Abstract
Early adversity such as maltreatment is associated with increased risk for psychopathology and atypical neurological development in children. The present study examined associations between depressive symptoms and error-related brain activity (the error-related negativity, or ERN) among children involved with Child Protective Services (CPS) and among comparison children. Results indicate that the relation between depressive symptoms and ERN amplitude depends on CPS involvement, such that depressive symptoms were associated with blunted ERNs only for CPS-referred children. The present study can inform future research investigating the mechanisms by which experiences of adversity affect the association between symptoms and error-related brain activity.
Keywords: maltreatment, depression, ERN
1. Introduction
Children who have experienced early adversity like maltreatment are at risk for problematic developmental outcomes such as depressive symptoms and atypical neurological patterns (Bick & Nelson, 2016; Goff & Tottenham, 2015; Pavlakis, Noble, Pavlakis, Ali, & Frank, 2015). From a Research Domain Criteria (RDoC) perspective, describing psychobiological processes that may characterize or predict symptoms is an important step towards understanding the mechanisms of psychopathology and ultimately developing targeted interventions (Cuthbert, 2014). The present study examined depressive symptoms among children referred by Child Protective Services (CPS) due to allegations of maltreatment and among comparison children, and explored how such symptoms relate to neurophysiology in these populations.
Event-related potential (ERP) components are measures of neural activity with precise temporal resolution, derived from electroencephalogram (EEG) recordings. One ERP component that may be relevant to depressive symptoms is the error-related negativity, or ERN. The ERN is a fronto-central negative deflection peaking about 50 ms after an error is made, and it can be elicited experimentally with a simple two-choice reaction time task (Gehring, Goss, Coles, Meyer, & Donchin, 1993). The ERN is reflective of early error processing, and although it is relatively heritable and stable over time (Olvet & Hajcak, 2008), it has been shown to change as children develop (Davies, Segalowitz, & Gavin, 2004a, 2004b; Meyer, Weinberg, Klein, & Hajcak, 2012), and it seems to be sensitive to early caregiving quality (Brooker & Buss, 2014; Meyer et al., 2015). This component provides a signal that an error has occurred and may also reflect the degree to which the error is perceived as a threat (Simons, 2010; Weinberg, Meyer, et al., 2016). In light of this work, the ERN has been proposed as a measure of performance monitoring as well as sustained threat (Weinberg, Dieterich, & Riesel, 2014). The ERN has also been linked to certain symptoms and disorders including depression (Olvet & Hajcak, 2008), and continued research on these associations may enhance our understanding of the role of performance monitoring and sustained threat in psychopathology.
Findings on the relation between depression and the ERN are mixed, and the bulk of this research has been conducted with adults. Some researchers have found that depression is associated with large ERNs in adults, whereas others have found the opposite – that depression is associated with small ERNs (Olvet & Hajcak, 2008; Olvet, Klein, & Hajcak, 2010; Weinberg, Kotov, & Proudfit, 2015). Part of this inconsistency may be explained by the implication of the ERN in both performance monitoring and threat processing systems. Specifically, it may be that depression is associated with reduced threat sensitivity (smaller ERN) but enhanced error monitoring (larger ERN), and these opposite effects on the ERN could lead to apparently mixed results in the literature. It is also plausible that mild or moderate depression is associated with heightened error monitoring and thus larger ERNs, but that symptoms like loss in motivation and environmental disengagement are associated with reduced threat sensitivity and blunted ERNs (Weinberg, Liu, & Shankman, 2016; Weinberg, Meyer, et al., 2016; Weinberg, Riesel, & Hajcak, 2012).
Less research is available on the ERN and its relation to depressive symptoms in children than in adults, but a growing body of evidence suggests that depression (major depressive disorder or elevated depressive symptoms) is linked to small or blunted ERNs in children and adolescents (Ladouceur et al., 2012; Weinberg, Meyer, et al., 2016). In addition to active depressive symptoms, risk of depression may be associated with blunted ERNs. For example, having a parent with a history of chronic or recurrent major depressive disorder (MDD) is a risk factor for developing depression and has been associated with a reduced ERN (Meyer, Bress, Hajcak, & Gibb, 2016). Although most evidence seems to suggest that depressive symptoms are associated with reduced error-related brain activity in children, depressive symptoms have also been associated with an enhanced ERN (Hanna et al., 2016), or even unrelated to the ERN (Bress, Meyer, & Hajcak, 2015). As the authors of a recent meta-analysis concluded (Moran, Schroder, Kneip, & Moser, 2017), additional research is needed to more fully understand the association between the ERN and depression in children.
Given that the developing brain is sensitive to the early environment, it follows that early experience may affect the development of and association between depressive symptoms and the ERN in children. Early experiences of adversity such as maltreatment are associated with atypical brain development, as well as elevated depressive symptoms (Bick & Nelson, 2016; Heim & Binder, 2012; Kaufman, 1991). Further, the anterior cingulate cortex (ACC), a hypothesized generator of the ERN (van Veen & Carter, 2002), is frequently found to be abnormal in both structure and function in people who have experienced maltreatment (Teicher, Samson, Anderson, & Ohashi, 2016), and ACC function seems to be altered in depressed individuals (Liotti & Mayberg, 2001). Though there is limited research on the ERN in children who experience maltreatment, there is emerging evidence that the developing ERN is sensitive to parenting behaviors (Brooker & Buss, 2014; Meyer et al., 2015). Additionally, early deprivation in the form of institutional care has been linked to smaller ERNs in children relative to those who were not institutionalized (Loman et al., 2013). Early adversity such as chronic maltreatment can certainly be characterized as sustained threat, and if the ERN is indeed involved in processing threatening stimuli, repeated over-activation of these neural networks during critical periods of development could result in lasting alterations to the ACC and threat processing. Atypical ACC development could then interact with other risk factors to produce elevated depressive symptomatology over time.
Notably, the ERN also seems to be sensitive to early intervention, suggesting considerable plasticity, at least in early childhood. In the Bucharest Early Intervention Project (BEIP), institutionalized children were randomized to receive foster care (an improvement in the quality of the early caregiving environment) or usual care, which involved continued orphanage care for at least some period of time (Zeanah et al., 2003). In a go/no-go task at age 8, children in the foster care group exhibited larger ERNs than did the children assigned to usual care, similar to a never-institutionalized comparison group (McDermott, Westerlund, Zeanah, Nelson, & Fox, 2012). This study replicated other findings that prolonged deprivation in the form of institutional care was associated with small ERNs (Loman et al., 2013) and demonstrated that intervention improving the caregiving environment normalized children’s ERNs. Children in the BEIP later completed a flanker task at age 12. Although more time spent in institutional care was associated with more behavior problems, ERN amplitude at this session interacted with time in institutional care to predict behavior problems, such that small ERNs enhanced risk and large ERNs seemed to be protective (Troller-Renfree, Nelson, Zeanah, & Fox, 2016). Overall, an inadequate early caregiving environment has been associated with blunted ERNs (potentially due to atypical ACC development), and small ERNs seem to signal risk for psychopathology for children who have experienced such deprivation.
The present study examined the association between depressive symptoms and the ERN in middle childhood among CPS-referred children and comparison children. Consistent with research on early life stress and maltreatment, we hypothesized that CPS-referred children would exhibit more depressive symptoms than comparison children. We also hypothesized that more depressive symptoms would be associated with a blunted ERN among all children. Though few studies have investigated depression and the ERN in children to date, those studies seem to point to a reduced ERN in depressed children (Moran et al., 2017). The present study also aimed to contribute to this literature by testing the association of depressive symptoms and the ERN in CPS-referred children and in comparison children without CPS involvement. It was hypothesized that the link between the ERN and depressive symptoms would be moderated by CPS involvement, such that depressive symptoms would be more strongly related to a blunted ERN in CPS-referred children than in comparison children. This hypothesis is supported by several previous studies which found that an inadequate early caregiving environment is associated with smaller ERNs in general, and that larger ERNs in these children are associated with better functioning (Loman et al., 2013; Troller-Renfree et al., 2016).
