Abstract
Background and Purpose
Several preclinical studies have demonstrated the unique profiles of levetiracetam (LEV), inhibits spontaneous absence epilepsy models but does not affect traditional convulsion models; however, the detailed pharmacological mechanisms of action of LEV remain to be clarified.
Experimental Approach
We determined the interaction between LEV and IFNγ regarding astroglial release of anti‐convulsive (kynurenic acid and xanthurenic acid), pro‐convulsive (quinolinic acid) and anti‐convulsive but pro‐absence (cinnabarinic acid) kynurenine‐pathway metabolites from rat cortical primary cultured astrocytes using ultra‐HPLC equipped with MS.
Key Results
IFNγ increased basal astroglial release of cinnabarinic acid and quinolinic acid but decreased that of kynurenic acid and xanthurenic acid. IFNγ enhanced inositol 1,4,5‐trisphosphate (IP3) receptor agonist (adenophostin A, AdA)‐induced astroglial release of kynurenine‐pathway metabolites, without affecting AMPA‐induced release. LEV increased basal astroglial release of kynurenic acid and xanthurenic acid without affecting cinnabarinic acid or quinolinic acid. Chronic and acute LEV administration inhibited AMPA‐ and AdA‐induced kynurenine‐pathway metabolite release. Upon chronic administration, LEV enhanced stimulatory effects of IFNγ on kynurenic acid and xanthurenic acid, and reduced its stimulatory effects on cinnabarinic acid and quinolinic acid. Furthermore, LEV inhibited stimulatory effects of chronic IFNγ on AdA‐induced release of kynurenine‐pathway metabolites.
Conclusions and Implications
This study demonstrated several mechanisms of LEV: (i) inhibition of AMPA‐ and AdA‐induced astroglial release, (ii) inhibition of IFNγ‐induced IP3 receptor activation and (iii) inhibition of release of cinnabarinic acid and quinolinic acid with activation of that of kynurenic acid induced by IFNγ. These combined actions of LEV may contribute to its unique profile.
Abbreviations
- 3OH‐kynurenine
3‐hydroxykynurenine
- ACSF
artificial CSF
- AdA
adenophostin A
- fDMEM
DMEM containing 10% FCS
- IP3
inositol 1,4,5‐trisphosphate
- KAT
kynurenine aminotransferase
- LEV
levetiracetam
- UHPLC/MS
ultra‐HPLC equipped with MS
- WAG/Rij
Wistar albino Glaxo rats of Rijswijk
Introduction
The anti‐convulsant drug (S)‐α‐ethyl‐2‐oxo‐pyrrolidine (http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6826, LEV) has neuroprotective effects and a wide clinical anti‐epileptic spectrum (Berkovic et al., 2007; De Smedt et al., 2007a; Belcastro et al., 2011). LEV also exhibits unique effects on animal models of convulsion and epilepsy. It is ineffective in traditional convulsion screening models, namely, maximal‐electroshock and pentylenetetrazol‐induced convulsion models (De Smedt et al., 2007b), whereas LEV provides protection in several spontaneous absence epilepsy animal models, such as Strasbourg and Wistar albino Glaxo rats of Rijswijk (WAG/Rij) (Privitter and Cavitt, 2007). These preclinical findings in traditional convulsion and spontaneous epilepsy models suggest that LEV is an anti‐absence drug but lacks anti‐convulsant activity.
The major mechanism of action of LEV is considered to involve binding to http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2634 (Lynch et al., 2004), although other pharmacological actions have also been demonstrated, such as inhibition of N‐type http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=80 + and http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=81, http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=75s and Ca2+ release from intracellular Ca2+ stores mediated by http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=123 (Carunchio et al., 2007; De Smedt et al., 2007b; Fukuyama et al., 2012). In particular, activation of the AMPA receptor plays important roles in the initiation of epileptic seizures and propagation of epileptic discharge (Rogawski, 2002), whereas hyper‐activation of the IP3 receptor contributes to the neuronal damage induced by epileptic seizure, seizure maintenance and dysfunction of GABA exocytosis during epileptic discharge (Pal et al., 2001; Fukuyama et al., 2012).
Reactive astrocytes accompany chronic neurological disease. Both clinical and preclinical studies have strongly indicated that several immunological reactions play important roles in the ictogenesis and development of epileptogenesis (Getts et al., 2007; Somera‐Molina et al., 2007). In particular, several clinical studies have suggested that pro‐inflammatory reactions contribute to the development of epileptogenesis (Sinha et al., 2008; Li et al., 2017). Despite these clinical and preclinical efforts, the mechanisms of development of epileptogenesis associated with pro‐inflammatory and anti‐inflammatory reactions have remained to be clarified. Astrocytes and microglia, the immune cells within the CNS, are extremely heterogeneous (Liddelow and Barres, 2017). Recently, it has been proposed that reactive astrocytes are composed of at least two phases, toxic ‘A1’ and trophic ‘A2’ reactive astrocytes, which parallel the ‘M1’ and ‘M2’ microglia respectively (Liddelow and Barres, 2017). Basically, reactive astrocytes might well have more than two states of polarization, which is an important feature for immunological research (Liddelow and Barres, 2017). Furthermore, trophic A2 reactive astrocytes up‐regulate some genes responsible for the induction of synapse formation; however, these changes might also result in unwanted synapses that lead toepilepsy (Eftekhari et al., 2014). Therefore, we should assume that the mechanisms of epileptogenesis are extremely complex. To understand the detailed mechanisms of epileptogenesis and ictogenesis, each specific chemical mediator reaction needs to be identified as well as the more complex interactions amongst immunological mediators on tripartite synaptic transmission that provide the numerous mechanisms behind the pathophysiology of epilepsy.
http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4968 is involved in seizure generation and the process of epileptogenesis (Getts et al., 2007; Somera‐Molina et al., 2007). It is also reported to increase the expression of IP3 receptors and enhance AMPA receptor‐induced neurotoxicity (Sahu et al., 2007; Park et al., 2009). Furthermore, activation of IFNγ is reported to enhance the activity of the kynurenine pathway in glia (Myint, 2012). Several preclinical studies have demonstrated that LEV reduces and promotes the expression of pro‐inflammatory and anti‐inflammatory cytokines respectively (Haghikia et al., 2008; Christensen et al., 2010; Kim et al., 2010; Stienen et al., 2011). However, chronic LEV intake does not affect cytokine levels in patients with epilepsy (Guenther et al., 2014).
