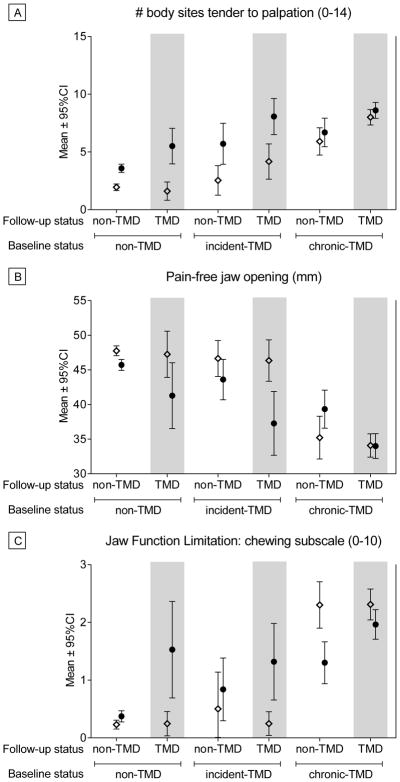
Mean scores at baseline and follow-up for each group for Clinical and Health Status variables are presented in Appendix Table 2; we present the means for selected variables at each time point for illustrative purposes in Figure 2. Adjusted mean change scores for each variable are presented in Table 2, along with p-values reflecting whether the change score differed significantly from zero after adjustment for study site and demographic variables. As can be seen, all groups showed an increase in the number of body sites painful to palpation from baseline to follow-up (Figure 2). All measures of jaw opening decreased over time for the Control group, the Control-to-TMD group, and the Incident TMD-to-TMD group. Pain-free opening increased for the TMD-to-Control group, while no changes in jaw opening emerged for the Chronic TMD-to-TMD group (Figure 2 shows pain-free opening for all groups). Scores on the JFLS indicated decreased jaw function over time for the Control-to-TMD and the Incident TMD-to-TMD groups; whereas JFLS scores improved for the Chronic TMD-to-Control and Chronic TMD-to-TMD groups (Figure 2). The number of non-specific face/jaw symptoms increased for Controls, Control-to-TMD, and Incident TMD-to-TMD groups, while decreasing for Chronic TMD-to-Control and Chronic TMD-to-TMD groups. SF12 Physical Component scores worsened for the Control-to-TMD group, but the scores improved for the Chronic TMD-to-TMD group. Also, the PSQI global sleep quality worsened for the Control-to-TMD group.
Figure 2.
Selected Clinical and Health Measures for Each Group at Baseline and Follow-Up. Three measures are presented for illustrative purposes to show observed patterns of change over time across groups. a) Number of body sites painful to palpation during examination; b) Pain-free jaw opening measured during examination, in which higher scores show greater range of motion; c) Jaw Function Limitation Scale chewing subscale score, when higher scores indicate greater functional limitations. Open diamonds (⋄) reflect Baseline values and closed circles (●) reflect Follow-Up values. No statistical inference testing was performed on these data, as our statistical approach modeled the change scores as presented in Tables 2, 3 and 4.
Table 2.
Clinical and Health Status Variables: Change Scores* (SEM) across Groups from Baseline to Follow-Up
| Baseline Classification | Non-TMD | Incident-TMD | Chronic-TMD | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Follow-Up Classification | Non-TMD | a | TMD | a | b | Non-TMD | a | b | TMD | a | b | Non-TMD | a | b | TMD | a | b |
| # Palpation Sites (0–14) | 2.2 (0.4) | X | 4.1 (0.8) | X | 3.5 (0.9) | X | 4.6 (0.8) | X | X | 1.5 (0.7) | X | 1.1 (0.5) | X | ||||
| Assisted Opening (mm) | −2.1 (0.4) | X | −2.0 (0.9) | X | −1.6 (1.0) | −3.7 (0.9) | X | −0.9 (0.8) | −0.5 (0.5) | X | |||||||
| Unassisted Opening (mm) | −2.1 (0.4) | X | −2.5 (0.9) | X | −1.7 (1.0) | −5.8 (1.0) | X | X | 0.0 (0.8) | X | −0.3 (0.5) | X | |||||
| Pain−Free opening (mm) | −2.5 (0.7) | X | −6.2 (1.6) | X | −2.7 (1.8) | −9.0 (1.6) | X | X | 3.8 (1.4) | X | X | −0.3 (0.9) | X | ||||
| JFLS-Chewing (0–10) | 0.1 (0.1) | 1.3 (0.3) | X | X | 0.1 (0.3) | 1.0 (0.3) | X | X | −1.0 (0.2) | X | −0.4 (0.2) | X | X | ||||
| JFLS-Opening (0–10) | 0.2 (0.1) | 1.2 (0.3) | X | X | 0.3 (0.3) | 0.8 (0.3) | X | −1.0 (0.2) | X | −0.3 (0.2) | X | ||||||
| JFLS-Verbal (0–10) | 0.1 (0.2) | 0.6 (0.2) | X | −0.1 (0.3) | 0.2 (0.2) | −0.8 (0.2) | X | −0.3 (0.1) | X | ||||||||
| JFLS-Total (0–10) | 0.1 (0.1) | 1.0 (0.2) | X | X | 0.1 (0.2) | 0.7 (0.2) | X | −0.9 (0.2) | X | X | −0.3 (0.1) | X | X | ||||
| OBC-Total (0–84) | 0.7 (0.9) | 9.3 (2.1) | X | X | 0.1 (2.2) | 9.8 (2.1) | X | X | −3.4 (1.7) | 0.0 (1.2) | |||||||
| Non-Specific Jaw Symptoms (0–6) | 0.3 (0.1) | X | 1.4 (0.3) | X | −0.03 (0.3) | 2.2 (0.3) | X | −2.2 (0.2) | X | −0.9 (0.2) | X | ||||||
| SF12 Physical Health (0–100) | 0.0 (0.8) | −4.3 (1.8) | X | 1.2 (1.9) | −2.0 (1.9) | 2.2 (1.5) | 2.4 (1.0) | X | |||||||||
| SF12 Mental Health (0–100) | 0.4 (1.0) | −3.2 (2.2) | 2.1 (2.4) | −2.6 (2.4) | 0.5 (1.9) | 0.5 (1.3) | |||||||||||
| PSQI Total (0–21) | 0.5 (0.3) | 3.2 (0.7) | X | 1.2 (0.7) | 1.2 (0.7) | 0.5 (0.6) | 0.6 (0.4) | ||||||||||
Change scores computed by subtracting Baseline value from Follow-Up value, such that positive change scores indicate increased values at follow-up. All means are unadjusted.