2. Method
2.1 Participants
Participants were children in middle childhood enrolled in a larger longitudinal study on the efficacy of the parenting intervention Attachment and Biobehavioral Catch-up (ABC; Bernard et al., 2012). For the study, two samples of children were recruited – a CPS-referred sample and a comparison sample. The CPS-referred sample was recruited when children were infants, and recruitment was conducted through referrals from Child Protective Services (CPS) due to allegations of maltreatment1 as part of a city-wide initiative designed to redirect children from foster care. At the time of recruitment, these families were randomized to receive either ABC or a control intervention (Developmental Education for Families; DEF). ABC is a ten session, home-based parenting intervention that is designed to enhance parent sensitivity. DEF was developed as a control intervention for ABC, and is delivered in the same format but focuses on teaching parents about child development rather than promoting specific parenting behaviors. In the present study, those children who received ABC or DEF are referred to as the CPS-referred sample. A comparison sample of children was recruited at age 8 through local community centers and schools. This sample was matched to the CPS-referred sample on race and gender, and children were ineligible for recruitment to the comparison sample if their families had ever been involved with CPS. Aside from this eligibility requirement, maltreatment was not formally assessed in the comparison group. Members of both the CPS-referred and comparison samples were invited to participate in middle childhood lab visits when children were about 8 years old (M = 8.46, SD = 0.36; 51.8% male). Eighty-four participants had usable EEG data (see data processing section for details) – 42 were from the CPS-referred sample, and 42 were from the comparison sample. Procedures were approved by the Institutional Review Board.
2.2 Measures
2.2.1 Depressive symptoms
Parents completed the Child Behavior Checklist (CBCL/6–18; Achenbach & Rescorla, 2001) in the lab as part of a battery of questionnaires. In the CBCL, parents are asked to what extent 113 emotional and behavior problems are true of their child, from 0 (“not true”) to 2 (“very true or often true”). The CBCL can be split into subscales that roughly map onto psychiatric disorders: anxious/depressed, withdrawn-depressed, somatic complaints, rule-breaking, aggressive behavior, social problems, thought problems, and attention problems. For the present study, two items related to self-harm and suicidality were removed from questionnaires. Sums were calculated for all subscales. The Withdrawn/Depressed subscale was selected as the variable of interest for the present study, as it captures central depressive symptoms such as not enjoying activities (anhedonia) and social withdrawal. The scale has 8 items, and reliability was acceptable considering the small number of items (α = .68).
2.2.2 Flanker task
Children completed a computerized version of the Eriksen Flanker Task (Eriksen & Eriksen, 1974) which was programmed and administered using Presentation software (Neurobehavioral Systems, Inc.) while EEG was continuously recorded. This task has been used in many studies with both children and adults to elicit a reliable ERN when participants make mistakes (Ladouceur, Dahl, Birmaher, Axelson, & Ryan, 2006; Meyer, Bress, & Proudfit, 2014; Meyer, Riesel, & Proudfit, 2013). On each trial, five angle brackets were presented. Participants were instructed to pay attention to only the middle bracket and to press the button on a button box that matched which way the bracket (“arrow”) was pointing. Half of the trials were congruent trials, in which all five brackets were pointing the same direction (e.g., > > > > >). The other half of the trials consisted of incongruent stimuli, in which the flanker brackets were pointing in the opposite direction of the middle bracket (e.g., > > < > >). The response deadline was 800 ms. Beginning after a response or 800 ms (whichever came first), an inter-trial interval (ITI) jittered between 900–1100 ms was used, during which time a fixation cross was displayed, followed by the brackets for 200 ms, and then the fixation cross immediately appeared again.
First, a research assistant explained the task to the child using stimuli that had been printed on paper so that instructions could be delivered slowly and so that the child could ask questions. The child then completed at least two computerized practice blocks before beginning the experimental blocks. During the first practice block, a research assistant provided corrective feedback to the child. Instructions emphasized accuracy over speed, though the child was encouraged to respond more quickly if he or she responded repeatedly after the response deadline (this only occurred occasionally). During the second practice block, a research assistant did not provide feedback but watched the child’s behavior to confirm that the child was performing the task (as opposed to pressing buttons arbitrarily). If the child did not appear to understand the task after the two standard practice blocks, additional practice blocks were administered as needed until the research assistant was confident that the child understood the task. Although we do not have a formal record of the number of practice blocks administered, most children did not require additional practice. After the practice blocks, the research assistant left the experiment room and the child completed six 50-trial blocks with short breaks between blocks. At each break, the child was given stickers and generic praise to maintain engagement in the task. On average, the task took about 30 minutes to complete, and it was the second of three tasks completed in a single recording session (the other two tasks are not included in the present study, and the order of the tasks was held constant for all participants). In data processing, children who performed at less than 60% accuracy were excluded from analyses in order to eliminate children who did not understand the task or who were performing around chance levels (see below). Participants were also excluded for having fewer than six artifact-free error trials (Olvet & Hajcak, 2009).
2.3 Electrophysiological Recording, Data Reduction, and Analysis
The EEG was recorded from 32 Ag/AgCl electrodes in an electrode cap with an average reference and forehead ground. Data were digitized at 1,024 Hz using ANT acquisition hardware (Advanced Neuro Technology, Enschede, The Netherlands) and all impedances were below 20 KΩ. Eye-blink correction was performed offline with ASA (Advanced Source Analysis) software from ANT. This correction procedure uses principal components analysis (PCA) to remove blink-related variance from continuous EEG recordings. After manual identification of several representative sample blinks, eye-blink correction was considered successful if the software was able to compute a single PCA factor that explained at least 96% of the noise subspace of the selected sample blinks.
The following EEG data processing steps were performed with custom MATLAB scripts and a MATLAB-based open source signal processing toolbox, FieldTrip (Oostenveld, Fries, Maris, & Schoffelen, 2011). This analysis procedure was previously reported by Palmwood et al. (2018). Continuous EEG data were re-referenced to the average of the mastoids. Epochs were extracted from 600 ms pre-response to 800 ms post-response onset. An 800 ms response deadline was used, such that only epochs with a button press made within 800 ms following stimulus presentation were extracted. Muscle and jump artifact rejection were then performed. Muscle artifact rejection was performed with the following steps: epochs were band-pass filtered with a 110–140 Hz Butterworth digital filter, Z-transformed, averaged across sensors, and epochs with Z values greater than 4 were rejected. The frequency range 110–140 Hz was selected to isolate myogenic energy because most muscle activity exceeds 100 Hz (Luck, 2014), and this frequency range is the standard recommended by the FieldTrip toolbox (Oostenveld, Fries, Maris, & Schoffelen, 2011). Jump artifact rejection was performed in the same way, except that data were not filtered and a Z value cutoff of 20 was used. For each epoch, average voltage between 600 ms pre-response and 400 ms pre-response served as the baseline. Lastly, the data were band-pass filtered from 0.2 to 30 Hz with a Butterworth digital filter.
Latency adjustment was performed on single trials to correct for increased ERP latency jitter associated with childhood. Developmental investigations of error ERPs have demonstrated that ERN amplitude increases nonlinearly from age 7 years to adulthood despite relatively no change in the error positivity (Pe), a positive voltage deflection typically observed shortly after the ERN peaks (Davies et al., 2004a, 2004b). Because the Pe has a longer time course than the ERN (due to reflecting activity in a lower frequency band; Luu, Tucker, & Makeig, 2004) and is therefore less susceptible to the effects of latency jitter, these developmental ERP results likely indicate that the time lag between error commission and error-related brain processes becomes more consistent as an individual matures. In fact, Lin, Gavin, and Davies (2015) showed that performing latency adjustment on single trial data from children as young as 7 years makes these data nearly indistinguishable from adult data in terms of ERN amplitude and measures of inter-trial phase synchronization.
