Abstract
Pancreatic cancer is the third leading cause of death in the US with a poor 5-year survival rate of 8.5%. A novel anti-cancer drug, dimethylamino-parthenolide (DMAPT), is the water-soluble analog of the natural sesquiterpene lactone, parthenolide. The putative modes of action of DMAPT are inhibition of the NFκB pathway and depletion of glutathione levels; the latter causing cancer cells to be more susceptible to oxidative stress-induced cell death. Actinomycin-D is a polypeptide antibiotic that binds to DNA and inhibits RNA and protein synthesis by inhibiting RNA polymerase II. A phase 2 clinical trial indicated that Actinomycin-D could be a potent drug against pancreatic cancer; however, it was not a favored drug due to toxicity issues. New drug entities and methods of drug delivery, used alone or in combination, are needed to treat pancreatic cancer more effectively. Thus, it was postulated that combining DMAPT and Actinomycin-D would result in synergistic inhibition of Panc-1 pancreatic cancer cell growth because DMAPT's inhibition of NFκB would enhance induction of apoptosis by Actinomycin-D, via phosphorylation of c-Jun, by minimizing NFκB inhibition of c-Jun phosphorylation. Combining these two drugs induced a higher level of cell death than each drug alone. A fixed drug ratio of DMAPT: Actinomycin-D (1200:1) was used. Data from metabolic (MTT) and colony formation assays were analyzed for synergism with CompuSyn software, which utilizes the Chou-Talalay equation. The analyses indicated synergism and moderate synergism at combination concentrations of DMAPT/Actinomycin-D of 12/0.01 and 18/0.015 µM, respectively.
Keywords: Pancreatic cancer, Panc-1 cells, DMAPT, Actinomycin-D, synergism, NFκB
Introduction
Pancreatic cancer is the third leading cause of cancer-related deaths in the United States (“SEER, Cancer Stat Facts: Pancreatic Cancer,” 2018). Of the 1,735,350 new cancer cases and 609,640 cancer deaths projected to occur in 2018 (Siegel, Miller, & Jemal, 2018), 55,440 of these new cancers and the 44,330 deaths will be attributable to pancreatic cancer (Siegel et al., 2018). Approximately 94% of new pancreatic cancers are exocrine (pancreatic ductal adenocarcinoma, PDAC) and 6% are endocrine carcinomas (Siegel et al., 2018). Due to poor diagnosis and prognosis, the 5 year survival rate for pancreatic cancer for all stages combined, is only 8.5% (“SEER, Cancer Stat Facts: Pancreatic Cancer,” 2018). Locally diagnosed cancers, which are relatively better controlled, have a 5 year survival rate of 32% and that for distantly diagnosed cancers is only 3% (American Cancer Society, 2018).
Symptoms of pancreatic cancer usually appear in the later stages of the disease, when it is too late for surgical resection; remaining treatment options at this stage of the disease are chemotherapy and radiation. Despite advances in both of these fields, treating pancreatic cancer remains difficult and ineffective because tumor cells develop treatment-resistance, and there is a limited understanding of the molecular biology associated with this issue (Thota, Pauff, & Berlin, 2014).
Currently, chemotherapeutic agents for pancreatic cancer used as single treatment agents have shown limited efficacy (Kroep, Pinedo, Van Groeningen, & Peters, 1999). Thus, treatment strategies combining two or more drugs are being explored (Gatzemeier et al., 2007; Giovannetti, Mey, Danesi, Mosca, & Tacca, 2004; Louvet et al., 2005; Meng et al., 2015; Rausch et al., 2010). Combining two anti-neoplastic drugs, e.g. gemcitabine and paclitaxel (Meng et al., 2015), has the potential to be effective enough to manage this aggressive cancer. Some drugs that enter clinical studies display great potential as effective anti-cancer agents; however, they are often not approved by the Food and Drug Administration (FDA) due to their poor therapeutic index and toxic side effects at the clinically utilized dose. Combining two or more anticancer drugs that act synergistically may provide effective disease treatment, with manageable side effects, at doses lower than those that produce toxicity with either drug alone.
The Actinomycins are antibiotic metabolites obtained from the bacterial family, Streptomyces (Hollstein, 1974), with Actinomycin-D (ActD) being especially effective against cancers. ActD is a cyclic polypeptide that acts by binding to DNA and inhibiting RNA synthesis (Hennings, Smith, Colburn, & Boutwell, 1968; Hollstein, 1974; Sobell, 1985), putatively by inhibiting RNA polymerase II (Sobell, 1985; Wadkins & Jovin, 1991). The c-Jun N-terminal kinase (JNK) pathway, which is involved in phosphorylation of c-Jun, is also activated by ActD, which leads to apoptosis (Kleeff, Kornmann, Sawhney, & Korc, 2000). ActD has been used as an experimental drug to treat Wilm’s tumor, soft tissue sarcomas, testicular cancer, and lymphomas (Grimm et al., 1980).
Parthenolide (PTL), a sesquiterpene lactone, is obtained from the Feverfew plant (Tanacetum parthenium). It has anti-inflammatory properties and has been used to treat migraines (Yip-Schneider et al., 2005) and rheumatoid arthritis (Guzman et al., 2007), but also has anti-cancer activity (Liu et al., 2009). Its exact mechanism(s) of action is not clearly understood, but evidence suggests that it kills tumor cells preferentially by inhibiting the NFκB pathway and by perturbing glutathione homeostasis (Sohma et al., 2011; W. Wang et al., 2006; Pei et al., 2013). PTL has poor oral bioavailability; hence, an equally effective, more water-soluble variant, dimethylaminoparthenolide (DMAPT), has been developed (Neelakantan, Nasim, Guzman, Jordan, & Crooks, 2009).
Rarely has a single anti-cancer agent been effective in PC therapy because of the many genetic and molecular alterations of this cancer and the unpredictable progression of the disease (D.Y. Lu, 2015). A drug ‘cocktail’, or mixture of multiple anti-cancer drugs, targeting more than one pathway or complementing the standard of care drug, may improve outcomes in current cancer therapies (D.Y. Lu, 2015; D.Y. Lu, Lu, & Cao, 2013). Studies show that drug combinations such as Sulindac and PTL (Yip-Schneider et al., 2005), or gemcitabine and DMAPT (Holcomb et al., 2012) enhanced the inhibition of PC cell growth, providing support for investigating other cancer drug combinations with sesquiterpene lactones such as DMAPT.
DMAPT has been studied as a potential therapeutic in many cancers, including non-small cell lung cancer, leukemia, prostate, bladder, pancreas and gastric, both in vitro (Guzman et al., 2007; Estabrook et al., 2011; Holcomb et al., 2012; Shanmugam et al., 2010, 2011) and in vivo (Guzman et al., 2007; Estabrook et al., 2011; Jenkins et al., 2008; Shanmugam et al., 2010). Experimental data indicate that DMAPT induces cancer cell death via apoptosis (Guzman et al., 2007; Holcomb et al., 2012; Shanmugam et al., 2011) and necrosis in a cell line-dependent manner (Shanmugam et al., 2010). More importantly, DMAPT has been shown to be non-toxic to normal hematopoietic cells and has no severe, systemic side effects (Guzman et al., 2007).
