Abstract
Fetal alcohol spectrum disorders (FASD) are associated with social interaction behavior and gastrointestinal (GI) abnormalities. These abnormal behaviors and GI abnormalities overlap with autism spectrum disorder (ASD). We investigated the effect of fetal alcohol exposure (FAE) on social interaction deficits (hallmark of autism). Evidence indicates that exogenous lipopolysaccharide (LPS) administration during gestation induces autism-like behavior in the litters. LPS regulates the expression of genes underlying differentiation, immune function, myelination and synaptogenesis in fetal brain by the LPS receptor, TLR-4-dependent mechanism. In this study, we evaluated the role of TLR-4 in FAE-induced social behavior deficit. WT and TLR4−/− pregnant mice were fed Lieber-DeCarli liquid diet with or without EtOH. Control group was pair-fed with isocaloric diet. Social behavior was tested in the adult litters at postnatal day 60. Frontal cortex mRNA expression of autistic candidate genes (Ube3a, Gabrb3, Mecp2) and inflammatory cytokine genes (IL-1β, IL-6, TNF-α) were measured by RT-qPCR. Adult male litters of EtOH-fed WT dams showed low birth weight compared to litters of pair-fed WT dams. However, their body weights at adulthood were greater compared to the body weights of litters of pair-fed WT dams. There were no body weight differences in litters of TLR4−/− dams. Social interaction deficit was observed only in male litters of EtOH-fed WT dams, but it was not observed in both male and female litters of EtOH-fed TLR4−/− dams. Expressions of autism candidate genes, Gabrb3 and Ube3a were elevated, while that of Mecp2 gene was suppressed in the frontal cortex of male, but not female litters of EtOH-fed WT mice. The expressions of inflammatory cytokine genes, IL-1β, IL-6 and TNF-α were also significantly increased in the frontal cortex of male, but not female, litters of EtOH-fed dams. The changes in the expression of autistic and cytokine genes were unaffected in the litters of EtOH-fed TLR4−/− dams. These data also indicate that TLR4 mediates FAE-induced changes in social interactions and gene expression in brain, suggesting that EtOH-induced LPS absorption from the maternal gut may be involved in gene expression changes in the fetal brain.
Keywords: FAE, ASD, Lipopolysaccharide, Behavior, Neuroinflammation
Introduction
Alcohol consumption during pregnancy causes a number of neurobehavioral abnormalities in children, collectively known as fetal alcohol spectrum disorder (FASD)(Coriale et al 2013). Social interaction deficits and reduced social memory, particularly in male have been shown in animal model of FASD. FASD affects as many as 2%–5% of school-aged children in the United States (May et al 2009). Study showed that 4.7% of North American women, who are pregnant, have alcoholism (McHugh et al 2014). According to Centers for Disease Control and Prevention, the prevalence of FAE in the US between 2006 and 2010 was 8%. It suggests that FAE is a leading cause of neurobehavioral abnormalities in growing children (Mizejewski 2010).
The worldwide population prevalence of autism is about 1% (Lai et al 2014). Number of FAE-induced neurobehavioral abnormalities overlap with autism spectrum disorder, which manifests social behavior deficits, developmental delay and mental retardation. Previously, we have shown that FAE affects social behavior and brain autistic candidate gene expression in male offspring’s (Tunc-Ozcan et al 2013).
Evidences suggest that lipopolysaccharide (LPS) from gram-negative bacteria can stimulate TLR-4 receptor and acts as a sensor for LPS (Poltorak et al 1998). Alcohol consumption increases the permeability of LPS from gastrointestinal tract to blood (Mathurin et al 2000). Evidence indicates that exogenous LPS administration during gestation induces autism-like behavior in the offspring’s (Kirsten et al 2015) and also serum levels of LPS were significantly higher in autistic patients (Emanuele et al 2010). LPS regulates the expression of genes underlying differentiation, immune function, myelination, and synaptogenesis in brain by TLR-4 dependent mechanism (Alfonso-Loeches et al 2010, Bilbo & Schwarz 2012, Pascual et al 2017). However, the role of LPS in FAE children is not known yet. In this study, we examined the FAE-induced social interaction deficits, inflammatory and autistic candidate gene expression in male and female offspring’s and determined the role of TLR-4 in these effects of FAE.
