Abstract
Objectives
This placebo-controlled randomized double-blinded clinical study assessed the analgesic efficacy of intramuscular morphine in TMD patients with myofascial pain and sex dependent responses of the morphine treatment.
Subjects and Methods
Men and women with TMD were treated with morphine (1.5 or 5 mg), lidocaine or saline in the masseter muscle. VAS of pain intensity, PPT and PPtol were compared between treatment groups and gender. An additional group was treated with morphine in the trapezius muscle to evaluate the systemic effect of morphine that may reduce pain in the masseter muscle.
RESULTS
There was a significant difference in VAS scores between the morphine 5 mg group and the saline group favoring morphine, but not between the morphine 5 mg and lidocaine. Morphine 1.5 mg and 5 mg treatments led to consistently and significantly elevated PPT and PPtol measures in men, but not in women. Morphine administered in the trapezius muscle did not affect the outcome measures.
Conclusion
A single dose intramuscular morphine produced analgesic effects up to 48 hrs in patients with myofascial pain. Intramuscular morphine elevated mechanical pain threshold and tolerance in the masseter only in male patients, suggesting sex differences in local morphine effects. No systemic effect of intramuscular morphine was detected.
Keywords: Peripheral, muscle pain, opioid receptor, trigeminal, masseter
Introduction
Despite strong analgesic effects, the use of conventional systemic opioids in the management of non-malignant pain has been marred by serious side effects including addiction, overdose, abuse, respiratory depression, and cognitive impairment (Stein, 2013). As an alternative, peripherally localized opioid receptor has been targeted to effectively attenuate pain and hyperalgesia, and an overwhelming amount of preclinical and clinical data supporting the role of peripheral opioid receptors in various pain models has been accumulated (Smith, 2008, Sehgal et al., 2011, Vadivelu et al., 2011, Spetea, 2013). More recent clinical studies continue to report effective analgesia with opioids delivered to local sites in various post-operative pain conditions (Faktorovich & Basbaum, 2010, Yari et al., 2013, Binning et al., 2011). Intra-articular morphine in the knee joint produces significant analgesia in patients with osteoarthritis (Beyaz, 2012, Gazi et al., 2008, Stein et al., 1999). The analgesic efficacy of intra-articular morphine in the temporomandibular joint (TMJ) has been also demonstrated in patients with temporomandibular disorders (TMD) (List et al., 2001, Ziegler et al., 2010). These studies indicate that intra-articular morphine at a dose as high as 10 mg produce analgesic efficiency lasting up to a week without obvious adverse effects. To our knowledge, the analgesic efficacy of intramuscular morphine in TMD patients has not been studied.
The basic premises of treating patients with either peripherally restricted opioids or systemically low doses of opioid agonists at the injured site are that opioid receptors are expressed in nociceptor terminals (Coggeshall et al., 1997, Wenk & Honda, 1999) and that the activation of those receptors results in the attenuation of pain under various pathological pain conditions (Stein, 2013). It is presumed that opioid receptors are also expressed in muscle nociceptors (Coggeshall et al., 1997, Lee et al., 2016). Preclinical studies provide compelling evidence that morphine treatment in the rat masseter muscle attenuates nocifensive responses under acute myositis conditions (Han et al., 2008, Sanchez et al., 2010, Nunez et al., 2007). Furthermore, direct injections of DAMGO ([D-Ala2, N-MePhe4, Gly-ol]-enkephalin), a specific agonist for μ-opioid receptor (MOR), in the masseter muscle effectively reversed complete Freund’s adjuvant (CFA)-induced persistent inflammatory mechanical hyperalgesia (Zhang et al., 2014). In an experimental muscle pain model in healthy human subjects, intravenous infusion of a low dose of morphine-6-β-glucuronide significantly reduced muscle hyperalgesia induced by a series of concentric and eccentric muscle contractions (Tegeder et al., 2003). However, the functional contribution of peripherally applied opioid agonists in patients with ongoing muscle pain has not been demonstrated.
Sex-differences in systemic opioid analgesia have been extensively studied (Craft, 2003, Fillingim & Gear, 2004). In contrast, there is only limited information on sex differences in peripherally mediated opioid analgesia. Morphine injection into the TMJ of male, but not female rats, significantly reduces glutamate-evoked jaw muscle activity in a dose-dependent manner (Cai et al., 2001). Consistent with this finding, DAMGO treatment in the masseter muscle significantly reverses inflammatory mechanical hyperalgesia in male, but not in female rats (Zhang et al., 2014). These preclinical data suggest that the peripheral application of opioids may lead to a greater pain relief in men than women with muscle pain conditions. The primary objective of the present study was to demonstrate the short-term analgesic efficacy of intramuscular morphine compared to saline and lidocaine in TMD patients with myofascial pain. The secondary objective was to investigate sex differences in intramuscular morphine treatments in TMD patients.
