Abstract
Purpose
Due to the complexity of hematopoietic cell transplant trial treatments, informed consent forms are often long and difficult to read. We evaluated a two-column easy-to-read informed consent (ETRIC) form that incorporates elements of health literacy and readability in participants and centers participating in Blood and Marrow Transplant Clinical Trials Network (BMT CTN) clinical trials.
Methods
In a randomized study, 198 adult patients from 25 centers potentially eligible to participate in four BMT CTN interventional trials were randomized to ETRIC or standard consent form for that trial. Both forms were written at ≤8th grade reading level. The primary endpoint was objective comprehension score on the Quality of Informed Consent Part-A (QuIC-A) instrument. In a parallel evaluation study, two moderators conducted semi-structured interviews of 49 investigators, research staff, and IRB members at 9 BMT CTN trial sites.
Results
The mean QuIC-A scores were comparable in 152 (77%) patients evaluable for the primary endpoint (80.5 ETRIC form, 81.8 standard form, P=0.37). In regression analysis, there was no significant association between the consent type and QuIC-A score. In the evaluation study, dominant themes identified on qualitative analyses included general comfort and willingness to utilize the ETRIC template and that its formatting and layout enhancements would offer additional value to research participants, investigators and IRBs. IRB language preferences and requirements, length, and prior experience with alternative consent formats were perceived as barriers.
Conclusion
Among patients considering participation in BMT CTN clinical trials, the formatting enhancements of the ETRIC form did not alter comprehension of the trial. Despite local challenges to implementation, trial sites generally viewed the ETRIC form favorably and expressed willingness to utilize it over standard consent form.
Keywords: Clinical Trials, Research Subjects, Informed Consent, Blood and Marrow
INTRODUCTION
Obtaining voluntary informed consent is the cornerstone of the ethical conduct of research. Improvements to the informed consent process that enhance participant understanding are essential, both to promote respect for autonomy and because increased comprehension leads to better protocol adherence and lower attrition.1, 2 Many cancer clinical trial participants have misconceptions about the trials in which they participate, including: overestimation of benefits, underestimation of the unproven nature of the study intervention, and failure to recognize the primary purpose of the trial.3–7 One key component of the informed consent process is a clear written consent form. Especially for cancer clinical trials, however, written consents have become longer, difficult to read, and more complex. Complex and lengthy written consents can compromise participant comprehension of clinical trials.8–11
Research to improve the consent process has included enhanced consent form interventions (e.g., shortening length, revising content, improving formatting, and adding graphics). Results of such interventions have been mixed with generally limited efficacy in enhancing participant understanding of clinical trials or increasing trial participation.12, 13 Few studies, however, have evaluated interventions to enhance consent forms for multicenter cancer clinical trials.14, 15 A non-randomized study assessing a standard Southwestern Oncology Group consent form or a simplified booklet form in 183 healthy individuals and cancer patients showed a significantly higher proportion preferred the simplified form, although no difference in their understanding of the information on the two forms was observed.14 In an Eastern Cooperative Oncology Group study, 226 patients in lung and breast cancer trials were randomized to a standard consent form or a consent form with readability adjusted to the 7th–8th grade level.15 Participants reported reduced anxiety and increased satisfaction with the easier to read consent form yet there was no difference in comprehension of the consent form.
Participant decision-making and the informed consent process for hematopoietic cell transplantation (HCT) clinical trials present unique challenges since HCT is a complex, ‘high-stakes’ medical treatment for life-threatening hematologic malignancies and other diseases where the potential for cure has to be balanced against the possibility of developing significant morbidity and mortality from the procedure itself.16, 17 Simplification of the consent process has been identified as a putative intervention to improve participant comprehension of HCT clinical trials.16, 18 The Blood and Marrow Transplant Clinical Trials Network (BMT CTN) previously proposed an easy-to-read informed consent (ETRIC) form template that follows evidence-based recommendations for health literacy, readability and processability, and the use of plain language specifically targeting specialized terminology related to HCT, while including all federally required elements for research informed consent.19 BMT CTN 1205 was a two-part study comprised of: (1) a randomized study comparing the effects of ETRIC and standard consent form templates on understanding and other outcomes among patients approached for enrollment on select BMT CTN clinical trials, and (2) a qualitative evaluation study to understand the barriers and facilitators to implementation of the ETRIC template at BMT CTN clinical trial sites.
