Abstract
Epidemiological studies suggest that binge drinking is prevalent among adolescents, and may result in neurobehavioral consequences. Animal models provide the experimental control to investigate the consequences of “binge” alcohol exposure during this neurodevelopmental epoch. The current study used an animal model that combined an intermittent pattern of alcohol vapor exposure with voluntary drinking of 20% unsweetened alcohol in adolescent male and female Wistar rats (P22-62), in order to test for potential differences in: behavioral changes, ethanol drinking, and hypocretin/orexin (Hcrt/OX) signaling associated with exposure status. Two weeks after discontinuation of the alcohol vapor exposure and drinking during adolescence, rats were tested in adulthood for anxiety-like behaviors using a modified open field conflict task, pre-pulse facilitation of startle response, Light/Dark box, and marble burying test. Adolescent alcohol exposure led to overall decreased startle response and increased behavioral arousal in the Light/Dark chamber during adulthood. Additionally, male rats demonstrated more disinhibited behavior during the conflict task compared to females, and female rats exhibited more rearing behavior during the Light/Dark test. Rats were also given a two-bottle choice test that resulted in adolescent alcohol exposed rats drinking significantly more alcohol in adulthood. Further, female rats also consumed more alcohol in adulthood compared to males. Estrous cycle phase did not account for any of the sex differences observed in the behavioral measures. Histological results indicated that adolescent alcohol did not alter Hcrt/OX-1 or Hcrt/OX-2 receptor mRNA expression levels in adult rats compared to control adults. However, female rats expressed a higher level of Hcrt/OX-1 and Hcrt/OX-2 receptor mRNA in the frontal cortex compared to males. These data suggest that our current model of intermittent ethanol exposure in adolescence can modestly impact both behavior and future consumption of alcohol and that Hcrt/OX receptor signaling differs between males and females.
Keywords: Adolescence, Alcohol drinking, Hypocretin/Orexin, Female, Wistar rats
Introduction
Adolescence is defined by both physiological and psychological developments, that occur over the entire second decade of life. While adolescence is associated with an increase in a number of behaviors such as peer-directed social interaction, novelty seeking and risk-taking (Spear, 2000), it is also associated with an increase in experimentation with recreational drugs (van Amsterdam & van den Brink, 2010). Alcohol is by far the most commonly used drug in adolescents with over 90% of youth having tried alcohol at some point in their lives (Anderson & Baumberg, 2007), and 10–40% of adolescents (depending on the country) have reported binge drinking (Assanangkornchai, Mukthong, & Intanont, 2009; Caamaño-Isorna, Corral, Parada, & Cadaveira, 2008; Miller, Naimi, Brewer, & Jones, 2007; Viner & Taylor, 2007; Wechsler et al., 2002). This common pattern of drinking, in adolescents, is generally defined as an episode of drinking in which five (four drinks for females) or more alcoholic beverages are consumed in a 2h period (Courtney & Polich, 2009; Parada et al., 2011). Adolescent drinking is also a risk factor for the later onset of alcohol use disorders. Early onset of intoxication, especially prior to the age of 15yrs, is in fact one of the strongest predictors of alcohol dependence, with a 4-fold increase in the likelihood of dependence in those who begin drinking before the age of 15 yr compared to those who began drinking as young adults (Chuo & Pickering, 1992; Dawson, Goldstein, Chou, Ruan, & Grant, 2008; DeWit, Adlaf, Offord, & Ogborne, 2000; Ehlers, Slutske, Gilder, Lau, & Wilhelmsen, 2006; Grant, 1998; Grant & Dawson, 1997, 1998). One hypothesis forwarded to explain this finding suggests that early binge drinking can alter the normal course of cognitive development leading to an increased risk for a number of psychopathologies including drug addiction (DeWit et al., 2000; York, 1999). However, an alternative theory suggests that a subset of adolescents may have an underlying propensity for making impulsive and potentially risky decisions including an early onset of drinking (Iacono, Carlson, Malone, & McGue, 2002; Jessor & Jessor, 1975). These opposing hypotheses are difficult to disentangle in human studies. However, the development of an animal model to study the effects of adolescent alcohol exposure on behavior in adulthood could provide support for the alcohol exposure hypothesis as well as provide a better understanding of the brain mechanisms underlying the effects of adolescent drinking on development.
A recent body of literature supports a prominent role of the hypothalamic peptide hypocretin/orexin (Hcrt/OX) in homeostatic control of a number of regulatory processes including sleep wake cycle, feeding and energy metabolism and reward/motivated behavior (España, 2012; Sutcliffe & de Lecea, 2002; Willie, Chemelli, Sinton, & Yanagisawa, 2001). Additionally, experience with drugs of abuse results in upregulation of Hcrt/OX and receptor expression (Dayas, McGranahan, Martin-Fardon, & Weiss, 2008; Kane et al., 2000; Pasumarthi, Reznikov, & Fadel, 2006; Sharf, Sarhan, & Dileone, 2008). Orexin-A (OX-A) and orexin-B (OX-B) (also known as hypocretin-1 and −2, respectively) are peptides produced by a small population of cells in the lateral and dorsomedial hypothalamus and perifornical area (de Lecea et al., 1998; Sakurai et al., 1998). These Hcrt/OX neurons have widespread projections throughout the brain (Peyron et al., 1998), including projections from the lateral hypothalamus to many areas of the mesolimbic ‘reward pathway’ (Di Chiara & Imperato, 1985; Koob & Bloom, 1988; Wise & Rompre, 1989). Additionally, both receptor subtypes and Hcrt/OX-containing nerve terminals are distributed extensively throughout the prefrontal cortex (PFC) (Cluderay, Harrison, & Hervieu, 2002; Fadel & Deutch, 2002; Hervieu, Cluderay, Harrison, Roberts, & Leslie, 2001). Recent evidence suggests a dichotomous role of Hcrt/OX 1 (Hcrt/OX 1R) and Hcrt/OX 2 (Hcrt/OX 2R) receptors in the brain. A number of studies have shown that signaling through the Hcrt/OX 1 and Hcrt/OX 1 receptors regulates alcohol seeking and consumption (Barson, Ho, & Leibowitz, 2015; Dayas et al., 2008; Jupp, Krivdic, Krstew, & Lawrence, 2011; Kim, Brown, & Lawrence, 2012; Lawrence, Cowen, Yang, Chen, & Oldfield, 2006; Moorman & Aston-Jones, 2009; Richards et al., 2008; Shoblock et al., 2011; Smith, See, & Aston-Jones, 2009; Srinivasan et al., 2012). Specifically, Hcrt/OX receptor agonists have been shown to increase EtOH consumption (Barson et al., 2015; Schneider, Rada, Darby, Leibowitz, & Hoebel, 2007), while Hcrt/OX receptor antagonists reduce drinking (Jupp et al., 2011; Lawrence, Cowen, Yang, Chen, & Oldfield, 2009; Moorman & Aston-Jones, 2009; Srinivasan et al., 2012). While this demonstrates a role of Hcrt/OX system in alcohol consumption, there is currently no research investigating the impact adolescent ethanol consumption has on the Hcrt/OX receptor expression, thus we sought to investigate this in a rat model of adolescent alcohol exposure.
In the current study, we combine voluntary drinking of 20% unsweetened alcohol with 4 day episodes of alcohol vapor exposure, during adolescence, to simulate intermittent high level “binge” alcohol exposure. We have shown that vapor exposure alone in Wistar rats, results in moderate BACs (@175mg%) and robust neurobehavioral changes (Ehlers, Oguz, Budin, Wills, & Crews, 2013), but does not increase drinking in adulthood (Ehlers, Criado, Wills, Liu, & Crews, 2011). Whereas, drinking alone results in low BACs (<50 mg%) and produces increased drinking but not robust neurobehavioral changes in adulthood (Amodeo, Kneiber, Wills, & Ehlers, 2017). Together, we expect that this dynamic model of alcohol exposure in male and female adolescents will result in both an increase in neurobehavioral changes and an increase in drinking in adulthood. Further, we investigated the potential influence of early alcohol exposure on Hcrt/OX signaling in adulthood.
Materials and Methods
Animals
Subjects were 48 male and 47 female Wistar rats (Charles River, Hollister, CA, USA) who arrived in the laboratory on PD 22 and were housed in same-sex groups of three. The colony room was kept under a 12-h light/dark cycle (lights on at 8:00 h) with behavioral testing conducted during the light phase. Rats were pair-housed prior to behavioral testing in adulthood. Lab chow was provided ad libitum all times, except 24h prior to the Open Field Conflict test. Subjects were cared for in accordance with the guidelines of the NIH Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute.