Materials and methods
Participants
TMD patients who participated in this study were referred to the Department of Orofacial Pain & Oral Medicine at the Kyung Hee University Dental Hospital. This pilot was conducted in accordance with the protocol approved by the Institutional Review Board at the Kyung Hee University Dental School, Seoul, Korea, and followed the guidelines of the Helsinki Declaration (2013). Written informed consent was obtained from all participants. The patient inclusion criteria were: a diagnosis of myalgia pain according to the Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) (Schiffman et al., 2014), pain upon palpation of the masseter muscle, and the age between 20–59 years old. The exclusion criteria were: diagnoses of systemic muscle pain disorders (e.g. fibromyalgia), systemic joint disease (e.g. rheumatoid arthritis), pain of dental origin, pregnancy, and high blood pressure. Patients who have been taking antidepressant, anticonvulsant, antianxiety agents, NSAIDs, opioids, or muscle relaxants for 3 months or more were excluded from the study.
Study groups and injection of test substances
The study used a randomized, double-blind and placebo-controlled design. All participants were randomly allocated using a computer program to generate numbers. The allocation was made by one of the researcher who examined the participants. The participants of each sex were randomly assigned to receive one of the following treatments: saline (NaCl 9 g/ 1000 ml, JW Pharmaceutical, Korea), morphine sulfate (BCworld, Korea) at 1.5 mg or 5 mg, or lidocaine HCl (2%/20 ml, Huons, Korea). The test substances and the doses were selected from previous studies with TMJ treatments. (List et al., 2001, Ziegler et al., 2010). The dose of morphine used for TMJ treatments ranges from 0.1 mg to 10 mg. We determined the two doses of morphine to assess dose-related effects within that range. For all participants, a single bolus injection of test substance was made into the most painful site of the superficial masseter muscle as determined by digital palpation. In order to determine whether intramuscular morphine produces analgesic responses via the systemic effect, we administered the higher dose of morphine (5 mg) in the trapezius muscle contralateral to the painful masseter in another group of patients. The injection was made over a 10-second period with a 27-gauge needle and a disposable syringe. All injections were made by the same investigator to ensure the stability of injection procedures. Neither the investigator who administered test substances nor the participants were aware of the contents of the injections. The contents of the treatment were revealed to the participants after the completion of the study for each subject. All injections were made in a 0.3 ml volume. No pain medication was allowed during the study period.
Pain Assessment
Thirty minutes prior to the injection, the participants were instructed to rate the intensity of the ongoing pain in the masseter muscle on a 0–100 mm visual analog scale (VAS) with end-points marked by 0 = ‘No pain’ and 100 = ‘Worst pain imaginable’. VAS scores were then assessed 10, 30 and 60 min following injection on the same day. The participants were brought back to the same clinic and were asked to rate the pain intensity on the same VAS 24 hr and 48 hr after injection.
Pressure Pain Threshold (PPT) and Pressure Pain Tolerance (PPtol)
PPT, defined as the minimum force (or pressure, kPa) which is first perceived as painful, and PPtol, defined as the amount of force which is perceived as intolerably painful (Svensson et al., 1995), were measured with a pressure algometer (Wagner Pain Test ™ - Model FPK/FPN Mechanical Algometer, CT, USA). Pressure was applied with a 1 cm diameter probe to the injection site of the masseter muscle at a constant speed. During assessment, the participants were instructed to keep their jaws at rest and raise their hand once their PPT or PPtol was reached. The PPT and PPtol measures were obtained before, 10, 30, 60 min, and 24 hrs and 48 hrs after injection. The PPT was always assessed before PPtol and the two assessments were separated by at least 3 min. The PPT and PPtol measures were always obtained after the assessment of the VAS score. Thus, the patients were tested in three sessions, i.e., the day of treatment, and 24 hrs and 48hrs later. Each session lasted no more than 2 hours including the preparation time. Another investigator who is unaware of test substances that the subjects received assessed VAS, PPT and PPtol measures.
Adverse Effects
On the first visit, the participants were monitored up to 2 hr following injection. The participants were monitored for any signs of adverse effects such as nausea, vomiting, and dizziness. We also monitored respiratory difficulties, allergic reactions, sedation and palpitations. The occurrence of any adverse effects at 24 hr and 48 hr after injection were determined from the participants’ verbal reports.