ActD is a potent anti-neoplastic agent. However, it has severe, dose-limiting toxic side effects that include hepatic toxicity (Hill et al., 2014), depression of bone marrow (Farber, Angio, Evans, & Mitus, 1954), low platelet and white blood cell count, and gastrointestinal issues (Philips, Schwartz, Sternberg, & Tan, 2006; Schein et al., 1978). In a phase 2 clinical trial for pancreatic carcinoma, ActD showed promise. However, there was lowered survival in ActD-treated patients when compared to other drugs studied in that trial, and it was postulated to be due to the toxicity of ActD (Schein et al., 1978). ActD has been evaluated in a number of drug combination studies to determine if it synergizes with other anticancer agents (Anderson et al., 2003; Guo et al., 2012; Olberding et al., 2010) in an effort to minimize its toxicity through dose reduction.
DMAPT is known to exhibit anti-cancer activity via the NFκB pathway (Holcomb et al., 2012) and ActD induces apoptosis by phosphorylating c-Jun via the JNK pathway (Kleeff et al., 2000). It has been reported that the pro-apoptotic JNK pathway is down-regulated by excessive NFκB activity (Nakano, 2017). Hence, by utilizing a combination DMAPT-ActD drug therapy, DMAPT inhibition of NFκB may enhance ActD-induced c-Jun JNK-mediated apoptosis, resulting in synergistic anti-pancreatic cancer activity. Consequently, we hypothesize that the combination of DMAPT and ActD will act synergistically to inhibit pancreatic cancer cell growth and cause cell death via apoptosis in Panc-1 cells. DMAPT is a relatively safe drug, and studies indicate that it can selectively target cancer cells (Guzman et al., 2007). Conversely, ActD can have severe, toxic sequela in normal tissues (Philips et al., 2006). We postulate that combining clinically acceptable levels of the relatively toxic ActD with a higher concentration of the better-tolerated DMAPT will yield a synergistic combination that will prove effective against pancreatic cancer cells without producing intolerable normal tissue toxicities. This in vitro study investigated the synergistic potential of combining ActD with DMAPT to treat Panc-1 human pancreatic carcinoma cells.
Materials and Methods
Cells and Treatments
Panc-1 cells were obtained from the American Type Culture Collection (ATCC) and cultured in Dulbecco's Modified Eagle Medium/F-12 (DMEM/F-12) media (GIBCO, Life Technologies, Carlsbad, CA) containing 10% iron-supplemented Bovine calf serum (BCS) (GE Hyclone, Logan, UT), 0.1 g/L of streptomycin sulfate and 0.07 g/L of penicillin G potassium (Pen/Strep), at 37°C in a 5% CO2 humidified incubator.
ActD (Sigma-Aldrich, St Louis, MO) was dissolved in dimethylsulfoxide (DMSO) as a 1 mg/mL stock solution and stored at −20°C. DMAPT was synthesized by reaction of PTL (Sigma-Aldrich, St. Louis, MO) with dimethylamine (Sigma-Aldrich, St. Louis, MO) and isolated as the fumarate salt (Neelakantan et al., 2009). A 200 µM stock solution was prepared by dissolving DMAPT in sterile water using sonication to facilitate dissolution. Stock solutions of the drugs were diluted serially to produce working solutions.
3.5×104 cells/cm2 were allowed to attach to the culture substrates for 24 hours, after which time drug solutions were added. Drug remained in contact with the cells for specified periods of time. Detached cells were collected with the media; attached cells were removed with trypsin containing 1.1mM EDTA, and the detached and the trypsinized cells combined for all further procedures. A drug solution volume to cell number ratio of 0.33mL of drug/3.5×104 cells/cm2 was maintained at the start of all drug treatments and for all drug concentrations tested. This ratio is based on the National Cancer Institute's (NCI) 60 human cancer cell line screening methodology (www.dtp.cancer.gov/discvery_development/nci-60).
3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium (MTT) assay
Cellular metabolism was measured utilizing the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) assay according to the instructions provided with a commercial kit (Cayman Chemical, Ann Arbor, MI). Panc-1 cells were seeded in a 96-well plate at a density of 3.5×104 cells/cm2 and drugs were added 24 hours later. The cells were exposed to ActD and/or DMAPT for 48 hours. The diagonally constant drug ratio combination table proposed by Chou and Talalay (T. Chou et al., 2010) was used to select concentrations. Combination drug concentrations were kept at a fixed ratio of DMAPT-ActD = 1200:1. Serial dilutions were made at concentrations 0.25, 0.5, 1.5 and 2 times that of the GI50 values of both drugs. Plates were then incubated with MTT for 3 hours to allow mitochondrial dehydrogenase activity to reduce the yellow MTT dye to a purple formazan product. The concentration of the formazan product is indicative of the amount of mitochondrial metabolism and is measured at 570 nm using a spectrophotometric plate reader.
Caspase assay
Panc-1 cells seeded in a 35mm dish were treated with DMAPT and/or ActD for 48 hours and then assayed for caspase activity using the CaspACE™ FITC-VAD-FMK In Situ Marker (Promega Corporation, Madison, WI). The media was removed and centrifuged (2.5g, 30–45 seconds) to collect detached cells. The remaining cells in the dish were removed using trypsin (containing 1.1mM EDTA) and added to the detached cells. The combined cell suspension was then diluted in cell culture media such that there were 1×106 cells per sample. Cells from the previous step were washed twice in CO2-independent media (Gibco, Life Technologies, Carlsbad, CA), resuspended in 1mL of CO2-independent media and analyzed by flow cytometry (488 nm excitation, 520 nm emission).
Live-Dead assay
Panc-1 cells were treated with a combination of fluorescein diacetate (FDA) and propidium iodide (PI) to identify live, dead and dying cells by flow cytometry (Jones & Senft, 1985; “Live/dead staining with FDA and PI,” 2015). Esterases in live cells cleave FDA to fluorescein acetate, which fluoresces green when excited by 488 nm light. PI enters dead cells with permeable membranes and then intercalates into the DNA of the cell and fluoresces red. One million cells from each sample were mixed with 2µL of the FDA solution (2µg/mL stock) and incubated at 37°C for 5 minutes on a tube rotator in CO2-independent media (Gibco, Life Technologies, Carlsbad, CA). Afterwards, 1µL of PI (12.5 mg/mL stock) was added and the cells were incubated at room temperature for 3 minutes prior to analyses. CO2-independent media was used to keep the pH of the media constant at 7.4 during incubation with FDA and PI.
Colony formation assay (CFA)
Media was removed from the drug-treated cells. Detached cells were pelleted from the media by centrifugation and cells still attached were detached from the substrate with trypsin. The pelleted, detached cells and trypsinized cells were combined and counted on a Z1 Coulter particle counter (Beckman Coulter Inc, Indianapolis, IN) and then diluted appropriately in serum-free media. CFAs were carried out in triplicate with 300 cells seeded into 25 cm2 flasks containing 5mL of DMEM/F-12 media supplemented with BCS and Pen/Strep. The flasks were plated with lethally irradiated A549 (a human lung carcinoma cell line) feeder cells 24 hours prior to adding the treated Panc-1 cells (Borrelli, Thompson, & Dewey, 1989).