Methods
Fetal alcohol exposure
C57BL/6, WT and TLR4−/− male and female mice were purchased from Jackson Laboratories. These mice were bred and the progeny genotyped to obtain wild-type and TLR4−/− mice. All animal experiments were performed according to the protocol approved by the University of Tennessee Health Science Center (UTHSC) Institutional Animal Care and Use Committee. Mice were housed in groups of 2–5 per cage, segregated by sex, in a room on a 123h/123h light/dark cycle (lights on at 6:00 AM, off at 6:00 PM) maintained at 22 ± 23°C. Pregnant WT and TLR4−/− mice were fed with or without EtOH (0% 2d, 1% 2d, 2% 2d, 4% 1 week and 5% 1 week) in Lieber-DeCarli liquid diet. Control group was pair-fed with isocaloric diet. The behavioral test and mRNA expression analysis were performed at postnatal day 60 of their age.
WT and TLR4−/− mice were fed with or without EtOH for 4 days (0% 1d, 1% 1d, 2% 1d and 5% 1d) in Lieber-DeCarli liquid diet and alcohol levels were measured in WT and TLR4−/− mice. Furthermore, in separate set of mice we administered a single dose of EtOH (5 g kg−1) and measured plasma alcohol levels in WT and TLR4−/− mice, 2 hr. after EtOH administration. We prepared Et-OH gavage solution by mixing 6.6 ml of 95% ethanol with 13.4 ml of water. The gavage volume (μl) of 31.5% (vol/vol) ethanol solution for each mouse = mouse body weight in grams × 20. This solution prepared just before administration to avoid changes in concentration resulting from evaporation (Bertola et al 2013).
Social Interaction Test
For social interaction test, each mouse was left alone in its home cage for 15 min. An unfamiliar C57BL/6J mouse of the same sex was then introduced. The behavior of the test mouse was video-recorded for 10 min and scored for active interaction (that is sniffing, allogrooming, mounting and following) and rearing behavior (Sato et al 2013).
RNA Isolation and Quantitative RT-PCR
Total RNA from frontal cortex (1.5 μg) was used for generation of cDNAs using the ThermoScript RT-PCR system for first strand synthesis (Invitrogen). Quantitative PCR (qPCR) reactions were performed using cDNA mix (cDNA corresponding to 35 ng RNA) with 300 nmoles of primers in a final volume of 25 μl of 2× concentrated RT2 Real-Time SYBR Green master mix (Qiagen) in an Applied Biosystems QuantStudio 6 Flex Real-Time PCR instrument (Norwalk, CT, USA). The cycle parameters were: 50 °C for 2 min, one denaturation step at 95 °C for 10 min and 40 cycles of denaturation at 95 °C for 10s followed by annealing and elongation at 60 °C. Relative gene expression of each transcript was normalized to GAPDH using the ΔΔCt method. Sequences of primers used for qPCR are provided in Table 2 (Shukla et al 2016).
Table 2.
Quantitative RT-PCR Primer Sequences
| Gene | Sequence 5′-3′ |
|---|---|
| IL-1β | F: GCAACTGTTCCTGAACTCAACT |
| R: ATCTTTTGGGGTCCGTCAACT | |
| TNF-α | F: CCCTCACACTCAGATCATCTTCT |
| R: GCTACGACGTGGGCTACAG | |
| IL-6 | F: TAGTCCTTCCTACCCCAATTTCC |
| R: TTGGTCCTTAGCCACTCCTTC | |
| Ub3a | F: ATCCCAGTCTGAGGACATTGA |
| R: GCACAAAACTCATTCGTGCAG | |
| Mecp2 | F: ATGGTAGCTGGGATGTTAGGG |
| R: TGAGCTTTCTGATGTTTCTGCTT | |
| Gabrb3 | F: CTGCTGCCAATCTGGCTTTC |
| R: CGTAGCCTTTCAACAGCTTGTC | |
| GAPDH | F: CTGCACCACCAACTGCTTAG |
| R: GGGCCATCCACAGTCTTCT |
Cytokine measurement by ELISA
Blood samples were centrifuged for 10 minutes at 3000 g and the supernatant was frozen at −70°C for quantification. Interleukin-1β (IL-1β), tumor necrosis factor α (TNF-α) and interleukin-6 (IL-6) levels were estimated using commercially available immunoassay ELISA kits for mice (R&D System, Minneapolis, MN, USA), according to the manufacturer’s instructions. The results are expressed as picograms of cytokine per milliliter of plasma.