METHODS
BMT CTN ETRIC Template
The ETRIC format was developed by a multi-stakeholder BMT CTN task force with expertise in institutional review board (IRB) regulations and procedures, research ethics, clinical trial design, health services research, health literacy, and patient advocacy. Existing BMT CTN clinical trial consent forms were reviewed for completeness, readability, length, and format, and based on guidance from the literature and feedback from BMT CTN investigators, coordinators, and site IRBs, recommendations were provided for an ETRIC form template.19 The overarching intent of the template was to simplify the process for development and review of informed consent documents, enhance potential participants’ understanding of their trials, and improve participant satisfaction with the informed consent process. An innovative feature was the layout of the consent document, with information provided in a two-column format and recommendations for layout, organization of text, typography, and plain language that can facilitate information location, comprehension, and identification of target words (Supplement Figure S1).19
Randomized Study
Study Design
We conducted a randomized multicenter study comparing ETRIC and standard consent forms to improve participant comprehension of BMT CTN clinical trials (protocol available at bmtctn.net [BMT CTN 1205 trial]). Patients who were potentially eligible for BMT CTN 0901, 1101, 1203 or 1301 (parent) clinical trials were first approached for participation on the ETRIC study. These trials reflect common indications and/or complications of HCT and the diversity and complexity of transplantation approaches and supportive care treatments (protocol details available at bmtctn.net). Participants who provided consent to BMT CTN 1205 were randomized 1:1 to the ETRIC or the standard consent form arms and provided the study-assigned written consent form for participation on the parent clinical trial. Participants who declined participation on the 1205 study could still undergo consent discussion and participate on the parent clinical trial using the standard consent form. The study was conducted under the guidance and approval of participating center IRBs. A waiver of the requirement to document consent to BMT CTN 1205 was obtained from site IRBs, where the informed consent for participating could be obtained verbally; however, participants signed informed consent forms (ETRIC or standard) prior to enrollment on the parent clinical trial. Participants were enrolled between November 2013 and September 2016. The study was registered on ClinicalTrials.gov database of clinical trials: https://clinicaltrials.gov/ct2/show/NCT02081248.
Participants and Interventions
Participants had to meet the eligibility criteria for the parent clinical trial for which they were being considered. In addition, they had to be adults (age ≥ 18 years) with adequate speaking and reading proficiency in English to complete the study assessments. The intervention arm received the ETRIC form described above.19 The standard consent form had a single column format and lacked the formatting and readability enhancements of the ETRIC form. Of note, both the ETRIC and standard consent forms were written in plain language (8th grade or lower reading level). The content of both forms was similar and contained all federally required elements of informed consent.
Assessments and Endpoints
Supplement Table S1 describes the BMT CTN 1205 study assessments. Participants were asked to complete them within 7 business days after the consent discussion for the parent clinical trial irrespective of enrollment on the parent trial. Assessments included: (1) health literacy (Rapid Estimate of Adult Literacy in Medicine [REALM]20 and Newest Vital Sign [NVS]21), (2) actual and perceived comprehension of the clinical trial presented (Quality of Informed Consent [QuIC]22 and Modified Deaconess Informed Consent Comprehension Test [DICCT]23), (3) anxiety related to the consent process (State Trait Anxiety Inventory [STAI]24), (4) satisfaction with the consent process (QuIC supplement and study specific questionnaire, and (5) information location (study-specific instrument). The QuIC incorporates basic elements of informed consent specified in federal regulations, assesses therapeutic misconception, and measures actual (part A) and perceived (part B) understanding of cancer clinical trials.22 This instrument has been developed specifically for assessing subject comprehension of cancer clinical trials, has good test-retest reliability (interclass correlation coefficient 0.66–0.77), and has been well validated in the literature The primary endpoint for the study was objective comprehension score on the QuIC-A instrument. Secondary endpoints included subjective comprehension of the clinical trial (QuIC-B and modified DICCT), anxiety and satisfaction with the consent process, time taken to locate selected information in the consent form, and consent rates to parent clinical trials.