The kynurenine pathway synthesizes various endogenous neuroactive metabolites from tryptophan (Figure 1) (Myint, 2012). http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2918, which is a broad‐spectrum endogenous neuroprotective antagonist of AMPA and http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=75 (Hilmas et al., 2001), inhibits absence seizures in WAG/Rij (Peeters et al., 1994; Kaminski et al., 2003). In contrast to kynurenic acid, quinolinic acid is a pro‐convulsive metabolite, since it produces convulsions and neuronal damage through activation of the NMDA receptor (Lapin, 1978; Stone, 2001). Recently, xanthurenic acid and cinnabarinic acid have been identified as endogenous agonists of group II (II‐mGlu) and group III (III‐mGlu) http://www.guidetopharmacology.org/GRAC/FamilyIntroductionForward?familyId=40s respectively (Fazio et al., 2012; Copeland et al., 2013; Fazio et al., 2017). Agonists of II‐mGlu and III‐mGlu receptors attenuate chemical‐induced convulsions, whereas antagonists of them abolish the anti‐convulsive effects of their agonists (Folbergrova et al., 2001; Folbergrova et al., 2003). Contrary to the findings in convulsion models, the activation of II‐mGlu and III‐mGlu receptors suppressed and enhanced absence seizures in spontaneous epileptic models respectively (Moldrich et al., 2001; Ngomba et al., 2008). These previous finidings suggest that kynurenic acid and xanthurenic acid are anti‐convulsive metabolites, while quinolinic acid is a pro‐convulsive one. Interestingly, the candidate endogenous III‐mGlu agonist cinnabarinic acid is probably an anti‐convulsive but pro‐absence metabolite.
Figure 1.
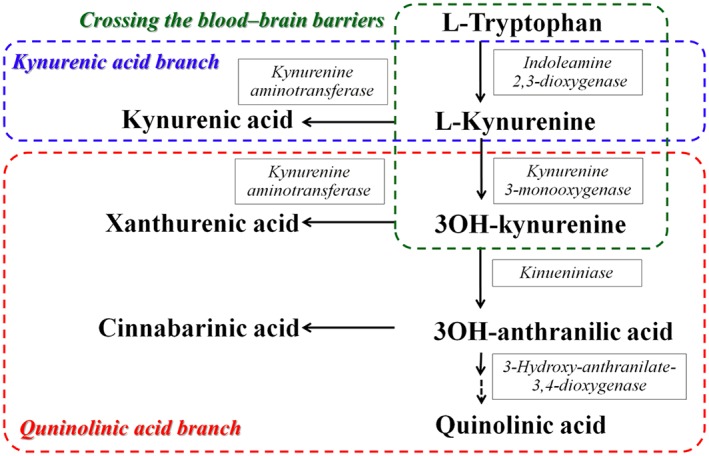
Kynurenine‐pathway of tryptophan metabolism. L‐kynurenine is synthesized from http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=717 by indoleamine 2,3‐dioxygenase. L‐kynurenine and 3OH‐kynurenine are transaminated by KAT into kynurenic acid and xanthurenic acid respectively. Kynurenine 3‐monooxygenase transforms L‐kynurenine into 3OH‐kynurenine. Kynureninase transforms 3OH‐kynurenine into 3‐hydroxyanthranilic acid. Quinolinic acid is formed from 3‐hydroxyanthranilic acid by 3‐hydroxyanthranilic acid oxygenase. Cinnabarinic acid is an endogenous oxidation product of 3OH‐anthranilic acid. Blue square indicates ‘kynurenic acid branch’ which is the major kynurenine‐pathway of astrocytes. In physiological conditions, astrocytes can synthesize mainly kynurenic acid but not 3OH‐kynurenine, since astrocytes contain KAT but not kynurenine 3‐monooxygenase (Guillemin et al., 2001). Red square indicates ‘quinolinic acid branch’, which is dormant during the physiological stage (Guillemin et al., 2001). Green square indicates the kynurenine‐pathway metabolites, which can be transported across the blood–brain barrier (Fukui et al., 1991).
Based on previous demonstrations, the mechanisms behind the anti‐absence effect but lack of anti‐convulsive action of LEV in animal models are considered to involve the regulation of IFNγ and astroglial release of kynurenine‐pathway metabolites (especially the inhibition of cinnabarinic acid); however, the effects of LEV on either astroglial release of kynurenine‐pathway metabolites or IFNγ‐induced astroglial release have not been clarified. Therefore, to clarify the mechanisms of the anti‐absence effect with a lack of anti‐convulsive action of LEV, the present study determined the effects of LEV on the astroglial release of kynurenine‐pathway metabolites (kynurenine, kynurenic acid, quinolinic acid, xanthurenic acid and cinnabarinic acid) and IFNγ‐induced astroglial release of kynurenine‐pathway metabolites from cortical primary cultured astrocytes using ultra‐HPLC equipped with MS (UHPLC/MS). Furthermore, to explore the mechanism of the neuroprotective action of LEV, we determined its effects on the astroglial release of kynurenine‐pathway metabolites induced by AMPA‐ and IP3‐receptors, since the activation of AMPA‐ and IP3‐receptors is reported to enhance the release of several gliotransmitters (Hamilton and Attwell, 2010; Tanahashi et al., 2012; Yamamura et al., 2013).