We therefore performed latency adjustment following procedures similar to those described by Woody (1967) and recommended by Luck (2014). Again, these procedures were previously reported by Palmwood et al. (2018). Because Woody-filtering requires the selection of a single recording site, several candidate fronto-central sites were considered (Cz, Fz, and Pz). A repeated measures analysis of variance (ANOVA) with electrode site as a within-subjects factor indicated that the ERN (calculated as difference in amplitude between error trials and correct trials) was largest at Cz for the full sample (F(1,83) = 8.85, p = .004). Cz was therefore selected as the electrode site of interest for latency adjustment and subsequent analyses.
The following steps were performed for each participant and trial type (error, correct): 1) trials of each type, recorded at Cz, were averaged together and the time window from response onset to 300 ms post-response was designated the template; 2) this average template was dragged along each single trial at Cz, from 300 ms pre-response to 300 ms post-response, one sample at a time; 3) at each sample, a Pearson correlation coefficient was computed between the template and the single trial data; 4) each trial was aligned in time with respect to the sample offset at which the Pearson correlation was maximal for that trial, such that 0 ms in the aligned single trial would correspond to 0 ms in the template (i.e., the button press); and 5) the aligned trials were averaged together, producing a distinct waveform for each participant and trial type. ERN scores were quantified as the mean voltage from 50 ms pre-response to 100 ms post-response at Cz for error trials, and CRN (correct response negativity) scores were calculated as the mean voltage of the same time-window for correct response trials.
3. Results
3.1 Data Processing and Preliminary Analyses
The ERP task was completed by 176 children. Sixty performed the task at less than 60% accuracy and were excluded from analyses. An additional 31 were excluded because their ERP data were too noisy to analyze. Of those 31, eye-blink artifact correction was impossible for 10 subjects, 15 subjects had fewer than six artifact-free error and correct response trials, and 6 subjects were removed after visual inspection of ERP waveforms revealed artifacts of virtually impossible amplitude (i.e., > 100 µV). One additional case was removed because the ERN amplitude was greater than three standard deviations above the mean. This left 84 children in the final sample (42 from the CPS-referred group and 42 from the comparison group). Chi-square analyses indicated the proportion excluded from the comparison group was not significantly different from that excluded from the CPS-referred group, χ2(1) = 1.02, p = .31.
Potential intervention differences and effects were considered. One-way ANOVAs indicated that there were no significant intervention differences (ABC vs. DEF) on the ERN (F(1,40) = 2.07, p = .16) or depressive symptoms (F(1,36) = 2.06, p = .16). In addition, as parenting has been associated with the ERN in previous studies (Brooker & Buss, 2014; Meyer et al., 2015), and ABC targets parenting behaviors, group differences in parent sensitivity were examined. Parent sensitivity was measured in an observer-coded parent-child interaction task conducted at the 8-year lab visit (ICC = .89). There were no intervention effects on parent sensitivity in the final sample of the present study (F(1,39) = .09, p = .77). Although parents of children in the comparison group were more sensitive than CPS-referred parents (F(1,77) = 15.12, p < .001), parent sensitivity was not significantly associated with ERN amplitude in the full sample (r = .05, p = .68).
Zero-order correlations, means, and standard deviations are reported in Tables 1 and 2. Notably, all of the CBCL scales were all significantly correlated with each other (r = .36 – .84, p-values ≤ .001). In addition, accuracy on the flanker task was positively correlated with age (r = .30, p = .001; older children were more accurate) and negatively correlated with sample (r = −.26 p = .005; comparison children were more accurate) and with the Withdrawn/Depressed scale (r = −.22, p = .02; children with fewer symptoms were more accurate). Gender was not associated with any study variables. Finally, in the full sample, the ERN was positively correlated with depressive symptoms, such that more symptoms were associated with smaller ERNs (r = .22, p < .05). In addition, slower reaction times were associated with smaller ERNs (r = .29, p = .008).
Table 1.
Correlations of study variables and covariates.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Age | -- | ||||||||||||||||
| 2. Gender | −.02 | -- | |||||||||||||||
| 3. CPS Involvement | −.15 | −.11 | -- | ||||||||||||||
| 4. CBCL Anxious/Depressed | −.02 | .17 | .06 | -- | |||||||||||||
| 5. CBCL Withdrawn/Depressed | −.15 | −.19 | .30** | .42*** | -- | ||||||||||||
| 6. CBCL DSM-Oriented Anxiety | −.02 | .22 | .01 | .84*** | .36** | -- | |||||||||||
| 7. CBCL Externalizing | −.07 | −.10 | .40*** | .53*** | .42*** | .46*** | -- | ||||||||||
| 8. Flanker Accuracy | .30** | .06 | −.26** | .10 | −.22* | .08 | −.02 | -- | |||||||||
| 9. Flanker Reaction Time - Overall | −.07 | .16 | −.09 | −.00 | .04 | .07 | .07 | .18* | -- | ||||||||
| 10. Flanker Reaction Time - Error | −.12 | .12 | −.14 | .04 | .10 | .07 | .04 | .12 | .74*** | -- | |||||||
| 11. Flanker Reaction Time - Correct | −.08 | .16 | −.07 | −.02 | .03 | .04 | .07 | .15 | .99*** | .71*** | -- | ||||||
| 12. Flanker PES - Traditional | .11 | .11 | −.14 | .02 | −.09 | .03 | .07 | .21* | .11 | .22* | .09 | -- | |||||
| 13. Flanker PES - Robust | .16† | −.01 | −.12 | .03 | .05 | .01 | .09 | .23* | .27** | .23* | .25** | .69*** | -- | ||||
| 14. Flanker Post-error Accuracy (PEA) | .21* | −.04 | −.19* | −.01 | −.11 | .05 | −.01 | .73*** | .19* | .11 | .16† | .25** | .20* | -- | |||
| 15. Flanker PEA - Difference Score | .05 | −.17† | −.04 | −.09 | .02 | .00 | −.02 | .16† | −.17† | −.12 | −.17† | .14 | −.02 | .73*** | -- | ||
| 16. ERN | −.19 | .04 | −.02 | −.03 | .22* | .07 | .06 | −.05 | .29** | .33** | .26* | −.03 | .07 | .07 | −.02 | -- | |
| 17. CRN | −.24* | .06 | .05 | −.15 | −.03 | −.04 | −.15 | .14 | .06 | .04 | .06 | .01 | −.05 | .04 | −.03 | .50** | -- |
p < .1;
p < .05;
p < .01;
p < .001.
Note: For the Gender variable, males were dummy coded as "1" and females were dummy coded as "2." For the CPS Involvement variable, the CPS-referred group was dummy coded as “1” and the comparison group was dummy coded as “0.” PES - Traditional: Post-error slowing is calculated by subtracting reaction time on post-correct trials from reaction time on post-error trials. PES - Robust: An average of the differences between reaction time on paired post-error trials and pre-error trials. CPS: Child Protective Services. CBCL: Child Behavior Checklist. ERN: Error-related negativity. CRN: Correct response negativity.
Table 2.