JFLS=Jaw Function Limitation Scale; OBC=Oral Behaviors Checklist; SF12=Short Form 12; PSQI=Pittsburgh Sleep Quality Inventory
The “a” column indicates whether or not the change score was statistically different from 0 for that study group at p < 0.05, adjusting for demographics and study site.
The “b” column indicates whether or not the change score for that study group differed significantly from the change score for the reference group (Non-TMD/Non-TMD) at p < 0.05, adjusting for demographics and study site.
Psychosocial Variables
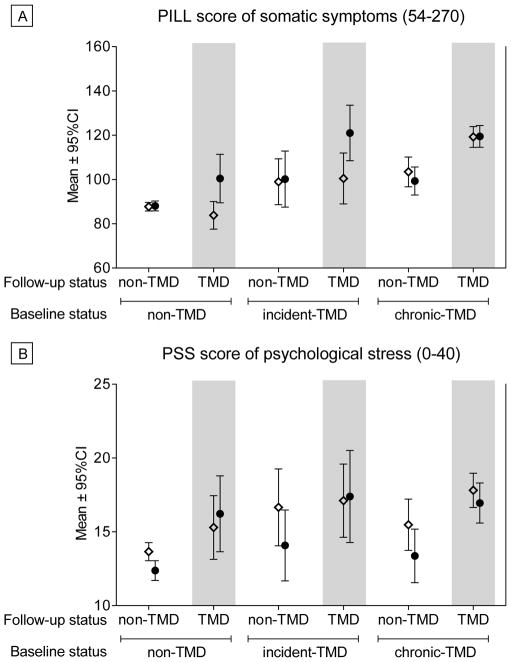
Mean scores at baseline and follow-up for each group for Psychosocial variables are presented in Appendix Table 3, and selected variables are shown for illustrative purposes in Figure 3. Adjusted mean change scores for each variable are presented in Table 3. In general, the Control-to-TMD and Incident TMD-to-TMD groups tended to worsen across several psychosocial measures, including the PILL score (Figure 3), SCL-90R Somatization subscale, and Trait Anxiety Inventory. The Control-to-TMD group also showed an increase in SCL-90R Depression scores, while the Incident TMD-to-TMD group increased in POMS Negative Affect. Controls also showed decreases in POMS Positive Affect and decreases in Negative Affect. Chronic TMD-to-Control individuals decreased in Pain Catastrophizing. Controls and Incident TMD-to-Controls showed reductions in Perceived Stress scores (Figure 3).
Figure 3.
Selected Psychosocial Measures for Each Group at Baseline and Follow-Up. Two measures are presented for illustrative purposes to show observed patterns of change over time across groups. a) Pennebaker Inventory of Limbic Languidness (PILL) scores, in which higher scores indicate greater endorsement of somatic symptoms; b) Perceived Stress Scale (PSS) scores, where higher scores indicate greater perceived stress. Open diamonds (⋄) reflect Baseline values and closed circles (●) reflect Follow-Up values. No statistical inference testing was performed on these data, as our statistical approach modeled the change scores as presented in Tables 2, 3 and 4.
Table 3.
Psychosocial Variables: Change Scores* (SEM) across Groups from Baseline to Follow-Up
| Baseline Classification | Non-TMD | Incident-TMD | Chronic-TMD | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Follow-Up Classification | Non-TMD | a | TMD | a | b | Non-TMD | a | b | TMD | a | b | Non-TMD | a | b | TMD | a | b |
| PILL Total Score (54–270) | 2.0 (1.9) | 17.3 (4.6) | X | X | 2.7 (4.7) | 20.9 (4.6) | X | X | −2.3 (3.9) | 1.7 (2.6) | |||||||
| SCL-90R Depression (0–4) | 0.1 (0.03) | 0.4 (0.1) | X | −0.01 (0.1) | 0.3 (0.1) | X | −0.1 (0.1) | −0.1 (0.04) | |||||||||
| SCL-90R Somatization (0–4) | 0.01 (0.1) | 0.3 (0.1) | X | X | −0.2 (0.1) | 0.1 (0.1) | X | −0.2 (0.1) | X | −0.01 (0.1) | X | ||||||
| Perceived Stress Scale (0–40) | −1.6 (0.6) | X | 0.6 (1.4) | −3.0 (1.5) | 0.02 (1.5) | −2.4 (1.2) | −1.1 (0.8) | ||||||||||
| POMS Negative Affect (30–120) | 3.7 (1.5) | X | 4.4 (3.5) | 2.1 (3.7) | 13.8 (3.6) | X | X | 2.7 (3.0) | 4.1 (2.0) | X | |||||||
| POMS Positive Affect (30–120) | −3.6 (1.3) | X | −4.9 (3.1) | 1.8 (3.3) | −4.7 (3.2) | 0.8 (2.6) | 0.9 (1.8) | X | |||||||||
| Trait Anxiety (20–80) | 0.7 (0.8) | 5.4 (1.9) | X | 0.2 (2.0) | 4.3 (2.0) | X | −2.4 (1.6) | 0.8 (1.1) | |||||||||
| CSQ Catastrophizing (0–6) | −0.1 (0.1) | 0.3 (0.2) | 0.4 (0.2) | 0.4 (0.2) | −0.6 (0.2) | X | X | −0.2 (0.1) | |||||||||
| CSQ Coping Statements (0–6) | −0.3 (0.1) | 0.1 (0.3) | −0.2 (0.3) | −0.3 (0.3) | −0.2 (0.3) | −0.2 (0.2) | |||||||||||
| CSQ Distancing (0–6) | −0.2 (0.1) | 0.2 (0.3) | −0.8 (0.3) | X | 0.5 (0.3) | −0.1 (0.3) | −0.1 (0.2) | ||||||||||
| CSQ Distraction (0–6) | −0.3 (0.1) | 0.2 (0.3) | −0.3 (0.3) | 0.5 (0.3) | 0.1 (0.3) | −0.2 (0.2) | |||||||||||
| CSQ Ignoring Pain (0–6) | −0.1 (0.1) | −0.1 (0.3) | −0.5 (0.3) | 0.4 (0.3) | −0.2 (0.3) | 0.0 (0.2) | |||||||||||
| CSQ Praying (0–6) | −0.3 (0.1) | 1.1 (0.3) | X | X | −0.3 (0.3) | 0.2 (0.3) | −0.3 (0.3) | −0.3 (0.2) | |||||||||
Change scores computed by subtracting Baseline value from Follow-Up value, such that positive change scores indicate increased values at follow-up.