Adolescent Intermittent Voluntary & Vapor Ethanol Exposure
Adolescent ethanol exposure occurred using two methods of administration; (1) voluntary intermittent 2-bottle access where animals could choose to self-administer alcohol and (2) intermittent vapor exposure where blood alcohol levels (BAL) were held constant over the duration of 4 days. Voluntary intermittent alcohol access occurred 24h before and 24h after each 4-day vapor session with 24h in between voluntary alcohol exposures. This cycle occurred 6 times and began on PD 22. Rats continued to receive five more voluntary access periods after the last vapor exposure on PD 62.
For the intermittent voluntary exposure, rats were given a choice between one bottle of tap water and one bottle with 20% ethanol for the EtOH group (male = 24, female = 23) or a second bottle of tap water for the control group (male = 24, female = 24). Immediately prior to the 2-bottle choice session, cage mates were divided by an acrylic glass divider and the solutions were presented in 100 ml graduated cylinders that were fitted with curved ball-point sipper tubes and bottle position was alternated daily to avoid any possible side preference. Two “leak” bottles, one for water and one for ethanol, were used to control for accidental sipper leakage during the experiment. Each bottle was weighed prior to drinking, 1 h, and 24 h after drinking commenced. After each 24-h session the pair housed animals were placed back together in their home cage.
Ethanol vapor (or air) exposure was delivered to standard rat cages that were sealed as previously described (Ehlers et al., 2011). The ethanol vapor was created by dripping 95% ethanol into 2000ml Erlenmeyer flasks that were maintained at 50°C. Alteration of the ethanol vapor concentration was accomplished by modulating the air flow carrying the ethanol vapor into the chamber. The animals were subjected to intermittent vapor exposure (14 hrs on /10 hrs off) over the course of 6, 4 day sessions between PD 23 and PD 62.
Each animal underwent two tail bleeds during each voluntary/vapor cycle to measure their BALs. The two bleeds occurred 1h before the offset of the intermittent vapor on the first day of the cycle and 1h before the offset of the last vapor on the fourth day of the cycle. Target BALs were 175–250 mg% across the exposure period and were determined by sampling blood collected from the tail weekly, generating a mean BAL for the exposure period. Following centrifugation, plasma ethanol levels were determined using the Analox micro-stat GM7 (Analox Instr. Ltd.; Lunenberg, MA).
Open Field Conflict Test
Adult behavioral testing began on PD80 for both male and female rats. Assessment of anxiety-like behavior/disinhibition was accomplished using a modified open field test previously described (Desikan, Wills, & Ehlers, 2014; Ehlers, Liu, Wills, & Crews, 2013; Ehlers et al., 2011). Rats were placed for five minutes in a modified open field with a 3×3 grid pattern identified on the bottom of the box (122cm × 122cm × 46cm). A single 5-gram food pellet coated with peanut butter (Jif brand, creamy) was placed in a plastic holder in the middle of the illuminated center grid square (100 lux). This test provided an assessment of anxiety (retreating from the illuminated center) and disinhibition (movement towards the center) in the context of a motivator (food pellet), thus producing a behavioral conflict situation.
At the start of each test, the rat was positioned away from the illuminated center, with the head directed towards the left bottom corner of the box. To ensure that the food pellet was a strong motivator, the animals were food deprived for 24 hours prior to testing. Rats were pre-exposed to the food pellets coated in peanut butter 4 days prior to testing to prevent novelty avoidance. Each food pellet with peanut butter was weighed before and after the experiment to assess how much the rat had eaten. Between subjects the apparatus was cleaned with a 3% acetic acid (vinegar) solution then water, and a new food pellet was placed in the holder. Animals were videotaped on a low light sensitive camera with a 3-watt infrared light and the videos were later analyzed with ANY-maze Video Tracking System (version 4.84, Wood Date, IL: Stoelting Co.). Latency to the first entry into the middle center square, total rearing behavior, total time in the middle center, total number of line crosses, mean distance from food pellet, and total food eaten were assessed.
Light/Dark Box
The methodology used to test animals in the Light/Dark box has been described previously (Ehlers et al., 2013; Slawecki, 2005). Briefly, the apparatus consisted of two chambers (34cm × 24cm × 24cm) joined together length-wise. Between the two chambers was a square opening (8cm × 8cm) to allow the animal to move between chambers. The Dark box was a chamber with black walls and floor with a black lid, while the Light box was a chamber with white walls, left uncovered, and was illuminated by a 60-lux light. The light was located approximately 120cm above the apparatus and there was no appreciable illumination within the dark box (i.e. < 2 lux). The rat was placed at the center of the Light box, with the head facing opposite of the square opening, and could freely explore both chambers for five minutes. Behaviors occurring in the Light box were recorded for further analysis. Between subjects the chambers were cleaned with a 3% acetic acid (vinegar) solution then water. Behaviors analyzed included latency to enter the Dark box, rearing behavior in the Light box, and latency to re-enter the Light box.
Acoustic Startle Response and Pre-pulse Facilitation
Acoustic startle response (ASR) and pre-pulse facilitation of the ASRs (PPF) has been previously demonstrated to be sensitive to ethanol exposure (Ehlers et al., 2011; Ehlers et al., 2013; Slawecki & Ehlers, 2005). On PD93-94, ASRs were measured in SR LAB Startle chambers (San Diego Instruments, San Diego, CA). Each session contained 45 trials consisting of 30 pulse trials [120 dB (white noise) pulse burst for 40 msec] and 15 pre-pulse + pulse trials (85 dB auditory pre-pulse burst for 20 msec followed 10 msec later by a 120 dB pulse burst). These two trial types were randomly presented with a variable time between each trial. Background white noise (70 dB) was presented for the entire session. The outcome variable was defined by: [(amplitude pre-pulse startle response – amplitude startle response)/amplitude startle response] × 100.
Marble Burying
The marble burying test, conducted on PD98-99, was used to measure anxiety- or irritability-induced defensive burying by placing a novel object in a home cage container environment. More specifically, a home cage fill with 5 cm of clean woodchip bedding (Sani-Chips®, Harlan-Teklad Lab) was placed in a dimly lit familiar room, previously used for Light/Dark behavior testing. 15 glass marbles (1.5 cm in diameter), arranged in 3 rows of 5, were placed on top of the bedding. A template was used to ensure that there was a consistent positioning of marbles. Each rat explored the container and marbles for 30 min. Marbles were considered buried if ≥ 2/3 of the surface area was covered in bedding. Between animals, marbles and container were thoroughly cleaned with 3% acetic acid (vinegar) solution then water and new bedding was used.
Two-Bottle Alcohol Choice Test in Adulthood
Beginning on PD 104, all rats (control and alcohol pre-exposed) were given an intermittent two-bottle choice test for 10 sessions beginning on PD 104. Identical to the procedure conducted in adolescence, rats were given a choice between one bottle of tap water and one bottle with 20% ethanol. Cage mates were again divided by acrylic glass and the bottle position was alternated each session. Two “leak” bottles were again used to control for accidental sipper leakage. Rats were given 24h where the bottles and dividers were removed between each session. Each bottle was weighed just before the start of the drinking session and 24 h later.
Identification of Stages of Estrous Cycle
To determine whether the phase of estrous cycle impacted performance in adulthood, stages were determined cytologically through vaginal lavage 30m after completing each behavioral task. Additionally, vaginal cells were also taken at the end of the first 24h drinking session (day 1) and the last 24h drinking session (day 10) in adulthood to determine whether the stage of estrous cycle significantly altered consumption levels. Rat vaginal secretions were collected with a micropipette containing 200 µL of 0.9% NaCl by inserting the tip into the vagina. Vaginal fluid was placed onto a clean glass slide and smeared. Unstained smears were observed with a Fisher Scientific Micromaster II and photomicrographs of the estrous cycle stages taken with a Laxco 3MP digital camera. Alternatively, smears were also stained with Cresyl Violet to further visualize differentiation of the stages. Proestrus was identified by the predominance of nucleated epithelial cells, estrus by the presence of dense sheets of cornified epithelial cells, and diestrus by mainly presence of leukocytes (Goldman, Murr, & Cooper, 2007).