Statistical Analyses
Baseline VAS scores and PPT and PPtol measures between treatment groups were compared with a one-way analysis of variance (ANOVA) or the Kruskal-Walis one-way ANOVA, depending on the outcome of the normality test. For each dependent measure, time dependent changes following the treatment were normalized to baseline values. To assess the effects of intramuscular morphine treatment, mean percent changes for each treatment condition were analyzed with a two-way ANOVA with repeated measures with treatment and time as the main effects. The effects of intramuscular lidocaine and trapezius morphine treatments were analyzed with a one-way ANOVA with repeated measures. For comparisons between men and women, mean percent changes in each dependent measure for each treatment condition were analyzed with a two-way ANOVA with repeated measures with sex and time as the main effects. Holm-Sidak method was used as a post-hoc analysis. The significance level of all statistical analyses was set at p < 0.05. The data are described as mean ± standard deviation (SD) in the text and tables and mean ± standard error of the mean (SEM) for graphic presentations.
Results
Fifty one TMD patients (27 men and 24 women, mean ± SD age 29 ± 6.3 and 28 ± 8.5 years, respectively, were divided into the following control and experimental groups: saline masseter (n=11, 6 men and 5 women), morphine 1.5 mg masseter (n=13, 8 men and 5 women), morphine 5 mg masseter (n=11, 5 men and 6 women), lidocaine masseter (n=11, 6 men and 5 women) and morphine 5 mg trapezius (n=5, 2men and 3 women). None of the participants showed any obvious signs of adverse effects due to treatment on the day of treatment. The participants reported no complaints throughout the duration of the study.
Intramuscular morphine effects
Mean VAS scores and mean PPT and PPtol measures for all patients under each condition during the observation period are described in Table 1. Mean baseline VAS scores were not significantly different amongst treatment groups. We therefore normalized the VAS scores following treatment to pre-treatment scores and analyzed percent changes in the VAS scores overtime with a two-way ANOVA with repeated measures. As shown in Fig 1A, there was a significant treatment effect (F=4.36, p<0.05) as well as a significant time effect (F=12.4, p<0.001). The post-hoc analysis further revealed that there was a significant difference in VAS scores between the patients treated with morphine 5 mg and those treated with saline. Significantly lower VAS scores at 30 min, 60 min, 24 hrs and 48 hrs were primarily derived from the patients treated with morphine 5 mg.
TABLE 1.
Descriptive statistics for pain and mechanical sensitivity measures
| Pre | 10min | 30min | 60min | 24hr | 48hr | |
|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
|
| M±SD | M±SD | M±SD | M±SD | M±SD | M±SD | |
| VAS (0–100) | ||||||
| Saline (n=11) | 42±21 | 39±21 | 40±21 | 36±23 | 34±25 | 31±21 |
| Mor 1.5mg (n=13) | 48±22 | 52±22 | 45±23 | 38±23 | 42±22 | 37±24 |
| Mor 5 mg (n=11) | 51±21 | 41±21 | 33±24 | 31±21 | 30±21 | 25±20 |
| PPT (kPa) | ||||||
| Saline (n=11) | 154±33 | 168±32 | 176±25 | 181±44 | 175±42 | 170±31 |
| Mor 1.5mg (n=13) | 152±25 | 167±28 | 191±40 | 200±49 | 189±39 | 188±39 |
| Mor 5 mg (n=11) | 181±48 | 209±57 | 219±70 | 235±84 | 209±80 | 229±84 |
| PPtol (kPa) | ||||||
| Saline (n=11) | 238±46 | 236±37 | 241±44 | 256±51 | 242±54 | 242±43 |
| Mor 1.5mg (n=13) | 225±24 | 231±41 | 251±50 | 265±55 | 256±47 | 248±47 |
| Mor 5 mg (n=11) | 261±57 | 271±66 | 288±80 | 319±85 | 292±86 | 301±86 |
Figure 1.
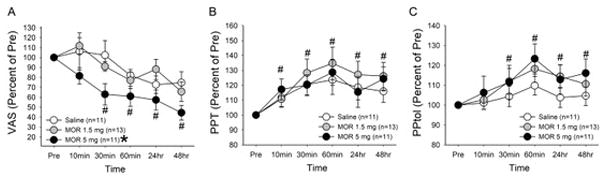
Effects of intramuscular treatments on (A) VAS scores, (B) PPT and (C) PPtol measures. Data were normalized to the baseline scores for each participant. Mean percent changes in the VAS scores, PPT and PPtol measures were plotted against time and compared between saline and morphine treatment conditions. * denotes significant treatment effect between morphine 5 mg and saline groups. # denotes significant changes compared to the baseline values for all three treatment groups over time. The data are represented as mean ± SEM.
Mean baseline PPT and PPtol measures were not significantly different amongst all groups. As with VAS scores, we normalized the PPT measures at each time point following treatment to pre-treatment scores and analyzed percent changes in the PPT measures overtime. There was an overall increase in PPT measures throughout the observation period (F=10.8, p<0.001, Fig 1B). Both morphine treated groups tended to show greater increases in PPT measures compared to those of saline treated group, but no significant treatment effect was observed (F=0.25, p>0.05).