PILL=Pennebaker Inventory of Limbic Languidness; SCL-90R=Symptom Checklist-90 Revised; POMS=Profile of Mood States; CSQ=Coping Strategies Questionnaire
The “a” column indicates whether or not the change score was statistically different from 0 for that study group at p < 0.05, adjusting for demographics and study site.
The “b” column indicates whether or not the change score for that study group differed significantly from the change score for the reference group (Non-TMD/Non-TMD) at p < 0.05, adjusting for demographics and study site.
QST and Cardiovascular Variables
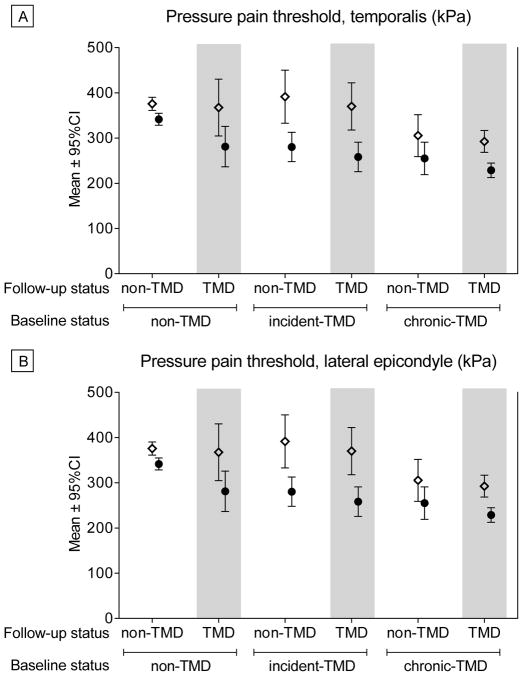
Mean scores at baseline and follow-up for each group for QST and Cardiovascular variables are presented in Appendix Table 4, and selected variables are shown for illustrative purposes in Figure 4. Adjusted mean change scores for each variable are presented in Table 4. Pressure pain thresholds decreased across all body sites for most groups, except for Chronic TMD-to-Control and Chronic TMD-to-TMD, though the latter group showed decreased PPTs for the lateral epicondyle only (Figure 4). Cutaneous mechanical pain threshold decreased for the Incident TMD-to-TMD group, and Mechanical Temporal Summation increased for the Control and Chronic TMD-to-TMD groups. Mechanical Pain Aftersensations decreased for the Incident TMD-to-Control group. Resting Systolic Blood pressure increased significantly over time for all groups, except for the Incident TMD-Control group.
Figure 4.
Selected Quantitative Sensory Testing Measures for Each Group at Baseline and Follow-Up. Two measures are presented for illustrative purposes to show observed patterns of change over time across groups. a) Pressure Pain Threshold at the temporalis muscle, a cranial site; b) Pressure Pain Threshold at the lateral epicondyle, a non-cranial site. For both measures, higher scores reflect greater pain thresholds, or lower sensitivity to pressure pain. Open diamonds (⋄) reflect Baseline values and closed circles (●) reflect Follow-Up values. No statistical inference testing was performed on these data, as our statistical approach modeled the change scores as presented in Tables 2, 3 and 4.
Table 4.