Real-time Polymerase Chain Reaction Procedures
Rats were decapitated and bilateral frontal cortex (FCTX) was extracted. The tissue was immediately frozen in dry ice and stored at −80C. The mRNA for each sample was isolated and purified using a RNA extraction kit (NucleoSpin RNA Plus, Macherey-Nagel #740984.250). The resulting RNA concentrations were calculated using the NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Inc.). Total RNA was reverse transcribed into complimentary DNA (cDNA) using the Maxima First Strand cDNA Synthesis Kit with dsDNase (Thermo Fisher Scientific Inc., #FERK1642) per manufacturer’s protocol. The cDNA templates were amplified via PCR using Taq polymerase enzyme (PowerUP Sybr Master Mix, Life Technologies, #A25777). The gene-specific predesigned forward and reverse PCR-primers respectively were obtained for Hcrt/OX 1R (5’TGGGCTGTGTCGCTGGCTG-3’; and 5’CGAAACATCCCAAACACTCT-3’), Hcrt/OX 2R (5’-TTGGGGTTCATCGTCGTCAA-3’ and 5’TCGGTCAATGTCCAATGTTC-3’), and the housekeeping gene β-actin (5’-CCTAGCACCATGAAGATCAAGAT-3’ and 5’- ACTCATCGTACTCCTGCTTGCT-3’). Biorad CFX 384 PCR System was used to perform the qPCR reactions.
Gene transcription of Hcrt/OX 1R and Hcrt/OX 2R were normalized to β-actin and comparative Cq (ΔΔCq) was calculated to quantify relative gene expression. Relative gene expression was expressed in arbitrary units using the following formula: R = 2−ΔΔCq (ΔΔCq = ΔCq Sample − ΔCq calibrator, ΔCq = Cq Target gene − Cq β-actin). Mann-Whitney tests were conducted to determine if relative gene expression varied across sex and ethanol groups. Values are presented as mean rank and significance was considered at p < 0.05.
Statistics
Statistical analyses were performed by using SPSS (IBM Corp, Armonk, NY). The effects of treatment (EtOH-exposed vs. control) and sex (male vs. female) on behavioral performance were assessed using two-way analysis of variance (ANOVA). Post-hoc analyses were conducted for significant analyses. Linear regressions were conducted to identify correlations for the mean 24h adolescent ethanol consumption and the mean 24h ethanol consumption in adulthood for male and female rats, separately. Values are presented as mean ± standard error of the mean (SEM) and significances were considered at p < 0.05.
The impact of estrous cycle phase in female rats was determined by planned comparisons using one-way ANOVAs. Analyses were conducted for each behavioral variable comparing rats in estrus and proestrus to those in diestrus alone. If statistical significance were found, further analyses were conducted. Two one-way ANOVAs (male vs. female) without estrus and proestrus were conducted to determine whether the estrous cycle significantly altered the overall model for each behavioral task.
Results
Weight Gain in Male and Female Rats across Development
As expected, weight for both male [F(30, 1380) = 2858, p<0.001] and female rats [F(30, 1350) = 1263, p<0.001] increased throughout the testing procedures. However, there was only a significant effect of exposure in males [F (1, 46) = 7.050, p=0.010] with lower mean weight for ethanol exposed males on 7 select days between PD 62 – 83 compared to male controls (Figure 1A). Ethanol exposure did not impact female weight gain compared to controls.
Figure 1. Intermittent Ethanol Vapor and Voluntary Consumption in Adolescence.
Adolescent ethanol exposure occurred using voluntary intermittent alcohol access occurred 24h before and 24h after each 4-day vapor binge session with 24h in between voluntary alcohol exposures. This exposure period occurred from PD 22–62. After discontinuation of the adolescent ethanol exposure, both male and female rats were tested on various neurobehavioral paradigms followed by 10 days of voluntary 2 bottle ethanol preference testing in adulthood. (A) Ethanol exposure reduced weight gain in male rats during PD 62–83 compared to controls. Exposure did not impact weight gain in female rats. (B) Female adolescent rats consumed more ethanol over the mean (SEM) 24h sessions compared to males. (C) BALs were taken 24h after the start and again at the end of the vapor binge session. While BALs between males and females differed on various days, BALs were consistent on the last 2 exposure periods. *p < 0.05 vs. male rats.
Adolescent Ethanol Exposure
While there was no significant difference between male and female adolescent consumption after the first hour of consumption, adolescent females consumed on average significantly more ethanol (mean = 6.17±0.40 g/kg) than males (mean = 4.52±0.40 g/kg) after 24h of drinking [F(1,43) = 77.57, p<0.001; Figure 1B]. This sex difference was also seen in overall average BALs taken at the end of select ethanol vapor exposure days in the females (mean = 238.72±7.73 mg/dL) compared to males [mean = 199.1±7.56 mg/dL; F(1,43) = 13.42, p=0.001; Figure 1C]. The BAL levels were significantly higher on select days for males on PD 27 compared to females, and for females between PD 31–41 and PD 48 compared to males. However, overall BAL levels in females obtained during vapor exposure, were not a significant indicator of ethanol consumption during adolescence (R2=0.04, p=0.350) or in adulthood (R2=.08, p=0.184).
Open Field Conflict in Adulthood
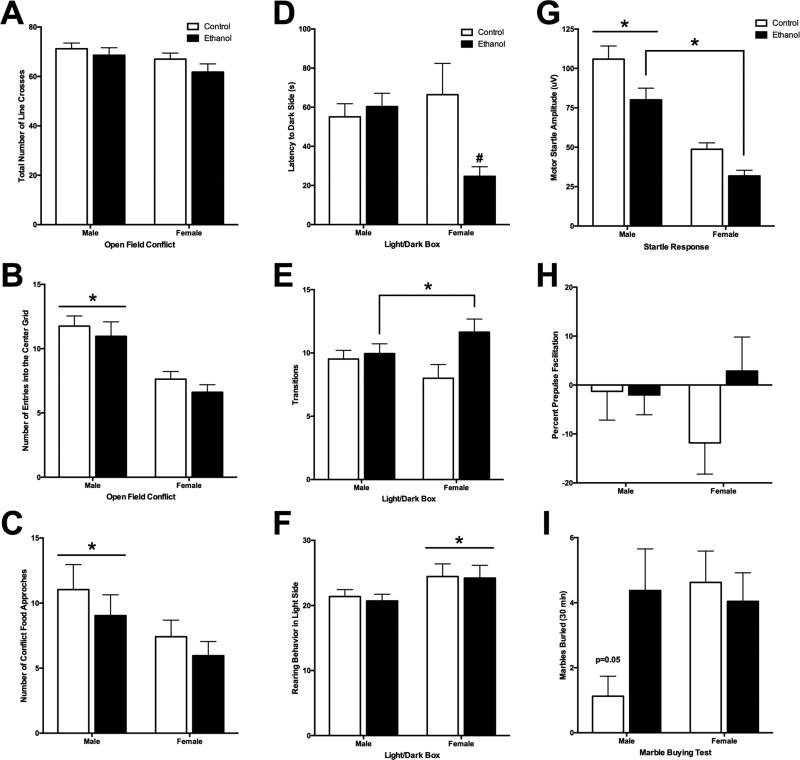
There were no differences in locomotor behavior (total number of line crosses) between the controls and ethanol animals (Figure 2A). However, male rats (48.55±6.77 sec) were quicker to initially enter the center grid compared to females [76.88±6.37 sec; F(1,91) =9.27, p=0.003], males (11.35±0.40) had overall more entries into the center grid than females [7.12±0.51; F(1,91) =27.42, p<0.000; Figure 2B] and spent more overall time in that center grid compared to females [male = 76.06±7.00 sec, female = 47.65±7.02 sec; F(1,89) = 6.58, p=0.010]. While males (mean = 10.04±1.00) made more contact with the food compared to female rats [mean = 6.69±0.73; F(1,91) = 4.92, p=0.030; Figure 2C], there was no significant difference in the amount of food consumed from the illuminated center of the field.
Figure 2. Adolescent Ethanol Exposure on Neurobehavioral Paradigms in Adulthood.