Plasma EtOH Concentration Analysis
Plasma samples were analyzed using the Colorimetric Alcohol Assay Kit (STA-620; Cell Biolabs, Inc, San Diego, CA, USA) according to vendor’s instructions. Briefly, the stock of EtOH (200mM) was diluted in a 1X assay buffer to produce standards in the concentration range of 0–200 μM. Next, all samples were diluted in 1X assay buffer (1:80). Following this, 10 μl of the diluted EtOH standards or samples were added to a 96-well microtiter plate. The reaction mixture (90ul) containing assay buffer, enzyme mixture and colorimetric probe was added to each well in a microtiter plate. The plate was incubated at 37 °C for 30 minutes and read at 570 nm. EtOH concentration of samples was calculated.
Statistical analysis
1–2 animal(s)/sex/litter were used for social behavior testing. Frontal cortex and plasma were collected from same mice after social behavior testing for mRNA expression and cytokine analysis. All data are expressed as Mean±SEM. The differences among multiple groups were first analyzed by one-way ANOVA (Prism 6.0). When a statistical significance was detected, Tukey’s t test was used to determine the statistical significance between multiple testing groups and the corresponding control. Statistical significance was established at 95%. Genotype and treatment effect was determined by a two-way ANOVA.
Results
There were no visible morphological changes in the newborns in different groups. Liquid diet intake was similar for the WT and TLR4−/− mice. The litter size and the birth weights of litters from EtOH-fed WT mothers were significantly low compared to those in litters from pair-fed WT mothers (Table 1). But, no differences in litter size or birth weights were recorded in litters of pair-fed and EtOH-fed TLR4−/− mice. The litters were allowed to grow for up to 60 days and were examined for social interactions. Male litters of EtOH-fed WT mice restored their body weights and were significantly greater than the body weights of corresponding litters of pair-fed WT mice on postnatal days 21 and 60 (Fig. 1A), but such a difference in the body weights was absent in female litters (Fig. 1B). In TLR4−/− mice, EtOH feeding during prenatal period had no effect on the body weights of litters. The maternal weight gain during gestation period was similar in the WT and TLR4−/− mice both with and without EtOH feeding (Fig. 1C).
Table 1. WT EtOH fed mothers gave birth to litter of decrease size and body weight.
There were no visible morphological changes in the newborn. Birth weights were recorded at postnatal day (P) 0. Birth weights did not vary by prenatal diet, except that pups of the WT EtOH mothers weighted less than pups of all other dams. Liquid diet intake was similar for the WT and TLR4−/− mice. Values are mean ± SEM (n = 7–10 litters). Asterisk indicates the value that is significantly (p <0.05) different from corresponding control value.
| Group | Litter size | Birth weight (g) | Diet intake (ml/mouse/day) |
|---|---|---|---|
| WT-PF | 9.0 ± 0.57 | 1.310 ± 0.073 | 18.75 ± 0.39 |
| WT-EF | 6.0 ± 0.47* | 1.157 ± 0.078* | 18.98 ± 0.22 |
| TLR-4−/−PF | 8.6 ± 0.32 | 1.422 ± 0.066 | 18.83 ± 0.39 |
| TLR-4−/−EF | 8.3 ± 0.33 | 1.350 ± 0.075 | 19.33 ± 0.25 |
Fig. 1. TLR4−/− mitigates FAE induced body weight decrease.
At P30 and P60 WT male litters of EtOH consuming mothers shows a significant increase in body weight compared to PF group. On other hand EtOH fed TLR4−/− dam’s weight were unaffected (A). WT and TLR4−/− female FAE pups body weight was unaffected both with and without EtOH treatment (B). The maternal weight gain during gestation period was similar in the WT and TLR4−/− mice both with and without EtOH treatment (C). Values are mean ± SEM (n = 7–10 litters). Asterisk indicates the value that is significantly (p <0.05) different from corresponding control value.
We observed a comparable increased plasma alcohol level in WT (73.4+ 10.3, mg/dl) and TLR4−/− mice (81.9+ 5.4) after 4 days of EtOH feeding. Single gavage of EtOH (5 g kg−1) also showed comparable increase in plasma EtOH levels in WT (93.4+ 9.53, mg/dl) and TLR4−/− 87.9+ 15.9) mice.