QST and Cardiovascular Variables: Change Scores* (SEM) across Groups from Baseline to Follow-Up
| Baseline Classification | Non-TMD | Incident-TMD | Chronic-TMD | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Follow-Up Classification | Non-TMD | a | TMD | a | b | Non-TMD | a | b | TMD | a | b | Non-TMD | a | b | TMD | a | b |
| Heat Pain Tolerance (°C) | −0.3 (0.2) | 0.3 (0.4) | −1.0 (0.5) | X | −0.3 (0.4) | −0.4 (0.4) | −0.02 (0.3) | ||||||||||
| Heat Temporal Summation (0–100) | −5.2 (3.1) | −6.5 (8.6) | 3.5 (8.4) | −3.4 (7.4) | 5.1 (6.4) | 3.5 (4.6) | |||||||||||
| Heat Pain Aftersensation (0–100) | −0.5 (1.7) | 5.8 (4.3) | −12.4 (4.8) | X | 4.0 (4.1) | −1.3 (3.6) | −2.4 (2.6) | ||||||||||
| Mechanical Pain Threshold (mN) | −23.2 (14.5) | −58.7 (34.6) | −70.3 (37.4) | −141.0 (35.6) | X | X | −29.1 (29.6) | −2.0 (20.0) | |||||||||
| Mechanical Temporal Summation (0–100) | 7.1 (1.8) | X | 7.0 (4.2) | 7.6 (4.6) | 6.8 (4.1) | 7.9 (3.5) | 3.0 (2.4) | ||||||||||
| Mechanical Pain Aftersensation (0–100) | −0.3 (1.3) | 0.6 (3.2) | 1.0 (3.5) | 2.0 (3.1) | 3.9 (2.6) | −2.1 (1.8) | |||||||||||
| PPT Temporalis (kPa) | −17.3 (6.0) | X | −62.5 (14.4) | X | X | −44.1 (15.4) | X | −74.9 (14.3) | X | X | −17.4 (12.5) | −11.7 (8.4) | |||||
| PPT Masseter (kPa) | −19.5 (5.4) | X | −51.0 (13.0) | X | −58.1 (13.8) | X | X | −75.5 (12.9) | X | X | −6.8 (11.3) | −9.9 (7.6) | |||||
| PPT TMJ (kPa) | −14.5 (5.2) | X | −56.8 (12.5) | X | X | −44.7 (13.2) | X | −59.2 (12.4) | X | X | −5.1 (11.0) | −8.0 (7.2) | |||||
| PPT Trapezius (kPa) | −29.6 (12.8) | X | −78.6 (30.3) | X | −64.5 (32.5) | X | −96.2 (31.0) | X | −4.6 (26.6) | −24.9 (18.0) | |||||||
| PPT Lateral Epicondyle (kPa) | −43.1 (11.5) | X | −96.0 (27.2) | X | −104.2 (29.3) | X | −90.2 (27.5) | X | −32.8 (23.9) | −51.7 (16.1) | X | ||||||
| Resting Heart Rate (bpm) | 1.3 (0.8) | −0.5 (2.0) | 1.3 (2.1) | 1.0 (2.0) | −0.7 (1.7) | −0.4 (1.1) | |||||||||||
| Resting Systolic Blood Pressure (mmHg) | 6.9 (1.1) | X | 10.5 (2.5) | X | 3.9 (2.7) | 9.9 (2.5) | X | 5.8 (2.2) | X | 6.8 (1.5) | X | ||||||
Change scores computed by subtracting Baseline value from Follow-Up value, such that positive change scores indicate increased values at follow-up.
PPT=Pressure Pain Threshold; TMJ=Temporomandibular Joint
The “a” column indicates whether or not the change score was statistically different from 0 for that study group at p < 0.05, adjusting for demographics and study site.
The “b” column indicates whether or not the change score for that study group differed significantly from the change score for the reference group (Non-TMD/Non-TMD) at p < 0.05, adjusting for demographics and study site.
Change Scores Compared to the Control Group
Clinical-Health Variables
Compared to Controls, Control-to-TMD and Incident TMD-to-TMD Groups showed greater worsening over time on several measures. Both groups showed greater increases in JFLS-Chewing, and the Control-to-TMD group also showed greater increases in JFLS-Opening and JFLS-Total scores, and greater reductions in sleep quality on the PSQI. Both groups also showed greater increases in OBC Scores, indicating increased oral parafunction compared to Controls. Both groups also showed greater increases in non-specific Face/Jaw Symptoms than Controls. The Incident TMD-to-TMD group also showed greater reductions in jaw opening and greater increases in the number of body sites painful to palpation than did Controls. In contrast, Chronic TMD-to-Control and Chronic TMD-to-TMD groups showed greater improvements than Controls across numerous measures, including JFLS Scores, Jaw Opening, and non-specific Face/Jaw Symptoms. The Chronic TMD-to-Control group also showed greater reductions in OBC scores, and the Chronic TMD-to-TMD group showed a greater improvement in SF-12 Physical Component Scores (see Table 2).
Psychosocial Variables
Compared to Controls, the Control-to-TMD group showed greater increases in somatic symptoms, on both the PILL and SCL-90R Somatization scale, and greater increases in Trait Anxiety and on the CSQ-Praying subscale. The Incident TMD-to-TMD group increased more in PILL Scores and in Negative Affect compared to Controls. Chronic TMD-to-Control participants showed greater reductions in SCL-90R Somatization and in CSQ-Catastrophizing compared to Controls, while the Chronic TMD-to-TMD decreased more in SCL-90R Somatization and showed slightly increased Positive Affect, which differed significantly from the decrease observed for Controls.
QST and Autonomic Variables
The Incident TMD-to-TMD group showed greater decreases in PPTs at all cranial sites and in Cutaneous Mechanical Pain Threshold compared to Controls. The Control-to-TMD group also showed greater decreases in PPTs for two of the cranial sites (temporalis, TMJ). The Incident TMD-to-Control group showed greater decreases in masseter PPT compared to Controls.
Discussion
This study recruited participants both with and without TMD and followed them over a period of several years. At both baseline and follow-up time points, we assessed TMD status as well as multiple biopsychosocial variables that have been associated with TMD in previous research. Several interesting findings emerged from our analysis. First, individuals transitioned from non-TMD to TMD status, as well as vice versa, providing the opportunity to examine changes in biopsychosocial functioning associated with the development and resolution of this painful condition. Second, individuals who transitioned from being TMD-free to a TMD state generally showed concomitant increases in symptoms and declines in function across multiple clinical and psychosocial domains. As one might expect, both self-reported and examiner-assessed jaw function declined among individuals who transitioned from being TMD-free to meeting case classification for TMD. Likewise, these individuals also reported increases in general somatic symptoms and trait anxiety over the observation period. They also tended to show increases in pressure pain sensitivity in the orofacial region, but not at other body sites. Third, a somewhat surprising finding was the degree of improvement in several measures of clinical and psychosocial status among individuals who had chronic TMD at enrollment and at follow-up. This included improved jaw function, reduced somatic symptoms, and slightly increased positive affect. Finally, amongst all subjects when change was observed, it was most apparent for clinical and psychosocial variables compared to QST and autonomic measures.
