Key Points
Question
How do patient-perceived voice changes compare with quantitative vocal measures during the first year after thyroidectomy?
Findings
In this mixed methods study of 42 patients with clinically node-negative papillary thyroid cancer, voice changes were perceived by 57% of participants 2 weeks after surgery. During semistructured interviews, most of those affected by voice symptoms reported continued voice-related quality-of-life consequences out to 1 year after surgery; these deficits were not captured by the Voice Handicap Index or other quantitative assessments.
Meaning
This study highlights the importance of directly querying patients about postoperative voice changes and questions the sensitivity of commonly used, validated patient-reported outcome measures and other quantitative assessments of voice.
Abstract
Importance
Voice changes after thyroidectomy are typically attributed to recurrent laryngeal nerve injury. However, most postoperative voice changes occur in the absence of clinically evident vocal fold paralysis. To date, no study has compared the prevalence, duration, and consequences of voice-related disability from the patient perspective with use of quantitative vocal measures.
Objectives
To assess the quality-of-life consequences of postthyroidectomy voice change from the perspective of patients with thyroid cancer and to compare patient-perceived voice changes with changes in quantitative vocal variables at 5 time points in the first postoperative year.
Design, Setting, and Participants
This prospective mixed methods observational study within a randomized clinical trial occurred at the University of Wisconsin Hospital and Clinics. Participants were 42 patients with clinically node-negative papillary thyroid cancer without a preexisting vocal cord paralysis who were recruited and enrolled from outpatient clinics between June 6, 2014, and March 6, 2017, as part of the ongoing randomized clinical trial.
Intervention
Total thyroidectomy.
Main Outcomes and Measures
Semistructured interviews, symptom prevalence, and instrumental voice evaluations (laryngoscopy, phonation threshold pressure, Dysphonia Severity Index, and Voice Handicap Index) occurred at baseline (n = 42) and 2-week (n = 42), 6-week (n = 39), 6-month (n = 35), and 1-year (n = 30) postoperative time points.
Results
Participants had a mean age of 48 years (interquartile range, 38-58 years; age range, 22-70 years) and were mostly female (74% [31 of 42]) and of white race/ethnicity (98% [41 of 42]). Impaired communication was the primary theme derived from patient interviews from before thyroidectomy to after thyroidectomy. Voice changes were perceived by 24 participants at 2 weeks after thyroidectomy. After surgery, voice symptoms were prevalent and persisted for 50% (21 of 42) of participants out to at least 1 year of follow-up. Quantitative vocal perturbations were detected in the Dysphonia Severity Index and Voice Handicap Index at the 2-week follow-up but returned to baseline levels by the 6-week follow-up visit.
Conclusions and Relevance
Voice changes are common after surgery for papillary thyroid cancer and affect quality of life for many patients out to 1 year of follow-up. Directly querying patients about postoperative voice changes and questioning whether commonly used aerodynamic and acoustic variables detect meaningful voice changes are important in identifying patients whose quality of life has been affected by postthyroidectomy dysphonia.
Trial Registration
ClinicalTrials.gov Identifier: NCT02138214
This mixed methods study assesses the quality-of-life consequences of postthyroidectomy voice change from the perspective of patients with thyroid cancer and compares patient-perceived voice changes with changes in quantitative vocal variables at 5 time points in the first postoperative year.
Introduction
Thyroid cancer remains the only noncutaneous head and neck malignant tumor that continues to increase in incidence, with a mean 5% increase per year in the United States.1 In fact, its incidence tripled between 1973 and 2007, from 3.6 cases per 100 000 to 11.9 cases per 100 000. Total thyroidectomy is the primary treatment for thyroid cancer and puts both superior and recurrent laryngeal nerves at risk. During thyroid surgery, surgical manipulation of these nerves is responsible for 46% of unilateral and 56% of bilateral vocal fold paralysis cases2,3,4 and can be associated with significant dysphonia,5,6 dysphagia,6,7 and dyspnea.6,8 However, voice changes are common after thyroidectomy even in the absence of overt vocal fold paralysis, but the nature of these changes is not well characterized.9,10,11
Rates of postthyroidectomy dysphonia range widely in the literature (14%-90%) because of different assessment criteria, study designs or ascertainment methods, and patient populations considered.12,13,14,15,16,17,18 Part of this variability relates to dysphonia being characterized using different methods during the instrumental voice evaluations, including patient-reported outcome measures9,14,19,20,21,22,23,24 and audioperceptual,10 acoustic and aerodynamic,10,13,14,19,22,24,25 and visuoperceptual (ie, videostroboscopy) measures.26 Despite growing interest, no study to date has investigated the actual patient experience of living with postthyroidectomy voice changes.
In general, patients with thyroid cancer are counseled that postthyroidectomy voice changes are rare and transient, despite growing evidence to the contrary.9,10,11,27,28 The objectives of this prospective mixed methods29 observational study within a randomized clinical trial were (1) to assess the quality-of-life consequences of postthyroidectomy voice change from the perspective of patients with thyroid cancer and (2) to compare patient-perceived voice changes with changes in quantitative vocal variables at 5 time points in the first postoperative year derived from the instrumental voice evaluations. The setting was the University of Wisconsin Hospital and Clinics.