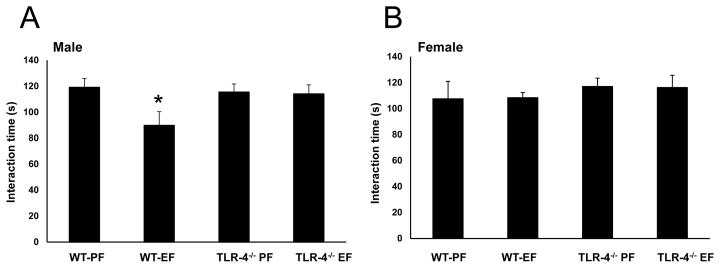
Social interaction behavioral tests were performed at the postnatal day 60. Social interaction scores were significantly low in male litters of EtOH-fed WT mice compared to those in male litters of pair-fed WT mice (Fig. 2A). Such a social interaction deficit was not recorded in female litters of EtOH-fed WT mice (Fig. 2B). Social interaction deficits were not recorded in male or female litters of EtOH-fed TLR4−/− mice, sex [F(1–24)=3.30, p<0.05]; sex x diet: [F(3–24)=6.18, p<0.05].
Fig. 2. TLR4−/− attenuates FAE induced social interaction deficit.
WT adult male litters of EtOH consuming mothers show social interaction deficits (A). These social interaction deficits were attenuated in TLR4−/− litters of EtOH consuming mothers (B). Values are mean ± SEM (n = 7–10 litters). Asterisk indicates the value that is significantly (p <0.05) different from corresponding control value.
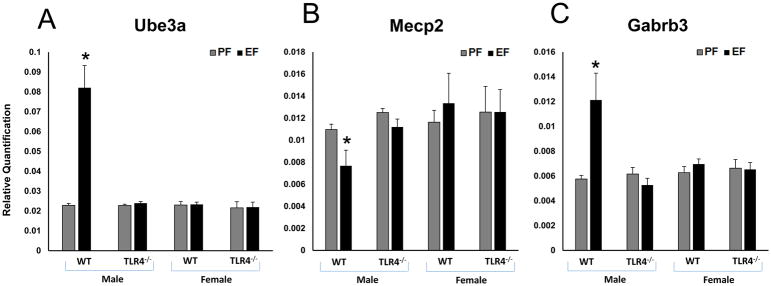
It is known that FAE affects some of the autistic genes in rat hippocampus (Tunc-Ozcan et al 2013). We evaluated the expression of Ube3a, Mecp2 and Gabrb3 in the frontal cortex of different litters groups. The levels of mRNA for Ube3a (Fig. 3A) [F(1–8)=27.03, p<0.05]; and Gabrb3 (Fig. 3C) [F(1–8)=7.84, p<0.05]; genes were significantly increased, whereas the mRNA level for Mecp2 gene (Fig. 3B) [F(1–8)=3.8, p<0.05]; was decreased in male litters of EtOH-fed WT dams compared to those in male litters of pair-fed WT dams. We observed a significant gender difference in WT litters. Ube3a, [F(1–12)=31.8, p<0.05], Gabrb3 [F(1–12)=7.8, p<0.05] and Mecp2 [F(1–12)=5.73, p<0.05]. There were no EtOH-induced differences in mRNA levels for these autism candidate genes in the frontal cortex of female litters of WT mice and male or female litters from TLR4−/− mice (Fig. 3A, 3B and 3C).
Fig. 3. WT male FAE litters showed autistic gene expression changes in the frontal cortex.
Relative quantification measured by real time RT-PCR indicates an increase in Ube3a (A), Gabrb3 (C) and decrease in Mecp2 (B) transcript levels in the frontal cortex of WT adult male litters of EtOH consuming mother. Ube3a (A), Gabrb3 (C) and Mecp2 (B) transcript levels were unaffected in TLR4−/− litters of EtOH consuming mothers. Values are mean ± SEM (n = 4 litters). Asterisk indicates the value that is significantly (p <0.05) different from corresponding control value. WT and TLR4−/− female FAE litters showed no effect on autistic gene expression in the frontal cortex. Relative quantification measured by real time RT-PCR indicates no effect on the mRNA expression levels of Ube3a, Mecp2 and Gabrb3 in the frontal cortex of WT and TLR4−/− adult female litters of EtOH consuming mother.