(A) There was no significant difference in mean (±SEM) number of line transitions that occurred during the open field conflict test in adulthood. (B) While there was no effect of ethanol exposure, adult male rats had more mean (±SEM) number of entries into the center grid of the open field box compared to female adult rats. (C) The mean (±SEM) amount of appetitive food consumed in the center grid of the open field box was no different between groups. (D) There was a significant interaction between ethanol exposure group and sex with latency (mean±SEM) to enter the dark chamber after being initially placed in the Light chamber during Light/Dark test. Females exposed to ethanol during adolescence demonstrating a quicker latency to enter the dark compared to female controls. (E) There was no significant differences in mean (±SEM) amount of time spent in the dark chamber during the Light/Dark test. (F) While there was no effect of ethanol exposure on mean (±SEM) number of rears in the light chamber during the Light/Dark test, adult female rats demonstrated more rearing behavior compared to male adult rats. (G) Ethanol exposure during adolescence resulted in reduced mean (±SEM) amplitude for motor startle response during ASR task compared to controls. Male rats also had a significantly greater motor startle response compared to female rats. (I) Percent Pre-pulse facilitation response (mean±SEM) was not significantly different between groups. (J) While trending, there was no significant effect of mean (±SEM) number of marbles buried in 30m during the Marble burying test. *p < 0.05 main effect of group, #p < 0.05 vs. control group.
Light/Dark Box in Adulthood
There was a significant interaction between ethanol exposure and sex in latency to enter the dark side after being placed on the light side [F(1,75) = 6.04, p=0.02; Figure 2D]. Ethanol-exposed females (mean = 24.67±10.26 sec) entered the dark side more quickly than female control rats (mean = 66.45±9.71 sec). However, there was no effect of sex or exposure groups in overall time spent in the dark chamber. Ethanol-exposed rats (mean = 10.80±0.63) had significantly more transitions between the chambers compared to control rats [mean = 8.76±0.62; F(1,75)=5.28, p=0.02; Figure 2E]. While rearing behavior was similar between exposure groups, females (mean = 24.21±1.16) reared in the light side more than males (mean = 20.93±1.14; Figure 2F). These results suggest that animals exposed to intermittent ethanol throughout adolescence demonstrate overall increased behavioral arousal during the Light/Dark test compared to controls.
Acoustic Startle Response and Pre-pulse Facilitation in Adulthood
There was a main effect of exposure group in the ASR amplitude [F(1,91)=71.51, p<0.001; Figure 2G]. Ethanol-exposed adolescents (mean = 55.88±4.44) exhibited a lower startle response amplitude during adulthood compared to controls (mean = 77.33±4.39). Overall, male rats (mean = 92.98±4.39) also had a significantly higher startle response amplitude compared to female rats [mean = 40.23±4.44; F(1,91)=11.83, p=0.001]. However, this sex difference might be due to size differences between males and females since the same apparatus was used for both sexes. No differences were observed in the PPF for sex or adolescent ethanol exposure group (Figure 2H).
Marble Burying in Adulthood
While there was a trend for adolescent alcohol exposure in males (mean = 4.38±1.28) to increase marble burying in adulthood compared to sex-matched controls [mean = 1.13±0.62; interaction F(1,91)=3.94, p=0.05], there was no significant effect of adolescent alcohol exposure condition or sex (Figure 2I).
Two-Bottle Alcohol Choice Test in Adulthood
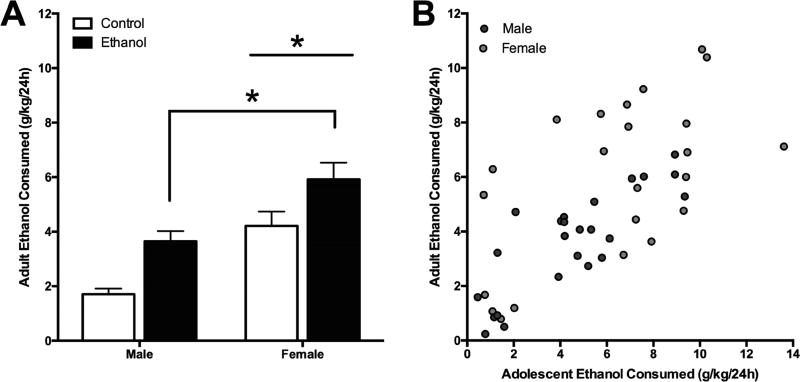
There was a significant main effect of adolescent ethanol exposure on 24h drinking in adulthood [F(1,91) = 16.02, p<0.001] with ethanol exposed rats (mean = 4.78±0.32 g/kg) consuming on average more than control rats (mean = 2.96±0.32 g/kg; Figure 3A). Female rats (mean = 5.32±0.33 g/kg) also consumed more overall during the 24h period than males (mean = 3.00±0.32 g/kg) in adulthood [F(1,91)=27.52, p<0.001]. There was also a significant difference in adolescent exposure [F(1,91)=11.40, p=0.001] and sex [F(1,91) = 24.22, p<0.001] after the first hour of ethanol consumption on each day. Further, Ethanol-exposed rats (mean = 0.59±0.04 g/kg) consumed more than controls (mean = 0.38±0.04 g/kg) and females (mean = 0.63±0.04 g/kg) consumed more than males (mean = 0.33±0.04 g/kg) after one hour access.
Figure 3. Adult Ethanol Consumption After Adolescent Ethanol Exposure.
(A) Mean (±SEM) ethanol consumption in adulthood was significantly higher in rats exposed to ethanol during adolescence compared to those who were not exposed. Additionally, female rats also consumed more overall during the 24h period than males in adulthood (B) Further analysis correlating overall 24h ethanol consumption in adolescence to 24h consumption levels in adulthood demonstrate a significant linear regression for both male and female rats. *p < 0.05 main effect of group.
Further analysis correlating overall 24h ethanol consumption in adolescence to 24h consumption levels in adulthood demonstrate a significant linear regression for both male [R2=0.67, F(1,22)=45.62, p<0.001] and female rats [R2=0.35, F(1,21)=11.19, p=0.003; Figure 3B]. The results of all the behavioral measures have been summarized in Table 1.
Table 1.
Summarized results of behavioral measures in male and female rats after adolescent ethanol exposure compared to controls.
| Behavioral Measure | Ethanol | Sex |
|---|---|---|
| Adolescent ethanol consumption | N/A | More ethanol consumption in females |
| Open field conflict | No differences | Less anxiety-like behavior in males |
| Light/Dark Box | More locomotor activity in ethanol-exposed | Females reared more |
| Acoustic Startle Response | Lower startle response in ethanol-exposed | Lower startle response in females* |
| Prepulse Facilitation | No differences | No differences |
| Marble Burying | No differences | No differences |
| Adult ethanol consumption | More ethanol consumption in ethanol-exposed | More ethanol consumption in females |
This sex difference might be due to body weight differences since the same apparatus was used for both sexes
Effect of Estrous Cycle Phase during Behavioral Tasks
To determine whether estrous cycle phase significantly impacted the behavioral results, planned comparisons were conducted including estrous cycle phase at the time of the behavioral task. For each behavioral task, rats in estrus and proestrus were compared to rats in diestrus alone. There were no significant differences in any measure of ASR, marble burying, or Light/Dark test. For the open field conflict task, there was a significant difference between estrus/proestrus compared to diestrus for time spend in the center grid [F(1,45)=4.20, p=0.046] and the number of times the rat came in contact with the food located in the center grid [F(1,45)=4.12, p=0.048]. Rats in estrus/proestrus spent more time in the center grid (estrus/proestrus = 53.34±7.49 sec, diestrus = 39.28±3.06 sec) and had a greater number of contacts with the center food (estrus/proestrus = 9.06±1.86, diestrus = 5.53±0.80). In adulthood, estrous cycle phase had no effect on the first day of drinking when comparing 24h ethanol consumption to estrous cycle phase as taken at the end of the drinking period.
To establish whether these differences significantly impacted overall behavioral performance, one-way ANOVAs for sex were run for those behavioral tasks that had significant differences between phases of estrous. In the original model for open field conflict, male rats had a significantly faster latency to the center grid, entered more and spent more time in the center grid and came in contact more with the center food compared to females. These same differences remained significant when rats in estrus and proestrus were removed from the model, [latency to center grid, F(1,75) = 7.22, p=0.009; center grid entries, F(1,75) = 20.17, p<0.001; time spent in center grid, F(1,75) = 8.59, p=0.004; contact with food, F(1,75) = 5.15, p=0.026], suggesting that estrous cycle phase does not account for this sex difference.
