Abstract
Objective
Relative to SLE, epidemiologic studies on chronic cutaneous lupus erythematosus (CCLE) are rare and limited to populations without racial diversity. We sought to provide minimum estimates of the incidence of primary CCLE (CCLE in absence of SLE) in a predominantly white and black population in the Southestern United States.
Methods
The Georgia Lupus Registry used multiple sources for case finding, including dermatology and rheumatology practices, multispecialty healthcare facilities, and dermatopathology reports. Cases with a clinical or clinical-histological diagnosis of CCLE were classified as definite. Cases ascertained exclusively from dermatopathology reports were categorized as probable. Age-standardized incidence rates stratified by sex and race were calculated for discoid lupus erythematosus (DLE) in particular and for CCLE in general.
Results
The overall age-adjusted estimates for combined (definite and probable) CCLE were 3.9/100,000 person-years (95% CI: 3.4,4.5). The overall age-adjusted incidence of definite and combined DLE were 2.9 (95% CI: 2.4,3.4) and 3.7 (95% CI: 3.2,4.3) per 100,000 person-years, respectively. With capture-recapture methods, the age-adjusted incidence of definite DLE increased to 4.0 (95% CI: 3.2,4.3). Black-to-white and female-to-male incidence ratios were 5.4 and 3.1 for definite DLE.
Conclusion
Our findings underscore striking racial disparities in the susceptibility for primary CCLE, with black people experiencing between three and five-fold increased incidence of CCLE in general and DLE in particular, compared to white people. Gender differences were consistent with those reported previously, with a three times higher risk of DLE in females compared to males.
Keywords: cutaneous lupus erythematosus, chronic cutaneous lupus, epidemiology, incidence, racial disparities
Cutaneous lupus erythematosus (CLE) comprises multiple dermatological disorders, which may be skin-limited or found in association with underlying systemic lupus erythematosus (SLE). CLE exhibits distinctive clinical and histopathological features, which are categorized as acute (ACLE), subacute (SCLE) or chronic (CCLE)1, 2. CCLE comprises discoid lupus erythematosus (DLE), lupus erythematosus profundus (LEP), lupus erythematous tumidus (LET) and chilblain lupus erythematosus (CHLE). CCLE subtypes are less likely to overlap with or progress to SLE than other CLE types2, 3, 4; however, they pose significant burden to individuals and the healthcare system. For instance, DLE, the hallmark of CCLE, represents 80% of the CLE conditions seen by dermatologists5–7. Characterized by erythematous indurated plaques with adherent scale that heals with atrophic scarring and dyspigmentation8, and largely found on the scalp, face and ears, DLE can cause scarring alopecia and facial disfigurement2, 5, 6, 9, with substantial impact on individuals’ quality of life10, 11.
DLE has a relatively characteristic clinical-pathological description and has been recognized in individuals of all races12–15. Although DLE is less likely associated with SLE2 than other CLE subtypes, only a few population-based studies have estimated the incidence of DLE in absence of SLE (or “primary” DLE)4, 15–17. Early reports suggest that similar to SLE, DLE might be more frequent among blacks, compared to whites18. However, recent incidence estimates were higher (3.6/100,000/year) in a predominantly white population of the United States than in the African-descendant population of French Guiana South America (nearly 2.6/100,000/year)16, 17. Methodological differences limit the comparability of both studies, and to our knowledge, no epidemiological studies have targeted black/white populations to assess racial disparities in the susceptibility of CCLE. We sought to determine minimum estimates for the incidence of CCLE in general and DLE in particular, in a black/white population of the Southeastern United States.
PATIENTS AND METHODS
Georgia Lupus Registry (GLR) data were examined to assess CCLE and DLE in absence of SLE. The GLR is a population-based registry designed to better estimate the incidence and prevalence of SLE in a large population with high proportion of high-risk individuals of black race. The GLR methodology has been described extensively19, 20. Briefly, GLR is one of five lupus registries funded by the Centers for Disease Control and Prevention21 to conduct more reliable surveillance of lupus in the US. The GLR catchment area, Fulton and DeKalb counties in Atlanta, encompassed a population of 1.5 million inhabitants with nearly even representation of whites and blacks. The Georgia Department of Public Health (GA DPH) allowed Emory University to collect private health information (PHI) and review medical records without patient consent using the health surveillance exemption to the the Health Insurance Portability and Accountability Act (HIPAA) privacy rules (45 CFR parts 160 and 164), a key authorization to ascertain and validate cases on a population level. The project was approved by the Institutional Review Boards of Emory University and the GA DPH.
Study population and period
The study population consisted of all residents of Fulton and DeKalb counties, the central counties in the Atlanta metropolitan area. The Bureau of the Census population estimates in 2002 for the two counties was 1,552,970 with 51.1% women, 49.3% blacks, and 46.4% whites21. Incidence rates for a diagnosis from January 1st 2002 through December 31st 2004 were estimated on a catchment area population of 4,742,264 person-years.
Ascertainment and validation of CCLE and DLE
Although the GLR was primarily designed to ascertain the full spectrum of SLE, registry efforts also entailed finding and validating individuals with a variety of lupus-related conditions, including primary CCLE20. GLR used multiple sources in the pluralistic US health care system to find potential cases. The primary sources included hospitals, rheumatology, nephrology and dermatology practices in and around the catchment area. As described elsewhere, 18 of 25 dermatology groups in the target area contributed, along with the other sources, to finding CCLE cases20. With the exception of one high yield practice, dermatology groups that declined to contribute to the registry efforts were either cosmetically oriented practices or self-reported to be very low yield for CCLE. Administrative databases were queried for the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnostic code 695.4 (discoid lupus), in addition to 710.0 (SLE), 710.8 (other specified connective tissue disease), and 710.9 (unspecified connective tissue disease).