CompuSyn Analysis
The median-effect equation was employed to determine synergism, additivity or antagonism of the DMAPT plus ActD treatments (fixed drug combination ratio. The Chou-Talalay method for drug combination effects was used, which is derived from the mass-action law principle. Combination index (CI) is calculated from the Chou-Talalay equation, which provides a quantitative method of measuring drug interaction. CI= [(D1)/(Dx)1] + [(D2)/(Dx)2]; (Dx) is for D1“alone” that inhibits a system x%; and (Dx)2 is for D2“alone” that inhibits a system x%. D is the dose (or concentration) of a drug (T.-C. Chou, 2006). The equation provides the following criteria: CI >1, CI=1 and CI<1, which indicates antagonism, additive effect, and synergism, respectively. CompuSyn is a third generation software employing the Chou-Talalay equation and is used specifically in drug combination studies (T. C. Chou, 2010). Data from the MTT and CFA were utilized in the CompuSyn program analysis.
Western blot analysis
Whole cell lysates were prepared from untreated (control) cells and cells treated with DMAPT and/or ActD for 24 hours. PhosSTOP phosphatase inhibitor and cOmplete protease inhibitor (Roche, Basel, Switzerland) were added to all samples, and the protein content of each sample was measured using the bicinchoninic acid (BCA) assay (Pierce, Indianapolis, IN). Western blot studies were done at 24 hours and not 48 hours because we wanted to observe cellular changes earlier in the drug-cell reaction process and capture events that could be leading to cell death. At 48 hours there are many dead cells and very sick/dying cells. The protein from these cells would be indicative of cell death processes that result from the drug-induced changes in the cell and not indicative of the drug-induced changes that lead to induction of cell death. 20µg protein samples were subjected to electrophoresis on 12% SDS-PAGE gels and electro-blotted onto PVDF membrane. Primary mouse monoclonal antibodies for c-Jun (G-4) (sc-74543) and p-c-Jun (KM-1) (sc-822) were obtained from Santa Cruz Biotechnology, Inc., Dallas, TX; monoclonal mouse anti-actin clone C4 was purchased from MP