Methods
This study was approved by the University of Wisconsin–Madison Health Sciences Institutional Review Board (HSIRB 2014-0391). Enrolled participants provided written informed consent and verbal consent before each interview and consented to have their responses recorded and published.
Patient Selection
Patients with clinically node-negative papillary thyroid cancer were recruited from endocrine surgery and otolaryngology clinics as part of an ongoing randomized clinical trial comparing surgical outcomes (trial protocol UW13115 [NCT02138214]) after total thyroidectomy with and without ipsilateral central neck dissection (CND). Patients were eligible if they met the following criteria: (1) age 21 to 73 years with a diagnosis of papillary thyroid cancer, (2) no preexisting vocal fold paralysis or immobility, (3) no laryngeal pathology that could affect vocal function and no abnormality or vocal difficulties on baseline flexible transnasal laryngoscopy and instrumental voice evaluations as determined by a laryngologist, (4) no preoperative evidence of cervical or distant metastases, (5) no evidence of nodal involvement, and (6) the ability to read and write in English.
Semistructured Interviews
Semistructured interviews were conducted after the instrumental voice evaluations by interviewers trained in qualitative research who were not part of the clinical staff (C.L.M., J.O., R.S.S., and N.P.C.) at 5 time points (baseline, 2-week postoperative, 6-week postoperative, 6-month postoperative, and 1-year postoperative). The interview guide, which was developed inductively based on pilot interviews and consultation with clinical staff, encouraged participants to discuss overall experiences with cancer from diagnosis through surgery and recovery. Using this method allowed exploration of the subjective effects of symptoms and permitted participants to discuss effects of voice changes on quality of life.30
Symptom Identification and Assessment
Two different interview methods were used to assess participant symptoms (ie, open-ended and prompted questions). After answering a series of open-ended questions regarding their physical and emotional health, participants were given a stack of cards listing a range of 38 common postthyroidectomy symptoms (eAppendix 1 and eAppendix 2 in the Supplement). Participants selected those symptoms that they were currently experiencing and also had the opportunity to report additional symptoms that they were having. While the cards covered a wide range of postoperative symptoms, 3 cards listed voice-related symptoms (ie, hard to talk, hoarse voice, and voice gets tired). Participants were prompted to reflect on any voice-related symptom cards that they were presented.
Instrumental Voice Evaluations
All participants underwent preoperative and 2-week postoperative instrumental voice evaluations, at which time they completed the Voice Handicap Index (VHI)31 and had their phonation threshold pressure (PTP)32 and Dysphonia Severity Index (DSI)33 measured. Instrumental voice evaluations, including flexible transnasal laryngoscopy, were repeated at later time points (eg, 6 weeks, 6 months, and 1 year) if “abnormal” voice evaluation findings were observed on at least 2 measures (PTP, DSI, and/or VHI) at the previous evaluation or if patients reported voice concerns at any postoperative time point. Abnormal was operationalized based on the following published norms: PTP exceeding 6 cm H2O25,34,35,36 and DSI less than 1.6 or DSI reduction of at least 2.49 from before surgery to after surgery.19,21,25,33,36,37,38 A VHI increase of at least 13 points was indicative of clinically important voice-related quality-of-life changes.14,23,39
Grounded Theory Analysis
Interviews were deidentified and transcribed verbatim. Research team members (C.L.M., J.O., R.S.S., and N.P.C.) performed line-by-line open coding of a subset of transcripts to ascertain emergent themes.30 The process yielded 327 focused codes, which were then applied to the entire data set using a software program (NVivo 11; QSR International) by trained coders, with excellent intracoder reliability (κ = 0.79).40 Voice-related codes were then analyzed inductively using interview data.
Statistical Analysis
Paired t tests and χ2 tests were used to examine differences in randomization groups for quantitative (PTP, DSI, and VHI) and nominal (normal vs abnormal PTP, DSI, and VHI; patient-perceived impaired communication; and symptom frequency) data at the 2-week postoperative time point. A mixed model, repeated-measures analysis of variance was used to examine treatment effects and time effects for all quantitative data (PTP, DSI, and VHI). Pairwise comparisons were examined using Fisher least significant difference test when P values for the omnibus analysis of variance were .1 or less. Cohen d values were calculated to determine effect size. The 95% CI around the effect was calculated to provide information on the precision of the effect size estimate and the range within which the true effect size is likely to be found. Cohen d effect sizes were interpreted as follows: small (d = 0.20), medium (d = 0.50), and large (d = 0.80) effect.41 McNemar test was used to examine nominal data (normal vs abnormal PTP, DSI, and VHI; symptom frequency; and frequency of impaired voice code) from the preoperative or 2-week postoperative time points to later postoperative time points. Linear regression was used to examine the relationship between primary voice evaluation data (PTP, DSI, and VHI) and patient-perceived impaired communication across the 5 study time points. The critical value for obtaining statistical significance was set at 1-tailed α = .05. Statistical software was used for analyses, including SAS version 9.4 (SAS Institute Inc), SPSS Statistics 23 (IBM), and Prism (GraphPad Inc).