While this analysis included a long-term prospective component, causal interpretations are not possible because changes in clinical, psychosocial and QST measures were assessed at the same time points at which case status was ascertained. It is plausible that changes in clinical and psychosocial variables contributed to development or resolution of TMD. For example, clinical and health status changes, such as declines in jaw function or reductions in sleep quality could heighten risk for development of TMD. Indeed, many of the clinical and health status variables examined in this study have been found to predict future development of TMD [26;41]. Similarly, the neural mechanisms that contribute to changes in psychosocial variables, including increased somatic symptoms and anxiety, could confer increased TMD risk, especially considering that these psychological factors when assessed premorbidly are associated with increased odds of incident TMD [1;9]. However, it seems at least equally likely that the observed clinical, psychosocial and QST changes may reflect consequences of developing TMD. For instance, reductions in jaw function and increases in orofacial pressure pain sensitivity would be expected after TMD onset. In fact, our previous analysis revealed that pressure pain sensitivity changed as a function of TMD status. Specifically, thresholds declined from their premorbid levels to the time of TMD onset, remaining low in TMD cases whose symptoms persisted, but trending toward normal values among cases whose symptoms remitted [49]. Moreover, development and resolution of TMD would be expected to result in worsening and improvement of psychosocial function, respectively. Regardless of the direction of effects, that biopsychosocial functioning and TMD status change in parallel has important practical implications. That is, the changes in clinical and psychosocial functioning that accompany TMD are likely to promote persistence and potentially exacerbation of symptoms. Hence, treatment is likely to be more successful if it addresses these broader biopsychosocial factors in addition to orofacial symptoms, consistent with other literature [32].
One notable observation is that the group who met criteria for TMD across both time points showed some tendency toward improved functioning in clinical, health status and psychological domains. Specifically, compared to controls, this group evinced improvements in jaw function, health-related quality of life, non-specific face and jaw symptoms, general somatic symptoms, and positive affect. These improvements in clinical and psychosocial profiles may be related to the significant reductions in clinical pain that emerged from baseline to follow-up. When combined with the group that transitioned from chronic TMD at baseline to TMD-free status at follow-up, our findings show that the majority of our chronic TMD cohort exhibited improvement in TMD symptoms as well as other clinical and psychosocial characteristics. These results are consistent with several other longitudinal studies that have followed people with TMD over long observational periods. For example, Ohrbach and Dworkin [27] observed that the majority of people with painful TMD at baseline reported no pain or substantially improved pain five years later, and these groups also showed reduced palpation sensitivity and psychological distress. Similarly, among individuals with TMD pain at age 50, less than half reported TMD pain 10 years later [17], and patients with myofascial TMD pain at baseline showed significant decreases in pain intensity and frequency over a five-year follow-up period [35]. These previously reported results, combined with our findings, suggest that the most common outcome for individuals with chronic TMD is improvement or resolution of symptoms. The phenomenon may result from successful coping or adaptation, perhaps reflecting the influence of resilience mechanisms, despite the persistence of pain. Community dwelling individuals not seeking care may show improved adjustment to pain via resilience processes, including adaptive coping, acceptance, optimism, and benefit finding [16;52]. Unfortunately, measurement of these resilience mechanisms was quite limited in our protocol, therefore, additional research will be needed to address these possibilities.
One unique aspect of our study was the inclusion of a large group of controls who remained TMD-free throughout the observational period, which provided a reference group against which biopsychosocial changes among the TMD-related groups could be compared. This turned out to be important for interpreting results across several variables. For example, systolic blood pressure increased across almost all TMD-related groups, but these changes were mirrored in the control group, suggesting that this may simply be a function of aging in the cohort. Similarly, almost all groups showed decreased pressure pain thresholds at non-cranial sites; however, none of these changes differed for TMD-related versus control groups. This contrasted with thresholds at condition-related cranial sites, where groups that transitioned to TMD at follow-up generally showed greater increases in pressure pain sensitivity than did controls. Thus, the availability of this reference group allows more informed interpretation of changes in the TMD-related groups, preventing misattribution of findings to changes in TMD status.
Our findings should be interpreted in light of several limitations of the study. First, while our long-term follow-up is a strength, the length of the observational period resulted in a low retention rate for our original cohort. This resulted in small sample sizes for several of our groups, and it also creates the possibility of sampling bias, which could impact our findings. Indeed, the sample that returned for follow-up differed in age, sex, and race/ethnicity compared to the initial sample, and retention differed considerably across study sites. Also, the retained sample differed significantly from those lost to follow-up on some study variables. While we controlled for study site and demographic factors in our analyses, other differences between retained participants and those lost to follow-up could bias the results. Second, as alluded to above, we assessed clinical, psychosocial and QST variables at the same time that TMD cases status was ascertained, which precludes causal interpretations. Data with greater temporal resolution would be informative in this regard, similar to that reported in our prior findings [38;48]. Third, our analysis did not include information on treatment seeking among individuals who experienced TMD at some point during the study. This may be particularly important for the participants who had chronic TMD at baseline, because they tended to show improvement in symptoms over time. However, Ohrbach and Dworkin [27] found that treatment seeking did not differ between TMD patients whose pain improved versus those whose pain persisted or worsened over a five-year period. Finally, we conducted numerous statistical tests without controlling for Type 1 error rate. While this may have generated some false positive findings, we prioritized highlighting potentially important results in this unique longitudinal study over the possibility of increased Type 2 error and the concomitant risk of overlooking valuable findings.
In summary, over a long-term observational period, we demonstrated changes across multiple clinical, health status, psychosocial and QST variables that occurred with the development and resolution of painful TMD. Despite the aforementioned limitations, strengths of our study include: multiple subgroups varying in TMD status over time, a long-term follow-up period, and extensive phenotyping across different areas of biopsychosocial function. Our findings showed that transition from being TMD-free to experiencing TMD was associated with increased symptoms and poorer function across multiple clinical and psychosocial domains, as well as increased cranial pressure pain sensitivity. In contrast, and to our surprise, people with chronic TMD at baseline tended to show improvement not only in their TMD symptoms, but also on multiple clinical and psychosocial variables, including better jaw function, reduced somatic symptoms, and increased positive affect. In general, clinical and psychosocial variables more frequently changed in parallel with TMD status compared to QST and autonomic measures. Taken together, these findings demonstrate substantial temporal variability in TMD and its biopsychosocial accompaniments. Future research is needed to elucidate the causal directionality and mechanisms of these results.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grants U01DE017018 (NIDCR) and UL1TR001427 (NCATS). The OPPERA program also acknowledges resources provided for this project by the participating institutions: Battelle Memorial Institute; University at Buffalo; University of Florida; University of Maryland; and University of North Carolina at Chapel Hill.