PPtol measures also gradually increased overtime following morphine treatments, but the differences were not statistically significant compared to those of saline group (F=0.7, p>0.05, Fig 1C). There was significant time effect with significant changes occurring at 30min, 60min, 24hr and 48hr (F=6.9, p<0.001).
Lidocaine injection in the masseter muscle gradually decreased VAS scores to the extent similar to that produced by morphine treatments (Fig 2A). However, the decrease in VAS scores by lidocaine did not reach a statistical significance. Lidocaine treatment lead to significant elevation of PPT measures (Fig 2B, F=5.1, p < 0.01), but PPtol measures were unaffected (Fig 2C). In Fig 2, the effects of morphine 5 mg were added for comparison with lidocaine effects. Although morphine treatment produced a greater decrease in VAS scores than that of lidocaine, the difference did not reach a statistical significance (F=2.6, p > 0.5). PPT and PPtol measures were not significantly different between morphine 5 mg and lidocaine treated groups (F=0.2, p > 0.5, F=0.98, p > 0.5, respectively). Intramuscular injection of morphine 5 mg to the trapezius muscle, a muscle distant from the painful masseter muscle, did not lead to significant changes in VAS, PPT and PPtol scores arising from the masseter muscle (Fig 2D–F).
Figure 2.
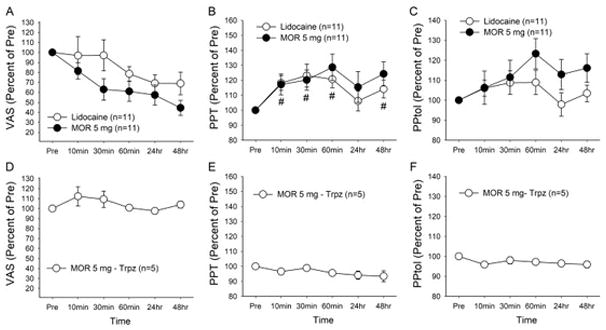
Effects of intra-masseter lidocaine on VAS scores (A), PPT (B) and PPtol (C) measures. Effects of morphine 5 mg shown in Fig 1 were re-plotted here for comparison. Mean percent changes in the VAS scores, PPT and PPtol measures assessed from the masseter muscle were plotted against time. # denotes significant changes compared to the baseline values only for lidocaine group. Effects of intra-trapezius morphine 5 mg on changes in the VAS scores (D), PPT(E) and PPtol (F) measures assessed from the masseter muscle. The data are represented as mean ± SEM. Trpz, trapezius
Sex differences in intramuscular morphine effects
Under saline treatment condition, there was neither a significant sex effect (F= 0.06, p=0.8) nor a time effect (F=2.1, p=0.08; Fig 3A). Morphine at both 1.5 mg and 5 mg doses significantly attenuated the mean percent VAS scores (F=4.4, p<0.01 and F=9.7, p<0.001, respectively). Post-hoc analyses revealed that morphine 1.5 mg significantly reduced the mean percent VAS scores only at 48hrs whereas morphine 5 mg lead to an immediate reduction of the mean percent VAS scores that persisted for the entire observation duration (Fig 3B, C). Morphine 5 mg tended to produce greater effect in women, but no statistically significant sex difference was observed in either morphine 1.5 mg or 5 mg group (F=0.08, p=0.79 and F=3.4, p=0.1, respectively).
Figure 3.
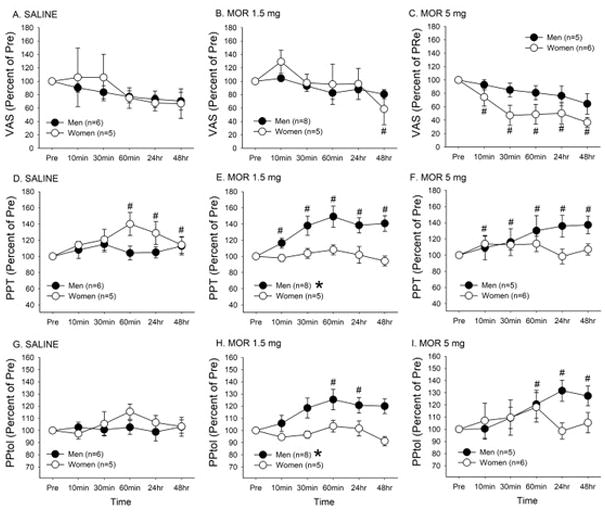
Mean percent changes in VAS scores (A–C), PPT measures (D–F), and PPtol measures (G–I) were plotted against time and compared between men and women for saline, morphine 1.5 mg, and morphine 5 mg treatment condition. # denotes significant changes in VAS scores compared to the baseline values. * denotes significant difference between men and women. The data are represented as mean ± SEM.