PCR Analysis of Hcrt/OX Receptors in the Adult Frontal Cortex
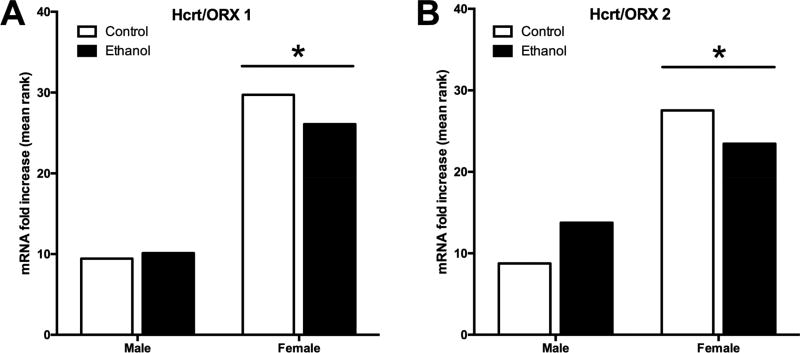
For the Hcrt/OX1 receptor mRNA expression in the frontal cortex, there was no significant difference between male ethanol exposed adolescent rats and controls in adulthood. Additionally, there was no difference in Hcrt/OX 1R mRNA expression between ethanol and control females in adulthood. However, there was an overall difference between male (mean rank = 9.76) and female (mean rank = 27.91) Hcrt/OX 1R mRNA, with a significantly higher mean rank for female Hcrt/OX 1R mRNA compared to males (U=13, p<.001, r=.79, Figure 4A). This trend held constant with the Hcrt/OX 2R mRNA expression with no significant difference between adolescent ethanol exposed and controls for both adult males and females. However, the mean rank of Hcrt/OX receptor mRNA expression was again significantly greater in adult females (mean rank = 25.50) compared to adult males (mean rank = 11.25; U=44, p<.001, r=.63, Figure 4B).
Figure 4. Hcrt/OX Receptor Expression in Adulthood After Adolescent Ethanol Exposure.
Real-time qPCR was conducted on frontal cortex tissue samples of adult rats exposed to ethanol in adolescence to determaine whether adolescent exposure to ethanol has lasting effects on Hcrt/OX receptor expression. (A) Ethanol exposure did not impact Hcrt/OX 1 receptor mRNA expression in adulthood, however female adult rats had overall more Hcrt/OX 1 receptor expression compared to males. (B) Hcrt/OX 2 receptor mRNA expression was again not significantly different between exposure groups. Female rats exhibited more mRNA Hcrt/OX 2 compared to male adult rats. *p<0.05 vs. male group.
Discussion
The present study utilized a dynamic model of alcohol exposure combining intermittent voluntary ethanol consumption and intermittent vapor exposure sessions to investigate the impact on voluntary consumption in adolescence and the persistent impact it may have on neurobehavioral functioning and Hcrt/OX receptor mRNA expression in frontal cortex in adulthood. While adolescent ethanol exposure resulted in minimal changes in the behavioral tasks, early exposure did lead to increased alcohol consumption in adulthood for both male and female rats. Further, female rats consumed more alcohol in adolescence, and subsequently more alcohol in adulthood compared to males. Additionally, female rats also exhibited overall higher levels of Hcrt/OX 1 and Hcrt/OX 2 receptor mRNA expression in frontal cortex in adulthood compared to males.
Adolescent ethanol exposure resulted in increased consumption during 10 sessions of intermittent 24h ethanol access during adulthood compared to those who never received alcohol during adolescence. This is consistent with previously studies showing early exposure to ethanol increases ethanol consumption in adulthood (Alaux-Cantin et al., 2013; Ho, Chin, & Dole, 1989; Strong et al., 2009; Yoshimoto et al., 2002). Exposure alone does not always increase ethanol preference which has been demonstrated by studies showing no effect of early ethanol exposure on future ethanol consumption (Füllgrabe, Vengeliene, & Spanagel, 2007; Siegmund, Vengeliene, Singer, & Spanagel, 2005; Slawecki & Ehlers, 2005; Vendruscolo et al., 2010; Vetter, Doremus-Fitzwater, & Spear, 2007). The results of this model suggest that a combined measure of intermittent involuntary vapor and voluntary consumption over adolescence results in increases in preference for consumption, similar to that seen with consumption alone (Amodeo et al., 2017) a finding that is not seen with adolescent vapor exposure alone (Slawecki & Betancourt, 2002). Additionally, adolescent females consumed significantly more ethanol on average during the 24h periods compared to males. This sex difference persisted into adulthood with females consuming more than males during ethanol access in adulthood. These results are consistent with the current literature demonstrating that female rats have greater ethanol intake compared to their male counterparts (Acevedo, Molina, Nizhnikov, Spear, & Pautassi, 2010; Doremus, Brunell, Rajendran, & Spear, 2005; Lancaster, Brown, Coker, Elliott, & Wren, 1996). There was a significant difference in BAL on select days between males and females during adolescence. This is due in part to abrupt changes in body weight for males resulting in lower BALs. However, BALs stabilized for the last third of the administration period. Further statistical analysis revealed that these select differences in BALs were not significantly correlated with ethanol intake in adolescence or adulthood. Overall, the current results indicate that females exhibit similar consumption patterns to males, however overall levels of consumption are consistently higher.
Adolescent alcohol use in male and female rats resulted in modest changes in neurobehavioral performance in adulthood. Ethanol rats demonstrated more behavioral arousal during the novel Light/Dark test. Additionally, ethanol rats had a reduced motor startle response compared to controls. Together, these results suggest that adolescent ethanol exposure has mild lasting behavioral changes in adulthood with some indication that exposure can lead to less inhibited behavior. Overall, sex differences in behavioral measures were far more robust. Male rats seemed more disinhibited on the open field conflict tasks with more transitions and time spent in the center grid compared to females. However there were no significant differences between ethanol groups which has been demonstrated with chronic vapor ethanol exposure during the same exposure period (Desikan et al., 2014). Female rats reared more during the Light/Dark task which may indicate an “escape” behavior. There was also a robust effect of startle response with male rats demonstrating a larger ASR compared to females. However, we did not account for differences in animal size which would result in tighter restraint for male rats then females which could potentially be a contributing factor in sex difference for ASR.
Prior research has addressed concerns about the impact of female reproductive cycle and fluctuating hormones on behavioral performance. The current results indicate that rats in estrus and proestrus have a decrease in anxiety-like behavior in the open field conflict task and an increase in ethanol consumption on the last, but not the first day, of administration in adulthood compared to rats in the diestrus phase. However, estrus and proestrus do not significantly alter the overall model (males vs. females). Overall these results indicate that estrous cycle phase can impact behavior but does not account for sex differences. This is consistent with a recent meta-analysis of normal male and female rats demonstrating that sex has no significant difference on behavioral performance (Becker, Prendergast, & Liang, 2016).
The Hcrt/OX system has been previously implicated in a variety of physiological processes including sleep, arousal, cognitive function, and alcohol consumption behavior (España, 2012; Sutcliffe & de Lecea, 2002; Willie et al., 2001). While we anticipated finding an increase in mRNA receptor expression due to the prior literature on ethanol consumption in adults, adolescent ethanol exposure did not result in difference in Hcrt/OX receptor expression in the frontal cortex. It may be that investigation of other brain areas in a larger group of rats could lead to significant results. We did however find sex differences in receptor expression in frontal cortex with females exhibiting more RNA expression in both Hcrt/ OX1 and Hcrt/OX 2 receptors compared to males. Sex difference in the Hcrt/OX 1 and Hcrt/OX 2 receptors have also been previously shown in both rats (Grafe, Cornfeld, Luz, Valentino, & Bhatnagar, 2017; Jöhren, Neidert, Kummer, & Dominiak, 2002; Zhou et al., 2012) and humans (Lu et al., 2017). Specifically, female rats demonstrate higher baseline levels of hypothalamus prepro-orexin mRNA (Grafe et al., 2017; Jöhren et al., 2002) which is not mediated by female gonadal hormones (Jöhren, Brüggemann, Dendorfer, & Dominiak, 2003). Female rats also have a higher activation of Hcrt/OX neurons and an increase in OXA concentrations in the cerebrospinal fluid compared to males (Grafe et al., 2017). One potential mechanism for sex-dependent difference in Hcrt/OX levels could be mediated through more active or prevalent transcription factors that result in the upregulation of Hcrt/OX receptors (Grafe et al., 2017).
This study provides a translational model of adolescent alcohol exposure by mimicking intermittent “weekend binging” pattern in humans by using a combination of involuntary exposure and voluntary consumption in rats. This model resulted in a modest increase in behavioral arousal and reduced startle in adulthood, as compared to larger changes that have been seen in a model of adolescent alcohol exposure that included more days of vapor exposure over adolescence. Strong correlations between adolescent consumption with adult consumption were also prominent in both males and females. These data suggest that our current model of intermittent ethanol exposure in adolescence can modestly impact both behavior and future consumption of alcohol and that Hcrt/OX receptor signaling differs between males and females.