References
- 1.Aggarwal VR, Macfarlane GJ, Farragher TM, McBeth J. Risk factors for onset of chronic oro-facial pain--results of the North Cheshire oro-facial pain prospective population study. Pain. 2010;149(2):354–359. doi: 10.1016/j.pain.2010.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bair E, Brownstein NC, Ohrbach R, Greenspan JD, Dubner R, Fillingim RB, Maixner W, Smith SB, Diatchenko L, Gonzalez Y, Gordon SM, Lim PF, Ribeiro-Dasilva M, Dampier D, Knott C, Slade GD. Study protocol, sample characteristics, and loss to follow-up: the OPPERA prospective cohort study. The journal of pain : official journal of the American Pain Society. 2013;14(12 Suppl):T2–19. doi: 10.1016/j.jpain.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bair E, Gaynor S, Slade GD, Ohrbach R, Fillingim RB, Greenspan JD, Dubner R, Smith SB, Diatchenko L, Maixner W. Identification of clusters of individuals relevant to temporomandibular disorders and other chronic pain conditions: the OPPERA study. Pain. 2016;157(6):1266–1278. doi: 10.1097/j.pain.0000000000000518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 5.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. JHealth SocBehav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- 6.Derogatis L. The SCL-90-R: administration, scoring and procedures manual. Minneapolis, MN: National Computer Systems, Inc; 1994. [Google Scholar]
- 7.Dworkin SF, LeResche L. Research diagnostic criteria for temporomandibular disorders. JCraniomandibDisord. 1992;6:302–355. [PubMed] [Google Scholar]
- 8.Epker J, Gatchel RJ, Ellis E. A model for predicting chronic TMD: practical application in clinical settings. Journal of the American Dental Association. 1999;130(10):1470–1475. doi: 10.14219/jada.archive.1999.0058. [DOI] [PubMed] [Google Scholar]
- 9.Fillingim RB, Ohrbach R, Greenspan JD, Knott C, Diatchenko L, Dubner R, Bair E, Baraian C, Mack N, Slade GD, Maixner W. Psychological factors associated with development of TMD: the OPPERA prospective cohort study. The journal of pain : official journal of the American Pain Society. 2013;14(12 Suppl):T75–90. doi: 10.1016/j.jpain.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fillingim RB, Ohrbach R, Greenspan JD, Knott C, Dubner R, Bair E, Baraian C, Slade GD, Maixner W. Potential psychosocial risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA case-control study. JPain. 2011;12(11 Suppl):T46–T60. doi: 10.1016/j.jpain.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fillingim RB, Slade GD, Diatchenko L, Dubner R, Greenspan JD, Knott C, Ohrbach R, Maixner W. Summary of findings from the OPPERA baseline case-control study: implications and future directions. JPain. 2011;12(11 Suppl):T102–T107. doi: 10.1016/j.jpain.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garofalo JP, Gatchel RJ, Wesley AL, Ellis E. Predicting chronicity in acute temporomandibular joint disorders using the research diagnostic criteria. Journal of the American Dental Association. 1998;129(4):438–447. doi: 10.14219/jada.archive.1998.0242. [DOI] [PubMed] [Google Scholar]
- 13.Glymour MM, Weuve J, Berkman LF, Kawachi I, Robins JM. When is baseline adjustment useful in analyses of change? An example with education and cognitive change. American journal of epidemiology. 2005;162(3):267–278. doi: 10.1093/aje/kwi187. [DOI] [PubMed] [Google Scholar]
- 14.Greenspan JD, Slade GD, Bair E, Dubner R, Fillingim RB, Ohrbach R, Knott C, Diatchenko L, Liu Q, Maixner W. Pain sensitivity and autonomic factors associated with development of TMD: the OPPERA prospective cohort study. The journal of pain : official journal of the American Pain Society. 2013;14(12 Suppl):T63–74. e61–66. doi: 10.1016/j.jpain.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greenspan JD, Slade GD, Bair E, Dubner R, Fillingim RB, Ohrbach R, Knott C, Mulkey F, Rothwell R, Maixner W. Pain sensitivity risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA case control study. JPain. 2011;12(11 Suppl):T61–T74. doi: 10.1016/j.jpain.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hassett AL, Finan PH. The Role of Resilience in the Clinical Management of Chronic Pain. Current pain and headache reports. 2016;20(6):39. doi: 10.1007/s11916-016-0567-7. [DOI] [PubMed] [Google Scholar]
- 17.Johansson A, Unell L, Carlsson GE, Soderfeldt B, Halling A. Differences in four reported symptoms related to temporomandibular disorders in a cohort of 50-year-old subjects followed up after 10 years. Acta Odontol Scand. 2008;66(1):50–57. doi: 10.1080/00016350801922775. [DOI] [PubMed] [Google Scholar]
- 18.LeResche L, Mancl LA, Drangsholt MT, Huang G, Von KM. Predictors of onset of facial pain and temporomandibular disorders in early adolescence. Pain. 2007;129(3):269–278. doi: 10.1016/j.pain.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LeResche L, Saunders K, Von Korff MR, Barlow W, Dworkin SF. Use of exogenous hormones and risk of temporomandibular disorder pain. Pain. 1997;69(1–2):153–160. doi: 10.1016/s0304-3959(96)03230-7. [DOI] [PubMed] [Google Scholar]
- 20.Liu GF, Lu K, Mogg R, Mallick M, Mehrotra DV. Should baseline be a covariate or dependent variable in analyses of change from baseline in clinical trials? Statistics in medicine. 2009;28(20):2509–2530. doi: 10.1002/sim.3639. [DOI] [PubMed] [Google Scholar]
- 21.Lorr M, McNair DM. Profile of Mood States: Bipolar Form. San Diego: Educational and Industrial Testing Service; 1988. [Google Scholar]