Means and standard deviations of study variables by sample.
| CPS-Referred | Comparison | |||
|---|---|---|---|---|
|
|
||||
| M | SD | M | SD | |
| CBCL Anxious/Depressed | 2.24 | 2.5 | 1.98 | 2.23 |
| CBCL Withdrawn/Depressed | 1.42 | 1.69 | .57 | 1.06 |
| CBCL DSM-Oriented Anxiety Problems | 1.35 | 1.46 | 1.40 | 1.71 |
| CBCL Externalizing Problems | 9.25 | 8.89 | 3.66 | 2.89 |
| Flanker Accuracy-Overall (%) | .77 | .11 | 0.83 | .10 |
| Flanker Accuracy-Congruent (%) | .85 | .09 | .88 | .09 |
| Flanker Accuracy-Incongruent (%) | .70 | .15 | .77 | .13 |
| Flanker Reaction Time (ms) | 365.88 | 86.52 | 381.21 | 92.27 |
| Flanker Reaction Time-Error (ms) | 259.75 | 85.99 | 283.10 | 82.76 |
| Flanker Reaction Time-Correct (ms) | 373.99 | 77.61 | 384.94 | 83.54 |
| Flanker Reaction Time-Congruent (ms) | 344.21 | 81.44 | 354.77 | 87.62 |
| Flanker Reaction Time-Incongruent (ms) | 388.79 | 96.00 | 409.62 | 100.08 |
| Flanker Post-error Reaction Time (ms) | 373.08 | 111.19 | 401.69 | 102.46 |
| Flanker Post-error Slowing (ms) - Traditional | 8.32 | 66.90 | 24.36 | 43.80 |
| Flanker Post-error Slowing (ms) - Robust | 49.15 | 65.69 | 64.81 | 63.46 |
| Flanker Post-error Accuracy (%) | .79 | .13 | .84 | .15 |
| Flanker Post-error Accuracy (%) - Difference | −.04 | .08 | −.03 | .12 |
| ERN (µV) | 5.4 | 10.04 | 5.78 | 8.97 |
| CRN (µV) | 18.05 | 9.98 | 17.21 | 8.24 |
| Age (years) | 8.48 | .37 | 8.52 | .33 |
| Gender (% Female) | .45 | -- | .49 | -- |
Note: Post-error slowing—traditional: Post-error slowing is calculated by subtracting reaction time on post-correct trials from reaction time on post-error trials. Post-error slowing—robust: An average of the differences between reaction time on paired post-error trials and pre-error trials. CBCL: Child Behavior Checklist. ERN: error-related negativity. CRN: correct response negativity.
Of the 60 excluded for their behavioral performance, 39 were from the CPS-referred group and 21 were from the comparison group. The proportions excluded from each group were significantly different, χ2(1)= 4.01, p = .045. In addition, CBCL symptoms differed between the included sample and the excluded sample, such that the included sample exhibited fewer problems on almost all scales. The included sample had significantly fewer behavior problems on the following CBCL scales: Withdrawn/Depressed (p = .04), Social Problems (p = .01), Attention (p = .01), Internalizing (p = .049), Externalizing (p = .04), and DSM Anxiety Problems (p = .04). Differences were marginally significant for Anxious/Depressed, Thought Problems, Rule Breaking, and Aggression.
Overall, as expected, these results indicate that the sample excluded from analyses due to their behavioral performance (because they were unable to learn and/or perform the task sufficiently well) had more behavior problems and were more likely to be from the CPS-referred group than the comparison group. However, because CPS-referred children were over-represented in the full sample relative to comparison children, the final sample was composed of comparably sized groups. In addition, the included group and excluded group did not specifically differ on the scale of interest (i.e., Withdrawn/Depressed), but rather differed on almost all scales on the CBCL.
3.2 Behavioral Data
Behavioral data are reported for all children who performed the task with at least 60% accuracy (n = 116; CPS-referred n = 57, comparison n = 59). To examine effects of trial type and group on behavioral performance, separate 2 (group: CPS-referred, comparison) × 2 (trial type: error vs. correct) mixed model ANOVAs were run predicting response time and accuracy. Each model tested group as a between-subjects factor, and one trial type (congruent vs. incongruent; post-error vs. post-correct) or response type (error vs. correct) as a within-subjects factor.
Main effects are reported first, followed by interactions. In all models there was a significant main effect of trial type, such that children were slower and less accurate on incongruent trials than on congruent trials (reaction time: F(1,114) = 230.02, p < .001; accuracy: F(1,114) = 152.61, p < .001), as well as on post-error response trials relative to post-correct response trials (reaction time: F(1,114) = 9.75, p = .002; accuracy: F(1,114) = 15.86, p < .001). In addition, there was a significant main effect of group for accuracy (congruent/incongruent: F(1,114) = 7.83, p = .006; post-error/post-correct: F(1,114) = 6.25, p = .010) such that CPS-referred children were less accurate overall than comparison children. There was no main effect of group on reaction time (congruent/incongruent: F(1,114) = .88, p = .35; error/correct: F(1,114) = 1.47, p = .23; post-error/post-correct: F(1,114) = 1.39, p = .24). There was a marginal interaction of group by trial type (congruent/incongruent) for accuracy (F(1,114) = 2.81, p = .09), such that the difference in accuracy between CPS-referred and comparison children was greater for incongruent trials than congruent trials. There was not a significant group by trial type interaction for congruent vs. incongruent trial response time, error vs. correct response trial response time, post-error vs. post-correct accuracy, or post-error vs. post-correct reaction time.
To understand the potential implications of post-error slowing (PES) on accuracy, post-error slowing was calculated two ways, referred to as traditional PES and robust PES (Dutilh et al., 2012). The traditional PES variable was defined as the difference in mean reaction time between post-error trials and post-correct trials, with larger values indicating more slowing after error trials relative to correct trials. The robust PES variable was created by first calculating the differences between reaction time on paired post-error trials and pre-error trials, and then averaging those differences for each participant, which accounts for global state changes over the task (Dutilh et al., 2012). Post-error accuracy was defined as the mean accuracy on trials immediately following errors. Indeed, post-error slowing was positively correlated with post-error accuracy (traditional PES: r = .25, p = .006; robust PES: r = .20, p = .03) for the full sample, suggesting that slowing may be an adaptive process. However, PES was not correlated with PEA when PEA was calculated as the difference in accuracy between post-error and post-correct trials (traditional PES: r = .14, p = .13; robust PES: r = −.02, p = .86).
Post hoc exploratory analyses examined group differences in this association. Although the correlation between post-error slowing and post-error accuracy remained significant or marginally significant in the CPS-referred group (traditional PES: r = .34, p = .01; robust PES: r = .25, p = .06), it was not significant for the comparison group (traditional PES: r = .12, p = .36; robust PES: r = .11, p = .40). When PEA was calculated as a difference score, it was not significantly correlated with traditional or robust PES for either group, but it was marginally correlated with traditional PES for the CPS-referred group (r = .23, p = .09). Regression models testing the interaction between group and PES predicting PEA were not significant. Interestingly, although the CPS-referred group was less accurate overall than the comparison group, the samples did not differ in post-error slowing (traditional PES: t(114) = −1.53, p = .13; robust PES: t(113) = −1.30, p = .20), and the earlier main effect of trial type indicated that the full sample responded more slowly following error trials than correct trials. This pattern of results suggests that although both groups adjusted their performance following errors at similar rates, it only was associated with improved accuracy among the CPS-referred group.
3.3. Electrophysiological Data
For preliminary analyses, both Woody-filtered and non-Woody-filtered ERP data were considered. Separate 2 (group: CPS-referred, comparison) × 2 (response type: error, correct) mixed model ANOVAs were run for the non-Woody- and Woody-filtered ERN data. There was a main effect of response type for both analyses such that ERNs were larger (more negative) than CRNs (non-Woody: F(1, 82) = 34.47, p < .001; Woody: F(1, 82) = 139.87, p < .001), and neither analysis produced a significant response type by group interaction (non-Woody: F(1,82) = .17, p = .68; Woody: F(1,82) = .36, p = .55). There was no main effect of group in either analysis (non-Woody: F(1,82) < .001, p = .99; Woody: F(1,82) = .02, p = .90). Finally, a bivariate correlation was run with the Woody-filtered and non-Woody-filtered ERNs to test whether they were significantly related, and there was a significant correlation between the two variables (r = .77, p < .001). As these preliminary analyses produced the same pattern of results for the Woody-filtered and the non-Woody filtered data, and the Woody-filtered data retained much of the variance from the non-Woody-filtered data, the Woody-filtered data were selected for subsequent analyses. Based on visual inspection of the grand average waveforms, latency adjustment seemed to successfully reduce noise resulting from the variable latency of the ERN in our sample, potentially increasing power for subsequent analyses and decreasing the likelihood of making a Type II error. Figure 1 depicts response-locked ERP waveforms by group and response type, for both the Woody-filtered and non-Woody-filtered data.