Results
Of 57 participants who consented, 42 patients with clinically node-negative papillary thyroid cancer without a preexisting vocal cord paralysis who were recruited and enrolled from outpatient clinics were randomized into the ongoing prospective clinical trial and completed semistructured interviews and instrumental voice evaluations (Figure 1) between June 6, 2014, and March 6, 2017. Five patients dropped out of the study before randomization, and papillary thyroid cancer could not be confirmed via pathology in 10 patients. At the 2-week postoperative time point, no statistically significant or clinically meaningful differences were observed between the 2 randomization groups (total thyroidectomy vs total thyroidectomy with ipsilateral CND) for any of the instrumental voice evaluations. Because no differences were detected between the randomization groups, the groups were combined to examine quantitative and qualitative measures of voice after surgery for the treatment of thyroid cancer across the 5 study time points.
Figure 1. Study Flow Diagram.
Participants had a mean age of 48 years (interquartile range, 38-58 years; range, 22-70 years) and were mostly female (74% [31 of 42]) and of white race/ethnicity (98% [41 of 42]). Sixty-nine percent (29 of 42) held a bachelor’s degree or higher, and 55% (23 of 42) were identified as vocal professionals, defined as those who use their voice regularly and with mastery in their career or cherished hobby. Tumors had a median size of 1.9 cm (interquartile range, 1.2-3.2 cm), and 51% (21 of 41) were multifocal (eTable 1 in the Supplement). One laryngologist, masked to the study and all study time points, diagnosed vocal fold paralysis or immobility in 8 participants (19%) using flexible transnasal laryngoscopy within 2 weeks after surgery (eTable 2 in the Supplement). Six of the 8 vocal fold paralysis or immobility cases resolved within the 1-year follow-up period. Specifically, 2 cases had resolved by the 6-week time point, and 4 cases had resolved by 6 months. One of the 8 participants with vocal fold paralysis or immobility that resolved was noted to have vocal fold intubation trauma with persistent voice sequelae out to the 1-year follow-up. One additional participant (2%) was suspected of having a potential superior laryngeal nerve injury. No further differences were observed between patients who experienced vocal fold paralysis or immobility and those who did not on any quantitative or qualitative voice measure.
Patient Experience
Impaired communication was the primary theme derived from qualitative analyses (Table). Before surgery, 2 participants (5%) discussed potential voice implications of thyroidectomy (Figure 2A). Awareness of voice-related disability increased over ensuing time points. For example, at 2 weeks after thyroidectomy, 24 participants (57%) experienced voice changes that affected interactions with family, friends, and coworkers. Many expressed frustration with the inability to communicate on the telephone. One participant explained that “A lot of people that have been calling me…especially after the surgery, I definitely didn’t speak to people on the phone…because of the hoarseness. There were times when I wanted to talk, but I couldn’t” (patient 13 in the Table at 2 weeks after surgery).
Table. Patient-Perceived Impaired Communication Over the First Postoperative Year.
| Time Point | Quotation |
|---|---|
| Baseline (n = 2) | I am very concerned about what voice I’m going to have when I come out…am I going to have a voice? Because that’s my career (patient 6). |
| The concerns are…at any time you go in for a procedure like this you run those small risks of something happening, could there be vocal cord damage, could I lose my voice, could I, even if I don’t lose it, could there be damage? That concerns me…I’d say of everything that’s what I’m most worried about (patient 28). | |
| 2 wk (n = 24) | It’s literally such an effort right now for me to talk…my voice isn’t strong like it was before…before the surgery, I wouldn’t have even thought about it…after the surgery it became much more relevant for me (patient 9). |
| When I was talking…I got to the point where I just didn’t want to talk anymore…I was tired from, it’s hard to explain I think just because…I’ve never had that happen in my life. It’s probably the only time it’ll ever happen (patient 11). | |
| A lot of people that have been calling me…especially after the surgery, I definitely didn’t speak to people on the phone…because of the hoarseness. There were times when I wanted to talk, but I couldn’t (patient 13). | |
| 6 wk (n = 17) | With my job, sometimes my voice gets strained and tired, and it becomes hard for me to talk, and I can’t manage the classroom…I can’t teach the material then, and that’s my livelihood…I need to have my voice (patient 19, glottic insufficiency). |
| That I don’t have a voice, yeah-that’s a major problem (patient 27, right VF immobility). | |
| Physically I feel good, it’s just so stressful because it’s taken away my livelihood. I work 14, 16 hour days and 90% of my job is talking on the phone, talking to clients, I can’t answer my phone…so it’s very frustrating to not be able to communicate well...it’s an effort to talk (patient 47, right VF paralysis, left VF paresis). | |
| 6 mo (n = 11) | [My voice] is just not perfect yet, I mean it’s very minimal, and I feel like it’s normal now in comparison to…immediately just after the surgery (patient 6). |
| My voice becomes weak by itself...I feel like I’m screaming or yelling, but other people can’t hear me (patient 12). | |
| I didn’t even know what to expect…and I think at the beginning I was surprised at how different my voice was, and how even if other people couldn’t hear it, it felt very different…I really didn’t have an expectation of what that was going to be like (patient 33). | |
| 1 y (n = 15) | By the time I get home, I could go the rest of the night without using it [my voice] …the joys of living alone and texting I guess...I don’t have to use it [my voice] until the next morning when I get to school again (patient 2). |
| There have been times where I’ve apologized to people saying, hey sorry my voice is, is sounding a little bit rough, or hey I feel a lot better than I sound…I’ve said that many times (patient 5). | |
| I did a lot of community theater…I was a soloist, and…I can’t even think about doing that…because I’m afraid of what came out…I don’t know what my voice is gonna do…that’s very frustrating because I know what it [my voice] sounds like and I don’t like it (patient 16). |
Abbreviation: VF, vocal fold.