Secondary sources for CCLE cases included the two largest dermatopathology laboratories in the target area, which were queried for the ICD-9 code 695.4 or for a wide range of key words in skin pathology reports (e.g. CLE, lupus, discoid, DLE, LE, tumidus, chilblain, panniculitis, lupus profundus).
After screening for residence in the catchment counties during the target period, medical records and pathology reports were requested for an extended period (e.g. 2001-2005), allowing for more complete capture of clinical information. Capturing PHI was required to avoid counting the same case multiple times. All available medical records for each case were fully abstracted for over 200 data elements, 36 of which corresponded to cutaneous manifestations (see Supplemental Data Dictionary Index). Abstractors also recorded the final diagnosis stated by the attending physician, type of physician (dermatologist, rheumatologist, nephrologist, other), the earliest date of diagnosis (CCLE, SLE), the ACR criteria for SLE, and earliest date of occurrence of each ACR criterion and skin manifestation. Demographic information, including race, was gathered from medical records. Detailed definitions for each data element were stated in a data dictionary. Abstractors were thoroughly trained and tested before entering the field, where they continued to undergo periodic quality assessments.
Case Definitions
The accepted diagnosis of CCLE is a characteristic clinical presentation with supporting histological features2, 8. This standard may not be achieved in every case, as biopsy may be unnecessary, particularly when lesions are classic in appearance or present on cosmetically sensitive areas. In these cases, diagnosis is based solely on clinical evaluation. Therefore, the requirement of histological confirmation for case definition in epidemiological studies may lead to underestimation of the population burden of CCLE.
Following the classification by Gilliam and Sontheimer22, we included as CCLE any of the following: DLE, lupus panniculitis, lupus profundus, lupus tumidus or chilblain lupus, or a combination of any of those conditions. Definitions of CCLE subtypes and keywords used to guide medical data abstraction are depicted as Suplemental Table 1. In order to estimate the incidence of primary CCLE, we excluded cases that either had a diagnosis of SLE by the treating dermatologist, rheumatologist, and/or nephrologist, or fulfilled the American College of Rheumatology (ACR) classification criteria for SLE23 within two months of the initial CCLE diagnosis. Two months was chosen as an appropriate timeframe to conduct clinical evaluation for possible systemic manifestations that may have occurred associated to the onset of CCLE.
CCLE cases were classified as DLE or other CCLE subtypes, and subdivided into either definite or probable categories. Definite CCLE were cases with a clinical-pathological or clinical diagnosis of a specific CCLE subtype documented in the medical records. Probable CCLE were cases ascertained through a dermatopathology report, in which both the presumed clinical diagnosis by the attending dermatologists and the histopathology findings were highly suggestive of either DLE, LEP or LET, but the original medical records from the attending dermatologist who ordered the biopsy were unavailable. Cases were pre-classified as probable DLE if they were either (i) submitted by the attending dermatologist to confirm DLE and had a histological description consistent with CLE, or (ii) submitted to rule out CLE, lupus, or a similar condition and had a histological description consistent with a discoid pattern of CLE (interface dermatitis at the dermal-epidermal junction, superficial and deep dermal perivascular and periadnexal lymphocytic infiltrate, +/- increased dermal mucin, and follicular hyperkeratosis)24. Similarly, cases with both a clinical assessment of probable CLE and a histological description suggestive of lupus erythematosus panniculitis (lobular lymphocytic panniculitis, paraseptal lymphoid follicles, hyaline degeneration of the fat, mucin deposition, +/- overlying features of DLE) or lupus erythematosus tumidus (interstitial mucin deposition, superficial and deep dermal lymphocytic perivascular and periadnexal infiltrate, with relative sparing of the dermal-epidermal junction) were pre-classified as probable LEP or LET, respectively24, 25. Next, two study dermatologists with extensive experience in CLE (SP, LDA) reviewed the dermatopathology reports for final case validation.
Incidence estimates were reported for three case definition categories: (i) definite DLE; (ii) “combined” definite and probable DLE; and (iii) “combined” definite and probable CCLE, which included definite and probable cases of all CCLE subtypes.
Statistical Analysis
Crude incidence rates and 95% confidence intervals (CI) as well as race- and sex-stratified incidence were estimated using methods based on the Poisson distribution26. The numerator consisted of cases with a first diagnosis in 2002-2004. Denominator data for DeKalb and Fulton counties were obtained from the post-censal population estimates for the years 2002–200427. Age-adjusted estimates and 95% CI were calculated using the standard 2000 projected age distribution by direct standardization, which calculates age standardized rates and “exact” CI based on the gamma distribution28. To estimate underascertainment of definite DLE cases, we conducted capture–recapture analysis accounting for the degree of overlap among multiple case-finding sources29. Community dermatologists, community rheumatologists & other specialists, and multispecialty healthcare facilities (e.g. community hospitals, Emory University Health System, Grady Health System, Kaiser Permanente) were chosen to be the primary sources of cases. Log-linear modeling was performed to estimate the number of persons with definite DLE who were missed in the population. The best fitting model was determined by goodness of fit statistics and the parsimony principle. Capture-recapture methods were implemented using SAS Proc Genmod.
RESULTS
Of 231 cases with a new diagnosis of CCLE between 2002 and 2004, 41 (17.5%) were excluded because they fulfilled ≥4 ACR criteria for SLE and the remaining 190 were primary CCLE. Among them, 147 with either a clinical-pathological or a clinical diagnosis of CCLE were classified as definite CCLE, and 43 that were ascertained through a pathology report were classified as probable CCLE (Figure 1). The overlap of individuals ascertained by sources are depicted in Figure 2.
Figure 1.