Figure 1.
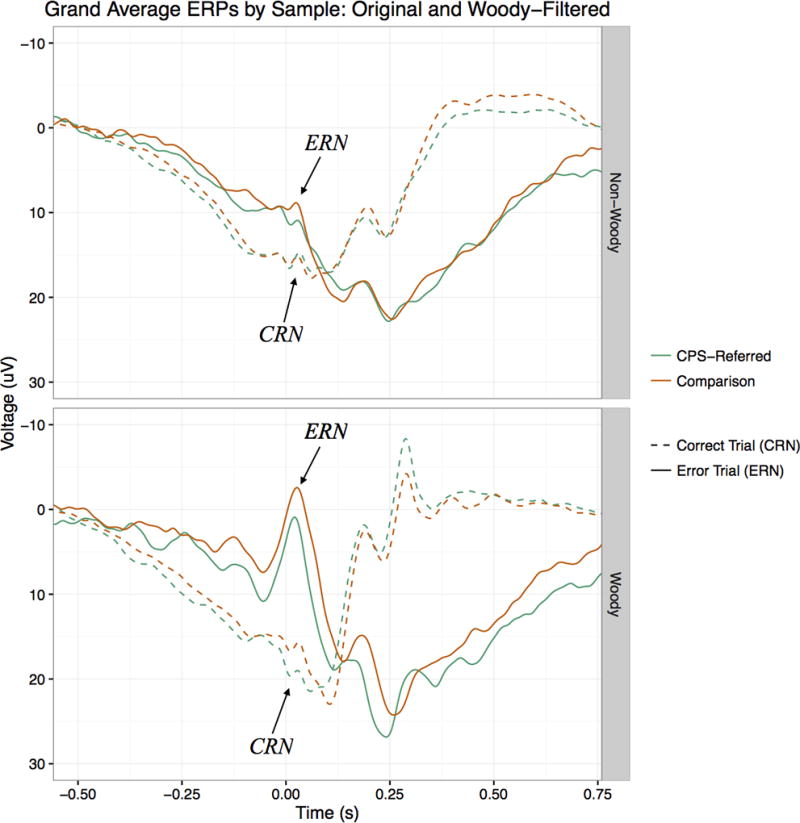
Response-locked ERPs by sample and response type, for Woody-filtered data (bottom figure) and non-Woody-filtered data (top figure). ERN and CRN peaks are labeled with arrows for visual clarity, but mean amplitude measures were used in analyses.
3.4 ERN and Depressive Symptoms
3.4.1 Preliminary analyses
Of the 84 children with usable ERP data, 80 children had complete CBCL data (38 in the CPS-referred group and 42 in the comparison group). Parents of children in the CPS-referred group reported significantly more depressive symptoms in their children (M = 1.42, SD = 1.69) than did parents of children in the comparison group (M = .57, SD = 1.06), t(61.22) = 2.66, p = .01. Zero-order correlations indicated that for the full sample, more depressive symptoms were associated with a smaller (more positive) ERN (r = .22, p < .05) than were fewer depressive symptoms.
Several possible covariates were considered for the following analyses. Age and gender were selected as covariates because, although they were largely uncorrelated with study variables in this sample, they have been associated with ACC development as well as the ERN and its relation to child behavior problems in other studies (Davies et al., 2004a, 2004b; Fischer, Danielmeier, Villringer, & Ullsperger, 2016; Larson, South, & Clayson, 2011; Meyer et al., 2012; Ruigrok et al., 2014). In order to isolate error-related activity, the CRN was considered as a covariate. As results did not differ between models using the ERN and models using the residualized ERN (i.e., the residual variance of the ERN after the variance of the CRN has been removed) as the outcome, the final model presented uses the ERN alone. Although intervention type was found to be unrelated to the ERN or depressive symptoms, it was considered as a covariate in the following regression analyses. As the inclusion of intervention type in the model did not alter the pattern of results, it was not included in the final model for parsimony. Likewise, although overall accuracy on the flanker task was associated with CPS involvement in zero-order correlations, including accuracy as a covariate did not affect regression results, and so was excluded from the final model.
In addition, the other CBCL scales were available for use as covariates, and the Anxious/Depressed, DSM-Oriented Anxiety, and Externalizing scales were considered. Because the ERN is more consistently associated with symptoms of anxiety than depression, and there is typically comorbidity with anxiety and depressive symptoms, anxiety (as measured by either CBCL scale) was tested as a covariate. In addition, as CPS involvement was associated more Externalizing problems (r = .40, p < .001), and externalizing problems have been linked to the ERN in previous studies (Stieben et al., 2007), externalizing problems were considered as a covariate. However, including either anxiety or externalizing problems (or both) did not alter the direction or significance of depression effects observed. Further, because the CBCL scales are highly correlated, removing the shared variance of these scales in the present analyses may make results more difficult to interpret and less ecologically valid (e.g. what does it mean to remove the “anxious” and “externalizing” variance from depressive symptoms?). Therefore, other CBCL scales are not included in the final model.
3.4.2 Testing moderation
After selecting covariates, CPS involvement was tested as a moderator of the association between depressive symptoms and the ERN using hierarchical multiple regression. In the first step, age and gender were entered as control variables predicting ERN amplitude. The first model was not significant (R2 = .04, p = .23), and neither age nor gender was a significant predictor. In the second step, mean-centered depressive symptoms and CPS involvement were entered as predictors of the ERN. This model was also not significant (R2 = .10, p = .12), depressive symptoms were significant (β = .26, p = .04), and CPS involvement was not significant (β = −.07, p = .56). This indicates that in the full sample, depressive symptoms were associated with a blunted ERN, when controlling for age, gender, and CPS involvement.
In the third and final step, an interaction term was added as the product of the two mean-centered predictors (CPS involvement and depressive symptoms). The final model was significant (R2 = .16, p = .03), as was the interaction term (B = 3.94, SE = 1.72, p = .03), and the R2 change due to the addition of the interaction term (ΔR2 = .06, p = .03). After including the interaction term in the model, depressive symptoms alone were no longer a significant predictor of ERN amplitude (see Table 3 for full results). To understand this interaction, simple slopes were calculated and plotted using the PROCESS macro in SPSS 24.0 (Hayes, 2012; see Figure 2). For the comparison group, the slope of the line was not significantly different from zero (B = −1.17, p = .43), indicating that there is not a significant relationship between depressive symptoms and the ERN in this group. However, the slope for the CPS-referred group was significant and positive (B = 2.77, p = .003), indicating that in this group, more depressive symptoms are associated with smaller (more positive) ERNs. The full model was re-run predicting the CRN instead of the ERN, and neither the overall model (R2 = .046 p = .48) nor any individual predictors were significant (p = .60 – .74), suggesting that the ERN results are specific to error-monitoring rather than performance-monitoring in general.
Table 3.
Hierarchical regression predicting the ERN from depressive symptoms and CPS involvement.
| Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|
| B | SE | β | B | SE | β | B | SE | |
| (Constant) | 47.47† | 27.11 | 39.56 | 27.36 | 45.05† | 26.67 | ||
| Age | −5.11 | 3.14 | −.19 | −4.23 | 3.14 | −.16 | −5.03 | 3.07 |
| Gender | 1.21 | 2.23 | .06 | 1.96 | 2.25 | .10 | 1.99 | 2.19 |
| Depressive Sx | 1.70 | .81 | .26* | −1.17 | 1.48 | |||
| CPS Involvement | −1.37 | 2.31 | –.07 | −.68 | 2.27 | |||
| Depression Sx * CPS Involvement | 3.94* | 1.72 | ||||||
| R2 | .04 | .10 | .16* | |||||
| F | 1.51 | 1.89 | 2.65 | |||||
| ΔR2 | .06 | .06* | ||||||
p < .1;
p < .05.