Figure 2. Patient-Perceived Vocal Impairments.
Shown are patient-perceived vocal disability (A) and voice symptoms (B-D) over the first year after thyroidectomy.
Reports of impaired communication remained common at 6 weeks (44% [17 of 39]), 6 months (31% [11 of 35]), and 1 year (50% [15 of 30]) after thyroidectomy (Figure 2A). The severity and consequence of voice changes varied. Patients became increasingly aware of their impaired ability to communicate and its effect on their daily life as perceived dysphonia persisted further from the inciting surgery. One participant shared “That I don’t have a voice, yeah-that’s a major problem” (patient 27 at 6 weeks after surgery). It continued to affect their ability to work: “With my job, sometimes my voice gets strained and tired, and it becomes hard for me to talk, and I can’t manage the classroom. I can’t teach the material then, and that’s my livelihood…I need to have my voice” (patient 19 at 6 weeks after surgery). Participants also communicated that they were not sufficiently educated about the potential risk of losing their voice: “I didn’t even know what to expect…and I think at the beginning I was surprised at how different my voice was, and how even if other people couldn’t hear it, it felt very different…I really didn’t have an expectation of what that was going to be like” (patient 33 at 6 months after surgery).
Prompted Questions
Similar to the open-ended interview results described above, voice symptoms (hard to talk, hoarse voice, and voice gets tired) increased from baseline to the 2-week postoperative interview. These symptoms decreased over the follow-up period but remained prevalent among up to 30% of the patients at 1 year after surgery (Figure 2B-D).
Phonation Threshold Pressure
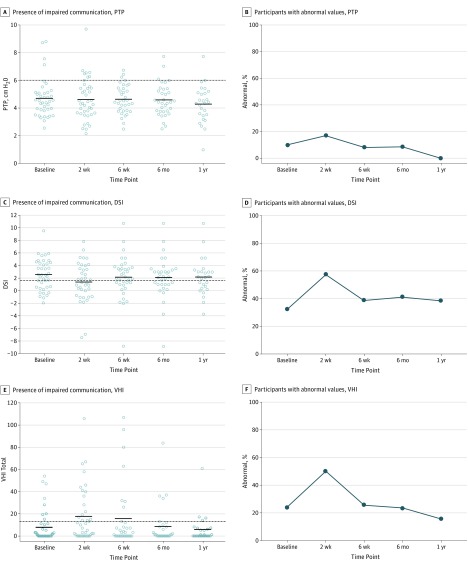
No difference was observed in PTP before surgery to after surgery (F4,131 = 0.95, P = .44): these Cohen d values were −0.05 (95% CI, −0.44 to 0.35) from baseline to 2 weeks after surgery and −0.05 (95% CI, −0.38 to 0.27) from 2 weeks to 6 months after surgery (Figure 3A). Similarly, there was no difference in the percentage of participants with an abnormal PTP (>6 cm H2O) at any preoperative or postoperative time point (Figure 3B). For all participants with baseline and 2-week postoperative values, 13% (95% CI, 2%-23%) went from a normal to an abnormal PTP, 3% (95% CI, −2% to 8%) improved, and the remaining patients stayed the same. When the 2-week to 6-month postoperative time points are examined, 6% (95% CI, −2% to 14%) of the participants went from a normal to an abnormal PTP, and 12% (95% CI, 1%-23%) improved to a normal PTP. At 1 year after surgery, no patients had gone from a normal to an abnormal PTP, and 15% (95% CI, 2%-29%) of the participants had improved to a normal PTP from the 2-week postoperative visit.
Figure 3. Instrumental Voice Evaluations.
Shown are quantitative voice measures (A, C, and E) and abnormal voice findings (B, D, and F) out to 1-year follow-up. Dashed lines denote the operational cutoff values used in the determination of abnormal vs normal for PTP, DSI, and VHI. DSI indicates Dysphonia Severity Index; PTP, phonation threshold pressure; and VHI, Voice Handicap Index.