Methods
All animal care and experimental procedures described in this report complied with the Ethical Guidelines established by the Institutional Animal Care and Use Committee at Mie University (No. 24–35). Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010).
Primary astrocyte culture
Astrocytes were prepared using a protocol adapted from previously described methods (Tanahashi et al., 2012; Yamamura et al., 2013; Fukuyama et al., 2014). Pregnant Sprague–Dawley rats (SLC, Shizuoka, Japan) were used, which were housed individually in cages in air‐conditioned rooms (temperature, 22 ± 2°C) under a 12 h light/dark cycle, and subsequently had ad libitum access to food and water. For the preparation of cultured astrocytes, cortical astrocyte cultures were prepared from neonatal Sprague–Dawley rats (n = 60) killed by decapitation at 0–24 h of age and subjected to the removal of cerebral hemispheres under a dissecting microscope. Tissue was chopped into fine pieces using scissors and then triturated briefly with a micropipette. The suspension was filtered using 70 μm nylon mesh (BD, Franklin Lakes, NJ) and centrifuged. Pellets were re‐suspended in 10 mL of DMEM containing 10% FCS (fDMEM) (repeated three times). After a 14 day culture (DIV14), contaminating cells were removed by shaking in a standard incubator for 16 h at 200 rpm. On DIV21, astrocytes were removed from flasks by trypsinization and seeded onto a translucent PET membrane (1.0 μm) with 12‐ or 24‐well plates (BD) directly at a density of 105 cells cm−2 for use in experiments (Tanahashi et al., 2012; Yamamura et al., 2013; Fukuyama et al., 2014).
During DIV21–DIV28, astrocytes in each well were incubated in culture medium containing various target agents [LEV, IFNγ, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5799 or 3‐hydroxykynurenine (3OH‐kynurenine)], which was changed twice a week for chronic administration (for 7 days) (detailed methods are described under ‘Treatment of astrocytes and study design’ section). The target agent in the culture medium was randomly set so as not to be exposed to the same drug within the same individual. There were 12 rats included in each of Studies 1–6. On DIV28, cultured astrocytes were washed out using artificial CSF (repeated three times) (ACSF: 130 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2 and 5.5 mM glucose, and buffered with 20 mM HEPES buffer to pH 7.3) (washout). The remaining adherent cells included 95% glial fibrillary acidic protein (GFAP) positive and A2B5‐negative cells, as detected using immunohistochemical staining (Tateishi et al., 2006).
After the washout, astrocytes were incubated in ACSF (100 μL per translucent PET membrane) at 35°C for 60 min in a CO2 incubator (pretreatment incubation). After this pretreatment incubation, astrocytes were incubated in ACSF containing agents (60 min) for acute administration, and ACSF was collected for analysis. Each 100 μL ACSF sample was filtered by Vivaspin 500‐3 K (Sartorius, Goettingen, Germany). Filtered samples were freeze‐dried and stored at −80°C until analyses. The freeze‐dried samples were treated with 50 μL of acetonitrile containing 5% ammonium (v·v−1) for UHPLC/MS analysis.
Treatment of astrocytes and study design
Each study was designed to ensure administration, including acute and chronic administrations, of different agents to astrocyte cultures prepared from the same rat.
Study 1: Concentration‐dependent effects of chronic IFNγ administration on astroglial release of kynurenine‐pathway metabolites
Astrocyte cultures were incubated in fDMEM containing IFNγ (0, 10, 30 and 100 U·mL−1) for 7 days (from DIV21 to DIV28). On DIV28, after washout and pretreatment incubation, astrocytes were incubated in ACSF for 60 min and ACSF was collected for later analysis.
Study 2: Effects of chronic IFNγ administration on kynurenine aminotransferase activity
To study the effects of chronic IFNγ administration on kynurenine aminotransferase (KAT) index, the KAT index was calculated as the ratio of kynurenic acid to kynurenine (Kocki et al., 2006; Myint et al., 2007) using data from Study 1.
To study the effects of the chronic administration of IFNγ on KAT activity, astrocyte cultures were incubated in fDMEM containing IFNγ (0, 10, 30 or 100 U·mL−1) plus kynurenine (1 μM) (Kocki et al., 2006) on a translucent PET membrane from DIV21 to DIV28. On DIV28, after washout and pretreatment incubation, astrocytes were incubated in ACSF for 60 min and ACSF was collected for analysis.
Study 3: Effects of chronic IFNγ administration on astroglial release of kynurenine‐pathway metabolites under the chronic application of 3OH‐kynurenine
Astrocyte cultures were incubated in fDMEM containing IFNγ (0, 10, 30 and 100 U·mL−1) plus 3OH‐kynurenine (1 μM) from DIV21 to DIV28. On DIV28, after washout and pretreatment incubation, astrocytes were incubated in ACSF for 60 min and ACSF was collected for analysis.
Study 4: Interaction between chronic administrations of IFNγ and LEV on astroglial release of kynurenine‐pathway metabolites
Astrocyte cultures were incubated in fDMEM containing LEV (0, 10, 30 and 100 μM), IFNγ (0 and 100 U·mL−1) without (for kynurenic acid) or with (for xanthurenic acid, cinnabarinic acid and quinolinic acid) 1 μM 3OH‐kynurenine from DIV21 to DIV28. On DIV28, after washout and pretreatment incubation, astrocytes were incubated in ACSF for 60 min and ACSF was collected for analysis.