Statistical Analysis
The study was designed to detect a 4-point difference in the mean QuIC-A comprehension scores between the study arms; this represents a 0.5 standard deviation (SD) and meaningful difference in comprehension.11, 22, 25 Based on a two-sided t-test, a sample size of 64 participants per arm was required to have sufficient power to detect this difference in group means (α=0.05, power=80%, SD=8). The sample size was inflated by 25% resulting in 80 participants per group to account for potential violation of the normality assumption under the Mann-Whitney test and also for possible dropouts. At a scheduled DSMB review, it was observed that the levels of missing assessments and drop-outs (i.e., participants who consented for the 1205 study but did not complete the consent process for the parent clinical trial or the 1205 study assessments) were higher than anticipated. To account for these levels, the final sample size was increased to 198 participants (99 in each arm). Analysis was performed using the intention-to-treat approach and all participants who completed sufficient questions of QuIC-A (≥7 of 13 domains) were included in the primary analysis. QuIC-A scores were compared using the two sample t-test after verification of normality. Secondary endpoints were analyzed using the Mann-Whitney test, except for consent rates which were compared using Fisher’s exact test. Regression analyses were used to control the effects of potentially confounding variables (age, gender, race/ethnicity, annual household income, education level, health literacy, and parent clinical trial). Analyses were also performed to test for interactions between QuIC-A and age, parent clinical trial, health literacy, household income, and education level. Analyses were performed with and without imputations for missing data; only the former are presented as the results were similar. All P-values were two-sided. Analyses were performed using SAS software v9.4 (SAS Institute, Cary, NC).
Evaluation Study
Study Design
A qualitative study was also conducted to understand clinical trial site-specific barriers and facilitators to implementing the ETRIC form. Centers were recruited from BMT CTN clinical trial core and affiliate sites, representing a diversity of geographic location and center size and a mix of centers that did and did not elect to participate in the 1205 randomized study. During a one-day visit, two trained moderators conducted 4–6 semi-structured interviews of investigators and research personnel involved in HCT trials and of IRB administrators and staff. Interviews lasted 30–45 minutes and examined: (1) willingness to utilize and acceptability of the ETRIC two-column format, (2) perceived barriers to implementation, (3) previous experience with alternative format informed consent forms or novel methods of obtaining consent, (4) perception of value of ETRIC form, and (5) helpfulness of sample resources to facilitate implementation of ETRIC form. Discussions were facilitated by an interview guide and audio recorded. Notes were taken during the interviews to record nonverbal behavior of participants and to provide a “back-up” record. Study participants were not provided any incentive to participate. The study had undergone review by the NMDP IRB and was determined to be exempt human subjects research as defined by the Common Rule. Verbal informed consent was obtained from each participant prior to the interview.
Qualitative Data Analysis
Sequential, transcript-based analysis was performed using NVivo 10 software.26 Two experienced reviewers familiar with the area of study and preliminary research independently analyzed the data. First, a codebook was developed and textual data was coded line by line (i.e., segmenting the data into meaningful analytical units). Reliability and validity of the coded data was assessed through inter- and intra-coder agreement measures.27 To correct for the possibility that coders might agree by chance, the kappa statistic was calculated.28 A kappa > 0.90 indicates agreement in the way codes are assigned.27 Coded textual data were explored inductively using content analysis to generate categories and explanations. This identified hierarchical relationships among codes; families of codes were created and were aggregated, reviewed, and analyzed at an increasingly general level.29 This process is referred to as content analysis for saturation of themes. Participant quotes are used to support key themes and to show the diversity of opinions gathered during the interviews.
RESULTS
Randomized Study
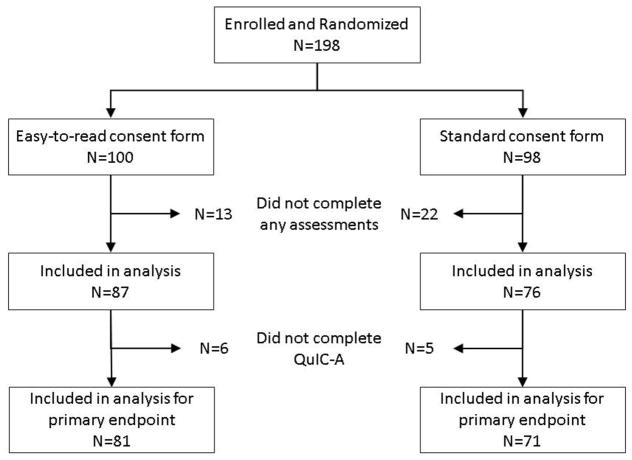
Twenty-five BMT CTN centers participated in the study and enrolled 198 patients (Figure 1). Thirty-five patients did not complete any study assessments (24 declined study assessments, 15 of whom also declined enrollment in or were ineligible for the parent trial, and consent discussion did not occur due to closure of parent trial for 1 participant). Among the remaining 163 participants who completed some assessments, 152 provided sufficient information on QuIC-A and were included in the analysis for the primary endpoint. Participant demographics including annual household income, education level and health literacy scores were well balanced between the two arms (Table 1).