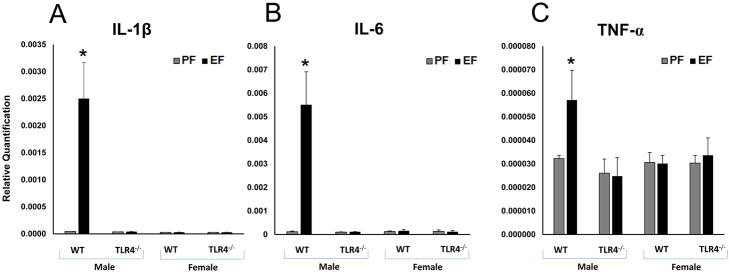
Expression of candidate pro-inflammatory cytokine genes were analyzed by measuring the specific mRNA levels in the frontal cortex. Levels of mRNA for IL-1β (Fig. 4A) [F(1–8)=13.44, p<0.05]; IL-6 (Fig. 4B) [F(1–8)=14.80, p<0.05]; and TNF-α (Fig. 4C) [F(1–8)=26.93, p<0.05]; were significantly increased in male litters of EtOH-fed WT dams compared to those in male litters of pair-fed WT dams. We observed a significant gender difference in WT litters. IL-1β [F(1–12)=15.7, p<0.05], IL-6 [F(1–12)=12.2, p<0.05] and TNF-α [F(1–12)=17.8, p<0.05]. There were no significant differences in transcript levels for these pro-inflammatory candidate genes in the frontal cortex of female litters of WT mice and male or female litters of TLR4−/− mice (Fig. 4A, 4B and 4C). We did not observe gender difference in TLR4−/− mice.
Fig. 4. WT male FAE litters showed increased proinflammatory cytokine gene expression in the frontal cortex.
Relative quantification measured by real time RT-PCR indicates an increase in IL-1β (A), IL-6 (B) and TNF-α (C) transcript levels in the frontal cortex of WT adult male litters of EtOH consuming mother. IL-1β (A), IL-6 (B) and TNF-α (C) transcript levels were unaffected in TLR4−/− litters of EtOH consuming mothers. Values are mean ± SEM (n = 4 litters). Asterisk indicates the value that is significantly (p <0.05) different from corresponding control value. WT and TLR4−/− female FAE litters showed no effect on inflammatory cytokine gene expression in the frontal cortex. IL-1β, IL-6 and TNF-α transcript levels were unaffected in WT and TLR4−/− litters of EtOH consuming mothers.
Plasma level of IL-1β (Fig. 5A) [F(1–6)=25.6, p<0.05], IL-6 (Fig. 5B), [F(1–6)=27.4, p<0.05] and TNF-α (Fig. 5C) [F(1–6)=20.4, p<0.05] were also significantly increased in the male litters of EtOH-fed WT dams compared to those in male litters of pair-fed WT dams. There were no significant differences in the plasma levels for these pro-inflammatory cytokines in the female litters of WT mice and male or female litters of TLR4−/− mice (Fig. 5A, 5B and 5C). We did not observe gender differences in the plasma level of cytokines in WT and TLR4−/− mice.
Fig. 5.
WT male FAE litters showed increased proinflammatory cytokine levels in the plasma. ELISA results indicate an increase in IL-1β (A), TNF-α (B) and IL-6 (C) levels in the plasma of WT adult male litters of EtOH consuming mother. IL-1β (A), TNF-α (B) and IL-6 (C) cytokine levels were unaffected in TLR4−/− litters of EtOH consuming mothers. Values are mean ± SEM (n = 4 litters). Asterisk indicates the value that is significantly (p <0.05) different from corresponding control value.