Study 5: Time‐dependent effects of acute LEV administration on AMPA‐ and AdA‐induced astroglial release of kynurenine‐pathway metabolites
After incubation in fDMEM without (for kynurenic acid) or with (for xanthurenic acid, cinnabarinic acid and quinolinic acid) 1 μM 3OH‐kynurenine, on DIV28, astrocyte cultures were washed out by ACSF (repeated three times). To obtain control data, after the pretreatment incubation for 60 min (repeated twice), astrocytes were incubated in ACSF containing http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4131 (100 μM) or http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4124 (AdA; 1 μM) for 60 min. To study the effects of acute LEV (100 μM) administration (for 1 h) on AMPA‐ and AdA‐induced release of kynurenine‐pathway metabolites, after pretreatment incubation for 60 min, astrocytes were incubated in ACSF containing LEV for 60 min. After pre‐incubation of LEV (1 h), astrocytes were incubated in ACSF containing LEV plus AMPA or AdA for 60 min. To study the effects of acute LEV (100 μM) administration (for 2 h) on AMPA‐ and AdA‐induced release of kynurenine‐pathway metabolites, after washout, astrocytes were incubated in ACSF containing LEV for 1 h (repeated twice). After pre‐incubation of LEV (2 h), astrocytes were incubated in ACSF containing LEV plus AMPA or AdA for 60 min.
Study 6: Interaction between chronic administrations of LEV and IFNγ on AMPA‐ and AdA‐induced astroglial release of kynurenine‐pathway metabolites
To study the interaction between chronic administrations of LEV and IFNγ on AMPA‐ and AdA‐induced astroglial release of kynurenic acid, astrocyte cultures were incubated in fDMEM without (control) or with IFNγ (100 U·mL−1), LEV (100 μM) or IFNγ (100 U·mL−1) plus LEV (100 μM) from DIV21 to DIV28. To study the interaction between chronic administrations of LEV and IFNγ on AMPA‐ and AdA‐induced astroglial release of xanthurenic acid, cinnabarinic acid and quinolinic acid, astrocytes were incubated in fDMEM containing 1 μM 3OH‐kynurenine without (control) or with IFNγ (100 U·mL−1), LEV (100 μM) or IFNγ (100 U·mL−1) plus LEV (100 μM) from DIV21 to DIV28. On DIV28, after washout and pretreatment incubation, astrocytes were incubated in ACSF containing AMPA (100 μM) or AdA (1 μM) for 60 min.
Determination of levels of kynurenine‐pathway metabolites
Levels of kynurenine, kynurenic acid, xanthurenic acid, cinnabarinic acid and quinolinic acid were determined by UHPLC (PU‐4185; Jasco, Tokyo, Japan) with MS (Acquity SQ detector; Waters, Milford, MA). Twenty microlitres of filtrated samples were injected using an auto‐sampler (AS‐4150; Jasco). The concentrations of kynurenine‐pathway metabolites were determined by UHPLC equipped with a Hypercarb column (particle 3 μm, 150 × 2.1 mm; Thermo, Waltham, MA) at 35°C, and the mobile phase was set at 500 μL·min−1. A linear gradient elution programme was performed over 10 min with mobile phases A (5 mM ammonium acetate buffer, pH 11) and B (acetonitrile). Nitrogen flows of desolvation and cone were set at 800 and 5 L·h−1, respectively, and desolvation temperature was set at 450°C. Cone voltages for the determination of kynurenine (m/z = 209.1), kynurenic acid (m/z = 190.2), xanthurenic acid (m/z = 207.1), cinnabarinic acid (m/z = 301.3) and quinolinic acid (m/z = 167.2) were 20, 25, 25, 40 and −10 V respectively. Where possible, we sought to randomize and blind sample data. In particular, for determination of the extracellular levels of kynurenine‐pathway metabolites, each sample was set on the auto‐sampler according to a table of random numbers.
Data analysis
All experiments were designed with equal sizes (n = 12) per group based on our previous studies (Tanahashi et al., 2012; Yamamura et al., 2013; Fukuyama et al., 2014). Values were expressed as mean ± SD. A P value less than 0.05 (P < 0.05) was considered statistically significant. Data were confirmed to be normally distributed using the Kolmogorov–Smirnov test (BellCurve for Excel; Social Survey Research Information Co., Ltd., Tokyo, Japan). There was no significant variance inhomogeneity as determined by Bartlett's test and Levene's test; the data were statistically analysed using one‐way or two‐way ANOVA respectively. When the F‐value was significant (P < 0.05), data were subsequently analysed by Tukey's post hoc test (BellCurve for Excel). The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2018).
Materials
LEV and AMPA (AMPA‐receptor agonist) were obtained from Wako Chemicals (Osaka, Japan). 3‐Hydroxykynurenine (3OH‐kynurenine) and adenophostin A (AdA) (IP3 receptor agonist) were purchased from Sigma‐Aldrich (St. Louis, MO). Rat recombinant IFNγ was purchased from Biolegend (San Diego, CA).
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017a,b,c,d,e).
Results
Concentration‐dependent effects of chronic IFNγ administration on astroglial release of kynurenine‐pathway metabolites (Study 1)
The levels of tryptophan and kynurenine in culture medium (fDMEM) were 73.9 ± 13.8 μM and 7.3 ± 0.8 nM respectively; however, the levels of other kynurenine‐pathway metabolites, kynurenic acid, 3OH‐kynurenine, xanthurenic acid, cinnabarinic acid and quinolinic acid, in fDMEM were not detectable.