Supplementary Material
Highlights.
Adolescent alcohol drinking and vapor exposure in rats causes increased drinking in adulthood.
Adolescent alcohol drinking and vapor exposure in rats causes decreased startle response in adulthood.
Female rats drank more alcohol than males and also had more rearing in the light-dark box.
Female rats expressed a higher level of Hypocretin 1 and 2 receptor mRNA in frontal cortex than males.
Acknowledgments
This work was supported by National Institute of Health (NIH) grants, U01 AA019969; R01 AA006059 to Cindy L. Ehlers from the National Institute on Alcohol Abuse and Alcoholism (NIAAA). The authors thank Phil Lau for help in statistical analyses.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
None
References
- Acevedo MB, Molina JC, Nizhnikov ME, Spear NE, Pautassi RM. High ethanol dose during early adolescence induces locomotor activation and increases subsequent ethanol intake during late adolescence. Developmental Psychobiology. 2010;52(5):424–40. doi: 10.1002/dev.20444. http://doi.org/10.1002/dev.20444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaux-Cantin S, Warnault V, Legastelois R, Botia B, Pierrefiche O, Vilpoux C, Naassila M. Alcohol intoxications during adolescence increase motivation for alcohol in adult rats and induce neuroadaptations in the nucleus accumbens. Neuropharmacology. 2013;67:521–31. doi: 10.1016/j.neuropharm.2012.12.007. http://doi.org/10.1016/j.neuropharm.2012.12.007 [DOI] [PubMed] [Google Scholar]
- Amodeo LR, Kneiber D, Wills DN, Ehlers CL. Alcohol drinking during adolescence increases consumptive responses to alcohol in adulthood in Wistar rats. Alcohol. 2017;59:43–51. doi: 10.1016/j.alcohol.2016.12.002. http://doi.org/10.1016/j.alcohol.2016.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P, Baumberg B. Alcohol Policy: Who should sit at the table? Addiction. 2007;102(2):335–336. doi: 10.1111/j.1360-0443.2006.01713.x. http://doi.org/10.1111/j.1360-0443.2006.01713.x [DOI] [PubMed] [Google Scholar]
- Assanangkornchai S, Mukthong A, Intanont T. Prevalence and patterns of alcohol consumption and health-risk behaviors among high school students in Thailand. Alcoholism, Clinical and Experimental Research. 2009;33(12):2037–46. doi: 10.1111/j.1530-0277.2009.01043.x. http://doi.org/10.1111/j.1530-0277.2009.01043.x [DOI] [PubMed] [Google Scholar]
- Barson JR, Ho HT, Leibowitz SF. Anterior thalamic paraventricular nucleus is involved in intermittent access ethanol drinking: role of orexin receptor 2. Addiction Biology. 2015;20(3):469–481. doi: 10.1111/adb.12139. http://doi.org/10.1111/adb.12139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Prendergast BJ, Liang JW. Female rats are not more variable than male rats: a meta-analysis of neuroscience studies. Biology of Sex Differences. 2016;7:34. doi: 10.1186/s13293-016-0087-5. http://doi.org/10.1186/s13293-016-0087-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caamaño-Isorna F, Corral M, Parada M, Cadaveira F. Factors Associated With Risky Consumption and Heavy Episodic Drinking Among Spanish University Students. Journal of Studies on Alcohol and Drugs. 2008;69(2):308–312. doi: 10.15288/jsad.2008.69.308. http://doi.org/10.15288/jsad.2008.69.308. [DOI] [PubMed] [Google Scholar]
- Chuo SP, Pickering RP. Early onset of drinking as a risk factor for lifetime alcohol-related problems. Addiction. 1992;87(8):1199–1204. doi: 10.1111/j.1360-0443.1992.tb02008.x. http://doi.org/10.1111/j.1360-0443.1992.tb02008.x [DOI] [PubMed] [Google Scholar]
- Cluderay JE, Harrison DC, Hervieu GJ. Protein distribution of the orexin-2 receptor in the rat central nervous system. Regulatory Peptides. 2002;104(1–3):131–144. doi: 10.1016/s0167-0115(01)00357-3. http://doi.org/10.1016/S0167-0115(01)00357-3 [DOI] [PubMed] [Google Scholar]
- Courtney KE, Polich J. Binge drinking in young adults: Data, definitions, and determinants. Psychological Bulletin. 2009;135(1):142–56. doi: 10.1037/a0014414. http://doi.org/10.1037/a0014414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DA, Goldstein RB, Chou SP, Ruan WJ, Grant BF. Age at first drink and the first incidence of adult-onset DSM-IV alcohol use disorders. Alcoholism, Clinical and Experimental Research. 2008;32(12):2149–60. doi: 10.1111/j.1530-0277.2008.00806.x. http://doi.org/10.1111/j.1530-0277.2008.00806.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayas CV, McGranahan TM, Martin-Fardon R, Weiss F. Stimuli Linked to Ethanol Availability Activate Hypothalamic CART and Orexin Neurons in a Reinstatement Model of Relapse. Biological Psychiatry. 2008;63(2):152–157. doi: 10.1016/j.biopsych.2007.02.002. http://doi.org/10.1016/j.biopsych.2007.02.002 [DOI] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, … Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(1):322–7. doi: 10.1073/pnas.95.1.322. http://doi.org/10.1073/pnas.1202526109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan A, Wills DN, Ehlers CL. Ontogeny and adolescent alcohol exposure in Wistar rats: open field conflict, light/dark box and forced swim test. Pharmacology Biochemistry and Behavior. 2014;122:279–285. doi: 10.1016/j.pbb.2014.04.011. http://doi.org/10.1016/j.pbb.2014.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWit DJ, Adlaf EM, Offord DR, Ogborne AC. Age at First Alcohol Use: A Risk Factor for the Development of Alcohol Disorders. American Journal of Psychiatry. 2000;157(5):745–750. doi: 10.1176/appi.ajp.157.5.745. http://doi.org/10.1176/appi.ajp.157.5.745 [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Ethanol preferentially stimulates dopamine release in the nucleus accumbens of freely moving rats. European Journal of Pharmacology. 1985;115(1):131–132. doi: 10.1016/0014-2999(85)90598-9. http://doi.org/10.1016/0014-2999(85)90598-9 [DOI] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Rajendran P, Spear LP. Factors Influencing Elevated Ethanol Consumption in Adolescent Relative to Adult Rats. Alcoholism: Clinical & Experimental Research. 2005;29(10):1796–1808. doi: 10.1097/01.alc.0000183007.65998.aa. http://doi.org/10.1097/01.alc.0000183007.65998.aa [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Criado JR, Wills DN, Liu W, Crews FT. Periadolescent ethanol exposure reduces adult forebrain ChAT+IR neurons: Correlation with behavioral pathology. Neuroscience. 2011;199:333–345. doi: 10.1016/j.neuroscience.2011.10.011. http://doi.org/10.1016/j.neuroscience.2011.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Liu W, Wills DN, Crews FT. Periadolescent ethanol vapor exposure persistently reduces measures of hippocampal neurogenesis that are associated with behavioral outcomes in adulthood. Neuroscience. 2013;244:1–15. doi: 10.1016/j.neuroscience.2013.03.058. http://doi.org/10.1016/j.neuroscience.2013.03.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Oguz I, Budin F, Wills DN, Crews FT. Peri-Adolescent Ethanol Vapor Exposure Produces Reductions in Hippocampal Volume that are Correlated with Deficits in Prepulse Inhibition of the Startle. Alcoholism: Clinical and Experimental Research. 2013;37(9):1466–1475. doi: 10.1111/acer.12125. http://doi.org/10.1111/acer.12125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Slutske WS, Gilder DA, Lau P, Wilhelmsen KC. Age at first intoxication and alcohol use disorders in Southwest California Indians. Alcoholism, Clinical and Experimental Research. 2006;30(11):1856–65. doi: 10.1111/j.1530-0277.2006.00222.x. http://doi.org/10.1111/j.1530-0277.2006.00222.x [DOI] [PubMed] [Google Scholar]