Discussion
FASD is a serious concern at the neonatal clinic and the mechanisms associated with the pathogenesis of this disorder is poorly understood. Alcohol is known to directly influence the fetus, but it is not clear if other injurious factors from the maternal circulation play a role in the development of FASD. Our study investigated the potential role of the LPS receptor, TLR4, in FAE-induced changes in gene expression in the frontal cortex of litters. The results indicate that EtOH feeding in mice during gestation induces inflammatory responses and autistic gene expression changes in the frontal cortex of litters, which is associated with the changes in social interaction by a gender-dependent and TLR4-dependent mechanism. A previous study showed that FAE reduced the litter size and lowered birth weights in rats (Tunc-Ozcan et al 2013). This previous study also showed that FAE causes impaired social interaction in adult male litters, but not in female litters. Our current study in mice confirms these findings on litter size and birth weights as well as social interactions in male litters, but not female litters. Although, maternal body weight gain during gestation period was similar in the WT and TLR4−/− mice both with and without Et-OH treatment. Interestingly, our present study demonstrates that FAE-induced reduction in litter size and birth weights and impaired social interactions were attenuated in TLR4−/− mice, indicating that TLR4 is involved in FAE-induced fetal growth defects and the social interactions of adult litters. In this study we did not evaluated maternal care but it has already been reported in one of FAE study with TLR4−/− and WT mice. That research group have assessed maternal behavior by evaluating maternal care by monitoring the time spent in the nest and grooming time. They have shown no difference between the groups in the percentage of time spent in the nest and the percent time grooming and care toward pups (Pascual et al 2017). LPS regulates the expression of genes underlying differentiation, immune function, myelination, and synaptogenesis in fetal brain by TLR4-dependent mechanism (Luan et al 2015, Pascual et al 2017, Salminen et al 2008, Shen et al 2016). Evidence indicates that exogenous LPS administration during gestation induces autism-like behavior in the litters (Kirsten et al 2012).
Chronic EtOH feeding has been shown to cause disruption of the colonic epithelial tight junctions, barrier dysfunction and endotoxemia in mice and rats (Chaudhry et al 2015, Rao 2009). The primary source of plasma LPS is the gut microflora. Disruption of colonic epithelial barrier function is expected to increase LPS absorption from the colon in pregnant animals. The maternal LPS is most likely the source of LPS in the fetal circulation. It is however, unclear at this point whether TLR4 in the maternal tissues or fetal tissues is responsible for the observed changes in the litters.
The gender differences in the present study is consistent with the high incidence of ASD such as autism in males compared to females (Lai et al 2014). There are no sex difference explicitly known in the social behavior of children and adolescents with FASD but males were significantly more with attention-deficit/hyperactivity disorder (ADHD) than FASD females (Herman et al 2008). Such a gender difference in FASD has not been reported in human subjects. The observed gender difference in the present mouse study may be species-dependent difference or in humans, secondary factors may precipitate FAE effects in females.
The FAE-induced alteration in social interaction suggested potential changes in expression autistic candidate genes. Our results demonstrate that FAE does increase the expression of Ube3a and Gabr3b in the frontal cortex of litters in a gender-dependent mechanism. These gene expression changes overlap with the FAE-induced social behavior changes, supporting the hypothesis that there is a common molecular mechanism between autism and EtOH-induced social interaction deficits. Mecp2 was significantly decreased in the frontal cortex of litters of EtOH-fed pregnant mice, which is consistent with the previous study in rat hippocampus (Samaco et al 2005).
The mechanism of FAE-induced changes in candidate autistic genes is unclear. Neuro-inflammation has been shown to be associated with autism (Bjorklund et al 2016), and LPS is likely to induce inflammatory responses in the fetus. Therefore, we examined the expression of candidate pro-inflammatory cytokines in the frontal cortex of fetal alcohol exposed litters. FAE litters showed significantly higher expression of IL-1β and IL-6 and TNF-α genes in the frontal cortex compared to that in litters of pair-fed mothers. Interestingly, FAE-induced IL-1β and IL-6 expressions were abrogated in TLR4−/− litters. FAE-induced TNF-α expression was significantly lesser in TLR4−/− litters compared to that in wild-type litters. Plasma cytokine levels were also comparable in these mice. Moderate prenatal alcohol exposure during gestation in rats showed a gender difference in the expression of proinflammatory cytokines in maternal serum, placenta and fetal brain (Terasaki & Schwarz 2016). A human study also demonstrated that IL-1β, IL-6 and TNF-α levels were higher in both neonatal and maternal blood in mothers who consumed alcohol compared to those did not (Ahluwalia et al 2000). This study explicitly indicated that maternal alcohol consumption increases cytokine exposure to the fetus. Microglial activation is one of the major indicators of neuroinflammation in ASD. One of the postmortem brain studies also suggested that these inflammatory cytokines were elevated in the individuals diagnosed with ASD (Vargas et al 2005).