After incubation in fDMEM with no agents (non‐treatment conditions), the astroglial release of kynurenine and kynurenic acid in ACSF was 60.7 ± 11.7 nM (n = 24) and 5.3 ± 1.0 nM, respectively, but that of xanthurenic acid, cinnabarinic acid and quinolinic acid was not detectable (Fukuyama et al., 2014). After incubation in fDMEM containing IFNγ (0, 10, 30 and 100 U·mL−1), the astroglial release of xanthurenic acid, cinnabarinic acid and quinolinic acid remained undetectable; however, the astroglial release of kynurenine and kynurenic acid was increased in an IFNγ concentration‐dependent manner (Figure 2).
Figure 2.
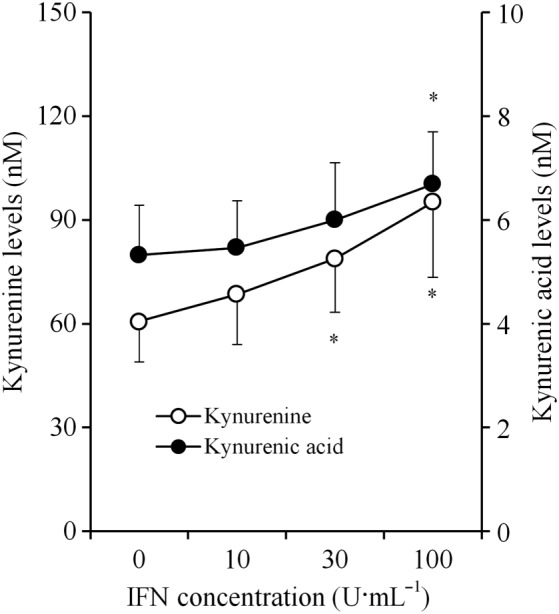
Concentration‐dependent effects of chronic IFNγ administration on astroglial release of kynurenine‐pathway metabolites. Concentration‐dependent effects of chronic IFNγ administration on astroglial release of kynurenine and kynurenic acid, after incubation in culture medium containing IFNγ (for 7 days), are indicated in the figure. Concentration‐dependent effects of chronic IFNγ administration on astroglial releases of kynurenine and kynurenic acid and KAT activity were analysed using one‐way ANOVA followed by Tukey's post hoc test. *P < 0.05 versus control (free IFNγ) (n = 12). In this study (Study 1), astrocyte cultures prepared from the same rat were administered different agents.
Effects of chronic IFNγ administration on kynurenine aminotransferase activity (Study 2)
The KAT activity index (ratio of kynurenic acid to kynurenine) (Kocki et al., 2006; Myint et al., 2007) was concentration‐dependently reduced by chronic IFNγ administration (Figure 3A). Similar to the KAT activity index, upon the chronic administration of IFNγ after incubation in fDMEM containing IFNγ with 1 μM kynurenine for 7 days (from DIV21 to DIV28), IFNγ concentration‐dependently reduced the astroglial release of kynurenic acid (Figure 3B).
Figure 3.
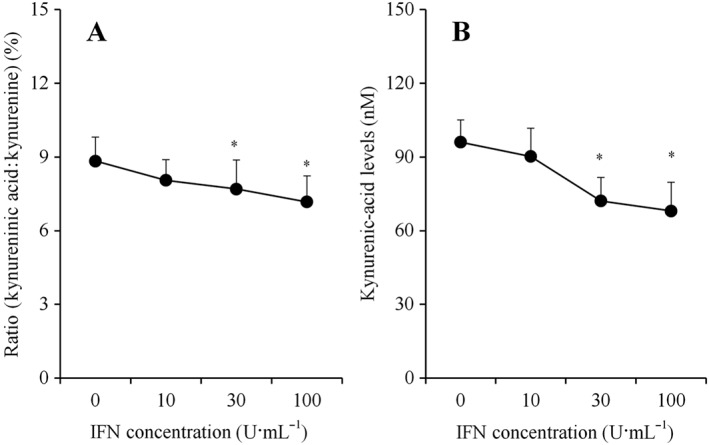
Effects of chronic IFNγ administration on KAT activity. Concentration‐dependent effects of chronic IFNγ administration (for 7 days) on KAT activity index is indicated in (A). KAT activity index was calculated as the ratio of kynurenic acid:kynurenine from results in Study 1. After incubation in culture medium containing 1 μM kynurenine plus IFNγ for 7 days, concentration‐dependent effect of chronic IFNγ administration (for 7 days) on astroglial kynurenic acid release is indicated in (B). Concentration‐dependent effects of chronic IFNγ administration on KAT activity index and astroglial kynurenic acid release were analysed using one‐way ANOVA followed by Tukey's post hoc test. *P < 0.05 versus control (free IFNγ) (N = 12). In this study (Study 2), astrocyte cultures prepared from the same rat were administered different agents.
Effects of chronic IFNγ administration on astroglial release of kynurenine‐pathway metabolites under the chronic application of 3OH‐kynurenine (Study 3)
After incubation in fDMEM containing IFNγ (0, 10, 30 and 100 U·mL−1) without 3OH‐kynurenine for 7 days, the astroglial release of xanthurenic acid, cinnabarinic acid and quinolinic acid was not detectable. After incubation in fDMEM containing 1 μM 3OH‐kynurenine for 7 days, the astroglial release of xanthurenic acid, cinnabarinic acid and quinolinic acid became detectable (Figure 4) without affecting that of kynurenine or kynurenic acid (data not shown) (Fukuyama et al., 2014). Chronic IFNγ administration increased the astroglial release of cinnabarinic acid and quinolinic acid but decreased that of xanthurenic acid (Figure 4).