Biomedicals, LLC, Santa Ana, CA; and monoclonal rabbit NFκB p65 (D14E12) and phospho-NFκB p65 (Ser 536) (93H1) were obtained from Cell Signaling Technology (Danvers, MA). The secondary antibodies were goat anti-mouse IgG, (H+L), peroxidase conjugated (Pierce Biotechnology, Rockford, IL) and anti-rabbit IgG, horseradish peroxidase (HRP)-linked antibody (Cell Signaling Technology, Danvers, MA). Membranes were probed with primary antibodies overnight in Tris-Tween buffered saline (TTBS) at a dilution of 1:850, with 0.3% BSA, at 4°C. β-Actin was used as a housekeeping protein and blots were probed with the β-Actin primary antibody diluted to 1:15,000. The blots were washed and exposed to secondary antibody with horse radish peroxidase at a 1:3,000 dilution for 1 hour at room temperature and labeled protein bands were detected by bioluminescence using an ECL kit (ChemiGlow West, Proteinsimple, San Jose, CA).
Results
DMAPT/ActD combinations enhance cell death in Panc-1 cells
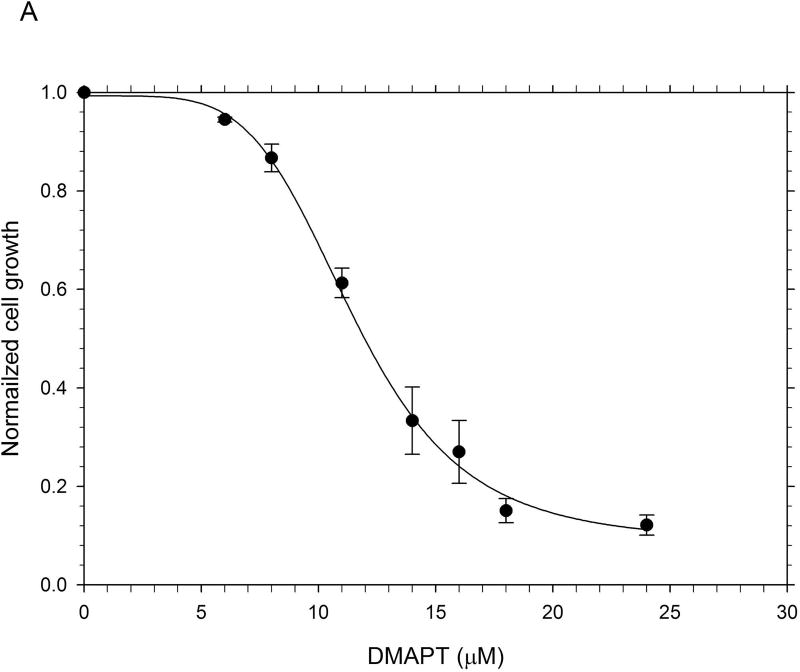
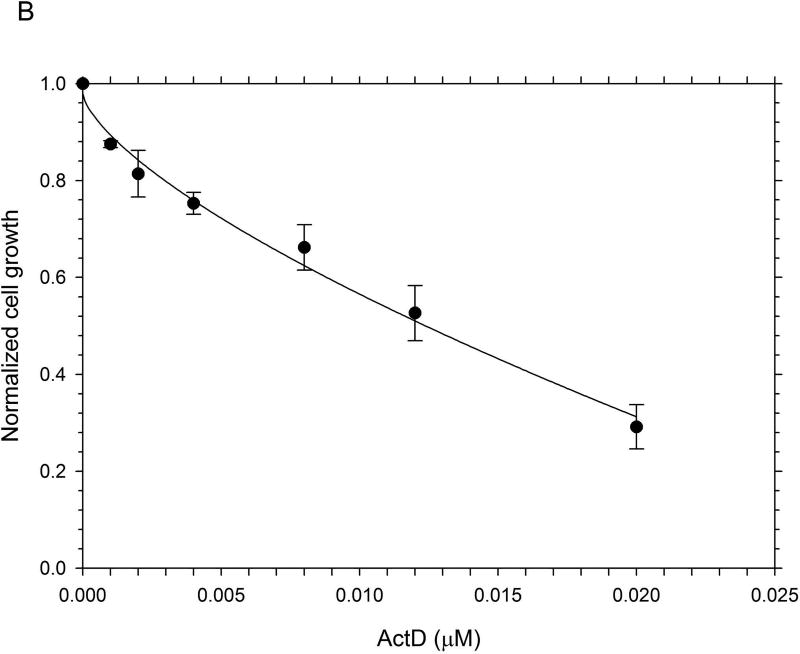
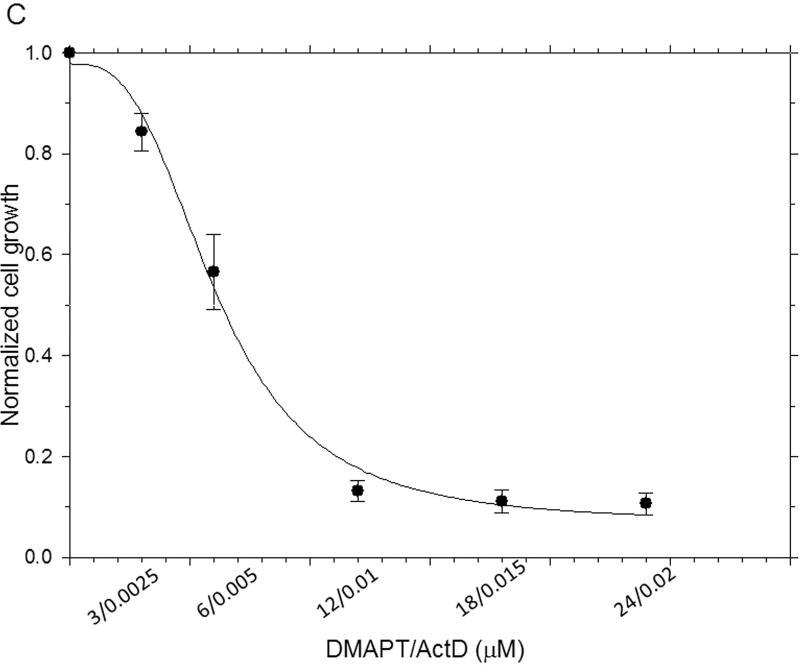
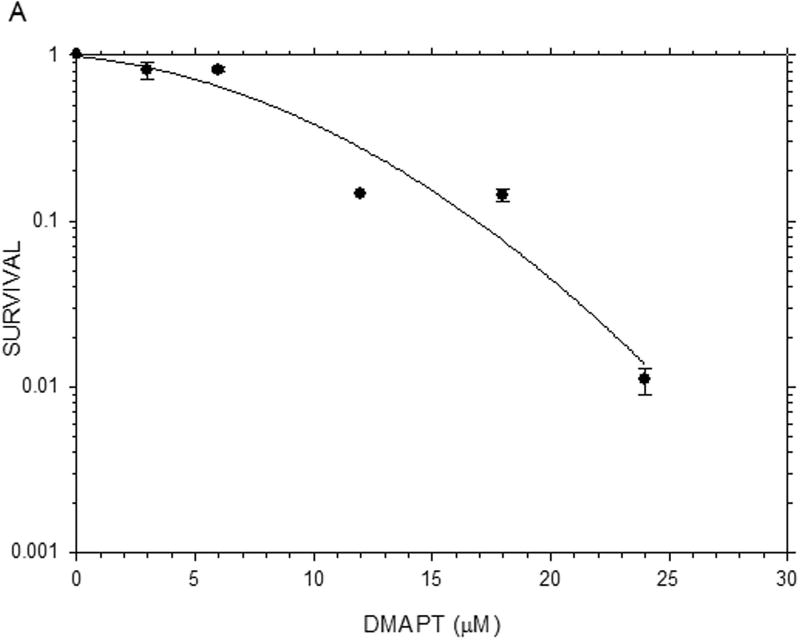
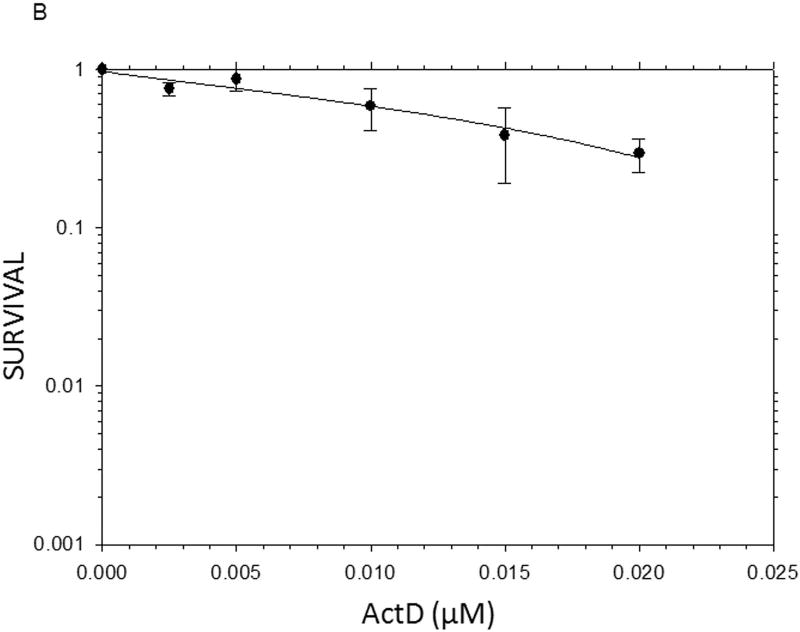
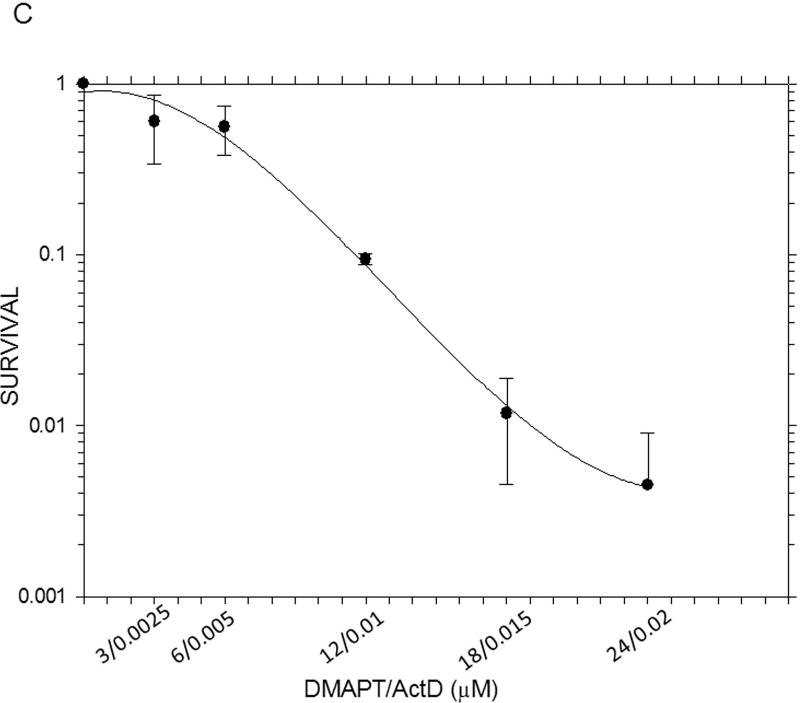
The results from the MTT assays demonstrated clearly that, on a molar basis, ActD was more toxic to Panc-1 cells than DMAPT (Fig. 1). The MTT assays yielded GI50 values (drug concentration causing 50% of maximal cell growth inhibition) of 12µM and 0.01 µM for DMAPT and ActD, respectively. However, when added in combination, at a ratio of 1200:1 (DMAPT: ActD), a more potent inhibition of Panc-1 cell metabolism was obtained, which lowered the GI50 value by approximately half of that for cells treated with DMAPT or ActD alone (Fig. 1).
Figure 1.
The inhibition of metabolic activity in Panc-1 cells measured by the MTT assay The metabolic activity of Panc-1 cells after treatment with different concentrations of (A) DMAPT (3–12µM) (B) ActD (0.0025–0.02 µM) (C) DMAPT/ActD (3/0.0025 – 24/0.02 µM). The MTT assay demonstrated that DMAPT, ActD and DMAPT/ActD combinations can inhibit in vitro cell metabolism in Panc-1 cells in a dose-dependent manner.