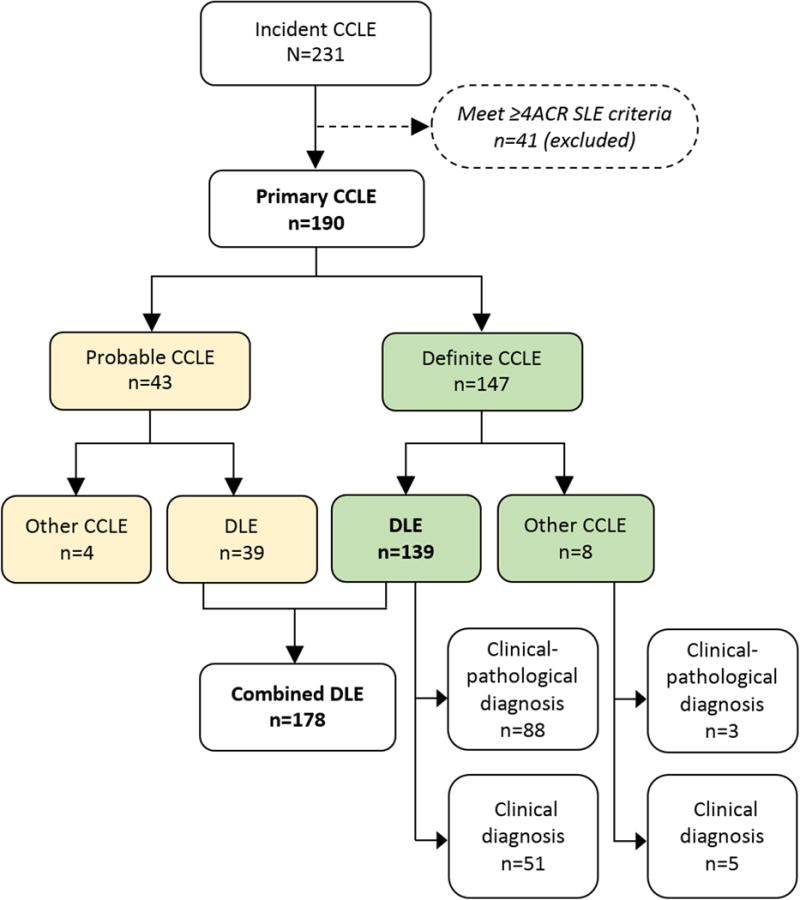
Case Ascertainment and Case Definition
Figure 2.
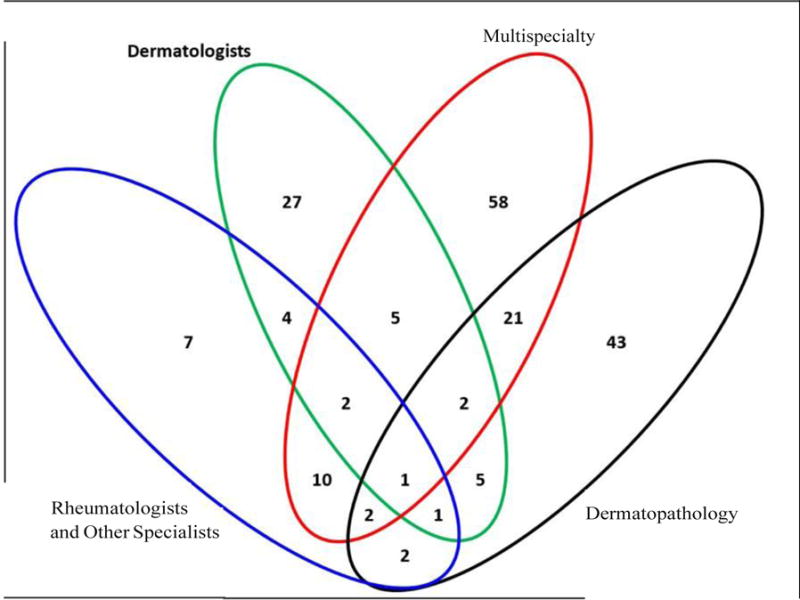
Sources of Case Ascertainment
Description of CCLE subtypes
DLE
There were 139 cases with definite DLE, of which 88 (63.3 %) had a clinical-pathological diagnosis of DLE by a dermatologist (Figure 1). Among the remaining 51, a clinical diagnosis of DLE was stated by a dermatologist in 30 (21.6%), a rheumatologist in 10 (7.2%), and other physicians in 11 (7.9%) cases. Among 43 probable CCLE, 39 were classified as DLE (Figure 1), of which 32 (78.6%) had a clinical diagnosis of DLE by a dermatologist and a histological description consistent with DLE (n=31) or CLE (n=1)24. Among the 7 remaining, 5 had a clinical diagnosis of CLE. The histopathological description was consistent with the discoid pattern of CLE as described in the Methods, in all those 7 cases. Overall, 178 cases had Combined DLE (definite or probable).
Other CCLE subtypes
There were 8 cases with definite CCLE different than DLE: 4 had LEP (1 with a consistent skin biopsy), 3 LET (1 with a consistent biopsy) and 1 had a clinical-pathological diagnosis of LET and LEP. Four cases ascertained through dermatopathology reports were classified as probable CCLE (1 with LEP and 3 with LET). The biopsies of these four cases were requested to rule out CLE, and the histological description and pathologist assessment were consistent with LEP and LET, as described in the Methods.