In summary, this study shows that EtOH feeding during gestation induces social interaction deficits only in male mice by a TLR4-dependent mechanism. We speculate that these effects of FAE on social interactions may involve altered expression of candidate autistic genes, and that pro-inflammatory cytokines such as IL-1β, IL-6 and TNF-α may be involved in FAE-induced autistic gene expression and social interaction changes.
Supplementary Material
Highlights.
Fetal alcohol exposure (FAE)-induced reduction in birth weight and social interaction deficit are restricted to male litters.
FAE alters autistic candidate genes in the brain frontal cortex.
Expression of inflammatory cytokine genes in brain frontal cortex was elevated by FAE.
FAE-induced altered expression of autistic and cytokine genes was observed in male, but not in female litters.
TLR4 mediates FAE-induced changes in social interactions and gene expression in frontal cortex.
Acknowledgments
This study was funded by NIH grant AA12307 to RKR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahluwalia B, Wesley B, Adeyiga O, Smith DM, Da-Silva A, Rajguru S. Alcohol modulates cytokine secretion and synthesis in human fetus: an in vivo and in vitro study. Alcohol. 2000;21:207–13. doi: 10.1016/s0741-8329(00)00076-8. [DOI] [PubMed] [Google Scholar]
- Alfonso-Loeches S, Pascual-Lucas M, Blanco AM, Sanchez-Vera I, Guerri C. Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage. J Neurosci. 2010;30:8285–95. doi: 10.1523/JNEUROSCI.0976-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertola A, Mathews S, Ki SH, Wang H, Gao B. Mouse model of chronic and binge ethanol feeding (the NIAAA model) Nat Protoc. 2013;8:627–37. doi: 10.1038/nprot.2013.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Schwarz JM. The immune system and developmental programming of brain and behavior. Front Neuroendocrinol. 2012;33:267–86. doi: 10.1016/j.yfrne.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorklund G, Saad K, Chirumbolo S, Kern JK, Geier DA, et al. Immune dysfunction and neuroinflammation in autism spectrum disorder. Acta Neurobiol Exp (Wars) 2016;76:257–68. doi: 10.21307/ane-2017-025. [DOI] [PubMed] [Google Scholar]
- Chaudhry KK, Samak G, Shukla PK, Mir H, Gangwar R, et al. ALDH2 Deficiency Promotes Ethanol-Induced Gut Barrier Dysfunction and Fatty Liver in Mice. Alcohol Clin Exp Res. 2015;39:1465–75. doi: 10.1111/acer.12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coriale G, Fiorentino D, Di Lauro F, Marchitelli R, Scalese B, et al. Fetal Alcohol Spectrum Disorder (FASD): neurobehavioral profile, indications for diagnosis and treatment. Riv Psichiatr. 2013;48:359–69. doi: 10.1708/1356.15062. [DOI] [PubMed] [Google Scholar]
- Emanuele E, Orsi P, Boso M, Broglia D, Brondino N, et al. Low-grade endotoxemia in patients with severe autism. Neurosci Lett. 2010;471:162–5. doi: 10.1016/j.neulet.2010.01.033. [DOI] [PubMed] [Google Scholar]
- Herman LE, Acosta MC, Chang PN. Gender and attention deficits in children diagnosed with a Fetal Alcohol Spectrum Disorder. Can J Clin Pharmacol. 2008;15:e411–9. [PubMed] [Google Scholar]
- Kirsten TB, Chaves-Kirsten GP, Chaible LM, Silva AC, Martins DO, et al. Hypoactivity of the central dopaminergic system and autistic-like behavior induced by a single early prenatal exposure to lipopolysaccharide. J Neurosci Res. 2012;90:1903–12. doi: 10.1002/jnr.23089. [DOI] [PubMed] [Google Scholar]
- Kirsten TB, Queiroz-Hazarbassanov N, Bernardi MM, Felicio LF. Prenatal zinc prevents communication impairments and BDNF disturbance in a rat model of autism induced by prenatal lipopolysaccharide exposure. Life Sci. 2015;130:12–7. doi: 10.1016/j.lfs.2015.02.027. [DOI] [PubMed] [Google Scholar]