Dysphonia Severity Index
There was an effect on the DSI over time (F4,128 = 1.95, P = .10) (Figure 3C). The DSI worsened from baseline to the 2-week postoperative time point (Cohen d, 0.36; 95% CI, 0.01-0.72) and improved from the 2-week to the 6-month postoperative time points (Cohen d, −0.31; 95% CI, −0.56 to −0.06). When the individual component measures of the DSI were examined, there was a significant difference in the variable “fundamental frequency high” such that this variable significantly decreased from 747.44 at baseline to 668.87 at 2 weeks after thyroidectomy (mean difference, −94.78; 95% CI, −163.78 to −25.78) and significantly increased from 668.87 at 2 weeks to 739.87 at 6 months after thyroidectomy (mean difference, 80.75; 95% CI, 29.29-132.20). For all patients with a preoperative DSI and a 2-week postoperative DSI, there was a significant increase in the percentage of patients with abnormal DSI values (34%; 95% CI, 19%-49%), while only 8% improved (95% CI, −1% to 17%), and the remaining patients stayed the same (Figure 3D). By 6 months, only 3% (95% CI, −3% to 9%) of the patients had worsened from a normal to an abnormal DSI value, and 21% (95% CI, 7%-34%) had improved to a normal DSI value. There were no new participants with abnormal DSI values at 1 year after thyroidectomy, and 15% (95% CI, 2%-29%) had improved to a normal DSI value.
Voice Handicap Index
The VHI values worsened (increased) from before surgery to 2 weeks after surgery and improved (decreased) at later postoperative time points (F4,126 = 4.31, P = .002) (Figure 3E). Specifically, the VHI values worsened from baseline to the 2-week postoperative assessment (Cohen d, −0.42; 95% CI, −0.78 to −0.07) and improved from 2 weeks to 6 months (Cohen d, 0.61; 95% CI, 0.30-0.92) and 1 year (Cohen d, 0.61; 95% CI, 0.26-0.96) after thyroidectomy (Figure 3E). Dichotomizing participants into those having a normal VHI value vs an abnormal VHI value (≥13), we found that 28% (95% CI, 14%-41%) of the patients who had a normal VHI value at baseline had an abnormal VHI value at the 2-week postoperative time point and that only 3% (95% CI, −2% to 7%) of the patients improved from the preoperative time point to a normal VHI value (Figure 3F). Notably, there were no new patients with an abnormal VHI value at 6 months or 1 year after surgery. Conversely, 31% (95% CI, 14%-48%) of the participants went from an abnormal VHI value at 2 weeks after surgery to a normal value VHI at the 6-month postoperative visit, and 36% (95% CI, 17%-55%) improved to a normal VHI value from the 2-week to the 1-year time points. All 4 participants with an abnormal VHI value at 1 year after surgery also had an abnormal preoperative VHI value, and 3 of the 4 participants did not have vocal fold paralysis or immobility.
Impaired Communication vs Quantitative Voice Variables
Overall, the presence of impaired communication strongly and significantly correlated with the DSI (r = −0.88; estimate of slope, −0.20 [95% CI, −0.04 to −0.00]) but did not significantly correlate with PTP (r = −0.35; estimate of slope, −0.00 [95% CI, −0.02 to 0.01]) or the VHI (r = 0.62; estimate of slope, 0.14 [95% CI, −0.19 to −0.47]) across all 5 study time points (Figure 4A). Although a moderate correlation was found between patient perception of impaired communication and the percentage of participants with an abnormal DSI value, it was not significant (r = 0.72; estimate of slope, 0.33 [95% CI, −0.25 to 0.91]). In addition, the percentage of participants with abnormal VHI and PTP values was only weakly correlated with patient-perceived impaired communication (r = 0.39; estimate of slope, 0.25 [95% CI, −0.83 to 1.33] for the VHI and r = 0.02; estimate of slope, 0.01 [95% CI, −0.54 to 0.55] for PTP) (Figure 4B).
Figure 4. Relationship Between Impaired Communication and Quantitative Voice Variables.
Shown is the relationship between impaired communication and quantitative voice variables (A) and the percentage of patients with abnormal voice values (B). DSI indicates Dysphonia Severity Index; PTP, phonation threshold pressure; and VHI, Voice Handicap Index.
Discussion
In this mixed methods observational study, we found that a high percentage of patients undergoing thyroidectomy for the treatment of thyroid cancer have new-onset dysphonia, a doubling from the preoperative visit even in the absence of vocal fold paralysis or immobility. Before surgery, few patients with thyroid cancer shared that postoperative dysphonia was a concern. In contrast, more than half of the patients perceived themselves as disabled by their vocal deficiencies at 2 weeks after surgery, and this high prevalence persisted out to at least 1 year of follow-up in 50% (21 of 42) of participants. Quantitative vocal perturbations were detected in the DSI and VHI at the 2-week follow-up but returned to baseline levels by the 6-week or 6-month visit. Notably, there were no differences in reported voice difficulties between those who underwent total thyroidectomy with vs without ipsilateral CND at the 2-week postoperative time point, which is the time point that is most sensitive in detecting vocal deficits on all quantitative and qualitative variables.