No significant sex effect on PPT measure was detected following saline treatment (F= 1.39, p=0.27). Saline treatment moderately, but significantly, increased the mean percent PPT measures (F=2.5, p<0.05), which was contributed primarily by women (Fig 3D). The treatment with morphine at 1.5 mg led to significant sex (F=8.6, p< 0.05) and time (F=5.89, p<0.001) effects. There was a gradual and significant increase in the mean percent PPT measures only in men (Fig 3E). There was a similar trend of responses with the higher dose (5 mg) of morphine. Sex difference was expected, but did not reach a statistical significance (F=4.9, p=0.055). At this dose, morphine tended to induce antihyperalgesic in both men and women. However, a significant interaction between sex and time were observed (F=2.83, p<0.05) as a gradual increase in the mean percent PPT measures was observed only in men (Fig 3F).
No significant sex (F=0.3, p>0.05) and time (F=1.2, p>0.05) effects on PPtol measures were detected following saline treatment (Fig 3G). Morphine treatment at 1.5 mg dose led to significant sex (F=6.3, p<0.05) and time (F=3.77, p<0.01) effects. A gradual and significant increase in the mean percent PPtol measures was observed only in men (Fig 3H). Morphine treatment at 5 mg also significantly increased the mean percent PPtol measures (F=2.8, p<0.05). There was no significant sex effect (F=0.6, p =0.45). However, there was a significant interaction between sex and time (F=2.9, p<0.05) as a significant increase in the mean percent PPtol measures compared to the baseline measure was observed at 24 and 48 hr time points only in men (Fig 3I).
Discussion
In the present study, we evaluated the effects of intramuscular administration of morphine in TMD patients diagnosed with myalgia on ongoing pain as measured by changes in the VAS scores. We found that direct intramuscular treatments with morphine led to a gradual and significant short-term reduction in the intensity of ongoing pain in both men and women. Control injection with saline did not significantly alter the VAS scores in either men or women. The significant analgesic effects could be observed as early as 30 minutes after the treatment and lasted for 48 hours. Our study was initially designed with the assumption that single intramuscular morphine treatment would produce relatively sustained effects that could last up to 48 hrs. It is possible that single injection of morphine may result in even more prolonged analgesic effects than we had anticipated. Despite high variability in VAS scores amongst women the analgesic effects were most pronounced with the higher dose of morphine (5 mg) for both men and women. It is possible that morphine at this dose may involve more than opioid receptor-mediated effects at the local injection site. However, it is unlikely that the dose of morphine used in our study produced its effects primarily via the opioid receptors in the CNS since none of our patients reported adverse effects. Intra-articular administration of morphine to TMD patients even at the 10 mg dose appears to produce no noticeable side effects (Ziegler et al., 2010). Furthermore, the higher dose of morphine (5 mg) used in this study did not produce any changes in masseter pain and sensitivity responses when administered into the distant muscle, ruling out the possibility of systemic effects. Thus, our results suggest that targeting local opioid receptors with intramuscular morphine has significant treatment effects on TMD patients with ongoing muscle pain.
Current therapy for pain related to TMD involves both non-pharmacological and pharmacological means. The major classes of drugs used to treat the TMD pain are NSAIDs, anti-depressants, anti-convulsants, muscle relaxants and opioids (Fricton, 2007, Kucuk, 2014). Unfortunately, many of these drugs are associated with unwanted side effects and do not offer reliable and persistent analgesia in patients. (Gallardo et al., 1975, Kimos et al., 2007, Rizzatti-Barbosa et al., 2003, Sharav et al., 1987, Singer & Dionne, 1997). Despite the strong analgesic effects provided by opioids, there are many unwanted side effects, such as nausea, vomiting, constipation, dependence, respiratory depression and opioid-induced hyperalgesia that often lead to poor compliance and rejection of therapy. Systemic opioids has no major effect on relieving chronic orofacial pain conditions and is better preserved for acute pain conditions and cancer/cancer therapy related pain The present findings add to the accruing clinical evidence that peripheral morphine provides effective analgesia in many pain conditions, including TMD (List et al., 2001, Ziegler et al., 2010). Our observation that a single treatment of low dose of morphine produced lasting analgesic effects up to 48hrs, effects that are not observed with intramuscular lidocaine treatment, presented a need for a larger scale study with a longer follow-up period to more conclusively determine the dosing and treatment paradigms for intramuscular morphine injections.
To our knowledge, there are no clinical or preclinical studies that directly compared sex differences in the effects of intramuscular morphine on spontaneous or non-evoked ongoing pain. Few pre-clinical studies that report sex differences in the effects of peripheral opioids in orofacial injury models assessed mechanical hyperalgesia, but not on-going pain, as the outcome measure (Bai et al., 2015, Zhang et al., 2014). The present study provided novel information that activation of peripheral opioid receptors in the masseter muscle in TMD patients with myalgia does not yield obvious sex differences. These results do not necessarily mean that there are no sex differences in peripheral opioid receptor mechanisms. It is possible that multiple factors beyond the nociceptor level contribute to verbal reports of ongoing muscle pain, which represent a composite sensory experience.