- 22.Macfarlane TV, Blinkhorn AS, Davies RM, Kincey J, Worthington HV. Predictors of outcome for orofacial pain in the general population: a four-year follow-up study. J Dent Res. 2004;83(9):712–717. doi: 10.1177/154405910408300911. [DOI] [PubMed] [Google Scholar]
- 23.Maixner W, Greenspan JD, Dubner R, Bair E, Mulkey F, Miller V, Knott C, Slade GD, Ohrbach R, Diatchenko L, Fillingim RB. Potential autonomic risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA case-control study. JPain. 2011;12(11 Suppl):T75–T91. doi: 10.1016/j.jpain.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Markiewicz MR, Ohrbach R, McCall WD., Jr Oral behaviors checklist: reliability of performance in targeted waking-state behaviors. J Orofac Pain. 2006;20(4):306–316. [PubMed] [Google Scholar]
- 25.Ohrbach R. Assessment and further development of RDC/TMD Axis II biobehavioural instruments: a research programme progress report. J Oral Rehabil. 2010;37(10):784–798. doi: 10.1111/j.1365-2842.2010.02144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohrbach R, Bair E, Fillingim RB, Gonzalez Y, Gordon SM, Lim PF, Ribeiro-Dasilva M, Diatchenko L, Dubner R, Greenspan JD, Knott C, Maixner W, Smith SB, Slade GD. Clinical orofacial characteristics associated with risk of first-onset TMD: the OPPERA prospective cohort study. The journal of pain : official journal of the American Pain Society. 2013;14(12 Suppl):T33–50. doi: 10.1016/j.jpain.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohrbach R, Dworkin SF. Five-year outcomes in TMD: relationship of changes in pain to changes in physical and psychological variables. Pain. 1998;74(2–3):315–326. doi: 10.1016/s0304-3959(97)00194-2. [DOI] [PubMed] [Google Scholar]
- 28.Ohrbach R, Dworkin SF. The Evolution of TMD Diagnosis: Past, Present, Future. J Dent Res. 2016;95(10):1093–1101. doi: 10.1177/0022034516653922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohrbach R, Fillingim RB, Mulkey F, Gonzalez Y, Gordon S, Gremillion H, Lim PF, Ribeiro-Dasilva M, Greenspan JD, Knott C, Maixner W, Slade G. Clinical findings and pain symptoms as potential risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA case-control study. JPain. 2011;12(11 Suppl):T27–T45. doi: 10.1016/j.jpain.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohrbach R, Larsson P, List T. The jaw functional limitation scale: development, reliability, and validity of 8-item and 20-item versions. J Orofac Pain. 2008;22(3):219–230. [PubMed] [Google Scholar]
- 31.Ohrbach R, List T. Psychometric properties of the Jaw Functional Limitation Scale. Journal of Dental Research. 2002;81(A) [Google Scholar]
- 32.Ohrbach R, List T. Predicting treatment responsiveness: somatic and psychologic factors. In: Greene CS, Laskin DM, editors. Treatment of TMDs: Bridging the Gap Between Advances in Research and Clinical Patient Management. Chicago: Quintessence; 2013. pp. 91–98. [Google Scholar]
- 33.Park JW, Clark GT, Kim YK, Chung JW. Analysis of thermal pain sensitivity and psychological profiles in different subgroups of TMD patients. International journal of oral and maxillofacial surgery. 2010;39(10):968–974. doi: 10.1016/j.ijom.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 34.Pennebaker JW. The psychology of physical symptoms. New York: Springer-Verlag; 1982. [Google Scholar]
- 35.Rammelsberg P, LeResche L, Dworkin S, Mancl L. Longitudinal outcome of temporomandibular disorders: a 5-year epidemiologic study of muscle disorders defined by research diagnostic criteria for temporomandibular disorders. J Orofac Pain. 2003;17(1):9–20. [PubMed] [Google Scholar]
- 36.Riley JL, III, Robinson ME. CSQ: five factors or fiction? ClinJPain. 1997;13(2):156–162. doi: 10.1097/00002508-199706000-00010. [DOI] [PubMed] [Google Scholar]
- 37.Rollman A, Visscher CM, Gorter RC, Naeije M. Improvement in patients with a TMD-pain report. A 6-month follow-up study. J Oral Rehabil. 2013;40(1):5–14. doi: 10.1111/joor.12009. [DOI] [PubMed] [Google Scholar]
- 38.Sanders AE, Akinkugbe AA, Bair E, Fillingim RB, Greenspan JD, Ohrbach R, Dubner R, Maixner W, Slade GD. Subjective Sleep Quality Deteriorates Before Development of Painful Temporomandibular Disorder. The journal of pain : official journal of the American Pain Society. 2016;17(6):669–677. doi: 10.1016/j.jpain.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanders AE, Akinkugbe AA, Fillingim RB, Ohrbach R, Greenspan JD, Maixner W, Bair E, Slade GD. Causal Mediation in the Development of Painful Temporomandibular Disorder. The journal of pain : official journal of the American Pain Society. 2017;18(4):428–436. doi: 10.1016/j.jpain.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanders AE, Jain D, Sofer T, Kerr KF, Laurie CC, Shaffer JR, Marazita ML, Kaste LM, Slade GD, Fillingim RB, Ohrbach R, Maixner W, Kocher T, Bernhardt O, Teumer A, Schwahn C, Sipila K, Lahdesmaki R, Mannikko M, Pesonen P, Jarvelin M, Rizzatti-Barbosa CM, Meloto CB, Ribeiro-Dasilva M, Diatchenko L, Serrano P, Smith SB. GWAS Identifies New Loci for Painful Temporomandibular Disorder. J Dent Res. 2017;96(3):277–284. doi: 10.1177/0022034516686562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanders AE, Slade GD, Bair E, Fillingim RB, Knott C, Dubner R, Greenspan JD, Maixner W, Ohrbach R. General health status and incidence of first-onset temporomandibular disorder: the OPPERA prospective cohort study. The journal of pain : official journal of the American Pain Society. 