Incidence of DLE
Crude and age-adjusted incidence rates of DLE were similar, overall and across demographic categories. The age-adjusted incidence rate was 2.9 (95% CI: 2.4,3.4) per 100,000 person-years for definite DLE and 3.7 (95% CI: 3.2,4.3) per 100,000 person-years for combined (definite and probable) DLE (Table 1). The highest age-adjusted incidence rates of definite and combined DLE were for black females (definite DLE: 6.6 [95% CI: 5.3,8.2] per 100,000 person-years; combined DLE: 7.9 [95% CI: 6.5,9.6] per 100,000 person-years). The female:male ratio was 3.1 for definite DLE and 2.8 for combined DLE. Incidence was also higher in blacks, with black:white ratios of 5.4 for definite DLE, and 4.1 for combined DLE. The lowest incident rates for all categories were in white males (0.2 [95% CI: 0.1,0.7] and 0.7 [95% CI: 0.4,1.4] per 100,000 person-years, for definite and combined DLE, respectively). Data on race were not available in 7 individuals, who were not included in the estimates by race.
Table 1.
Incidence Rates of DLE and CCLE in Fulton/DeKalb, Georgia (01/01/2002-12/31/2004)
| Definite DLE | Combined (definite and probable) DLE | Combined (definite and probable) CCLE | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| Race/Sex | Catchment Population (person-years) | N Cases | Crude Rate (95% CI) | Age-adjusted Rate (95% CI) | N Cases | Crude Rate (95% CI) | Age-adjusted Rate (95% CI) | # Cases | Crude Rate (95% CI) | Age-adjusted Rate (95% CI) |
|
|
||||||||||
| Overall | 4742264 | 139 | 2.9 (2.5,3.5) | 2.9 (2.4,3.4) | 178 | 3.8 (3.2,4.3) | 3.7 (3.2,4.3) | 190 | 4.0 (3.5,4.6) | 3.9 (3.4,4.5) |
| Female | 2424592 | 105 | 4.3 (3.6,5.2) | 4.3 (3.5,5.2) | 131 | 5.4 (4.6,6.4) | 5.3 (4.5,6.3) | 137 | 5.7 (4.8,6.7) | 5.6 (4.7,6.6) |
| Male | 2317672 | 34 | 1.5 (1.0,2.0) | 1.4 (1.0,2.0) | 47 | 2.0 (1.5,2.7) | 1.9 (1.4,2.6) | 53 | 2.3 (1.7,3) | 2.2 (1.7,2.9) |
| Black | 2321302 | 113 | 4.9 (4.0,5.9) | 4.9 (4.1,5.9) | 135 | 5.8 (4.9,6.9) | 5.8 (4.9,6.9) | 143 | 6.2 (5.2,7.3) | 6.2 (5.3,7.3) |
| Black Female | 1239819 | 83 | 6.7 (5.4,8.3) | 6.6 (5.3,8.2) | 100 | 8.1 (6.6,9.8) | 7.9 (6.5,9.6) | 104 | 8.4 (6.9,10.2) | 8.3 (6.8,10) |
| Black Male | 1081483 | 30 | 2.8 (1.9,4.0) | 2.8 (1.9,4.0) | 35 | 3.2 (2.3,4.5) | 3.3 (2.4,4.5) | 39 | 3.6 (2.6,4.9) | 3.7 (2.7,5) |
| White | 2210389 | 20 | 0.9 (0.6,1.4) | 0.9 (0.6,1.4) | 33 | 1.5 (1.1,2.1) | 1.4 (1,2) | 37 | 1.7 (1.2,2.3) | 1.6 (1.2,2.3) |
| White Female | 1082131 | 17 | 1.6 (1.0,2.5) | 1.6 (1,2.5.0) | 24 | 2.2 (1.5,3.3) | 2.2 (1.5,3.3) | 26 | 2.4 (1.6,3.5) | 2.4 (1.6,3.5) |
| White Male | 1128258 | 3 | 0.3 (0.1,0.8) | 0.2 (0.1,0.7) | 9 | 0.8 (0.4,1.5) | 0.7 (0.4,1.4) | 11 | 1.0 (0.5,1.7) | 0.9 (0.5,1.6) |
Rates are per 100,000 person-years (95% confidence intervals [95%CI]). Age-adjusted rates used the 2000 projected US population. The definite definition for discoid lupus erythematosus (DLE) consisted of cases with a clinical or clinical-pathological diagnosis of DLE. The combined definition for DLE included cases validated as definite and those ascertained through pathology reports (probable). Combined chronic cutaneous lupus (CCLE) included either definite or probable cases with all types of CCLE, including DLE. Three cases of Asian race and 7 of unknown race were not included in the estimates by race
Using capture-recapture methods, 53 additional definite DLE cases were ascertained, rendering the age-adjusted incidence for definite DLE to 4.0 (95% CI: 3.5,4.7) (Table 2). There were 35 and 6 definite DLE cases missed for blacks and whites, respectively. Capture-recapture analyses yielded incidence estimates per 100,000 person-years of 6.4 (95% CI: 5.4,7.5) and 1.2 (95% CI: 0.8,1.7) for blacks and whites, respectively, with a black:white ratio of 5.3.
Table 2.
Incidence Rates of DLE in Fulton/DeKalb, Georgia (01/01/2002-12/31/2004) Adjusted by Capture-recapture Methods
| Capture-recapture*
|
||||||
|---|---|---|---|---|---|---|
| Race | Catchment Area Population (person-years) | No. of Cases | Crude Rate (95% CI) | Age-adjusted Rate (95% CI) | No. of Cases Missed (95% CI) | Capture–recapture Adjusted Rate (95% CI) |
| Total | 4742264 | 139 | 2.9 (2.5,3.5) | 2.9 (2.4,3.4) | 53 (23,119) | 4.0 (3.5,4.7) |
| Black | 2321302 | 113 | 4.9 (4,5.9) | 4.9 (4.1,5.9) | 35 (12,100) | 6.4 (5.4,7.5) |
| White | 2210389 | 20 | 0.9 (0.6,1.4) | 0.9 (0.6,1.4) | 6 (1,36) | 1.2 (0.8,1.7) |
Values are the estimated number of definite DLE cases that were missed and the capture–recapture–adjusted incidence rate
Incidence of CCLE
Crude and age-adjusted incidence rates for all CCLE subtypes, including cases with definite and probable CCLE were 4.0 (95% CI: 3.5,4.6) and 3.9 (95% CI: 3.4,4.5) per 100,000 person-years, respectively. Crude and age-adjusted rates were similar across demographic categories. The highest age-adjusted incidence rates were for black females (8.3 [95% CI: 6.8,10] per 100,000 person-years) and the lowest for white males (0.9 [95% CI: 0.5,1.6] per 100,000 person-years). The female:male and the black:white ratios were 2.5 and 3.9, respectively.