Even more notable, quantitative (PTP, DSI, and VHI) and qualitative (impaired communication and voice symptom frequency) data collected from patients did not consistently correlate throughout the first postoperative year. This suggests that the VHI and other objective measures were not sensitive to detect voice changes that affected patients’ quality of life. For example, PTP values did not differ before to after thyroidectomy. Patients’ DSI and VHI values were worse at the 2-week postoperative visit but returned to normal levels by 6 months after thyroidectomy. However, patient-perceived impaired communication and patient-reported symptoms remained a major concern out to 1 year, while their quantitative vocal variables either did not change (PTP) or returned to baseline levels within 6 weeks (DSI) or 6 months (VHI) of the surgery.
The mixed methods approach used allowed the comparison of subjective patient-reported symptoms with the more quantitative vocal variables of PTP, DSI, and VHI derived from the instrumental voice evaluations. Rigorous serial qualitative interviews are rarely performed in surgical cohorts because they are time intensive to perform and analyze. Using this approach allowed us to more completely understand the voice-related quality-of-life consequences of thyroidectomy. The theme of impaired communication emerged at the 2-week postoperative visit and persisted out to 1 year after surgery. Notably, patients often did not voice symptoms with their physician at early postoperative visits unless asked directly. It is likely that patients expected their voice to improve over time, and perhaps this seemed a minor concern in the setting of their cancer care. However, as fears of cancer recurrence and other symptoms and concerns (difficulty swallowing, tingling, medication management, scar appearance, and low energy) diminished, voice-related issues became among the more prominent concerns, which contributes to perceived impaired communication and voice symptoms at 1 year after surgery. These data highlight the importance of physicians specifically asking patients with thyroid cancer about their voice symptoms.
If we tracked improvements in voice using traditional quantitative methods alone, such as PTP, DSI, and VHI, our conclusion would have been that postsurgical dysphonia is limited to a few postoperative weeks or months. Instead, we found that these measures did not capture the burden and duration of dysphonia perceived by patients. The VHI, which is purported to measure voice-related handicap from the patient’s perspective, did not correlate with patient-reported voice impairments described during interviews at all postoperative time points.
Limitations and Future Directions
This study has limitations. First, although the incidence of suspected recurrent laryngeal nerve injury owing to observed vocal fold immobility is higher in our participant population than that reported in the literature,3 it is likely an adverse effect of the instrumental voice evaluations, including laryngoscopy, being performed in all participants at early time points after surgery (eg, 2 weeks). Notably, their PTP, DSI, and VHI measures were not distinguishable from those of participants who did not have a paresis or paralysis at all postoperative time points. Second, thoroughly interviewing participants at multiple time points may have increased ascertainment of voice symptoms that may not be particularly troublesome to patients. Therefore, this approach risks overestimating the severity and consequence of symptoms. Third, because this is an ongoing clinical trial, participant enrollment at the 1-year time point is reduced compared with earlier postoperative time points. However, power analyses suggest that a group size of 30 is capable of detecting meaningful quantitative differences derived from the voice evaluation over time. In addition, qualitative data saturation was achieved, with no new voice information or vocal codes being derived from interviews with the ascertainment of additional participants across all study time points. Fourth, although all patients were treated with thyroid hormone replacement, we do not know their level of response or how long it took to achieve the target thyrotropin levels. This may have affected voice-related patient outcomes.
Our findings have implications for the management of voice before and after thyroidectomy. In 2013, published clinical guidelines for improving voice outcomes after thyroidectomy highlighted the need for patient education on voice outcomes and the importance of using preoperative and postoperative quantitative measures of vocal function.42 Data herein suggest that the recommended quantitative measures are limited in their ability to identify meaningful dysphonia in patients after thyroidectomy. Instead, our results argue that commonly used, validated quantitative vocal measurements, while important in some instances, have insufficient sensitivity and that surgeons and other clinicians need to directly and systematically query patients about vocal changes after thyroidectomy. Moreover, these findings highlight the need for preoperative education and counseling about potential postoperative vocal deficits, as well as the importance of early referral in patients with postoperative dysphonia. Acting early may assuage potential long-term quality-of-life voice sequelae related to thyroidectomy.
Conclusions
Postthyroidectomy voice changes are common even in the absence of vocal fold paralysis or immobility and can persist at least out to 1 year of follow-up. Patients with thyroid cancer who were interviewed indicated impaired communication after surgery because of voice changes. The high prevalence of significant voice changes was captured only through direct patient interviews. If we relied on traditional quantitative methods alone, such as PTP, DSI, and VHI, our conclusion would have been different. Specifically, we would have concluded that postthyroidectomy dysphonia is limited to a few postoperative weeks. Instead, we found that the quantitative measures PTP, DSI, and VHI inadequately capture the burden and duration of dysphonia experienced by patients with thyroid cancer after thyroidectomy.