Major symptoms of TMD are characterized by localized myalgia, tenderness upon manual palpation, reduced force output and limited range of motion(Hedenberg-Magnusson et al., 1997, Lund et al., 1991, Molin, 1972), all of which could result from sensitization of nociceptors (Mense, 1993). Thus, a prominent feature of persistent muscle pain conditions is a change in the mechanical sensitivity of muscle tissue. Our data showed that the intramuscular morphine treatment consistently led to more pronounced anti-hyperalgesic effects over saline. These observations suggest that locally administered morphine increases mechanical thresholds of muscle nociceptors via the opioid receptors expressed in masseter afferents, which could serve as important knowledge base for designing effective treatment strategies for mechanically evoked muscle pain conditions.
Interestingly, the profound morphine effects on masseter sensitivity observed in men were not present in women. These data are consistent with a recent animal study that demonstrated sex differences in the effects of intramuscular DAMGO on evoked pain or mechanical hyperalgesia (Zhang et al., 2014). Available studies indicate that sex differences in peripheral opioid analgesia involve multiple mechanisms (Holtman & Wala, 2006, Saloman et al., 2011). Inflammation in the masseter muscle significantly upregulates MOR in trigeminal ganglia of male, but not female, rats (Zhang et al., 2014). The MOR regulation in trigeminal ganglia is testosterone dependent: the inflammation-induced upregulation of MOR could not be observed in gonadectomized male rats and a testosterone replacement in these rats restored peripheral DAMGO effects (Zhang et al., 2014). Since androgen receptors function as a transcription factor that directly regulates cannabinoid receptors in trigeminal sensory neurons (Lee et al., 2013), it is possible that the testosterone-androgen receptor mediated regulation of MOR in sensory neurons contributes to the common mechanisms of sex differences in peripheral analgesia, which we propose as the underlying cellular basis for our findings.
Despite our observation that single intramuscular morphine treatment did not result in obvious adverse effects, any future studies should take into consideration that the exposure to repeated intramuscular injections over time might increase the risk of unwanted side effects. We also acknowledge that the small sample size of our study with limited power does not allow us to make firm conclusions. However, here we report the first clinical evidence on sex differences in peripheral intramuscular morphine effects that are consistent with recent animal studies, which offer clear mechanistic bases for sex differences in peripheral analgesia. Therefore, our findings should have potentially important clinical implications for the design of treatment strategies targeting peripheral opioid receptors that should take sex differences into account when considering TMD patient populations.
Acknowledgments
This study was supported by a grant from the Kyung Hee University (KHU-20161388) and NIH grant RO1 DE19448 (JYR).
References
- Bai X, Zhang X, Li Y, Lu L, Li B, He X. Sex differences in peripheral mu-opioid receptor mediated analgesia in rat orofacial persistent pain model. PloS one. 2015;10:e0122924. doi: 10.1371/journal.pone.0122924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyaz SG. Comparison of efficacy of intra-articular morphine and steroid in patients with knee osteoarthritis. Journal of anaesthesiology, clinical pharmacology. 2012;28:496–500. doi: 10.4103/0970-9185.101940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binning AR, Przesmycki K, Sowinski P, Morrison LM, Smith TW, Marcus P, Lees JP, Dahan A. A randomised controlled trial on the efficacy and side-effect profile (nausea/vomiting/sedation) of morphine-6-glucuronide versus morphine for post-operative pain relief after major abdominal surgery. European journal of pain. 2011;15:402–8. doi: 10.1016/j.ejpain.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Cai BB, Cairns BE, Sessle BJ, Hu JW. Sex-related suppression of reflex jaw muscle activity by peripheral morphine but not GABA. Neuroreport. 2001;12:3457–60. doi: 10.1097/00001756-200111160-00016. [DOI] [PubMed] [Google Scholar]
- Coggeshall RE, Zhou S, Carlton SM. Opioid receptors on peripheral sensory axons. Brain research. 1997;764:126–32. doi: 10.1016/s0006-8993(97)00446-0. [DOI] [PubMed] [Google Scholar]