2013;14(12 Suppl):T51–62. doi: 10.1016/j.jpain.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schiffman E, Ohrbach R, Truelove E, Look J, Anderson G, Goulet JP, List T, Svensson P, Gonzalez Y, Lobbezoo F, Michelotti A, Brooks SL, Ceusters W, Drangsholt M, Ettlin D, Gaul C, Goldberg LJ, Haythornthwaite JA, Hollender L, Jensen R, John MT, De Laat A, de Leeuw R, Maixner W, van der Meulen M, Murray GM, Nixdorf DR, Palla S, Petersson A, Pionchon P, Smith B, Visscher CM, Zakrzewska J, Dworkin SF. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: Recommendations of the International RDC/TMD Consortium Network* and Orofacial Pain Special Interest Groupdagger. Journal of oral & facial pain and headache. 2014;28(1):6–27. doi: 10.11607/jop.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schiffman EL, Ohrbach R, Truelove EL, Tai F, Anderson GC, Pan W, Gonzalez YM, John MT, Sommers E, List T, Velly AM, Kang W, Look JO. The Research Diagnostic Criteria for Temporomandibular Disorders. V: methods used to establish and validate revised Axis I diagnostic algorithms. J Orofac Pain. 2010;24(1):63–78. [PMC free article] [PubMed] [Google Scholar]
- 44.Slade GD, Bair E, By K, Mulkey F, Baraian C, Rothwell R, Reynolds M, Miller V, Gonzalez Y, Gordon S, Ribeiro-Dasilva M, Lim PF, Greenspan JD, Dubner R, Fillingim RB, Diatchenko L, Maixner W, Dampier D, Knott C, Ohrbach R. Study methods, recruitment, sociodemographic findings, and demographic representativeness in the OPPERA study. JPain. 2011;12(11 Suppl):T12–T26. doi: 10.1016/j.jpain.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Slade GD, Bair E, Greenspan JD, Dubner R, Fillingim RB, Diatchenko L, Maixner W, Knott C, Ohrbach R. Signs and symptoms of first-onset TMD and sociodemographic predictors of its development: the OPPERA prospective cohort study. The journal of pain : official journal of the American Pain Society. 2013;14(12 Suppl):T20–32. e21–23. doi: 10.1016/j.jpain.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Slade GD, Fillingim RB, Sanders AE, Bair E, Greenspan JD, Ohrbach R, Dubner R, Diatchenko L, Smith SB, Knott C, Maixner W. Summary of findings from the OPPERA prospective cohort study of incidence of first-onset temporomandibular disorder: implications and future directions. The journal of pain : official journal of the American Pain Society. 2013;14(12 Suppl):T116–124. doi: 10.1016/j.jpain.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Slade GD, Ohrbach R, Greenspan JD, Fillingim RB, Bair E, Sanders AE, Dubner R, Diatchenko L, Meloto CB, Smith S, Maixner W. Painful Temporomandibular Disorder: Decade of Discovery from OPPERA Studies. J Dent Res. 2016;95(10):1084–1092. doi: 10.1177/0022034516653743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Slade GD, Sanders AE, Ohrbach R, Bair E, Maixner W, Greenspan JD, Fillingim RB, Smith S, Diatchenko L. COMT Diplotype Amplifies Effect of Stress on Risk of Temporomandibular Pain. J Dent Res. 2015;94(9):1187–1195. doi: 10.1177/0022034515595043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Slade GD, Sanders AE, Ohrbach R, Fillingim RB, Dubner R, Gracely RH, Bair E, Maixner W, Greenspan JD. Pressure pain thresholds fluctuate with, but do not usefully predict, the clinical course of painful temporomandibular disorder. Pain. 2014;155(10):2134–2143. doi: 10.1016/j.pain.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith SB, Mir E, Bair E, Slade GD, Dubner R, Fillingim RB, Greenspan JD, Ohrbach R, Knott C, Weir B, Maixner W, Diatchenko L. Genetic variants associated with development of TMD and its intermediate phenotypes: the genetic architecture of TMD in the OPPERA prospective cohort study. The journal of pain : official journal of the American Pain Society. 2013;14(12 Suppl):T91–101. e101–103. doi: 10.1016/j.jpain.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spielberger CD, Gorusch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory (Form Y1) Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 52.Sturgeon JA, Zautra AJ. Resilience: a new paradigm for adaptation to chronic pain. Current pain and headache reports. 2010;14(2):105–112. doi: 10.1007/s11916-010-0095-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tu YK, Gilthorpe MS. Revisiting the relation between change and initial value: a review and evaluation. Statistics in medicine. 2007;26(2):443–457. doi: 10.1002/sim.2538. [DOI] [PubMed] [Google Scholar]
- 54.Van Breukelen GJ. ANCOVA versus change from baseline: more power in randomized studies, more bias in nonrandomized studies [corrected] J Clin Epidemiol. 2006;59(9):920–925. doi: 10.1016/j.jclinepi.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 55.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Initiative S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 56.Von Korff M, Dworkin SF, Le Resche L. Graded chronic pain status: an epidemiologic evaluation. Pain. 1990;40:279–291. doi: 10.1016/0304-3959(90)91125-3. [DOI] [PubMed] [Google Scholar]
- 57.Von Korff M, Ormel J, Keefe FJ, Dworkin SF. Grading the severity of chronic pain. Pain. 1992;50:133–149. doi: 10.1016/0304-3959(92)90154-4. [DOI] [PubMed] [Google Scholar]
- 58.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. MedCare. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.