Blacks developed CCLE nearly 4 years earlier on average compared to whites (p= 0.11). The mean age at onset for CCLE and DLE are depicted in Table 3.
Table 3.
Age at diagnosis in incident DLE and CCLE by Black and White Race
| Case Definition | Descriptor | Black | White | P value |
|---|---|---|---|---|
| Combined DLE | Count (N) | 135 | 33 | |
| Mean ± SD | 43.6 ± 13.1 | 47.5 ± 14.2 | 0.13 | |
| Median (IQR) | 43.6 (36.0–51.1) | 44.3 (37.7–60.5) | ||
| Range | (9.4–83.5) | (20.5–76.5) | ||
|
| ||||
| Combined CCLE | Count (N) | 143 | 37 | |
| Mean ± SD | 43.9 ± 13.2 | 47.2 ± 14.9 | 0.19 | |
| Median (IQR) | 43.6 (36.0–52.3) | 44.3 (37.7–60.5) | ||
| Range | (9.4–83.5) | (15.9–76.5) | ||
Abbreviations: DLE, discoid lupus erythematosus; CCLE, chronic cutaneous lupus erythematosus
Age-specific incidence rates of combined DLE were significantly higher for black individuals aged 30-59, compare to their white counterparts (Figure 3). While the incidence peak for blacks was 13.7/100,000 at age 40-49, whites showed two peaks of 3.2 and 2.9/100,000 person-years at 40-49 and ≥60, respectively. Moreover, age-specific incidence rates reached 10.3/100,000 person-years at age 40-49 in black females, as opposed to 2.6 and 2.5/100,000 person-years, at age 40-49 and >60, respectively in white females (data not shown). The incidence of DLE peaked at ages 40-49 and 50-59 (3.4 and 3.6/100,000 person-years, respectively) in black males, in contrast to approximately 0.5/100,000 persons-year in white males for all age groups older than 20 (data not shown).
Figure 3.
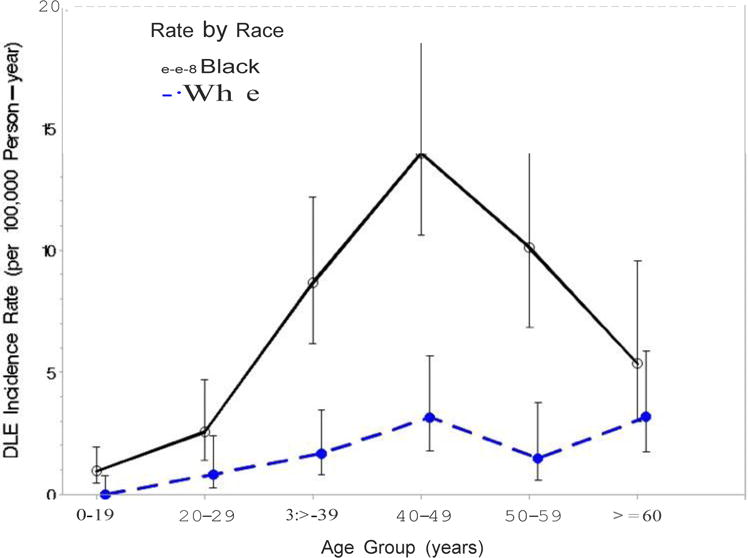
Incidence of DLE by Age in Blacks and Whites
Progression to SLE
9 and 16 cases progressed to SLE at 1 and 3 years since diagnosis, respectively. The progression rate was 5.3% (95% CI 2.8-10.0) and 12.3% (95% CI 7.5-20.1) at 1 and 3 years, respectively.
DISCUSSION
This study leveraged the population-based Georgia Lupus Registry (GLR) to report minimum incidence estimates of CCLE in a predominantly black/white population in the Southeastern US. Overall incidence rates of definite DLE, combined DLE (definite and probable), and combined CCLE (all CCLE disorders, including definite and probable) were 2.9, 3.7, and 3.9 per 100,000 person-years, respectively. Using capture-recapture analysis, the number of new definite DLE cases increased from 139 to 192, rendering an age-adjusted incidence of 4.0 per 100,000 person-years.
Substantial racial disparities in the susceptibility for CCLE in general, and DLE in particular, were uncovered. The black:white ratios were 3.4, 3.9, and 5.4 for combined CCLE, combined DLE, and definite DLE, respectively. Interestingly, the black:white ratios of primary CCLE reported in this study are similar to that for SLE reported by our group in the same geographic area20.