- Craft RM. Sex differences in opioid analgesia: “from mouse to man”. The Clinical journal of pain. 2003;19:175–86. doi: 10.1097/00002508-200305000-00005. [DOI] [PubMed] [Google Scholar]
- Faktorovich EG, Basbaum AI. Effect of topical 0.5% morphine on postoperative pain after photorefractive keratectomy. Journal of refractive surgery. 2010;26:934–41. doi: 10.3928/1081597X-20100212-06. [DOI] [PubMed] [Google Scholar]
- Fillingim RB, Gear RW. Sex differences in opioid analgesia: clinical and experimental findings. European journal of pain. 2004;8:413–25. doi: 10.1016/j.ejpain.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Fricton J. Myogenous temporomandibular disorders: diagnostic and management considerations. Dental clinics of North America. 2007;51:61–83. vi. doi: 10.1016/j.cden.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Gallardo F, Molgo J, Miyazaki C, Rossi E. Carisoprodol in the treatment of myofascial pain-dysfunction syndrome. J Oral Surg. 1975;33:655–8. [PubMed] [Google Scholar]
- Gazi MB, Sakata RK, Issy AM. Intra-articular morphine versus bupivacaine for knee motion among patients with osteoarthritis: randomized double-blind clinical trial. Sao Paulo medical journal = Revista paulista de medicina. 2008;126:309–13. doi: 10.1590/S1516-31802008000600003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SR, Lee MK, Lim KH, Yang GY, Jeon HJ, Ju JS, Yoon YW, Kim SK, Ahn DK. Intramuscular administration of morphine reduces mustard-oil-induced craniofacial-muscle pain behavior in lightly anesthetized rats. European journal of pain. 2008;12:361–70. doi: 10.1016/j.ejpain.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Hedenberg-Magnusson B, Ernberg M, Kopp S. Symptoms and signs of temporomandibular disorders in patients with fibromyalgia and local myalgia of the temporomandibular system. A comparative study. Acta odontologica Scandinavica. 1997;55:344–9. doi: 10.3109/00016359709059198. [DOI] [PubMed] [Google Scholar]
- Holtman JR, Jr, Wala EP. Characterization of the antinociceptive effect of oxycodone in male and female rats. Pharmacology, biochemistry, and behavior. 2006;83:100–8. doi: 10.1016/j.pbb.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Kimos P, Biggs C, Mah J, Heo G, Rashiq S, Thie NM, Major PW. Analgesic action of gabapentin on chronic pain in the masticatory muscles: a randomized controlled trial. Pain. 2007;127:151–60. doi: 10.1016/j.pain.2006.08.028. [DOI] [PubMed] [Google Scholar]
- Kucuk BB, Kaya ST, Motro PK, Oral MK. Pharmacotherapeutic agents used in temporomandibular disorders. Oral Diseases. 2014;20:740–743. doi: 10.1111/odi.12255. [DOI] [PubMed] [Google Scholar]
- Lee KS, Asgar J, Zhang Y, Chung MK, Ro JY. The role of androgen receptor in transcriptional modulation of cannabinoid receptor type 1 gene in rat trigeminal ganglia. Neuroscience. 2013;254:395–403. doi: 10.1016/j.neuroscience.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KS, Zhang Y, Asgar J, Auh QS, Chung MK, Ro JY. Androgen receptor transcriptionally regulates mu-opioid receptor expression in rat trigeminal ganglia. Neuroscience. 2016;331:52–61. doi: 10.1016/j.neuroscience.2016.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- List T, Tegelberg A, Haraldson T, Isacsson G. Intra-articular morphine as analgesic in temporomandibular joint arthralgia/osteoarthritis. Pain. 2001;94:275–82. doi: 10.1016/S0304-3959(01)00361-X. [DOI] [PubMed] [Google Scholar]
- Lund JP, Donga R, Widmer CG, Stohler CS. The pain-adaptation model: a discussion of the relationship between chronic musculoskeletal pain and motor activity. Canadian journal of physiology and pharmacology. 1991;69:683–94. doi: 10.1139/y91-102. [DOI] [PubMed] [Google Scholar]
- Mense S. Nociception from skeletal muscle in relation to clinical muscle pain. Pain. 1993;54:241–89. doi: 10.1016/0304-3959(93)90027-M. [DOI] [PubMed] [Google Scholar]
- Molin C. Vertical isometric muscle forces of the mandible. A comparative study of subjects with and without manifest mandibular pain dysfunction syndrome. Acta odontologica Scandinavica. 1972;30:485–99. doi: 10.3109/00016357209002499. [DOI] [PubMed] [Google Scholar]
- Nunez S, Lee JS, Zhang Y, Bai G, Ro JY. Role of peripheral mu-opioid receptors in inflammatory orofacial muscle pain. Neuroscience. 2007;146:1346–54. doi: 10.1016/j.neuroscience.2007.02.024. [DOI] [PubMed] [Google Scholar]
- Rizzatti-Barbosa CM, Nogueira MT, de Andrade ED, Ambrosano GM, de Barbosa JR. Clinical evaluation of amitriptyline for the control of chronic pain caused by temporomandibular joint disorders. Cranio : the journal of craniomandibular practice. 2003;21:221–5. doi: 10.1080/08869634.2003.11746254. [DOI] [PubMed] [Google Scholar]