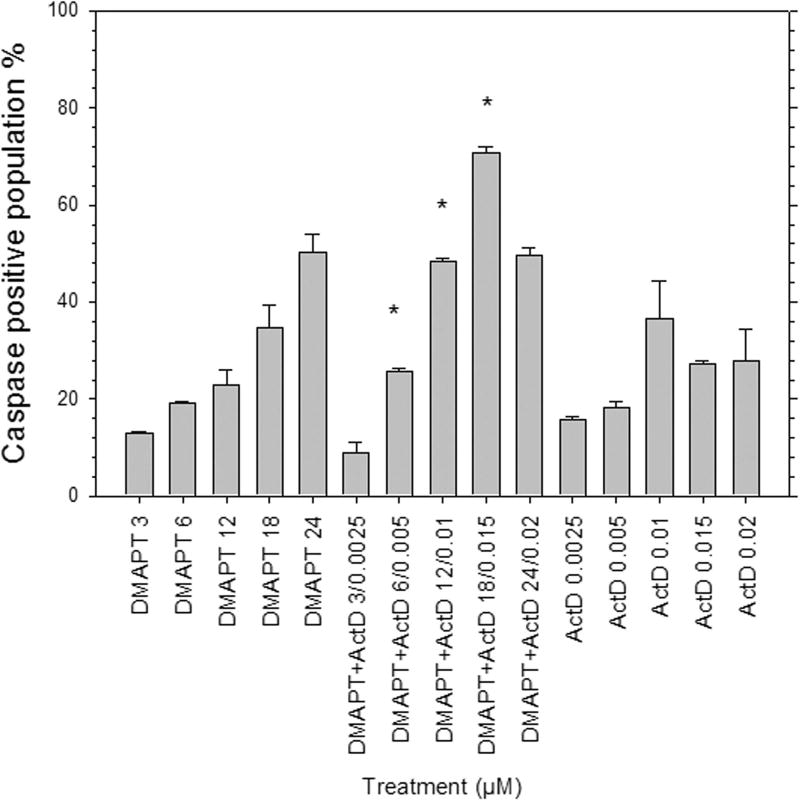
Caspase positive Panc-1 cell populations increased when treated with DMAPT/ActD combinations of 6 µM/0.005µM, 12 µM/0.01 µM and 18 µM/0.015 µM when compared with Panc-1 cell populations of individual drug treatments (Fig. 2). Although the caspase levels increased, this result appears to be an additive rather than a synergistic effect. After treatment with 3µM/0.0025µM DMAPT/ActD, caspase positive populations were lower compared to those resulting from treatment with 3µM DMAPT or 0.0025µM ActD alone, in this one case. Conversely, for all other drug combinations (6/0.005 µM to 24/0.02 µM), the caspase positive population is higher than for cells treated with each drug individually, using the concentration of each individual drug that was used in the drug pairings. Consequently, the caspase activation assay data suggest strongly that apoptosis induction is influenced by a threshold concentration of DMAPT and/or ActD to attain an additive effect with the drug combination.
Figure 2.
Caspase activation in Panc-1 cells 48h post drug treatment was determined by flow cytometry. Treatment concentrations were chosen based on the respective GI50 values of DMAPT and ActD, and maintaining a constant ratio for the drug combination concentrations. Activated caspases were detected by CaspACE™ FITC-VAD-FMK In Situ Marker.
(*p<0.05 vs each individual drug)
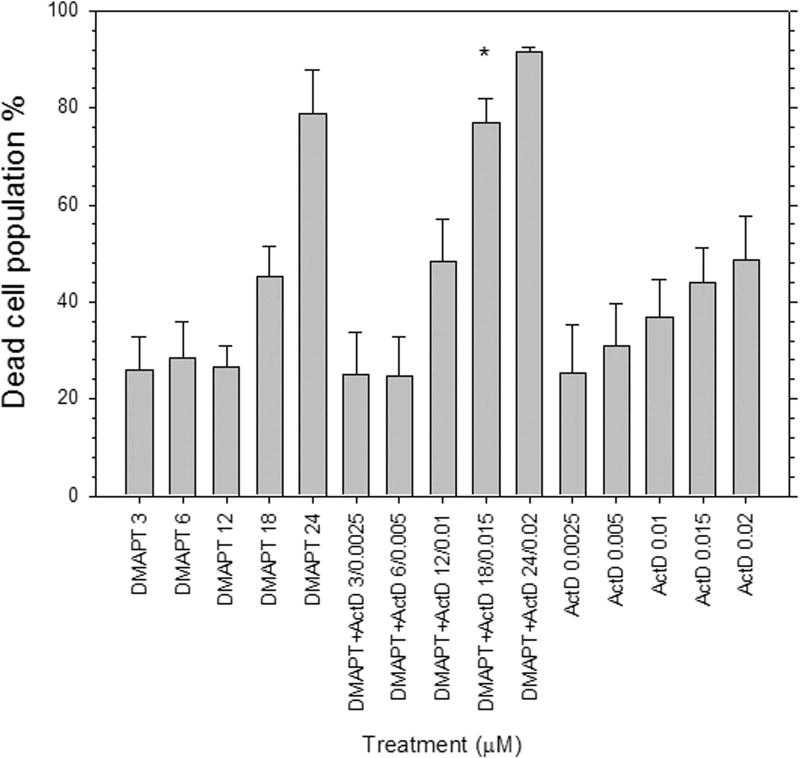
The fraction of PI positive Panc-1 cells increased in a dose-dependent manner and was greater for cells treated with DMAPT/ActD combinations than treatment with either drug alone (Fig. 3), except for the lower two DMAPT/ActD drug concentration combinations (i.e. 3µM/0.0025µM and 6µM/0.005µM). These results also suggest that a threshold concentration for DMAPT and/or ActD is required for these two drugs to work additively or synergistically to cause Panc-1 cell death.
Figure 3.
Live-Dead FDA-PI assay in Panc-1 cells 48h post drug treatment was determined by flow cytometry. Treatment concentrations were chosen based on the respective GI50 values of DMAPT and ActD, and maintaining a constant ratio for the drug combination concentrations. Live and dead cell populations were detected by incubating Panc-1 cells with FDA at 37°C and PI at room temperature, respectively, in CO2-independent media.
(*p<0.05 vs each individual drug)
The survival levels for each drug concentration combination were lower than the respective individual drug concentrations (Table 1), as determined by CFA (Fig 4). For 3/0.0025, 12/0.01, 18/0.015 and 24/0.02 µM drug combinations the CFA data indicates synergism.
Table 1.
Survival fraction of DMAPT, ActD and DMAPT/ActD combination treatment groups (48 hour drug exposure) assessed in Panc-1 cells by colony formation assay
| DMAPT (µM) |
Survival Fraction |
ActD (µM) |
Survival Fraction |
DMAPT/ActD (µM) |
Survival Fraction |
|---|---|---|---|---|---|
| 0 | 1 | 0 | 1 | 0 | 1 |
| 3 | 0.808 | 2.50E-03 | 0.757 | 3/0.0025 | 0.600 |
| 6 | 0.817 | 5.00E-03 | 0.866 | 6/0.005 | 0.558 |
| 12 | 0.146 | 0.01 | 0.586 | 12/0.01 | 0.093 |
| 18 | 0.142 | 0.015 | 0.383 | 18/0.015 | 0.0118 |
| 24 | 0.011 | 0.02 | 0.295 | 24/0.02 | 4.50E-03 |
Figure 4.