Figure 4.
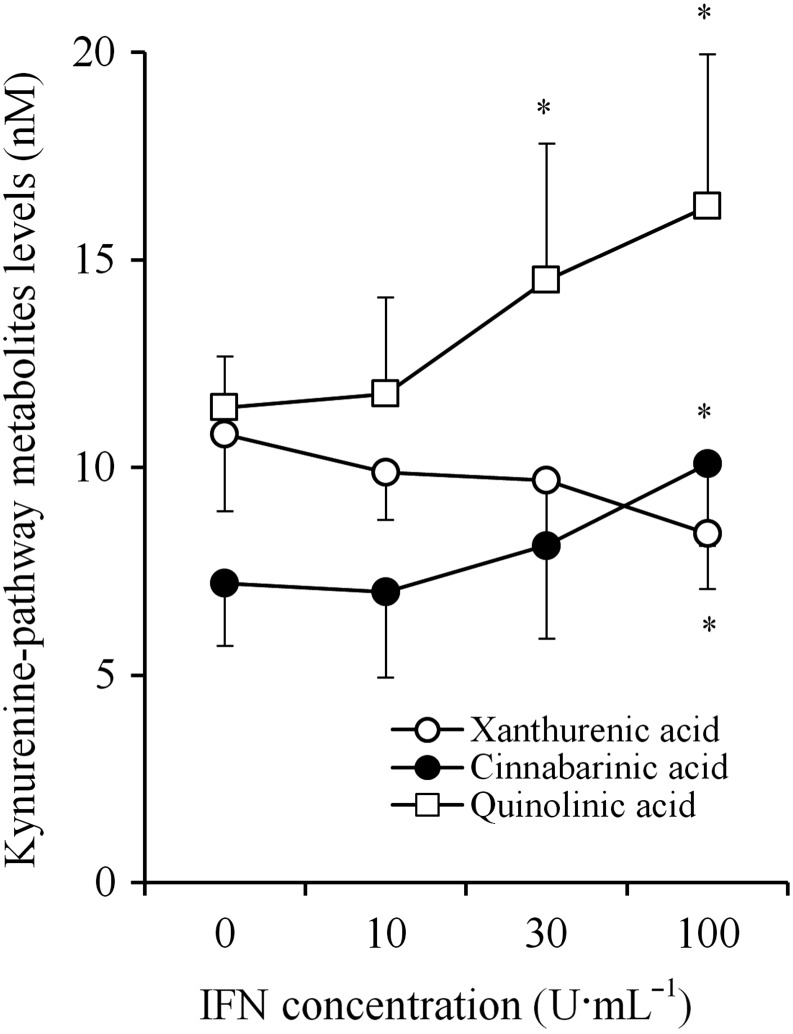
Concentration‐dependent effects of chronic IFNγ administration on astroglial release of kynurenine‐pathway metabolites in quinolinic acid branch under the chronic application of 3OH‐kynurenine. Concentration‐dependent effects of chronic IFNγ administration (for 7 days) on astroglial release of xanthurenic acid, cinnabarinic acid and quinolinic acid, after incubation in culture medium containing 1 μM 3OH‐kynurenine plus IFNγ for 7 days, are indicated. Concentration‐dependent effects of chronic IFNγ administration on astroglial releases of kynurenine‐pathway metabolites were analysed using one‐way ANOVA followed by Tukey's post hoc test. *P < 0.05 versus control (free IFNγ) (n = 12). In this study (Study 3), astrocyte cultures prepared from the same rat were administered different agents.
Interaction between chronic administrations of IFNγ and LEV on astroglial release of kynurenine‐pathway metabolites (Study 4)
LEV (0, 10, 30 and 100 μM) concentration‐dependently increased the astroglial release of kynurenic acid (Figure 5B) without affecting that of kynurenine (Figure 5A). IFNγ (100 U·mL−1) increased the astroglial release of both kynurenine and kynurenic acid (Figure 5A, B). Interestingly, IFNγ enhanced the concentration‐dependent stimulatory effects of LEV on kynurenic acid release (Figure 5B).
Figure 5.
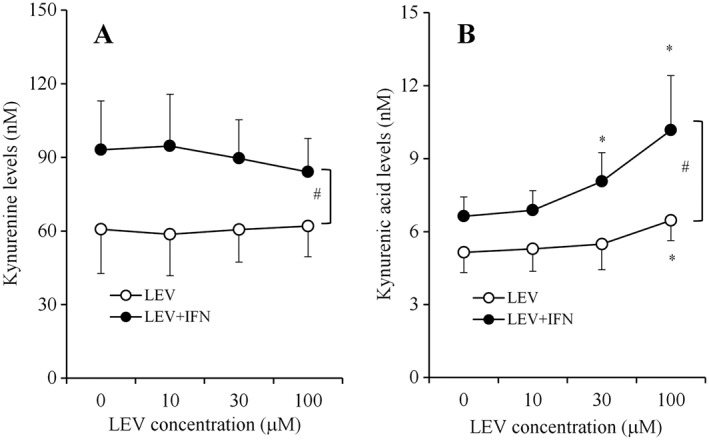
Interaction between chronic administration of IFNγ and LEV on astroglial release of kynurenine‐pathway metabolites. After incubation in culture medium containing LEV (10, 30 and 100 μM) with or without 100 U·mL−1 IFNγ for 7 days; concentration‐dependent effects of LEV (0, 10, 30 and 100 μM) on astroglial release of kynurenine and kynurenic acid are indicated in (A) and (B) respectively. Concentration‐dependent effects of LEV on astroglial releases of kynurenine‐pathway metabolites were analysed using two‐way ANOVA followed by Tukey's post hoc test. *P < 0.05 versus control (free LEV), #P < 0.05 versus LEV + IFNγ (n = 12). In this study (Study 4), different agents were administered to astrocyte cultures prepared from the same rat.