Note: For the Gender variable, males were dummy coded as "1" and females were dummy coded as "2." For the CPS Involvement variable, the CPS-referred group was dummy coded as “1” and the comparison group was dummy coded as “0." CPS: Child Protective Services.
Figure 2.
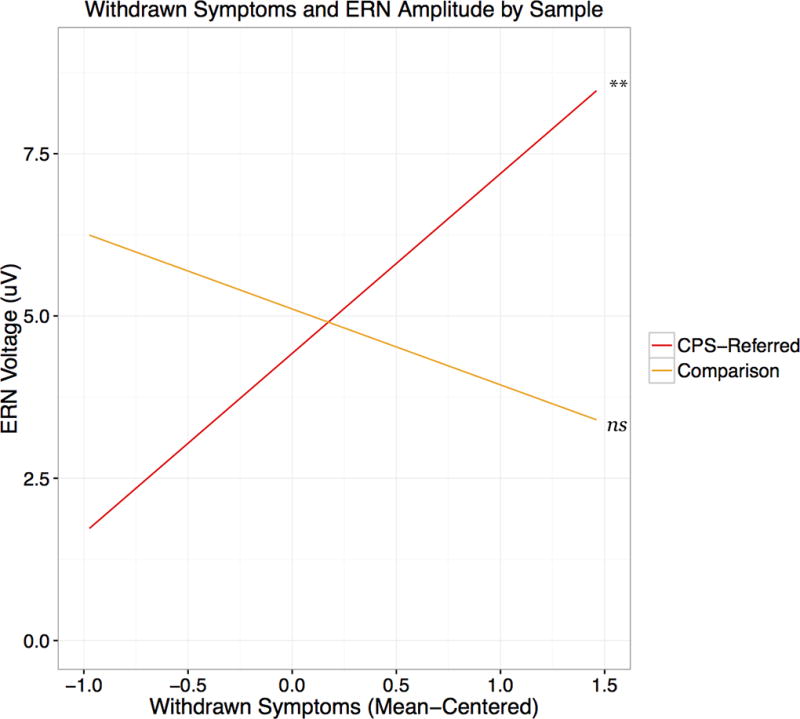
Simple slopes of the interaction of depressive (withdrawn) symptoms and ERN amplitude by sample. Note: A more positive ERN amplitude indicates a smaller ERN.
4. Discussion
The present study considered the relation between depressive symptoms and the ERN in CPS-referred and comparison children. No association was found between depressive symptoms and the ERN in the comparison group, but a significant association was found in the CPS-referred group such that more depressive symptoms were associated with a smaller, or blunted, ERN. This pattern provides support for the notion that the association between depression and the ERN may differ depending on sample characteristics, a hypothesis proposed in a recent meta-analytic study (Moran et al., 2017). A critical next step in refining theories of basic neurophysiological processes and development will be to incorporate environmental risk as well as other aspects of individual identity and experiences in future research.
It remains unclear why there was no main effect of group on the ERN, despite the finding that CPS-referred children exhibited significantly more depressive symptoms than comparison children. In addition, it is not clear why ERN amplitude was not significantly related to depressive symptoms in the comparison group. One possibility is that limited range and variance in depressive symptoms in the comparison group could reduce the power to detect a relation between symptoms and the ERN, but both groups reported relatively few symptoms (compared to what would be expected in a clinically depressed population, for example). Another possibility relates to associations between maltreatment and brain development (Teicher et al., 2016). Specifically, if the development of neural structures and networks that generate the ERN is altered (accelerated or slowed, for example) by an inadequate early caregiving environment, it is plausible that CPS-referred children would show a different pattern of psychophysiological associations than that observed in comparison children. Additional research on the association between the ERN and psychopathology in CPS-referred children, ideally with detailed referral information, is necessary in order to fully explicate this link. Incorporating neural measures in research on the development of psychopathology in maltreated and non-maltreated children may shed light on how experiences of inadequate early caregiving are related to psychopathology and even why some individuals who experience early adversity like maltreatment do not develop psychopathology.
In addition, due to a lack of access to detailed referral information, the present study grouped together children who had been likely neglected and/or abused into one CPS-referred sample. As previous research suggests that neglect and abuse may have differential effects on children’s developmental outcomes (McLaughlin, Sheridan, & Lambert, 2014), grouping children at risk for abuse and neglect together may reduce the likelihood of detecting group differences in the ERN. Future research could aim to investigate how these early experiences might uniquely relate to the ERN and psychopathology for children. This is particularly relevant if the ERN is conceptualized as an indicator of threat processing, as abuse may be more likely to be experienced as “threatening” than neglect, and so abuse may more directly affect the developing threat processing system. However, as the sample includes children who may have experienced either abuse or neglect (or both), associations between specific experiences and the ERN remain unclear.
It is noteworthy that the measure of depression used in this study was the Withdrawn/Depressed subscale of the CBCL. Anxiety and depression are often comorbid (a phenomenon that is reflected in another CBCL subscale, “Anxious/Depressed”), but the items in this scale are more characteristic of depression alone – especially anhedonia and social withdrawal. Although children in this sample did not exhibit clinical levels of depressive symptoms at the time of data collection, those with blunted ERNs may indeed be at greater risk for developing major depressive disorder. Future research may investigate whether the severity of specific symptoms like anhedonia is related to ERN amplitude, as well as whether blunted ERNs at age 8 may be a risk factor for major depressive disorder later on, even if they are not exhibiting clinical levels of depressive symptoms in middle childhood.
There were several differences between the groups in their behavioral performance on the flanker task. First, the comparison group was more accurate overall, but this effect was stronger for incongruent trials than for congruent trials. Children who have experienced maltreatment may have particular difficulty with maintaining focused attention while ignoring distractions. This fits with a large body of literature demonstrating that early adversity is associated with cognitive deficits (Pechtel & Pizzagalli, 2011). In terms of post-error behavior, the present study found that slowing (traditional and robust PES) was positively correlated with accuracy for the full sample, but not when accuracy was calculated as the difference between post-error accuracy and post-correct accuracy. Additionally, in post hoc analyses, slowing was only significantly correlated with accuracy in the CPS-referred group. That is, after making errors, children from the CPS-referred group who were able to utilize cognitive control to slow down improved their performance on subsequent trials, but slowing did not seem to affect accuracy for comparison children. It is not clear why slowing did not correspond to greater accuracy in the comparison group, but other studies have found that post-error slowing is not associated with improved post-error performance (Danielmeier & Ullsperger, 2011; Ullsperger, Danielmeier, & Jocham, 2014; see also Valadez & Simons, 2017). An important caveat to these interpretations of group differences in PES and PEA is that the association did not hold when PEA was calculated as a difference score (although the correlation between traditional PES and PEA as a difference score was marginally significant for the CPS-referred group). Though the present study was underpowered to combine CPS involvement, depressive symptoms, the ERN, and behavioral performance in a single model, future research with a larger sample may help explain these findings.
There are several limitations of the present study. First, the effect sizes and samples were relatively small. Replication is needed in order to determine the robustness of the relation between depressive symptoms and the ERN. A larger sample size would also allow for the construction of more complex models including multiple moderators and mediators like gender or parenting. Further, we were unable to test the association between the ERN and depressive symptoms in children who could not adequately perform the flanker task, and so our included sample was relatively higher functioning (i.e., had fewer emotional and behavioral problems) than the excluded sample. Although the Flanker task (with arrows) has been used successfully with children of this age group before, modifications may be made to adapt it for younger children, and our experience suggests that these modifications may be useful for high-risk children in middle childhood. Future research with similar samples should consider piloting an easier task in order to retain more children for analyses, but also elicit a sufficient number of errors to obtain reliable ERNs.