Prior population-based studies of the incidence were conducted in two predominantly Caucasian populations of the US (Olmsted County, Minnessotta) and Sweden, as well as in an African-descendant population in French Guiana, South America4, 16, 17. DLE annual incidence of 1.4 per 100,000 for white individuals in our catchment area is lower than previous estimates in Sweden (3.2/100,000) and Olmsted County, US (3.6/100,000)4, 16. Operational differences may account for our lower estimates in whites. For instance, the DLE definition in the Swedish study was based on ICD-10 codes, which could lead to overestimated incidence4. In contrast, nearly 80% of DLE cases in our study were validated through medical records review. Moreover, because we targeted CCLE without co-existing SLE, we excluded cases that fulfilled the ACR criteria for the classification of SLE around the diagnosis of DLE. Such approach differs from the Swedish study, in which 24% of DLE cases had co-existing SLE4. The US study, in contrast, used data from the Rochester Epidemiology Project, which can more efficiently retrieve medical information from multiple sources for Olmsted County residents16. Moreover, Olmsted residents face lower healthcare access barriers than people in the US Southern states. Consequently, we cannot exclude underascertainment associated to undiagnosis as a potential explanation of the lower incidence among whites in our study. Additionally, variability in biological (e.g. DNA methylation) and environmental (e.g. sun exposure, early diagnosis/treatment) factors between populations and geographic areas can account for differences in DLE estimates30.
Notably, the incidence of CCLE in the Atlanta black population was 6.2, as opposed to 2.6 in French Guiana, where 90% of the population are African-descendent17. However, methodological differences limit the comparability of both studies, and whether socio-environmental factors play a role in the higher risk of CCLE in blacks from the Southeast US deserve further research.
Our findings suggest that black/white disparities may also occur in relation to the age at diagnosis, with blacks tending to develop CCLE at earlier ages compared to whites, as noted with SLE in the same population20. Incident rates for combined DLE in black individuals aged 30-59 were significantly higher than in their white counterparts. However, the difference in mean age at DLE diagnosis by race (43.6 and 47.5 years for blacks and whites, respectively) was not statistically significant, which can be potentially explained by the small number of whites in our registry. The mean age at DLE diagnosis was 48.5 and 53 years-old in the predominantly Caucasian populations of Olmstead County and Sweden, respectively4, 16, and 32 years-old in the African-descendent population of French Guiana17. These findings support racial differences in the natural history of primary CCLE, analogous to SLE.
The overall incidence rates of CCLE in general, and DLE in particular, are relatively lower than the rates that we recently reported for SLE in the same catchment area20. While the GLR overall age-adjusted incidence rate for SLE was 5.6/100,000 person-years, rates of DLE and CCLE were 3.7 and 3.9/100,000, respectively. Our findings differ from those from the largely white Olmsted County population, where the incidence of CLE and SLE were similar15. However, in addition to CCLE, the Rochester study targeted bullous and SCLE, conditions linked to the HLA-B8, DR3 haplotype, which in turn disproportionately strike Caucasian individuals31.
Other factors can potentially explain the relatively lower incidence of primary CCLE compared to SLE in the GLR catchment area. First, socio-environmental factors might increase the risk of co-existing SLE and CCLE or progression from primarily cutaneous to systemic phenotypes30. GLR data support a higher rate of progression from primary CCLE to SLE in our area compared to Olmsted county16, 32. While the 5-year cumulative incidence of SLE among primary CLE in Olmsted County was 5%31, we reported 5% and 12% SLE progression at 1 and 3 years. Additionally, 15% of incident SLE patients ascertained in our catchment area had co-existing DLE, as opposed to only 7% in Olmsted Country16, 20. Secondly, disproportionaltely greater underascertainment may have occurred for CCLE/DLE than SLE in our study. Although the majority of collaborating dermatologists were medical, cosmetic practices were not included as sources of case finding, potentially leading to missed cases. Underascertainment may have also occurred because dermatology offices were contacted for case finding between 4-6 years after the surveillance dates of interest. This time discrepancy may have resulted in underreporting CCLE cases due to limited accessibility of records. To overcome this limitation, we conducted capture-recapture analysis. This method rendered an overall age-adjusted incidence for definite DLE of 4.0 per 100,000 person-years. The number of cases missed were estimated to be 35 and 6 among black and white people, respectively, raising the incidence of definite DLE from 4.0 to 6.5 and from 0.9 to 1.2 for blacks and whites, respectively. The black:white ratio after adjustment remained over 5, stressing out the greater predisposition for this condition in blacks. Additionally, because GLR data were collected from medical records, we can not exclude that some cases with overlapping CCLE and SLE may have been misclassified as primary CCLE. However, data abstraction entailed periodic audits to ensure consistency and accuracy in abstracting ACR criteria and other clinical data. Additionally, almost all rheumatology practices in the catchment area served as sources of cases, and all available medical records for each incident case were fully abstracted. As a result, the number of underreported incident SLE cases rendered by capture-recapture methods was very low (n=31), suggesting that the majority of overlapping SLE/CCLE cases were captured by GLR methods20. Second, because our case definition relied on medical records review and we assumed that attending physicians knew how to differentiate CCLE/DLE from other major forms of CLE and other autoimmune conditions, we cannot exclude misclassification of CCLE cases. However, these are limitations of population-based studies in general, where individual study physician assessment is not feasible13, 15–17, 33. Third, while a clinical or clinical-histological documentation of the diagnosis of CCLE was obtained in nearly 80% of our cases, medical records were unavailable for 43 individuals who were further classified and analyzed separately as probable CCLE based on data from dermatopathology reports. Fourth, patients with SCLE were not included because only 8 cases with a dermatologist-confirmed diagnosis of primary SCLE were ascertained. Fifth, it is possible that DLE may have been underdiagnosed by physicians among whites, a diagnostic challenge faced by many clinicians. Additionally, we cannot exclude underascertainment of whites with CCLE due to lower participation of dermatology practices located in areas with higher concentration of whites. Sixth, the results of this study are best generalized to white and black individuals in the Southeastern US. Because race was assigned based primarily on the physician’s assessment documented in the medical record, it may not reflect the patient’s true self-identity, particularly among multiracial individuals.