After incubation in fDMEM containing 1 μM 3OH‐kynurenine, LEV (0, 10, 30 and 100 μM) and IFNγ (100 U·mL−1) for 7 days, interactions between LEV and IFNγ regarding the astroglial release of xanthurenic acid, cinnabarinic acid and quinolinic acid were detected (Figure 6A–C). LEV concentration‐dependently increased the astroglial release of xanthurenic acid release (Figure 6A) but did not affect that of cinnabarinic acid or quinolinic acid (Figure 6B, C). In contrast to LEV, IFNγ (100 U·mL−1) decreased the astroglial release of xanthurenic acid (Figure 6A) but increased that of cinnabarinic acid and quinolinic acid (Figure 6B, C). Interestingly, under the IFNγ‐free conditions, 100 μM LEV did not affect the astroglial release of cinnabarinic acid or quinolinic acid; however, upon incubation in IFNγ with LEV, LEV concentration‐dependently reduced the astroglial release of cinnabarinic acid and quinolinic acid (Figure 6B, C). The concentration‐dependent stimulatory effect of LEV on xanthurenic acid release was enhanced by IFNγ (Figure 6A).
Figure 6.
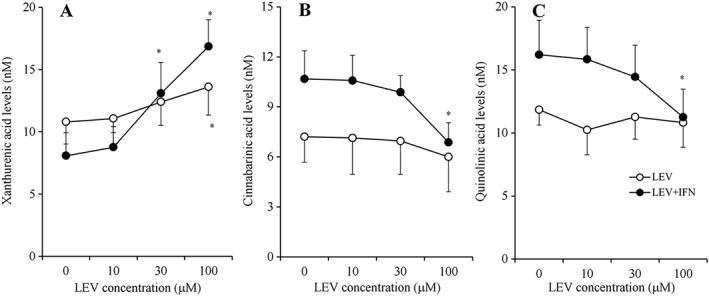
Interaction between chronic administration of IFNγ and LEV on astroglial release of kynurenine‐pathway metabolites under the application of 3OH‐kynurenine. After incubation in culture medium containing 1 μM 3OH‐kynurenine, LEV (10, 30 and 100 μM) with or without 100 U·mL−1 IFNγ for 7 days; concentration‐dependent effects of chronic administration of LEV (10, 30 and 100 μM) on astroglial release of xanthurenic acid, cinnabarinic acid and quinolinic acid are indicated in (A–C) respectively. Concentration‐dependent effects of LEV on astroglial release of kynurenine‐pathway metabolites were analysed using two‐way ANOVA followed by Tukey's post hoc test (n = 12). *P < 0.05 versus control (free LEV) (n = 12). In this study (Study 4), different agents were administered to astrocyte cultures prepared from the same rat.
Time‐dependent effects of acute LEV administration on AMPA‐ and AdA‐induced astroglial release of kynurenine‐pathway metabolites (Study 5)
The acute administration of both 100 μM AMPA (AMPA receptor agonist) and 1 μM AdA (IP3 receptor agonist) for 60 min increased the astroglial release of kynurenic acid, xanthurenic acid, cinnabarinic acid and quinolinic acid (control) (Figure 7A–D). AMPA‐ and AdA‐induced release was calculated by subtraction of the basal release level (open column: astroglial release before administration of 100 μM AMPA or 1 μM AdA) from the increased astroglial release following the administration of 100 μM AMPA and 1 μM AdA respectively.
Figure 7.
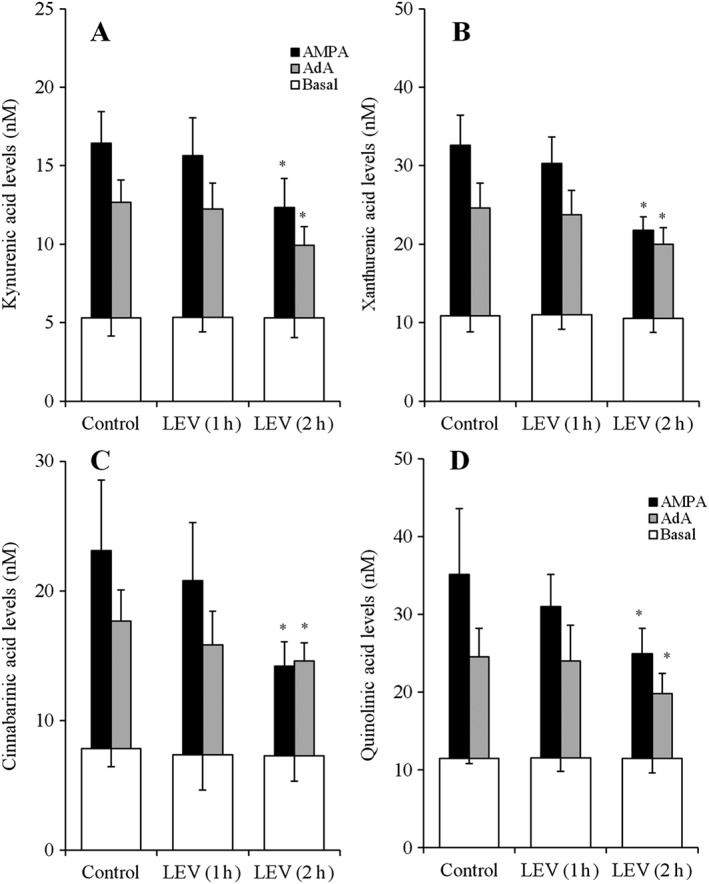
Time‐dependent effects of acute LEV administration on AMPA‐ and AdA‐induced astroglial release of kynurenine‐pathway metabolites. Time‐dependent effects of acute 100 μM LEV administration on 100 μM AMPA‐ and 1 μM AdA‐induced astroglial release of kynurenic acid, xanthurenic acid, cinnabarinic acid and quinolinic acid are indicated in (A–D) respectively. AMPA‐ and AdA‐evoked release of kynurenine‐pathway metabolites were calculated by subtraction of basal release from the astroglial release after AMPA‐ and AdA‐evoked stimulation (for 60 min). *P < 0.05 versus control by two‐way ANOVA followed by Tukey's post hoc test (n = 12). In this study (Study 5), different agents were administered to astrocyte cultures prepared from the same rat.