- Saloman JL, Niu KY, Ro JY. Activation of peripheral delta-opioid receptors leads to anti-hyperalgesic responses in the masseter muscle of male and female rats. Neuroscience. 2011;190:379–85. doi: 10.1016/j.neuroscience.2011.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez EM, Bagues A, Martin MI. Contributions of peripheral and central opioid receptors to antinociception in rat muscle pain models. Pharmacology, biochemistry, and behavior. 2010;96:488–95. doi: 10.1016/j.pbb.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Schiffman E, Ohrbach R, Truelove E, Look J, Anderson G, Goulet JP, List T, Svensson P, Gonzalez Y, Lobbezoo F, Michelotti A, Brooks SL, Ceusters W, Drangsholt M, Ettlin D, Gaul C, Goldberg LJ, Haythornthwaite JA, Hollender L, Jensen R, John MT, De Laat A, de Leeuw R, Maixner W, van der Meulen M, Murray GM, Nixdorf DR, Palla S, Petersson A, Pionchon P, Smith B, Visscher CM, Zakrzewska J, Dworkin SF International Rdc/Tmd Consortium Network IafDR and Orofacial Pain Special Interest Group IAftSoP. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: recommendations of the International RDC/TMD Consortium Network* and Orofacial Pain Special Interest Groupdagger. J Oral Facial Pain Headache. 2014;28:6–27. doi: 10.11607/jop.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal N, Smith HS, Manchikanti L. Peripherally acting opioids and clinical implications for pain control. Pain physician. 2011;14:249–58. [PubMed] [Google Scholar]
- Sharav Y, Singer E, Schmidt E, Dionne RA, Dubner R. The analgesic effect of amitriptyline on chronic facial pain. Pain. 1987;31:199–209. doi: 10.1016/0304-3959(87)90036-4. [DOI] [PubMed] [Google Scholar]
- Singer E, Dionne R. A controlled evaluation of ibuprofen and diazepam for chronic orofacial muscle pain. Journal of orofacial pain. 1997;11:139–46. [PubMed] [Google Scholar]
- Smith HS. Peripherally-acting opioids. Pain physician. 2008;11:S121–32. [PubMed] [Google Scholar]
- Spetea M. Opioid receptors and their ligands in the musculoskeletal system and relevance for pain control. Current pharmaceutical design. 2013;19:7382–90. doi: 10.2174/13816128113199990363. [DOI] [PubMed] [Google Scholar]
- Stein A, Yassouridis A, Szopko C, Helmke K, Stein C. Intraarticular morphine versus dexamethasone in chronic arthritis. Pain. 1999;83:525–32. doi: 10.1016/S0304-3959(99)00156-6. [DOI] [PubMed] [Google Scholar]
- Stein C. Opioids, sensory systems and chronic pain. European journal of pharmacology. 2013;716:179–87. doi: 10.1016/j.ejphar.2013.01.076. [DOI] [PubMed] [Google Scholar]
- Svensson P, Arendt-Nielsen L, Nielsen H, Larsen JK. Effect of chronic and experimental jaw muscle pain on pain-pressure thresholds and stimulus-response curves. Journal of orofacial pain. 1995;9:347–56. [PubMed] [Google Scholar]
- Tegeder I, Meier S, Burian M, Schmidt H, Geisslinger G, Lotsch J. Peripheral opioid analgesia in experimental human pain models. Brain : a journal of neurology. 2003;126:1092–102. doi: 10.1093/brain/awg115. [DOI] [PubMed] [Google Scholar]
- Vadivelu N, Mitra S, Hines RL. Peripheral opioid receptor agonists for analgesia: a comprehensive review. Journal of opioid management. 2011;7:55–68. doi: 10.5055/jom.2011.0049. [DOI] [PubMed] [Google Scholar]
- Wenk HN, Honda CN. Immunohistochemical localization of delta opioid receptors in peripheral tissues. The Journal of comparative neurology. 1999;408:567–79. doi: 10.1002/(sici)1096-9861(19990614)408:4<567::aid-cne10>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Yari M, Saeb M, Golfam P, Makhloogh Z. Analgesic efficacy of intra-articular morphine after arthroscopic knee surgery in sport injury patients. Journal of injury & violence research. 2013;5:84–8. doi: 10.5249/jivr.v5i2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhang Y, Asgar J, Niu KY, Lee J, Lee KS, Schneider M, Ro JY. Sex differences in mu-opioid receptor expression in trigeminal ganglia under a myositis condition in rats. European journal of pain. 2014;18:151–61. doi: 10.1002/j.1532-2149.2013.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler CM, Wiechnik J, Muhling J. Analgesic effects of intra-articular morphine in patients with temporomandibular joint disorders: a prospective, double-blind, placebo-controlled clinical trial. Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons. 2010;68:622–7. doi: 10.1016/j.joms.2009.04.049. [DOI] [PubMed] [Google Scholar]