Colony formation assay with Panc-1 cells: Cell survival as a function of concentration of drug, alone or in combination, for Panc-1 cells treated for 48 hours with (A) DMAPT (B) ActD (C) DMAPT/ActD. Each point represents the mean ± standard error of the mean for at least three experiments. The DMAPT/ActD combination was more toxic to Panc-1 cells than individual drug treatments. Survival fraction at 12/0.01µM was 0.6 and 0.16 times lower than 12µM DMAPT and 0.01µM ActD, respectively.
CompuSyn analysis provides synergistic to moderate additivity CI values for DMAPT/ActD combinations
CompuSyn provides a CI that assigns numerical values to the levels of synergism/additivity/antagonism. For drug combinations of 12µM/0.01µM and 18µM/0.01µM DMAPT/ActD the CI values indicate synergism, and the CI for 24µM/0.02µM DMAPT/ActD indicates slight synergism to near additivity (Table 2) from the MTT assay data. For the lower drug combination concentrations (i.e. 3µM/0.0025µM and 6µM/0.005µM) the CI values were 1.64 and 1.26, indicating slight to moderate antagonism. However, for the colony formation assay data, CI values were in the range of synergism to moderate additivity, except for the 6µM/0.005µM drug combination concentration (Table 2).
Table 2.
Combination index (CI) values for DMAPT + ActD fixed ratio treatments in Panc-1 cells
| Drug combination (DMAPT/ActD) (µM) |
Combination Index (CI) with MTT data |
Combination Index (CI) with CFA data |
|---|---|---|
| 3/0.0025 | 1.64 | 0.81 |
| 6/0.005 | 1.26 | 1.48 |
| 12/0.01 | 0.62 | 0.89 |
| 18/0.015 | 0.73 | 0.55 |
| 24/0.02 | 1.03 | 0.51 |
DMAPT/ActD combination inhibits NFκB p65 phosphorylation and increases c-Jun activation
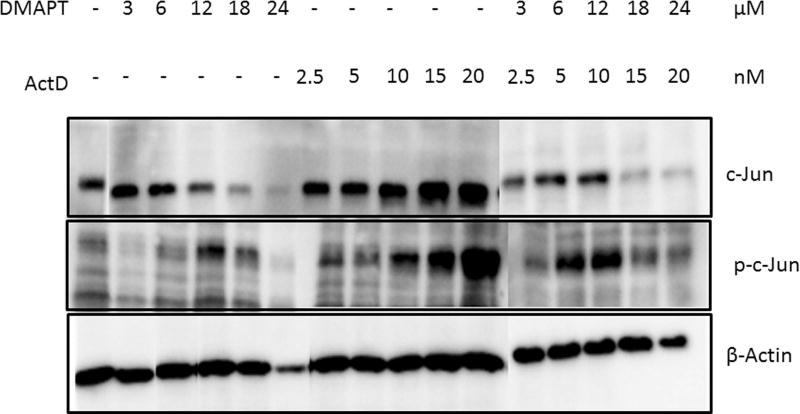
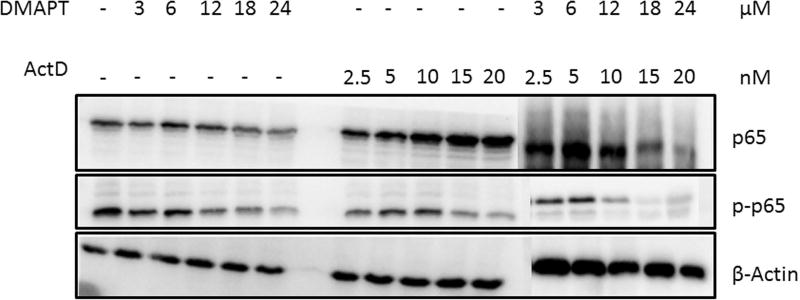
We hypothesized that the mechanistic basis of synergism with DMAPT/ActD drug combinations is that inhibition of the NFκB pathway would enhance the induction of c-Jun mediated apoptosis. Panc-1 cells were exposed to the two drugs, alone and in combination, for 24 hours and expression of c-Jun, p-c-Jun, p65 and p-p65 were detected by Western blotting. With increasing concentrations of each drug alone, and in combination, phosphorylation of c-Jun increased in a dose-dependent manner. There was also a gradual decrease in the levels of phosphorylated p65 in the DMAPT/ActD group when compared to control and individual drug treatment groups (Fig. 5). These observations are consistent with the above hypothesis.
Figure 5.
Apoptosis induced by (A) induction of c-Jun and (B) inhibition of p65 by DMAPT and ActD treatment, alone and in combination, in Panc-1 cells monitored by Western blot. Panc-1 cells were exposed to DMAPT (3–12µM), ActD (0.0025–0.02 µM) and DMAPT/ActD (3/0.0025 – 24/0.02 µM) for 24 hours. β-Actin is the housekeeping gene.
Discussion
The combination of DMAPT and ActD treatment resulted in additive to slightly synergistic cell killing in Panc-1 cells, depending on the drug concentration combinations used. The Western blot data confirmed these interactive effects of DMPAT and ActD. However, the increase in efficacy of the combined DMAPT/ActD treatments was much less than anticipated, which casts doubt on this drug combination being one that might have a positive impact in the clinic for improving chemotherapeutic treatment of pancreatic cancers. The most encouraging support for this drug combination was the CFA data, indicating additive to mildly synergistic cell killing. Conversely, the Live-Dead assay and caspase activity data indicated additive cell killing. The caspase and live-dead assays measure acute death; whereas the CFA detects clonogenic death from both acute and more subtle or latent effects of the drugs. The CFA is the more relevant assay with regard to chemotherapeutic treatments because if a cancer cell cannot proliferate it is no longer a threat to the host. Our data can be summed up by stating that: with regard to the acute killing of the cancer cells the effects of ActD and DMAPT are additive. However, the drugs act synergistically to induce clonogenic cell death, which, as argued above, is more relevant from a clinical perspective. The nature of the drugs and the patterns of the drug interactions suggest that it is a threshold concentration of ActD that is required for synergism with DMAPT to occur. Thus, both the caspase and the Live-Dead assay can underestimate total drug-induced cytotoxicity because they cannot account for later developing, drug-induced damage that can lead to cell death.
In determining CI values involved using data from the two assays, i.e. the MTT assay and the CFA, the colony formation data provided CI values in the synergistic range, except for the 6/0.005µM drug combination concentration. The MTT data provided synergistic to nearly additive CI values (0.62 to 1.03), and even values reflecting slight antagonism (CI=1.64). It is therefore important to note that assay selection and end-point determination are consequential, as results may vary based on different assays.
Based upon the results from this study, potential new combinations of ActD with the abundantly available sesquiterpene lactones and their analogs are worthy of investigation to determine whether synergistic drug combinations can be achieved. A number of newer sesquiterpene lactone-based drugs have been developed which are more cytotoxic to cancer cells than either DMAPT or PTL (Bommagani et al., 2017; Janganati, Ponder, Thakkar, Jordan, & Crooks, 2017). This might lead to numerous opportunities for exploring combination drug treatment regimens of ActD with these newer SLs. However, caution should be exercised when combining ActD with a more toxic sesquiterpene lactone, because this could increase the risk of normal tissue cytotoxicity.