Additionally, the available measures limited our ability to detect an association between anxiety symptoms and the ERN. First, the literature suggests that only certain symptom profiles of anxiety have been consistently associated with an enhanced ERN (e.g. checking behaviors, obsessive-compulsive disorder), and the anxiety scales on the CBCL (Anxious/Depressed and DSM-Oriented Anxiety) include multiple types of anxiety symptoms. Further, the Anxious/Depressed scale also includes depressive symptoms. If anxiety symptoms are associated with an enhanced ERN and depressive symptoms with a blunted ERN, those opposing effects would obfuscate each other. Future research could use more sensitive and specific measures of anxiety and depressive symptoms, in addition to including multiple reporters, to better understand these associations.
Despite these limitations, the present study provides meaningful contributions to the small literature on the ERN and depression in children. It offers new evidence on the relation between the ERN and depressive symptoms in CPS-referred children, and opens the door to new questions about why the effect may differ between CPS-referred children and children without CPS involvement. Considering sample characteristics such as maltreatment in research on the ERN makes it possible to assess the generalizability of previous findings, as well as further develop theory about the nature and role of the ERN in psychological processes. Additionally, as these data were collected as part of an ongoing longitudinal study, future research can test developmental trajectories of the ERN and depressive symptomatology over time. Ultimately, this research could determine the utility of the ERN as a biomarker of risk for psychopathology.
Acknowledgments
This work was supported by funding from the National Institute of Mental Health (NIMH), grant number R01MH074374 to the sixth author.
Footnotes
The authors have no conflicts of interest to disclose.
Detailed referral information is not available for this sample, so we are unable to distinguish specific types or severity of maltreatment in the present study. However, children with substantiated and unsubstantiated maltreatment reports seem to be at similar risk for negative behavioral and developmental outcomes (Hussey et al., 2009).
References
- Achenbach TM, Rescorla LA. Manual for ASEBA School Age Forms & Profiles. Burlington, VT: University of Vermont Research Center for Children, Youth and Families; 2001. [Google Scholar]
- Bernard K, Dozier M, Bick J, Lewis-Morrarty E, Lindhiem O, Carlson E. Enhancing attachment organization among maltreated children: Results of a randomized clinical trial. Child Development. 2012;83(2):623–636. doi: 10.1111/j.1467-8624.2011.01712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bick J, Nelson CA. Early adverse experiences and the developing brain. Neuropsychopharmacology. 2016;41(1):177–196. doi: 10.1038/npp.2015.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bress JN, Meyer A, Hajcak G. Differentiating anxiety and depression in children and adolescents: Evidence from event-related brain potentials. Journal of Clinical Child and Adolescent Psychology. 2015;44(2):238–249. doi: 10.1080/15374416.2013.814544. [DOI] [PubMed] [Google Scholar]
- Brooker RJ, Buss KA. Harsh parenting and fearfulness in toddlerhood interact to predict amplitudes of preschool error-related negativity. Developmental Cognitive Neuroscience. 2014;9:148–159. doi: 10.1016/j.dcn.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert BN. The RDoC framework: Facilitating transition from ICD/DSM to dimensional approaches that integrate neuroscience and psychopathology. World Psychiatry. 2014;13(1):28–35. doi: 10.1002/wps.20087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielmeier C, Ullsperger M. Post-error adjustments. Frontiers in Psychology. 2011;2:233. doi: 10.3389/fpsyg.2011.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PL, Segalowitz SJ, Gavin WJ. Development of error-monitoring event-related potentials in adolescents. Annals of the New York Academy of Science. 2004b;1021:324–328. doi: 10.1196/annals.1308.039. [DOI] [PubMed] [Google Scholar]
- Davies PL, Segalowitz SJ, Gavin WJ. Development of response-monitoring ERPs in 7- to 25-year-olds. Developmental Neuropsychology. 2004b;25(3):355–376. doi: 10.1207/s15326942dn2503_6. [DOI] [PubMed] [Google Scholar]
- Dutilh G, van Ravenzwaaij D, Nieuwenhuis S, van der Maas HL, Forstmann BU, Wagenmakers EJ. How to measure post-error slowing: a confound and a simple solution. Journal of Mathematical Psychology. 2012;56(3):208–216. doi: 10.1016/j.jmp.2012.04.001. [DOI] [Google Scholar]
- Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a nonsearch task. Perception and Psychophysics. 1974;16(1):143–149. doi: 10.3758/BF03203267. [DOI] [Google Scholar]
- Fischer AG, Danielmeier C, Villringer A, Klein TA, Ullsperger M. Gender influences on brain responses to errors and post-error adjustments. Scientific Reports. 2016:6. doi: 10.1038/srep24435. [DOI] [PMC free article] [PubMed]
- Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychological Science. 1993;4(6):385–390. doi: 10.1111/j.1467-9280.1993.tb00586.x. [DOI] [Google Scholar]
- Goff B, Tottenham N. Early-life adversity and adolescent depression: mechanisms involving the ventral striatum. CNS spectrums. 2015;20(4):337–345. doi: 10.1017/S1092852914000674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna GL, Liu Y, Isaacs YE, Ayoub AM, Torres JJ, O’Hara NB, Gehring WJ. Withdrawn/depressed behaviors and error-related brain activity in youth with obsessive-compulsive disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2016;55(10):906–913.e2. doi: 10.1016/j.jaac.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes A. PROCESS: A versatile computational tool for observed variable mediation, moderation, and conditional process modeling. 2012 [White paper]. Retreived from http://www.afhayes.com/public/process2012.pdf.
- Heim C, Binder EB. Current research trends in early life stress and depression: Review of human studies on sensitive periods, gene–environment interactions, and epigenetics. Experimental Neurology. 2012;233(1):102–111. doi: 10.1016/j.expneurol.2011.10.032. [DOI] [PubMed] [Google Scholar]
- Hussey JM, Marshall JM, English DJ, Knight ED, Lau AS, Dubowitz H, Kotch JB. Defining maltreatment according to substantiation: Distinction without a difference? Child Abuse & Neglect. 2005;29:479–492. doi: 10.1016/j.chiabu.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Kaufman J. Depressive disorders in maltreated children. Journal of the American Academy of Child and Adolescent Psychiatry. 1991;30(2):257–65. doi: 10.1097/00004583-199103000-00014. [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Dahl RE, Birmaher B, Axelson DA, Ryan ND. Increased error-related negativity (ERN) in childhood anxiety disorders: ERP and source localization. Journal of Child Psychology and Psychiatry. 2006;47(10):1073–1082. doi: 10.1111/j.1469-7610.2006.01654.x. [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Slifka JS, Dahl RE, Birmaher B, Axelson DA, Ryan ND. Altered error-related brain activity in youth with major depression. Developmental Cognitive Neuroscience. 2012;2(3):351–362. doi: 10.1016/j.dcn.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson MJ, South M, Clayson PE. Sex differences in error-related performance monitoring. Neuroreport. 2011;22(1):44–48. doi: 10.1097/WNR.0b013e3283427403. [DOI] [PubMed] [Google Scholar]
- Lin M, Gavin W, Davies P. Developmental trend of error-related negativity (ERN) in 7-to 25-year-olds after adjusting for trial-to-trial variability; Poster presented at the annual meeting of the Society for Psychophysiological Research; Seattle. 2015. Oct, [Google Scholar]
- Liotti M, Mayberg HS. The role of functional neuroimaging in the neuropsychology of depression. Journal of Clinical and Experimental Neuropsychology. 2001;23(1):121–136. doi: 10.1076/jcen.23.1.121.1223. [DOI] [PubMed] [Google Scholar]