eAppendix 1. Interview Guides for All Preoperative and Postoperative Time Points
eAppendix 2. Participant Data Sheet Collected at Preoperative Time Point
eTable 1. Patient Demographics
eTable 2. Laryngoscopy Results of Patients With Voice Complications
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7-30. [DOI] [PubMed] [Google Scholar]
- 2.Rosenthal LH, Benninger MS, Deeb RH. Vocal fold immobility: a longitudinal analysis of etiology over 20 years. Laryngoscope. 2007;117(10):1864-1870. [DOI] [PubMed] [Google Scholar]
- 3.Francis DO, Pearce EC, Ni S, Garrett CG, Penson DF. Epidemiology of vocal fold paralyses after total thyroidectomy for well-differentiated thyroid cancer in a Medicare population. Otolaryngol Head Neck Surg. 2014;150(4):548-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Francis DO, Williamson K, Hovis K, et al. . Effect of injection augmentation on need for framework surgery in unilateral vocal fold paralysis. Laryngoscope. 2016;126(1):128-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paniello RC, Edgar JD, Kallogjeri D, Piccirillo JF. Medialization versus reinnervation for unilateral vocal fold paralysis: a multicenter randomized clinical trial. Laryngoscope. 2011;121(10):2172-2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Francis DO, McKiever ME, Garrett CG, Jacobson B, Penson DF. Assessment of patient experience with unilateral vocal fold immobility: a preliminary study. J Voice. 2014;28(5):636-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leder SB, Suiter DM, Duffey D, Judson BL. Vocal fold immobility and aspiration status: a direct replication study. Dysphagia. 2012;27(2):265-270. [DOI] [PubMed] [Google Scholar]
- 8.Brunner E, Friedrich G, Kiesler K, Chibidziura-Priesching J, Gugatschka M. Subjective breathing impairment in unilateral vocal fold paralysis. Folia Phoniatr Logop. 2011;63(3):142-146. [DOI] [PubMed] [Google Scholar]
- 9.Stojadinovic A, Henry LR, Howard RS, et al. . Prospective trial of voice outcomes after thyroidectomy: evaluation of patient-reported and clinician-determined voice assessments in identifying postthyroidectomy dysphonia. Surgery. 2008;143(6):732-742. [DOI] [PubMed] [Google Scholar]
- 10.Akyildiz S, Ogut F, Akyildiz M, Engin EZ. A multivariate analysis of objective voice changes after thyroidectomy without laryngeal nerve injury. Arch Otolaryngol Head Neck Surg. 2008;134(6):596-602. [DOI] [PubMed] [Google Scholar]
- 11.Hong KH, Kim YK. Phonatory characteristics of patients undergoing thyroidectomy without laryngeal nerve injury. Otolaryngol Head Neck Surg. 1997;117(4):399-404. [DOI] [PubMed] [Google Scholar]
- 12.Hillel AD. Voice changes after thyroidectomy without recurrent laryngeal nerve injury. J Am Coll Surg. 2005;200(5):813-813. [DOI] [PubMed] [Google Scholar]
- 13.Stojadinovic A, Shaha AR, Orlikoff RF, et al. . Prospective functional voice assessment in patients undergoing thyroid surgery. Ann Surg. 2002;236(6):823-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Pedro Netto I, Fae A, Vartanian JG, et al. . Voice and vocal self-assessment after thyroidectomy. Head Neck. 2006;28(12):1106-1114. [DOI] [PubMed] [Google Scholar]
- 15.Debruyne F, Ostyn F, Delaere P, Wellens W. Acoustic analysis of the speaking voice after thyroidectomy. J Voice. 1997;11(4):479-482. [DOI] [PubMed] [Google Scholar]
- 16.Lombardi CP, Raffaelli M, D’Alatri L, et al. . Voice and swallowing changes after thyroidectomy in patients without inferior laryngeal nerve injuries. Surgery. 2006;140(6):1026-1032. [DOI] [PubMed] [Google Scholar]
- 17.Sinagra DL, Montesinos MR, Tacchi VA, et al. . Voice changes after thyroidectomy without recurrent laryngeal nerve injury. J Am Coll Surg. 2004;199(4):556-560. [DOI] [PubMed] [Google Scholar]
- 18.Soylu L, Ozbas S, Uslu HY, Kocak S. The evaluation of the causes of subjective voice disturbances after thyroid surgery. Am J Surg. 2007;194(3):317-322. [DOI] [PubMed] [Google Scholar]
- 19.Vicente DA, Solomon NP, Avital I, et al. . Voice outcomes after total thyroidectomy, partial thyroidectomy, or non-neck surgery using a prospective multifactorial assessment. J Am Coll Surg. 2014;219(1):152-163. [DOI] [PubMed] [Google Scholar]
- 20.Maeda T, Saito M, Otsuki N, et al. . Voice quality after surgical treatment for thyroid cancer. Thyroid. 2013;23(7):847-853. [DOI] [PubMed] [Google Scholar]