This study evaluated the combined effect of DMAPT and ActD on Panc-1 cell cytotoxicity and determined that ActD alone is more toxic to Panc-1 cells than DMAPT. However, the cells proved to be significantly more sensitive to DMAPT-ActD combinations than to treatment with each of the individual drugs. The best combinations of DMAPT and ActD were in the range of synergism to moderate additivity at combined concentrations of 12/0.01µM and 18/0.015µM, suggesting that combinations of DMAPT and ActD can improve the killing of Panc-1 cells in culture, relative to treatment with either drug alone. Although the cooperative killing of pancreatic cancer cells exhibited by the combined DMAPT/ActD treatments is significant and synergistic, it is likely that this increased cytotoxicity is not great enough to warrant further pre-clinical testing in an in vivo pancreatic cancer tumor model.
Acknowledgments
We are grateful to the NIH (grants CA158275, AG012411 and TR000039), the UAMS Department of Radiology, the UAMS CTSA Grant and the Arkansas Research Alliance for financial support.
Peter A. Crooks is a founding member of Leuchemix, Inc., and is an inventor on U.S. patent no. 8716329; he also has research funding through NIH grant no. CA 158275.
Footnotes
Conflict of Interest:
All other authors have no competing interests.
References
- American Cancer Society. Cancer Facts & Figures 2018. American Cancer Society. 2018 doi: 10.3322/caac.21442. [DOI]
- Anderson KM, Alrefai W, Bonomi P, Seed TM, Dudeja P, Hu Y, Harris JE. Cell Death Due to Actinomycin D and MK 886. 2003 doi: 10.1177/153537020322800807. [DOI] [PubMed] [Google Scholar]
- Bommagani S, Ponder J, Penthala NR, Janganati V, Jordan CT, Borrelli MJ, Crooks PA. Indole carboxylic acid esters of melampomagnolide B are potent anticancer agents against both hematological and solid tumor cells. European Journal of Medicinal Chemistry. 2017;136:393–405. doi: 10.1016/j.ejmech.2017.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrelli MJ, Thompson LL, Dewey WC. Evidence that the feeder effect in mammalian cells is mediated by a diffusible substance. International Journal of Hyperthermia: The Official Journal of European Society for Hyperthermic Oncology, North American Hyperthermia Group. 1989;5(1):99–103. doi: 10.3109/02656738909140436. [DOI] [PubMed] [Google Scholar]
- Chou T-C. Theoretical Basis, Experimental Design, and Computerized Simulation of Synergism and Antagonism in Drug Combination Studies. Pharmacological Reviews. 2006;58(3):621 LP–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- Chou TC. Drug combination studies and their synergy quantification using the chou-talalay method. Cancer Research. 2010;70(2):440–446. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- Chou T, Kurin E, Mučaji P, Nagy M, Hidalgo M, Sánchez-Moreno C, Liu Z. Flavonoid–flavonoid interaction and its effect on their antioxidant activity. Journal of Food Composition and Analysis. 2010;67(3):621–681. doi: 10.1124/pr.58.3.10. [DOI] [Google Scholar]
- Estabrook NC, Chin-Sinex H, Borgmann AJ, Dhaemers RM, Shapiro RH, Gilley D, Mendonca MS. Inhibition of NF-κB and DNA double-strand break repair by DMAPT sensitizes non-small-cell lung cancers to X-rays. Free Radical Biology and Medicine. 2011;51(12):2249–2258. doi: 10.1016/j.freeradbiomed.2011.09.029. [DOI] [PubMed] [Google Scholar]
- Farber S, Angio GD, Evans A, Mitus A. Clinical Studies of Actinomycin D With Special Reference to Wilm’s Tumor in Children. Journal of Urology. 1954;168(6):2560–2561. doi: 10.1016/S0022-5347(05)64213-9. [DOI] [PubMed] [Google Scholar]
- Gatzemeier U, Pluzanska A, Szczesna A, Kaukel E, Roubec J, De Rosa F, Von Pawel J. Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: The Tarceva lung cancer investigation trial. Journal of Clinical Oncology. 2007;25(12):1545–1552. doi: 10.1200/JCO.2005.05.1474. [DOI] [PubMed] [Google Scholar]
- Giovannetti E, Mey V, Danesi R, Mosca I, Tacca M Del. Synergistic Cytotoxicity and Pharmacogenetics of Gemcitabine and Pemetrexed Combination in Pancreatic Cancer Cell Lines Synergistic Cytotoxicity and Pharmacogenetics of Gemcitabine and Pemetrexed Combination in Pancreatic Cancer Cell Lines. 2004;10:2936–2943. doi: 10.1200/JCO.2003.11.136. [DOI] [PubMed] [Google Scholar]
- Grimm RA, Muss HB, White DR, Richards F, Cooper MR, Stuart JJ, Spurr CL. Actinomycin D in the treatment of advanced breast cancer. Cancer Chemotherapy and Pharmacology. 1980;4(3):195–197. doi: 10.1007/BF00254018. [DOI] [PubMed] [Google Scholar]
- Guo L, Fan L, Ren J, Pang Z, Ren Y, Li J, Jiang X. Combination of TRAIL and actinomycin D liposomes enhances antitumor effect in non-small cell lung cancer. Int J Nanomedicine. 2012;7:1449–1460. doi: 10.2147/IJN.S24711\rijn-7-1449. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman ML, Rossi RM, Neelakantan S, Li X, Corbett CA, Hassane DC, Jordan CT. An orally bioavailable parthenolide analog selectively eradicates acute myelogenous leukemia stem and progenitor cells. Blood. 2007;110(13):4427–4435. doi: 10.1182/blood-2007-05-090621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennings H, Smith HC, Colburn NH, Boutwell RK. Inhibition by actinomycin D of DNA and RNA synthesis and of skin carcinogenesis initiated by 7,12-dimethylbenz[a]anthracene or beta-propiolactone. Cancer Research. 1968;28(3):543–552. [PubMed] [Google Scholar]
- Hill CR, Cole M, Errington J, Malik G, Boddy AV, Veal GJ. Characterisation of the clinical pharmacokinetics of actinomycin D and the influence of ABCB1 pharmacogenetic variation on actinomycin D disposition in children with cancer. Clinical Pharmacokinetics. 2014;53(8):741–751. doi: 10.1007/s40262-014-0153-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holcomb BK, Yip-Schneider MT, Waters JA, Beane JD, Crooks PA, Schmidt CM. Dimethylamino Parthenolide Enhances the Inhibitory Effects of Gemcitabine in Human Pancreatic Cancer Cells. Journal of Gastrointestinal Surgery. 2012;16(7):1333–1340. doi: 10.1007/s11605-012-1913-7. [DOI] [PubMed] [Google Scholar]
- Hollstein U. Actinomycin. Chemistry and mechanism of action. Chem. Rev. 1974;74(6):625–652. doi: 10.1021/cr60292a002. [DOI] [Google Scholar]