Our study has several strengths. First, this is the first report of the incidence of primary CCLE and its DLE subtype in a large black/white population from the same geographic area. With the exception of the study in French Guiana17, prior epidemiologic studies have focused on overall CLE and targeted primarily Caucasian populations. Furthermore, the GLR allowed for acquisition of clinical data (including earliest date of each ACR criteria) from medical records across multiple facilities, reducing misclassification of incident SLE case as primary CCLE and vice versa. Moreover, most facilities provided medical records for an extended period (e.g. 2001-2005) and all available medical charts from multiple sources were fully abstracted. Thus, our efforts entailed the collection of comprehensive clinical information since the disease onset throughout 12/31/2004, potentially reducing lost to followup. The GLR also cross-referenced records from multiple sources, avoiding double-counting of cases.
In conclusion, this is the first epidemiologic description of primary CCLE in a predominantly black/white population and finds that CCLE disproportionately affects black individuals, paralleling the disparities observed in SLE in this region20 and other US populations30, 34.
Supplementary Material
Significance and Innovations.
- There are no studies that directly compare racial differences of the incidence of CCLE in a single population.
- The incidence of CCLE in general and DLE in particular in a large and predominantly black/white population of the Southeastern US was found to be between three and five-fold increased in black compared to white people.
- The GLR black/white disparities in the incidence of CCLE are analogous to those described for SLE in the same geographic area, suggesting that these two extremes of the lupus spectrum may share common biological and environmental pathways that contribute to the higher risk in black individuals.
- Whether black populations are also disproportionally stricken by more severe CCLE phenotypes and poorer outcomes, as it has been described in SLE, and whether black individuals with CCLE are at higher risk of progression from cutaneous to systemic lupus phenotypes, are questions that warrant further research.
Acknowledgments
Financial Support: This research was supported by the CDC, and by cooperative agreement CDC-RFA-DP08-806 and earlier by cooperative agreement PA03022 from the CDC. C.D. and S.S.L. are supported by the NIH (R01AR065493-01; R01MD010455-01; R01AR070898-01) and the CDC (U01DP005119).
Footnotes
DR. CRISTINA DRENKARD (Orcid ID: 0000-0002-6832-7291)
CDC Disclaimer: The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Chong BF, Werth VP. Skin Disease in Cutaneous Lupus Erythematosus. In: Wallace DJ, Hahn BH, editors. Dubois’ Lupus Erythematosus and Related Syndromes. 7th. Philadelphia: Lippincott Williams & Wilkin; 2007. pp. 310–32. [Google Scholar]
- 2.Werth VP. Clinical manifestations of cutaneous lupus erythematosus. Autoimmun Rev. 2005;4(5):296–302. doi: 10.1016/j.autrev.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Chong BF. Understanding how cutaneous lupus erythematosus progresses to systemic lupus erythematosus. JAMA Dermatol. 2014;150(3):296. doi: 10.1001/jamadermatol.2013.9030. [DOI] [PubMed] [Google Scholar]
- 4.Gronhagen CM, Fored CM, Granath F, Nyberg F. Cutaneous lupus erythematosus and the association with systemic lupus erythematosus: a population-based cohort of 1088 patients in Sweden. Br J Dermatol. 2011;164(6):1335–41. doi: 10.1111/j.1365-2133.2011.10272.x. [DOI] [PubMed] [Google Scholar]
- 5.Obermoser G, Sontheimer RD, Zelger B. Overview of common, rare and atypical manifestations of cutaneous lupus erythematosus and histopathological correlates. Lupus. 2010;19(9):1050–70. doi: 10.1177/0961203310370048. [DOI] [PubMed] [Google Scholar]
- 6.Rothfield N, Sontheimer RD, Bernstein M. Lupus erythematosus: systemic and cutaneous manifestations. Clin Dermatol. 2006;24(5):348–62. doi: 10.1016/j.clindermatol.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 7.Prystowsky SD, Gilliam JN. Discoid lupus erythematosus as part of a larger disease spectrum. Correlation of clinical features with laboratory findings in lupus erythematosus. Arch Dermatol. 1975;111(11):1448–52. [PubMed] [Google Scholar]
- 8.Walling HW, Sontheimer RD. Cutaneous lupus erythematosus: issues in diagnosis and treatment. Am J Clin Dermatol. 2009;10(6):365–81. doi: 10.2165/11310780-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 9.Wolff K, Johnson RA, Fitzpatrick TB. Fitzpatrick’s color atlas and synopsis of clinical dermatology. 6th. New York: McGraw-Hill Medical; 2009. p. xxxvii.p. 1114. [Google Scholar]
- 10.Klein R, Moghadam-Kia S, Taylor L, Coley C, Okawa J, LoMonico J, et al. Quality of life in cutaneous lupus erythematosus. J Am Acad Dermatol. 2011;64(5):849–58. doi: 10.1016/j.jaad.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teske NM, Cardon ZE, Ogunsanya ME, Li X, Adams-Huet B, Chong BF. Predictors of low quality of life in patients with discoid lupus. Br J Dermatol. 2017;177(4):e147–e9. doi: 10.1111/bjd.15490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bae YI, Yun SJ, Lee JB, Kim SJ, Won YH, Lee SC. A clinical and epidemiological study of lupus erythematosus at a tertiary referral dermatology clinic in Korea. Lupus. 2009;18(14):1320–6. doi: 10.1177/0961203309345769. [DOI] [PubMed] [Google Scholar]