- España RA. Hypocretin/orexin involvement in reward and reinforcement. Vitamins and Hormones. 2012;89:185–208. doi: 10.1016/B978-0-12-394623-2.00010-X. http://doi.org/10.1016/B978-0-12-394623-2.00010-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadel J, Deutch A. Anatomical substrates of orexin-dopamine interactions: lateral hypothalamic projections to the ventral tegmental area. Neuroscience. 2002;111(2):379–387. doi: 10.1016/s0306-4522(02)00017-9. http://doi.org/10.1016/S0306-4522(02)00017-9 [DOI] [PubMed] [Google Scholar]
- Füllgrabe MW, Vengeliene V, Spanagel R. Influence of age at drinking onset on the alcohol deprivation effect and stress-induced drinking in female rats. Pharmacology, Biochemistry, and Behavior. 2007;86(2):320–6. doi: 10.1016/j.pbb.2006.10.004. http://doi.org/10.1016/j.pbb.2006.10.004 [DOI] [PubMed] [Google Scholar]
- Goldman JM, Murr AS, Cooper RL. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Research Part B: Developmental and Reproductive Toxicology. 2007;80(2):84–97. doi: 10.1002/bdrb.20106. http://doi.org/10.1002/bdrb.20106 [DOI] [PubMed] [Google Scholar]
- Grafe LA, Cornfeld A, Luz S, Valentino R, Bhatnagar S. Orexins Mediate Sex Differences in the Stress Response and in Cognitive Flexibility. Biological Psychiatry. 2017;81(8):683–692. doi: 10.1016/j.biopsych.2016.10.013. http://doi.org/10.1016/j.biopsych.2016.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF. The impact of a family history of alcoholism on the relationship between age at onset of alcohol use and DSM-IV alcohol dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. Alcohol Health and Research World. 1998;22(2):144–7. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/15706789. [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the national longitudinal alcohol epidemiologic survey. Journal of Substance Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. http://doi.org/10.1016/S0899-3289(97)90009-2 [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age of onset of drug use and its association with DSM-IV drug abuse and dependence: Results from the national longitudinal alcohol epidemiologic survey. Journal of Substance Abuse. 1998;10(2):163–173. doi: 10.1016/s0899-3289(99)80131-x. http://doi.org/10.1016/S0899-3289(99)80131-X [DOI] [PubMed] [Google Scholar]
- Hervieu GJ, Cluderay JE, Harrison DC, Roberts JC, Leslie RA. Gene expression and protein distribution of the orexin-1 receptor in the rat brain and spinal cord. Neuroscience. 2001;103(3):777–797. doi: 10.1016/s0306-4522(01)00033-1. http://doi.org/10.1016/S0306-4522(01)00033-1 [DOI] [PubMed] [Google Scholar]
- Ho A, Chin AJ, Dole VP. Early experience and the consumption of alcohol by adult C57BL/6J mice. Alcohol. 1989;6(6):511–515. doi: 10.1016/0741-8329(89)90060-8. http://doi.org/10.1016/0741-8329(89)90060-8 [DOI] [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Malone SM, McGue M. P3 Event-Related Potential Amplitude and the Risk for Disinhibitory Disorders in Adolescent Boys. Archives of General Psychiatry. 2002;59(8):750. doi: 10.1001/archpsyc.59.8.750. http://doi.org/10.1001/archpsyc.59.8.750 [DOI] [PubMed] [Google Scholar]
- Jessor R, Jessor SL. Adolescent development and the onset of drinking. A longitudinal study. Journal of Studies on Alcohol. 1975;36(1):27–51. doi: 10.15288/jsa.1975.36.27. http://doi.org/10.15288/jsa.1975.36.27. [DOI] [PubMed] [Google Scholar]
- Jöhren O, Brüggemann N, Dendorfer A, Dominiak P. Gonadal Steroids Differentially Regulate the Messenger Ribonucleic Acid Expression of Pituitary Orexin Type 1 Receptors and Adrenal Orexin Type 2 Receptors. Endocrinology. 2003;144(4):1219–1225. doi: 10.1210/en.2002-0030. http://doi.org/10.1210/en.2002-0030 [DOI] [PubMed] [Google Scholar]
- Jöhren O, Neidert SJ, Kummer M, Dominiak P. Sexually dimorphic expression of prepro-orexin mRNA in the rat hypothalamus. Peptides. 2002;23(6):1177–1180. doi: 10.1016/s0196-9781(02)00052-9. http://doi.org/10.1016/S0196-9781(02)00052-9 [DOI] [PubMed] [Google Scholar]
- Jupp B, Krivdic B, Krstew E, Lawrence AJ. The orexin1 receptor antagonist SB-334867 dissociates the motivational properties of alcohol and sucrose in rats. Brain Research. 2011;1391:54–59. doi: 10.1016/j.brainres.2011.03.045. http://doi.org/10.1016/j.brainres.2011.03.045 [DOI] [PubMed] [Google Scholar]
- Kane JK, Parker SL, Matta SG, Fu Y, Sharp BM, Li MD. Nicotine Up-Regulates Expression of Orexin and Its Receptors in Rat Brain 1. Endocrinology. 2000;141(10):3623–3629. doi: 10.1210/endo.141.10.7707. http://doi.org/10.1210/endo.141.10.7707 [DOI] [PubMed] [Google Scholar]
- Kim AK, Brown RM, Lawrence AJ. The role of orexins/hypocretins in alcohol use and abuse: an appetitive-reward relationship. Frontiers in Behavioral Neuroscience. 2012;6:78. doi: 10.3389/fnbeh.2012.00078. http://doi.org/10.3389/fnbeh.2012.00078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G, Bloom F. Cellular and molecular mechanisms of drug dependence. Science. 1988;242(4879):715–723. doi: 10.1126/science.2903550. http://doi.org/10.1126/science.2903550 [DOI] [PubMed] [Google Scholar]
- Lancaster FE, Brown TD, Coker KL, Elliott JA, Wren SB. Sex Differences in Alcohol Preference and Drinking Patterns Emerge during the Early Postpubertal Period in Sprague-Dawley Rats. Alcoholism: Clinical and Experimental Research. 1996;20(6):1043–1049. doi: 10.1111/j.1530-0277.1996.tb01945.x. http://doi.org/10.1111/j.1530-0277.1996.tb01945.x [DOI] [PubMed] [Google Scholar]
- Lawrence AJ, Cowen MS, Yang H-J, Chen F, Oldfield B. The orexin system regulates alcohol-seeking in rats. British Journal of Pharmacology. 2006;148(6):752–9. doi: 10.1038/sj.bjp.0706789. http://doi.org/10.1038/sj.bjp.0706789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence AJ, Cowen MS, Yang H-J, Chen F, Oldfield B. The orexin system regulates alcohol-seeking in rats. British Journal of Pharmacology. 2009;148(6):752–759. doi: 10.1038/sj.bjp.0706789. http://doi.org/10.1038/sj.bjp.0706789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Zhao J, Balesar R, Fronczek R, Zhu Q-B, Wu X-Y, … Swaab DF. Sexually Dimorphic Changes of Hypocretin (Orexin) in Depression. EBioMedicine. 2017;18:311–319. doi: 10.1016/j.ebiom.2017.03.043. http://doi.org/10.1016/j.ebiom.2017.03.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JW, Naimi TS, Brewer RD, Jones SE. Binge Drinking and Associated Health Risk Behaviors Among High School Students. Pediatrics. 2007;119(1):76–85. doi: 10.1542/peds.2006-1517. http://doi.org/10.1542/peds.2006-1517 [DOI] [PubMed] [Google Scholar]
- Moorman DE, Aston-Jones G. Orexin-1 receptor antagonism decreases ethanol consumption and preference selectively in high-ethanol-preferring Sprague-Dawley rats. Alcohol. 2009;43(5):379–386. doi: 10.1016/j.alcohol.2009.07.002. http://doi.org/10.1016/j.alcohol.2009.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada M, Corral M, Caamaño-Isorna F, Mota N, Crego A, Rodríguez Holguín S, Cadaveira F. Definición del concepto de consumo intensivo de alcohol adolescente (binge drinking) Adicciones. 2011;23(1):53. http://doi.org/10.20882/adicciones.167. [PubMed] [Google Scholar]