Figure 1.
Participant flow diagram for randomized study
Table 1.
Demographics of participants enrolled on the randomized study
| Characteristic | ETRIC Form (N=100) | Standard Consent Form (N=98) | P-value† |
|---|---|---|---|
| Age, years (Median, range) | 61 (21–76) | 61 (27–73) | 0.5093 |
| Gender (N, %) | 0.7750 | ||
| Female | 43 (43) | 45 (46) | |
| Male | 57 (57) | 53 (54) | |
| Ethnicity (N, %) | |||
| Hispanic/Latino | 2 (2) | 5 (5) | 0.3187 |
| Not Hispanic/Latino | 94 (94) | 87 (89) | |
| Declined/missing | 4 (4) | 6 (6) | |
| Race (N, %) | 0.8954 | ||
| White | 88 (88) | 85 (87) | |
| Black | 8 (8) | 7 (7) | |
| Other | 2 (2) | 2 (2) | |
| Declined/missing | 2 (2) | 4 (4) | |
| Annual household income (N, %) | 0.3446 | ||
| <$40,000 | 19 (19) | 11 (11) | |
| $40,000–$79,999 | 20 (20) | 25 (26) | |
| ≥$80,000 | 26 (26) | 22 (22) | |
| Declined/missing | 35 (35) | 40 (41) | |
| Education level (N, %) | 0.1872 | ||
| High school or less | 35 (35) | 20 (20) | |
| More than high school but less than graduate degree | 27 (27) | 29 (30) | |
| Graduate degree or more | 11 (11) | 15 (15) | |
| Other | 6 (6) | 5 (5) | |
| Declined/missing | 21 (21) | 29 (30) | |
| Health literacy, REALM (N, %) | 0.3967 | ||
| Score <8 | 17 (17) | 15 (15) | |
| Score 8 | 69 (69) | 62 (63) | |
| Declined/missing | 14 (14) | 21 (21) | |
| Health literacy, NVS (N, %) | 0.3837 | ||
| Score <4 | 16 (16) | 11 (11) | |
| Score 4 | 52 (52) | 53 (54) | |
| Score 5 | 18 (18) | 13 (13) | |
| Declined/missing | 14 (14) | 21 (21) | |
| Parent clinical trial (N, %)* | 0.9188 | ||
| BMT CTN 0901 | 11 (11) | 10 (10) | |
| BMT CTN 1101 | 22 (22) | 25 (26) | |
| BMT CTN 1203 | 51 (51) | 50 (51) | |
| BMT CTN 1301 | 16 (16) | 13 13) |
BMT CTN – Blood and Marrow Transplant Clinical Trials Network; REALM – Rapid Estimate of Adult Literacy in Medicine; NVS – Newest Vital Sign
Parent clinical trials were: BMT CTN 0901 – Phase III randomized trial of full versus reduced intensity conditioning regimens in myelodysplastic syndromes and acute myeloid leukemia; BMT CTN 1101 – Phase III randomized trial of double unrelated umbilical cord blood versus HLA-haploidentical related bone marrow for hematologic malignancies; BMT CTN 1203 – Phase II randomized trial of novel approaches for graft-versus-host disease prevention; BMT CTN 1301 – Phase III randomized trial of calcineurin inhibitor-free interventions for prevention of graft-versus-host disease (all trial protocols are available at bmtctn.net)
Fisher exact test P-value with the exception of age which represents P-value from two sample t-test with pooled variance
There was no significant difference in the mean QuIC-A scores between the two study arms (80.5 for ETRIC form versus 81.8 for standard consent, P=0.37; Table 2). In unadjusted analyses that evaluated the association of the primary outcome with potential confounding variables, only the parent clinical trial was observed to be significantly associated with QuIC-A score (P=0.046). In subsequent multivariate analyses adjusted for the parent trial, there was no significant association between consent type and QuIC-A score (P=0.57). We also did not find any difference between arms in subjective comprehension, anxiety, satisfaction, information location or consent rates on parent clinical trials (Table 2). A priori, we tested for and found no significant interactions between the consent type and age, household income, education level, health literacy scores or parent clinical trial protocol. Similarly, there was no significant random center effect on QuIC-A scores, nor did find any significant nested random effect of center within clinical trial protocol on QuIC-A.