- Janganati V, Ponder J, Thakkar S, Jordan CT, Crooks PA. Succinamide derivatives of melampomagnolide B and their anti-cancer activities. Bioorganic & Medicinal Chemistry. 2017;25(14):3694–3705. doi: 10.1016/j.bmc.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins C, Hewamana S, Gilkes A, Neelakantan S, Crooks P, Mills K, Burnett A. Nuclear factor-kappaB as a potential therapeutic target for the novel cytotoxic agent LC-1 in acute myeloid leukaemia. British Journal of Haematology. 2008 Oct;143:661–671. doi: 10.1111/j.1365-2141.2008.07392.x. [DOI] [PubMed] [Google Scholar]
- Jones KH, Senft JA. An improved method to determine cell viability by simultaneous staining with fluorescein diacetate-propidium iodide. Journal of Histochemistry & Cytochemistry. 1985;33(1):77–79. doi: 10.1177/33.1.2578146. [DOI] [PubMed] [Google Scholar]
- Kleeff J, Kornmann M, Sawhney H, Korc M. Actinomycin D induces apoptosis and inhibits growth of pancreatic cancer cells. International Journal of Cancer. Journal International Du Cancer. 2000;86(3):399–407. doi: 10.1002/(SICI)1097-0215(20000501)86:3<399::AID-IJC15>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Kroep JR, Pinedo HM, Van Groeningen CJ, Peters GJ. Experimental drugs and drug combinations in pancreatic cancer. Annals of Oncology. 1999;10(SUPPL. 4):234–238. [PubMed] [Google Scholar]
- Liu Z, Liu S, Xie Z, Pavlovicz RE, Wu J, Chen P, Chan KK. Modulation of DNA methylation by a sesquiterpene lactone parthenolide. The Journal of Pharmacology and Experimental Therapeutics. 2009;329(2):505–14. doi: 10.1124/jpet.108.147934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Live/dead staining with FDA and PI. 2015 Nov;:2–5. 2015. [Google Scholar]
- Louvet C, Labianca R, Hammel P, Lledo G, Zampino MG, André T, De Gramont A. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: Results of a GERCOR and GISCAD phase III trial. Journal of Clinical Oncology. 2005;23(15):3509–3516. doi: 10.1200/JCO.2005.06.023. [DOI] [PubMed] [Google Scholar]
- Lu D-Y. Anticancer Drug Combinations, Studies from Different Pathways. Cell & Developmental Biology. 2015;04(03):3–4. doi: 10.4172/2168-9296.1000166. [DOI] [Google Scholar]
- Lu D-Y, Lu T-R, Cao S. Drug Combinations in Cancer Treatments. Clinical & Experimental Pharmacology. 2013;3(4):1–3. doi: 10.4172/2161-1459.1000134. [DOI] [Google Scholar]
- Meng H, Wang M, Liu H, Liu X, Situ A, Wu B, Nel AE. Use of a Lipid-Coated Mesoporous Silica Nanoparticle Platform for Synergistic Gemcitabine and Paclitaxel Delivery to Human Pancreatic Cancer in Mice. Meng Et Al. 2015;9(4):3540–3557. doi: 10.1021/acsnano.5b00510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano H. Signaling crosstalk between NF-kB and JNK. Trends in Immunology. 2017;25(8):402–405. doi: 10.1016/j.it.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Neelakantan S, Nasim S, Guzman ML, Jordan CT, Crooks PA. Aminoparthenolides as novel anti-leukemic agents: Discovery of the NF-κB inhibitor, DMAPT (LC-1) Bioorganic & Medicinal Chemistry Letters. 2009;19(15):4346–4349. doi: 10.1016/j.bmcl.2009.05.092. [DOI] [PubMed] [Google Scholar]
- Olberding KE, Wang X, Zhu Y, Pan J, Rai SN, Li C. Actinomycin D synergistically enhances the efficacy of the BH3 mimetic ABT-737 by downregulating Mcl-1 expression. Cancer Biology and Therapy. 2010;10(9):918–929. doi: 10.4161/cbt.10.9.13274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips FS, Schwartz HS, Sternberg SS, Tan CTC. The Toxicity of Actinomycin-D. Annals of the New York Academy of Sciences. 2006;89(2):348–360. doi: 10.1111/j.1749-6632.1960.tb20158.x. [DOI] [PubMed] [Google Scholar]
- Rausch V, Liu L, Kallifatidis G, Baumann B, Mattern J, Gladkich J, Herr I. Synergistic activity of sorafenib and sulforaphane abolishes pancreatic cancer stem cell characteristics. Cancer Research. 2010;70(12):5004–5013. doi: 10.1158/0008-5472.CAN-10-0066. [DOI] [PubMed] [Google Scholar]
- Schein PS, Lavin PT, Moertel CG, Frytak S, Hahn RG, O’Connell MJ, Knowlton A. Randomized phase II clinical trial of adriamycin, methotrexate, and actinomycin-d in advanced measurable pancreatic carcinoma. A gastrointestinal tumor study group report. Cancer. 1978;42(1):19–22. doi: 10.1002/1097-0142(197807)42:1<19::AID-CNCR2820420103>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- SEER, Cancer Stat Facts: Pancreatic Cancer. 2018:1–9. [Google Scholar]
- Shanmugam R, Kusumanchi P, Appaiah H, Cheng L, Crooks P, Neelakantan S, Sweeney CJ. A water soluble parthenolide analog suppresses in vivo tumor growth of two tobacco-associated cancers, lung and bladder cancer, by targeting NF-kB and generating reactive oxygen species. International Journal of Cancer. 2011;128(10):2481–2494. doi: 10.1002/ijc.25587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugam R, Kusumanchi P, Cheng L, Crooks P, Neelakantan S, Matthews W, Sweeney CJ. A water-soluble parthenolide analogue suppresses in vivo prostate cancer growth by targeting NFkB and generating reactive oxygen species. Prostate. 2010;70(10):1074–1086. doi: 10.1002/pros.21141. [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA: A Cancer Journal for Clinicians. 2018;68(1):7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- Sobell HM. Actinomycin and DNA transcription. Proceedings of the National Academy of Sciences of the United States of America. 1985;82(16):5328–31. doi: 10.1073/pnas.82.16.5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohma I, Fujiwara Y, Sugita Y, Yoshioka A, Shirakawa M, Moon J-H, Doki Y. Parthenolide, an NF-κB inhibitor, suppresses tumor growth and enhances response to chemotherapy in gastric cancer. Cancer Genomics & Proteomics. 2011;8(1):39–47. [PubMed] [Google Scholar]
- Thota R, Pauff JM, Berlin JD. Treatment of metastatic pancreatic adenocarcinoma: a review. Oncology (Williston Park, N.Y.) 2014;28(1):70–74. [PubMed] [Google Scholar]
- Wadkins RM, Jovin TM. Actinomycin D and 7-aminoactinomycin D binding to single-stranded DNA. Biochemistry. 1991;30(39):9469–78. doi: 10.1021/bi00103a012. [DOI] [PubMed] [Google Scholar]
- Wang W, Adachi M, Kawamura R, Sakamoto H, Hayashi T, Ishida T, Shinomura Y. Parthenolide-induced apoptosis in multiple myeloma cells involves reactive oxygen species generation and cell sensitivity depends on catalase activity. Apoptosis. 2006;11(12):2225–2235. doi: 10.1007/s10495-006-0287-2. [DOI] [PubMed] [Google Scholar]
- Yip-Schneider MT, Nakshatri H, Sweeney CJ, Marshall MS, Wiebke Ea, Schmidt CM. Parthenolide and sulindac cooperate to mediate growth suppression and inhibit the nuclear factor-kappa B pathway in pancreatic carcinoma cells. Molecular Cancer Therapeutics. 2005;4(4):587–594. doi: 10.1158/1535-7163.MCT-04-0215. [DOI] [PubMed] [Google Scholar]