- Lai MC, Lombardo MV, Baron-Cohen S. Autism. Lancet. 2014;383:896–910. doi: 10.1016/S0140-6736(13)61539-1. [DOI] [PubMed] [Google Scholar]
- Luan R, Cheng H, Li L, Zhao Q, Liu H, et al. Maternal Lipopolysaccharide Exposure Promotes Immunological Functional Changes in Adult Offspring CD4+ T Cells. Am J Reprod Immunol. 2015;73:522–35. doi: 10.1111/aji.12364. [DOI] [PubMed] [Google Scholar]
- Mathurin P, Deng QG, Keshavarzian A, Choudhary S, Holmes EW, Tsukamoto H. Exacerbation of alcoholic liver injury by enteral endotoxin in rats. Hepatology. 2000;32:1008–17. doi: 10.1053/jhep.2000.19621. [DOI] [PubMed] [Google Scholar]
- May PA, Gossage JP, Kalberg WO, Robinson LK, Buckley D, et al. Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Dev Disabil Res Rev. 2009;15:176–92. doi: 10.1002/ddrr.68. [DOI] [PubMed] [Google Scholar]
- McHugh RK, Wigderson S, Greenfield SF. Epidemiology of substance use in reproductive-age women. Obstet Gynecol Clin North Am. 2014;41:177–89. doi: 10.1016/j.ogc.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizejewski GJ. Can prenatal screening for fetal alcohol spectrum disorder be justified? A commentary. Gynecol Obstet Invest. 2010;69:128–30. doi: 10.1159/000263460. [DOI] [PubMed] [Google Scholar]
- Pascual M, Montesinos J, Montagud-Romero S, Forteza J, Rodriguez-Arias M, et al. TLR4 response mediates ethanol-induced neurodevelopment alterations in a model of fetal alcohol spectrum disorders. J Neuroinflammation. 2017;14:145. doi: 10.1186/s12974-017-0918-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–8. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- Rao R. Endotoxemia and gut barrier dysfunction in alcoholic liver disease. Hepatology. 2009;50:638–44. doi: 10.1002/hep.23009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A, Paananen R, Vuolteenaho R, Metsola J, Ojaniemi M, et al. Maternal endotoxin-induced preterm birth in mice: fetal responses in toll-like receptors, collectins, and cytokines. Pediatr Res. 2008;63:280–6. doi: 10.1203/PDR.0b013e318163a8b2. [DOI] [PubMed] [Google Scholar]
- Samaco RC, Hogart A, LaSalle JM. Epigenetic overlap in autism-spectrum neurodevelopmental disorders: MECP2 deficiency causes reduced expression of UBE3A and GABRB3. Hum Mol Genet. 2005;14:483–92. doi: 10.1093/hmg/ddi045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A, Mizuguchi M, Ikeda K. Social interaction test: a sensitive method for examining autism-related behavioral deficits. 2013 http://dx.doi.org/10.1038/protex.2013.046
- Shen Y, Qin H, Chen J, Mou L, He Y, et al. Postnatal activation of TLR4 in astrocytes promotes excitatory synaptogenesis in hippocampal neurons. J Cell Biol. 2016;215:719–34. doi: 10.1083/jcb.201605046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla PK, Chaudhry KK, Mir H, Gangwar R, Yadav N, et al. Chronic ethanol feeding promotes azoxymethane and dextran sulfate sodium-induced colonic tumorigenesis potentially by enhancing mucosal inflammation. BMC Cancer. 2016;16:189. doi: 10.1186/s12885-016-2180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasaki LS, Schwarz JM. Effects of Moderate Prenatal Alcohol Exposure during Early Gestation in Rats on Inflammation across the Maternal-Fetal-Immune Interface and Later-Life Immune Function in the Offspring. J Neuroimmune Pharmacol. 2016;11:680–92. doi: 10.1007/s11481-016-9691-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunc-Ozcan E, Ullmann TM, Shukla PK, Redei EE. Low-dose thyroxine attenuates autism-associated adverse effects of fetal alcohol in male offspring’s social behavior and hippocampal gene expression. Alcohol Clin Exp Res. 2013;37:1986–95. doi: 10.1111/acer.12183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005;57:67–81. doi: 10.1002/ana.20315. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.