Table 2.
Study endpoints by treatment arms
| Endpoint | ETRIC Form | Standard Consent Form | P-value | ||
|---|---|---|---|---|---|
| N | Estimate | N | Estimate | ||
| Objective Comprehension* | |||||
| Quality of Informed Consent (Part A), Mean (SD) | 81 | 80.5 (8.7) | 71 | 81.8 (9.5) | 0.3681† |
| Subjective Comprehension | |||||
| Quality of Informed Consent (Part B), Median (IQR) | 81 | 96.4 (89.3– 100.0) | 71 | 98.2 (91.9– 100.0) | 0.3893‡ |
| Modified Deaconess Informed Consent Comprehension Test, Median (IQR) | 84 | 15 (14–17) | 73 | 15 (13–17) | 0.1660‡ |
| Anxiety | |||||
| State anxiety score on STAI, Median (IQR) | 80 | 2.5 (2.3–2.6) | 72 | 2.5 (2.3–2.6) | 0.2085‡ |
| Trait anxiety score on STAI, Median (IQR) | 80 | 2.3 (2.1–2.5) | 72 | 2.4 (2.2–2.5) | 0.2474‡ |
| Satisfaction | |||||
| Study-specific survey, Median (IQR) | 82 | 4.1 (3.9–4.7) | 72 | 4.2 (3.7–4.9) | 0.8035‡ |
| Information Location# | |||||
| Finding main goal for study, Median (IQR) | 85 | 30 (11–63) | 76 | 22 (11–60) | 0.7333‡ |
| Finding who to contact for questions, Median (IQR) | 85 | 20 (10–45) | 76 | 15 (7–34) | 0.2641‡ |
| Finding risks and benefits section, Median (IQR) | 85 | 20 (10–40) | 76 | 20 (14–30) | 0.7368‡ |
| Finding how to leave the study, Median (IQR) | 85 | 30 (11–60) | 76 | 24 (15–60) | 0.5638‡ |
| Finding study procedures, Median (IQR) | 85 | 36 (18–96) | 76 | 43 (21–83) | 0.7934‡ |
| Consent Rate On Parent Trial | |||||
| BMT CTN 0901, % | 11 | 27.3 | 10 | 20.0 | 1.0000§ |
| BMT CTN 1101, % | 22 | 45.5 | 25 | 52.0 | 0.7726§ |
| BMT CTN 1203, % | 51 | 49.0 | 50 | 44.0 | 0.6914§ |
| BMT CTN 1301, % | 16 | 43.8 | 13 | 69.2 | 0.2642§ |
ETRIC – Easy-to-read informed consent; IQR – Inter-quartile range; STAI – State Trait Anxiety Inventory; BMT CTN – Blood and Marrow Transplant Clinical Trials Network
Primary endpoint for the study
Reported as seconds; participants who were not able to identify a given section were assigned the maximum time allotted to find each section (180 seconds)
P-value based on t-test
P-value based on Wilcoxon-Mann-Whitney test
P-value based on Fisher exact test
Evaluation Study
Nine BMT CTN transplant centers participated in the qualitative study; among these, 5 participated in the ETRIC randomized study while 4 elected not to participate. Overall, 49 semi-structured interviews were conducted among site investigators (N=13), research staff (N=27), IRB members (N=5), legal/regulatory staff (N=3) and a patient advocate (N=1), who collectively had a median of 13 (range, 0.5–38) years of experience working within the area of informed consent. A large proportion of interviewees were familiar with the ETRIC format (33/49 [67%]), which reflects their familiarity with BMT CTN trials and initiatives. On content analysis, dominant themes focused on five main areas 1) a willingness to utilize and acceptability of the ETRIC template; 2) perceived value of the ETRIC template, including usefulness in facilitating informed consent for study staff and PIs; 3) perceived barriers to implementation, such as concerns about implementation and whether IRBs would accept this template; 4) previous experience with alternative consent formats; and 5) educational resources for clinical trial researchers, staff and IRB members (Table 3 and Supplement Table S2).
Table 3.