- 13.Deligny C, Marie DS, Clyti E, Arfi S, Couppie P. Pure cutaneous lupus erythematosus in a population of African descent in French Guiana: a retrospective population-based description. Lupus. 2012;21(13):1467–71. doi: 10.1177/0961203312458167. [DOI] [PubMed] [Google Scholar]
- 14.Green A. Discoid erythematosus in Australian aborigines. Australas J Dermatol. 1995;36(3):175–7. doi: 10.1111/j.1440-0960.1995.tb00966.x. [DOI] [PubMed] [Google Scholar]
- 15.Jarukitsopa S, Hoganson DD, Crowson CS, Sokumbi O, Davis MD, Michet CJ, Jr, et al. Epidemiology of systemic lupus erythematosus and cutaneous lupus erythematosus in a predominantly white population in the United States. Arthritis Care Res (Hoboken) 2015;67(6):817–28. doi: 10.1002/acr.22502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Durosaro O, Davis MD, Reed KB, Rohlinger AL. Incidence of cutaneous lupus erythematosus, 1965–2005: a population-based study. Arch Dermatol. 2009;145(3):249–53. doi: 10.1001/archdermatol.2009.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deligny C, Clyti E, Sainte-Marie D, Couppie P, Huong du LT, Piette JC, et al. Incidence of chronic cutaneous lupus erythematosus in French Guiana: a retrospective population-based study. Arthritis Care Res (Hoboken) 2010;62(2):279–82. doi: 10.1002/acr.20079. [DOI] [PubMed] [Google Scholar]
- 18.Hochberg MC. Systemic lupus erythematosus. Rheum Dis Clin North Am. 1990;16(3):617–39. [PubMed] [Google Scholar]
- 19.Lim SS, Drenkard C, McCune WJ, Helmick CG, Gordon C, Deguire P, et al. Population-based lupus registries: advancing our epidemiologic understanding. Arthritis Rheumatism. 2009;61(10):1462–6. doi: 10.1002/art.24835. [DOI] [PubMed] [Google Scholar]
- 20.Lim SS, Bayakly AR, Helmick CG, Gordon C, Easley KA, Drenkard C. The incidence and prevalence of systemic lupus erythematosus, 2002–2004: The Georgia Lupus Registry. Arthritis Rheumatol. 2014;66(2):357–68. doi: 10.1002/art.38239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.CDC. Compiled from 1990–1999 bridged-race intercensal population estimates and 2000–2009 (Vintage 2009) bridged-race postcensal population estimates. Centers for Disease Control and Prevention. National Center for Health Statistics; 2009. Bridged-race population estimates, United States July 1st resident population by state, county, age, sex, bridged-race, and Hispanic origin. http://wonder.cdc.gov/bridged-race-v2009. [Google Scholar]
- 22.Gilliam JN, Sontheimer RD. Distinctive cutaneous subsets in the spectrum of lupus erythematosus. J Am Acad Dermatol. 1981;4(4):471–5. doi: 10.1016/s0190-9622(81)80261-7. [DOI] [PubMed] [Google Scholar]
- 23.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40(9):1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 24.Weedon D. The Lichenoid Reaction Pattern, chapter 3. In: Weedon D, editor. Weedon’s Skin Pathology. 3rd. London: Churchill Livingstone Elsevier; 2010. pp. 58–59. [Google Scholar]
- 25.Weedon D. Panniculitis, chapter 17. In: Weedon D, editor. Weedon’s Skin Pathology. 3rd. London: Churchill Livingstone Elsevier; 2010. p. 468. [Google Scholar]
- 26.Altman D, Machin D, Bryant T, Gardner M. Statistics with confidence. 2nd. London: British Medical Journal Books; 2000. [Google Scholar]
- 27.Klein RJ, Schoenborn CA. Age adjustment using the 2000 projected U.S. population. Healthy People 2010 Stat Notes. 2010:1–10. [PubMed] [Google Scholar]
- 28.Fay MP, Feuer EJ. Confidence intervals for directly standardized rates: a method based on the gamma distribution. Stat Med. 1997;16(7):791–801. doi: 10.1002/(sici)1097-0258(19970415)16:7<791::aid-sim500>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 29.Wittes JT, Colton T, Sidel VW. Capture-recapture methods for assessing the completeness of case ascertainment when using multiple information sources. J Chronic Dis. 1974;27(1):25–36. doi: 10.1016/0021-9681(74)90005-8. [DOI] [PubMed] [Google Scholar]
- 30.Lim SS, Drenkard C. The Epidemiology of Lupus. In: Wallace DJ, Hahn BH, editors. Dubois’Lupus Erythematosus and Related Syndromes. 8th. Philadelphia: Elsevier; 2012. pp. 8–24. [Google Scholar]
- 31.Sontheimer RD. Subacute cutaneous lupus erythematosus: 25-year evolution of a prototypic subset (subphenotype) of lupus erythematosus defined by characteristic cutaneous, pathological, immunological, and genetic findings. Autoimmun Rev. 2005;4(5):253–63. doi: 10.1016/j.autrev.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 32.Drenkard C, Shenvi N, Easley K, Lim SS. The Georgia Lupus Registry: A population-based estimate of the incidence of SLE in patients with chronic cutaneous lupus. Lupus. 2010;19(Suppl 1):10. Suppl 1. [Google Scholar]
- 33.Wieczorek IT, Propert KJ, Okawa J, Werth VP. Systemic symptoms in the progression of cutaneous to systemic lupus erythematosus. JAMA Dermatol. 2014;150(3):291–6. doi: 10.1001/jamadermatol.2013.9026. [DOI] [PubMed] [Google Scholar]
- 34.Somers EC, Marder W, Cagnoli P, Lewis EE, DeGuire P, Gordon C, et al. Population-based incidence and prevalence of systemic lupus erythematosus: the Michigan Lupus Epidemiology and Surveillance program. Arthritis Rheumatol. 66(2):369–78. doi: 10.1002/art.38238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