- 21.Lee JC, Breen D, Scott A, et al. . Quantitative study of voice dysfunction after thyroidectomy. Surgery. 2016;160(6):1576-1581. [DOI] [PubMed] [Google Scholar]
- 22.Lee DY, Lee KJ, Hwang SM, et al. . Analysis of temporal change in voice quality after thyroidectomy: single-institution prospective study. J Voice. 2017;31(2):195-201. [DOI] [PubMed] [Google Scholar]
- 23.Solomon NP, Helou LB, Henry LR, et al. . Utility of the Voice Handicap Index as an indicator of postthyroidectomy voice dysfunction. J Voice. 2013;27(3):348-354. [DOI] [PubMed] [Google Scholar]
- 24.Henry LR, Helou LB, Solomon NP, et al. . Functional voice outcomes after thyroidectomy: an assessment of the Dsyphonia Severity Index (DSI) after thyroidectomy. Surgery. 2010;147(6):861-870. [DOI] [PubMed] [Google Scholar]
- 25.Solomon NP, Helou LB, Makashay MJ, Stojadinovic A. Aerodynamic evaluation of the postthyroidectomy voice. J Voice. 2012;26(4):454-461. [DOI] [PubMed] [Google Scholar]
- 26.Rueger RG. Benign disease of the thyroid gland and vocal cord paralysis. Laryngoscope. 1974;84(6):897-907. [DOI] [PubMed] [Google Scholar]
- 27.Neri G, Castiello F, Vitullo F, De Rosa M, Ciammetti G, Croce A. Post-thyroidectomy dysphonia in patients with bilateral resection of the superior laryngeal nerve: a comparative spectrographic study. Acta Otorhinolaryngol Ital. 2011;31(4):228-234. [PMC free article] [PubMed] [Google Scholar]
- 28.Kuhn MA, Bloom G, Myssiorek D. Patient perspectives on dysphonia after thyroidectomy for thyroid cancer. J Voice. 2013;27(1):111-114. [DOI] [PubMed] [Google Scholar]
- 29.Creswell JW. Research Design: Qualitative, Quantitative, and Mixed Methods Approaches. Thousand Oaks, CA: SAGE Publications; 2013. [Google Scholar]
- 30.Charmaz K. Constructing Grounded Theory. Thousand Oaks, CA: SAGE Publications; 2014. [Google Scholar]
- 31.Jacobson BH, Johnson A, Grywalski C, et al. . The Voice Handicap Index (VHI). Development and Validation. 1997;6(3):66-70. Am J Speech Lang Pathol. 1997;6:66-70. doi: 10.1044/1058-0360.0603.66 [DOI] [Google Scholar]
- 32.Titze IR. Phonation threshold pressure: a missing link in glottal aerodynamics. J Acoust Soc Am. 1992;91(5):2926-2935. [DOI] [PubMed] [Google Scholar]
- 33.Wuyts FL, De Bodt MS, Molenberghs G, et al. . The Dysphonia Severity Index: an objective measure of vocal quality based on a multiparameter approach. J Speech Lang Hear Res. 2000;43(3):796-809. [DOI] [PubMed] [Google Scholar]
- 34.Zhuang P, Swinarska JT, Robieux CF, Hoffman MR, Lin S, Jiang JJ. Measurement of phonation threshold power in normal and disordered voice production. Ann Otol Rhinol Laryngol. 2013;122(9):555-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Plexico LW, Sandage MJ, Faver KY. Assessment of phonation threshold pressure: a critical review and clinical implications. Am J Speech Lang Pathol. 2011;20(4):348-366. [DOI] [PubMed] [Google Scholar]
- 36.Solomon NP, DiMattia MS. Effects of a vocally fatiguing task and systemic hydration on phonation threshold pressure. J Voice. 2000;14(3):341-362. [DOI] [PubMed] [Google Scholar]
- 37.Goy H, Fernandes DN, Pichora-Fuller MK, van Lieshout P. Normative voice data for younger and older adults. J Voice. 2013;27(5):545-555. [DOI] [PubMed] [Google Scholar]
- 38.Lee K, Kletzien H, Connor NP, Schultz E, Chamberlain CS, Bless DM. Effects of aging on thyroarytenoid muscle regeneration. Laryngoscope. 2012;122(12):2800-2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Lierde K, D’Haeseleer E, Wuyts FL, Baudonck N, Bernaert L, Vermeersch H. Impact of thyroidectomy without laryngeal nerve injury on vocal quality characteristics: an objective multiparameter approach. Laryngoscope. 2010;120(2):338-345. [DOI] [PubMed] [Google Scholar]
- 40.Mandrekar JN. Measures of interrater agreement. J Thorac Oncol. 2011;6(1):6-7. [DOI] [PubMed] [Google Scholar]
- 41.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed London, England: Routledge; 1988. [Google Scholar]
- 42.Chandrasekhar SS, Randolph GW, Seidman MD, et al. ; American Academy of Otolaryngology–Head and Neck Surgery . Clinical practice guideline: improving voice outcomes after thyroid surgery. Otolaryngol Head Neck Surg. 2013;148(6)(suppl):S1-S37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Interview Guides for All Preoperative and Postoperative Time Points
eAppendix 2. Participant Data Sheet Collected at Preoperative Time Point
eTable 1. Patient Demographics
eTable 2. Laryngoscopy Results of Patients With Voice Complications