Themes related to barriers and facilitators to use of ETRIC format identified on evaluation study (See supplemental Table S2 for representative quotes from interviewees to support themes)
| Interview Domain | Themes |
|---|---|
| Willingness to utilize and acceptability of ETRIC form |
|
| Perceived value of ETRIC form |
|
| Perceived barriers to implementation of ETRIC form |
|
| Previous experience with alternative consent forms |
|
| Educational resources for implementing ETRIC |
|
ETRIC – Easy-to-read informed consent; IRB – Institutional Review Board
DISCUSSION
There is general agreement about the critical need for reforming the informed consent process for cancer clinical trials by making it more patient-centric. However, studies investigating novel consent form interventions and generating high-quality evidence in this area are generally lacking. Compared to prior studies, our study was innovative in that it was a mixed methods study with both quantitative and qualitative methods. With this approach, we addressed the impact of our ETRIC form both on participants enrolling on BMT CTN sponsored HCT clinical trials as well as institution specific stakeholders whose engagement is essential for successfully implementing any interventions related to the informed consent process. Overall, the randomized study demonstrated no statistical improvement in understanding, anxiety, time to information location, and consent rates among participants assigned to the ETRIC versus standard format consent form. On the other hand, considering the impact that the ETRIC template had on all stakeholders in the informed consent process, including study staff, our qualitative data suggests that the ETRIC form represents an improvement in the informed consent process.
There are several potential reasons for not finding an association between the ETRIC form and participant comprehension and other endpoints on the randomized study. As noted above, the lay language enhancements of the ETRIC template had already been adopted by the BMT CTN. Thus, the main difference between the standard consent template and the ETRIC form were the formatting enhancements such as more white space, layout that was more readable and the two-column format. Furthermore, the trial was conducted at BMT CTN participating sites that are accustomed to educating and counselling participants about complex HCT clinical trials, and hence, the consent form itself did not have a major impact on our study endpoints. Also, the health literacy scores of our study population were relatively high and participants reviewing the consent form may not have benefitted as much from the formatting enhancements of the ETRIC form. We do acknowledge this as a limitation of the study as well, and we did not have data on health literacy scores for patients who did not enroll on our study to compare and determine generalizability of our findings. It is clear, though, that the formatting elements of the ETRIC template did not improve understanding and the other domains of interest.
That IRB chairs, IRB staff, and site investigators and research staff perceive the ETRIC template as helpful for not just participants, but researchers as well, is an important and novel finding not only for the ETRIC template, but for informed consent research in general. Discussions of informed consent often focus on the entire consent process, not merely the signing of the piece of paper, and rightly so. However, discussions of informed consent for research have thus far focused on the research participants in that process, ignoring the other stakeholders. Since PIs, study staff, and IRB chairs and staff are all involved in the consent process, it is essential to better understand their roles and their preferences in the informed consent process. To be clear, research participants are the most important stakeholders in the informed consent process; but they are not the only stakeholders.
In conclusion, the ETRIC template did not influence understanding, anxiety, time to information location, and consent rates for patients considering enrollment on BMT CTN clinical trials. However, it was viewed favorably by IRB chairs, staff, PIs and study staff, and may facilitate their roles in the consent process. The BMT CTN is transitioning towards a central IRB mechanism, which will facilitate implementation and utilization of the ETRIC form. In the meantime, continued research is needed to investigate alternative interventions to enhance participant understanding of HCT clinical trials.
Supplementary Material
HIGHLIGHTS.
A two-column easy-to-read informed consent form incorporating elements of health literacy and readability in participants was viewed favorably by clinical trial sites.
However, it did not alter comprehension of the trial among participants considering participation in Blood and Marrow Transplant Clinical Trials Network clinical trials.