- Loman MM, Johnson AE, Westerlund A, Pollak SD, Nelson CA, Gunnar MR. The effect of early deprivation on executive attention in middle childhood. Journal of Child Psychology and Psychiatry. 2013;54(1):37–45. doi: 10.1111/j.1469-7610.2012.02602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ. An introduction to the Event-Related Potential Technique. 2. Cambridge, MA: The MIT Press; 2014. [Google Scholar]
- Luu P, Tucker DM, Makeig S. Frontal midline theta and the error-related negativity: Neurophysiological mechanisms of action regulation. Clinical Neurophysiology. 2004;115(8):1821–1835. doi: 10.1016/j.clinph.2004.03.031. [DOI] [PubMed] [Google Scholar]
- McDermott JM, Westerlund A, Zeanah CH, Nelson CA, Fox NA. Early adversity and neural correlates of executive function: Implications for academic adjustment. Developmental Cognitive Neuroscience. 2012;2(SUPPL. 1):S59–S66. doi: 10.1016/j.dcn.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Lambert HK. Childhood adversity and neural development: deprivation and threat as distinct dimensions of early experience. Neuroscience & Biobehavioral Reviews. 2014;47:578–591. doi: 10.1016/j.neubiorev.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Bress JN, Hajcak G, Gibb BE. Maternal depression is related to reduced error-related brain activity in child and adolescent offspring. Journal of Clinical Child and Adolescent Psychology. 2016:1–12. doi: 10.1080/15374416.2016.1138405. [DOI] [PMC free article] [PubMed]
- Meyer A, Bress JN, Proudfit GH. Psychometric properties of the error-related negativity in children and adolescents. Psychophysiology. 2014;51(7):602–610. doi: 10.1111/psyp.12208. [DOI] [PubMed] [Google Scholar]
- Meyer A, Proudfit GH, Bufferd SJ, Kujawa AJ, Laptook RS, Torpey DC, Klein DN. Self-reported and observed punitive parenting prospectively predicts increased error-related brain activity in six-year-old children. Journal of Abnormal Child Psychology. 2015;43(5):821–829. doi: 10.1007/s10802-014-9918-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Riesel A, Proudfit GH. Reliability of the ERN across multiple tasks as a function of increasing errors. Psychophysiology. 2013;50(12):1220–1225. doi: 10.1111/psyp.12132. [DOI] [PubMed] [Google Scholar]
- Meyer A, Weinberg A, Klein DN, Hajcak G. The development of the error-related negativity (ERN) and its relationship with anxiety: Evidence from 8 to 13 year-olds. Developmental Cognitive Neuroscience. 2012;2(1):152–161. doi: 10.1016/j.dcn.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran TP, Schroder HS, Kneip C, Moser JS. Meta-analysis and psychophysiology: A tutorial using depression and action-monitoring event-related potentials. International Journal of Psychophysiology. 2017;111:17–32. doi: 10.1016/j.ijpsycho.2016.07.001. [DOI] [PubMed] [Google Scholar]
- Olvet DM, Hajcak G. The error-related negativity (ERN) and psychopathology: Toward an endophenotype. Clinical Psychology Review. 2008;28(8):1343–54. doi: 10.1016/j.cpr.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olvet DM, Hajcak G. The stability of error-related brain activity with increasing trials. Psychophysiology. 2009;46(5):957–961. doi: 10.1111/j.1469-8986.2009.00848.x. [DOI] [PubMed] [Google Scholar]
- Olvet DM, Klein DN, Hajcak G. Depression symptom severity and error-related brain activity. Psychiatry Research. 2010;179(1):30–37. doi: 10.1016/j.psychres.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen JM. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Computational Intelligence and Neuroscience. 2011;2011:1–9. doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmwood EN, Valadez EA, Zajac L, Griffith A, Simons RF, Dozier M. Early exposure to intimate partner violence predicts hypervigilant error monitoring. 2018 doi: 10.1016/j.ijpsycho.2022.01.006. Manuscript submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlakis AE, Noble K, Pavlakis SG, Ali N, Frank Y. Brain imaging and electrophysiology biomarkers: Is there a role in poverty and education outcome research? Pediatric Neurology. 2015;52(4):383–388. doi: 10.1016/j.pediatrneurol.2014.11.005. [DOI] [PubMed] [Google Scholar]
- Pechtel P, Pizzagalli DA. Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacology. 2011;214(1):55–70. doi: 10.1007/s00213-010-2009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruigrok AN, Salimi-Khorshidi G, Lai MC, Baron-Cohen S, Lombardo MV, Tait RJ, Suckling J. A meta-analysis of sex differences in human brain structure. Neuroscience & Biobehavioral Reviews. 2014;39:34–50. doi: 10.1016/j.neubiorev.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons RF. The way of our errors: Theme and variations. Psychophysiology. 2010;47(1):1–14. doi: 10.1111/j.1469-8986.2009.00929.x. [DOI] [PubMed] [Google Scholar]
- Stieben J, Lewis MD, Granic I, Zelazo PD, Segalowitz S, Pepler D. Neurophysiological mechanisms of emotion regulation for subtypes of externalizing children. Development and Psychopathology. 2007;19(2):455–480. doi: 10.1017/S0954579407070228. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Samson JA, Anderson CM, Ohashi K. The effects of childhood maltreatment on brain structure, function and connectivity. Nature Reviews Neuroscience. 2016;17(10):652–666. doi: 10.1038/nrn.2016.111. [DOI] [PubMed] [Google Scholar]
- Troller-Renfree S, Nelson CA, Zeanah CH, Fox NA. Deficits in error monitoring are associated with externalizing but not internalizing behaviors among children with a history of institutionalization. Journal of Child Psychology and Psychiatry. 2016;57(10):1145–1153. doi: 10.1111/jcpp.12604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullsperger M, Danielmeier C, Jocham G. Neurophysiology of performance monitoring and adaptive behavior. Physiological Reviews. 2014;94(1):35–79. doi: 10.1152/physrev.00041.2012. [DOI] [PubMed] [Google Scholar]
- Valadez EA, Simons RF. The power of frontal midline theta and post-error slowing to predict performance recovery: Evidence for compensatory mechanisms. Psychophysiology. 2017 doi: 10.1111/psyp.13010. http://doi.org/ https://doi.org/10.1111/psyp.13010. [DOI] [PubMed]
- van Veen V, Carter CS. The timing of action-monitoring processes in the anterior cingulate cortex. Journal of Cognitive Neuroscience. 2002;14(4):593–602. doi: 10.1162/08989290260045837. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Dieterich R, Riesel A. Error-related brain activity in the age of RDoC: A review of the literature. International Journal of Psychophysiology. 2014;98(2):276–299. doi: 10.1016/j.ijpsycho.2015.02.029. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Kotov R, Proudfit GH. Neural indicators of error processing in generalized anxiety disorder, obsessive-compulsive disorder, and major depressive disorder. Journal of Abnormal Psychology. 2015;124(1):172–185. doi: 10.1037/abn0000019. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Liu H, Shankman SA. Blunted neural response to errors as a trait marker of melancholic depression. Biological Psychology. 2016;113:100–107. doi: 10.1016/j.biopsycho.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A, Meyer A, Hale-Rude E, Perlman G, Kotov R, Klein DN, Hajcak G. Error-related negativity (ERN) and sustained threat: Conceptual framework and empirical evaluation in an adolescent sample. Psychophysiology. 2016;53(3):372–385. doi: 10.1111/psyp.12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A, Riesel A, Hajcak G. Integrating multiple perspectives on error-related brain activity: The ERN as a neural indicator of trait defensive reactivity. Motivation and Emotion. 2012;36(1):84–100. doi: 10.1007/s11031-011-9269-y. [DOI] [Google Scholar]
- Woody CD. Characterization of an adaptive filter for the analysis of variable latency neuroelectric signals. Medical and Biological Engineering. 1967;5(6):539–554. doi: 10.1007/BF02474247. [DOI] [Google Scholar]
- Zeanah CH, Nelson CA, Fox NA, Smyke AT, Marshall P, Parker SW, Koga S. Designing research to study the effects of institutionalization on brain and behavioral development: The Bucharest Early Intervention Project. Development and Psychopathology. 2003;15(4):885–907. doi: 10.1017/S0954579403000452. [DOI] [PubMed] [Google Scholar]