- Pasumarthi RK, Reznikov LR, Fadel J. Activation of orexin neurons by acute nicotine. European Journal of Pharmacology. 2006;535(1–3):172–176. doi: 10.1016/j.ejphar.2006.02.021. http://doi.org/10.1016/j.ejphar.2006.02.021 [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 1998;18(23):9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9822755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JK, Simms JA, Steensland P, Taha SA, Borgland SL, Bonci A, Bartlett SE. Inhibition of orexin-1/hypocretin-1 receptors inhibits yohimbine-induced reinstatement of ethanol and sucrose seeking in Long-Evans rats. Psychopharmacology. 2008;199(1):109–17. doi: 10.1007/s00213-008-1136-5. http://doi.org/10.1007/s00213-008-1136-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, … Yanagisawa M. Orexins and Orexin Receptors: A Family of Hypothalamic Neuropeptides and G Protein-Coupled Receptors that Regulate Feeding Behavior. Cell. 1998;92(4):573–585. doi: 10.1016/s0092-8674(00)80949-6. http://doi.org/10.1016/S0092-8674(00)80949-6 [DOI] [PubMed] [Google Scholar]
- Schneider ER, Rada P, Darby RD, Leibowitz SF, Hoebel BG. Orexigenic peptides and alcohol intake: differential effects of orexin, galanin, and ghrelin. Alcoholism, Clinical and Experimental Research. 2007;31(11):1858–65. doi: 10.1111/j.1530-0277.2007.00510.x. http://doi.org/10.1111/j.1530-0277.2007.00510.x [DOI] [PubMed] [Google Scholar]
- Sharf R, Sarhan M, Dileone RJ. Orexin mediates the expression of precipitated morphine withdrawal and concurrent activation of the nucleus accumbens shell. Biological Psychiatry. 2008;64(3):175–83. doi: 10.1016/j.biopsych.2008.03.006. http://doi.org/10.1016/j.biopsych.2008.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoblock JR, Welty N, Aluisio L, Fraser I, Motley ST, Morton K, … Galici R. Selective blockade of the orexin-2 receptor attenuates ethanol self-administration, place preference, and reinstatement. Psychopharmacology. 2011;215(1):191–203. doi: 10.1007/s00213-010-2127-x. http://doi.org/10.1007/s00213-010-2127-x [DOI] [PubMed] [Google Scholar]
- Siegmund S, Vengeliene V, Singer MV, Spanagel R. Influence of Age at Drinking Onset on Long-Term Ethanol Self-Administration With Deprivation and Stress Phases. Alcoholism: Clinical & Experimental Research. 2005;29(7):1139–1145. doi: 10.1097/01.alc.0000171928.40418.46. http://doi.org/10.1097/01.ALC.0000171928.40418.46 [DOI] [PubMed] [Google Scholar]
- Slawecki CJ. Comparison of anxiety-like behavior in adolescent and adult sprague-dawley rats. Behavioral Neuroscience. 2005;119(6):1477–1483. doi: 10.1037/0735-7044.119.6.1477. http://doi.org/10.1037/0735-7044.119.6.1477 [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Betancourt M. Effects of adolescent ethanol exposure on ethanol consumption in adult rats. Alcohol. 2002;26(1):23–30. doi: 10.1016/s0741-8329(01)00192-6. http://doi.org/10.1016/S0741-8329(01)00192-6 [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Ehlers CL. Enhanced Prepulse Inhibition Following Adolescent Ethanol Exposure in Sprague-Dawley Rats. Alcoholism: Clinical & Experimental Research. 2005;29(10):1829–1836. doi: 10.1097/01.alc.0000183024.47167.27. http://doi.org/10.1097/01.alc.0000183024.47167.27 [DOI] [PubMed] [Google Scholar]
- Smith RJ, See RE, Aston-Jones G. Orexin/hypocretin signaling at the orexin 1 receptor regulates cue-elicited cocaine-seeking. The European Journal of Neuroscience. 2009;30(3):493–503. doi: 10.1111/j.1460-9568.2009.06844.x. http://doi.org/10.1111/j.1460-9568.2009.06844.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000 doi: 10.1016/s0149-7634(00)00014-2. http://doi.org/10.1016/S0149-7634(00)00014-2 [DOI] [PubMed]
- Srinivasan S, Simms JA, Nielsen CK, Lieske SP, Bito-Onon JJ, Yi H, … Bartlett SE. The dual orexin/hypocretin receptor antagonist, almorexant, in the ventral tegmental area attenuates ethanol self-administration. PloS One. 2012;7(9):e44726. doi: 10.1371/journal.pone.0044726. http://doi.org/10.1371/journal.pone.0044726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong MN, Yoneyama N, Fretwell AM, Snelling C, Tanchuck MA, Finn DA. " Binge " drinking experience in adolescent mice shows sex differences and elevated ethanol intake in adulthood. Hormones and Behavior. 2009;58:82–90. doi: 10.1016/j.yhbeh.2009.10.008. http://doi.org/10.1016/j.yhbeh.2009.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe JG, de Lecea L. The hypocretins: Setting the arousal threshold9l-;.[ Nature Reviews Neuroscience. 2002;3(5):339–349. doi: 10.1038/nrn808. http://doi.org/10.1038/nrn808 [DOI] [PubMed] [Google Scholar]
- van Amsterdam J, van den Brink W. Ranking of drugs: a more balanced risk-assessment. Lancet (London, England) 2010;376(9752):1524–5. doi: 10.1016/S0140-6736(10)62000-4. http://doi.org/10.1016/S0140-6736(10)62000-4 [DOI] [PubMed] [Google Scholar]
- Vendruscolo LF, Gueye AB, Vendruscolo JCM, Clemens KJ, Mormède P, Darnaudéry M, Cador M. Reduced alcohol drinking in adult rats exposed to sucrose during adolescence. Neuropharmacology. 2010;59(6):388–94. doi: 10.1016/j.neuropharm.2010.05.015. http://doi.org/10.1016/j.neuropharm.2010.05.015 [DOI] [PubMed] [Google Scholar]
- Vetter CS, Doremus-Fitzwater TL, Spear LP. Time course of elevated ethanol intake in adolescent relative to adult rats under continuous, voluntary-access conditions. Alcoholism, Clinical and Experimental Research. 2007;31(7):1159–68. doi: 10.1111/j.1530-0277.2007.00417.x. http://doi.org/10.1111/j.1530-0277.2007.00417.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viner RM, Taylor B. Adult outcomes of binge drinking in adolescence: findings from a UK national birth cohort. Journal of Epidemiology & Community Health. 2007;61(10):902–907. doi: 10.1136/jech.2005.038117. http://doi.org/10.1136/jech.2005.038117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler H, Lee JE, Kuo M, Seibring M, Nelson TF, Lee H. Trends in College Binge Drinking During a Period of Increased Prevention Efforts: Findings from 4 Harvard School of Public Health College Alcohol Study Surveys: 1993-2001. Journal of American College Health. 2002;50(5):203–217. doi: 10.1080/07448480209595713. http://doi.org/10.1080/07448480209595713 [DOI] [PubMed] [Google Scholar]
- Willie JT, Chemelli RM, Sinton CM, Yanagisawa M. To Eat or to Sleep? Orexin in the Regulation of Feeding and Wakefulness. Annual Review of Neuroscience. 2001;24(1):429–458. doi: 10.1146/annurev.neuro.24.1.429. http://doi.org/10.1146/annurev.neuro.24.1.429 [DOI] [PubMed] [Google Scholar]
- Wise RA, Rompre PP. Brain Dopamine and Reward. Annual Review of Psychology. 1989;40(1):191–225. doi: 10.1146/annurev.ps.40.020189.001203. http://doi.org/10.1146/annurev.ps.40.020189.001203 [DOI] [PubMed] [Google Scholar]
- York JL. Clinical Significance of Alcohol Intake Parameters at Initiation of Drinking. Alcohol. 1999;19(1):97–99. doi: 10.1016/s0741-8329(99)00020-8. http://doi.org/10.1016/S0741-8329(99)00020-8 [DOI] [PubMed] [Google Scholar]
- Yoshimoto K, Hori M, Sorimachi Y, Watanabe T, Yano T, Yasuhara M. Increase of rat alcohol drinking behavior depends on the age of drinking onset. Alcoholism, Clinical and Experimental Research. 2002;26(8 Suppl):63S–65S. doi: 10.1097/01.ALC.0000026977.19902.61. http://doi.org/10.1097/01.ALC.0000026977.19902.61 [DOI] [PubMed] [Google Scholar]
- Zhou L, Ghee SM, Chan C, Lin L, Cameron MD, Kenny PJ, See RE. Orexin-1 receptor mediation of cocaine seeking in male and female rats. The Journal of Pharmacology and Experimental Therapeutics. 2012;340(3):801–9. doi: 10.1124/jpet.111.187567. http://doi.org/10.1124/jpet.111.187567 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.