Acknowledgments
FUNDING
Research Support: Support for this study was provided by grant #U10HL069294 to the Blood and Marrow Transplant Clinical Trials Network from the National Heart, Lung, and Blood Institute (NHLBI) and the National Cancer Institute and #U10HL069294-12S1 from the NHLBI. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
We are grateful to the administrative support provided for this study by BMT CTN Data Coordinating Center Protocol Coordinators Alyssa Ramirez and Cathryn Mudrick, MPH. The Writing Committee would like to thank the following transplant centers for their participation on this study: Blood and Marrow Transplant Program at Northside Hospital, Atlanta, GA; City of Hope National Medical Center, Duarte, CA; Cleveland Clinic Foundation, Cleveland, OH; Duke University Medical Center, Durham, NC; Emory University, Atlanta, GA; Florida Hospital Cancer Institute, Orlando, FL; H. Lee Moffitt Cancer Center, Tampa, FL; Johns Hopkins/Sidney Kimmel Comprehensive Cancer Center, Baltimore, MD; Kansas University Medical Center, Kansas City, KS; Karmanos Cancer Institute, Detroit, MI; Loyola University Medical Center, Chicago, IL; Mayo Clinic, Rochester, MN; Medical College of Wisconsin, Milwaukee, WI; The Jewish Hospital/OHC, Cincinnati, OH; The Ohio State University/Arthur G. James Cancer Hospital, Columbus, OH; University Hospitals of Cleveland/Case Western, Cleveland, OH; University of Florida Shands Hospital, Gainesville, FL; University of Minnesota, Minneapolis, MN; University of Nebraska Medical Center, Omaha, NE; University of North Carolina, Chapel Hill, NC; University of Pennsylvania Cancer Center, Philadelphia, PA; Emory University; Washington University in St. Louis/Barnes Jewish Hospital, St Louis, MO; West Virginia University Hospital, Morgantown, WV
Footnotes
Transplant Clinical Trials Network: Hematopoietic cell transplantation
Clinical Trial Information: NCT02081248
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of Interest Statement: None of the authors have a relevant conflict of interest related to this study.
Presentation: This study was presented in part at the American Society of Hematology annual meeting in December 2017 at Atlanta, GA
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Singer DA, Donnelly MB, Messerschmidt GL. Informed consent for bone marrow transplantation: identification of relevant information by referring physicians. Bone Marrow Transplant. 1990;6:431–437. [PubMed] [Google Scholar]
- 2.von Wagner C, Semmler C, Good A, Wardle J. Health literacy and self-efficacy for participating in colorectal cancer screening: The role of information processing. Patient Educ Couns. 2009;75:352–357. doi: 10.1016/j.pec.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 3.Aaronson NK, Visser-Pol E, Leenhouts GH, et al. Telephone-based nursing intervention improves the effectiveness of the informed consent process in cancer clinical trials. J Clin Oncol. 1996;14:984–996. doi: 10.1200/JCO.1996.14.3.984. [DOI] [PubMed] [Google Scholar]
- 4.Joffe S, Cook EF, Cleary PD, Clark JW, Weeks JC. Quality of informed consent in cancer clinical trials: a cross-sectional survey. Lancet. 2001;358:1772–1777. doi: 10.1016/S0140-6736(01)06805-2. [DOI] [PubMed] [Google Scholar]
- 5.Kuehn BM. Patients’ unrealistic hopes for cancer trial benefits may hinder consent. JAMA. 2011;305:1186–1187. doi: 10.1001/jama.2011.317. [DOI] [PubMed] [Google Scholar]
- 6.Lidz CW, Appelbaum PS, Grisso T, Renaud M. Therapeutic misconception and the appreciation of risks in clinical trials. Soc Sci Med. 2004;58:1689–1697. doi: 10.1016/S0277-9536(03)00338-1. [DOI] [PubMed] [Google Scholar]
- 7.Mills EJ, Seely D, Rachlis B, et al. Barriers to participation in clinical trials of cancer: a meta-analysis and systematic review of patient-reported factors. Lancet Oncol. 2006;7:141–148. doi: 10.1016/S1470-2045(06)70576-9. [DOI] [PubMed] [Google Scholar]
- 8.Beardsley E, Jefford M, Mileshkin L. Longer consent forms for clinical trials compromise patient understanding: so why are they lengthening? J Clin Oncol. 2007;25:e13–14. doi: 10.1200/JCO.2006.10.3341. [DOI] [PubMed] [Google Scholar]
- 9.Berger O, Gronberg BH, Sand K, Kaasa S, Loge JH. The length of consent documents in oncological trials is doubled in twenty years. Ann Oncol. 2009;20:379–385. doi: 10.1093/annonc/mdn623. [DOI] [PubMed] [Google Scholar]
- 10.Sharp SM. Consent documents for oncology trials: does anybody read these things? Am J Clin Oncol. 2004;27:570–575. doi: 10.1097/01.coc.0000135925.83221.b3. [DOI] [PubMed] [Google Scholar]
- 11.Truong TH, Weeks JC, Cook EF, Joffe S. Outcomes of informed consent among parents of children in cancer clinical trials. Pediatr Blood Cancer. 2011;57:998–1004. doi: 10.1002/pbc.22983. [DOI] [PubMed] [Google Scholar]
- 12.Nishimura A, Carey J, Erwin PJ, Tilburt JC, Murad MH, McCormick JB. Improving understanding in the research informed consent process: a systematic review of 54 interventions tested in randomized control trials. BMC medical ethics. 2013;14:28. doi: 10.1186/1472-6939-14-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flory J, Emanuel E. Interventions to improve research participants’ understanding in informed consent for research: a systematic review. JAMA. 2004;292:1593–1601. doi: 10.1001/jama.292.13.1593. [DOI] [PubMed] [Google Scholar]
- 14.Davis TC, Holcombe RF, Berkel HJ, Pramanik S, Divers SG. Informed consent for clinical trials: a comparative study of standard versus simplified forms. J Natl Cancer Inst. 1998;90:668–674. doi: 10.1093/jnci/90.9.668. [DOI] [PubMed] [Google Scholar]
- 15.Coyne CA, Xu R, Raich P, et al. Randomized, controlled trial of an easy-to-read informed consent statement for clinical trial participation: a study of the Eastern Cooperative Oncology Group. J Clin Oncol. 2003;21:836–842. doi: 10.1200/JCO.2003.07.022. [DOI] [PubMed] [Google Scholar]
- 16.D’Souza A, Pasquini M, Spellecy R. Is ‘informed consent’ an ‘understood consent’ in hematopoietic cell transplantation? Bone Marrow Transplant. 2015;50:10–14. doi: 10.1038/bmt.2014.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Massimo L. Ethical problems in bone marrow transplantation in children. Bone Marrow Transplant. 1996;18(Suppl 2):8–12. [PubMed] [Google Scholar]
- 18.Stevens PE, Pletsch PK. Ethical issues of informed consent: mothers’ experiences enrolling their children in bone marrow transplantation research. Cancer Nurs. 2002;25:81–87. doi: 10.1097/00002820-200204000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Denzen EM, Santibanez ME, Moore H, et al. Easy-to-read informed consent forms for hematopoietic cell transplantation clinical trials. Biol Blood Marrow Transplant. 2012;18:183–189. doi: 10.1016/j.bbmt.2011.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davis TC, Long SW, Jackson RH, et al. Rapid estimate of adult literacy in medicine: a shortened screening instrument. Fam Med. 1993;25:391–395. [PubMed] [Google Scholar]
- 21.Weiss BD, Mays MZ, Martz W, et al. Quick assessment of literacy in primary care: the newest vital sign. Ann Fam Med. 2005;3:514–522. doi: 10.1370/afm.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joffe S, Cook EF, Cleary PD, Clark JW, Weeks JC. Quality of informed consent: a new measure of understanding among research subjects. J Natl Cancer Inst. 2001;93:139–147. doi: 10.1093/jnci/93.2.139. [DOI] [PubMed] [Google Scholar]
- 23.Miller CK, O’Donnell DC, Searight HR, Barbarash RA. The Deaconess Informed Consent Comprehension Test: an assessment tool for clinical research subjects. Pharmacotherapy. 1996;16:872–878. [PubMed] [Google Scholar]
- 24.Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 25.Hoffner B, Bauer-Wu S, Hitchcock-Bryan S, Powell M, Wolanski A, Joffe S. “Entering a Clinical Trial: Is it Right for You?”: a randomized study of The Clinical Trials Video and its impact on the informed consent process. Cancer. 2012;118:1877–1883. doi: 10.1002/cncr.26438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krueger RA, Casey MA. Focus Groups: A Practical Guide for Applied Research. 3. Sage Publications, Inc; Thousand Oaks, CA: 2000. [Google Scholar]
- 27.Carey JW, Morgan M, Oxtoby MJ, Carey JW, Morgan M, Oxtoby MJ. Intercoder agreement in analysis of responses to open-ended interview questions: Examples from tuberculosis research. Field Methods. 1996;8:1–5. [Google Scholar]
- 28.Gorden RL. Basic Interviewing Skills. Waveland Press Inc; Long Grove, IL: 1998. [Google Scholar]
- 29.MacQueen KM, McLellan E, Kay K, Milstein B. Codebook development for team-based qualitative analysis. Cultural Anthropology Methods. 1998;10:31–36. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.