Acute LEV (100 μM) did not affect the astroglial release of kynurenine‐pathway metabolites (Figure 7A–D). Acute LEV administration time‐dependently inhibited the AMPA‐induced release of kynurenic acid (Figure 7A), xanthurenic acid (Figure 7B), cinnabarinic acid (Figure 7C) and quinolinic acid (Figure 7D). Acute LEV administration also time‐dependently inhibited the AdA‐induced release of kynurenic acid (Figure 7A), xanthurenic acid (Figure 7B), cinnabarinic acid (Figure 7C) and quinolinic acid (Figure 7D). In particular, 1 h of LEV administration did not affect AMPA‐ or AdA‐induced release of kynurenine‐pathway metabolites; however, 2 h of LEV administration inhibited both AMPA‐ and AdA‐induced release of kynurenine‐pathway metabolites (Figure 7A–D).
Interaction between chronic administrations of LEV and IFNγ on AMPA‐ and AdA‐induced astroglial release of kynurenic acid (Study 6)
After incubation in fDMEM containing IFNγ (100 U·mL−1), LEV (100 μM) or IFNγ (100 U·mL−1) plus LEV (100 μM) for 7 days, significant interactions between LEV and IFNγ on the AMPA‐ and AdA‐induced astroglial release of kynurenic acid, xanthurenic acid, cinnabarinic acid and quinolinic acid were detected (Figure 8A–D).
Figure 8.
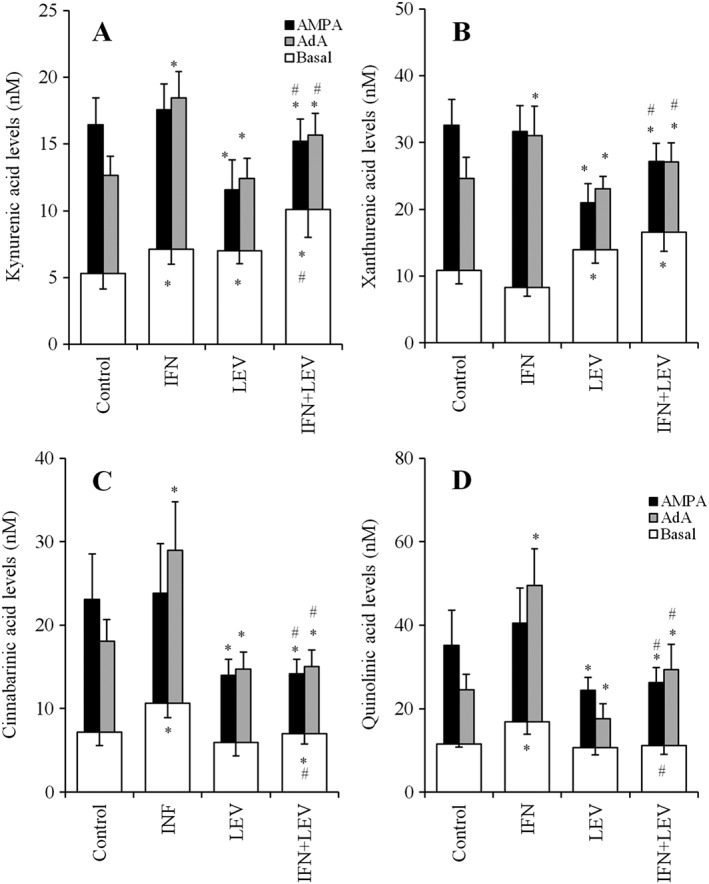
Interaction between chronic administration of LEV and IFNγ on AMPA‐ and AdA‐induced astroglial release of kynurenine‐pathway metabolites. Interaction between incubation of 100 U·mL−1 IFNγ and 100 μM LEV for 7 days on 100 μM AMPA‐ and 1 μM AdA‐induced astroglial release of kynurenic acid, xanthurenic acid, cinnabarinic acid and quinolinic acid are indicated in (A–D) respectively. AMPA‐ and AdA‐evoked release were calculated by subtraction of basal release (opened columns) from the astroglial release during AMPA‐ and AdA‐evoked stimulation. *P < 0.05 versus control, #P < 0.05 versus IFNγ by two‐way ANOVA followed by Tukey's post hoc test (n = 12). In this study (Study 6), different agents were administered to the astrocyte cultures prepared from the same rat.
IFNγ enhanced the AdA‐induced release of all kynurenine‐pathway metabolites, namely, kynurenic acid, xanthurenic acid, cinnabarinic acid and quinolinic acid, but did not affect AMPA‐induced release (Figure 8A–D). LEV inhibited both AMPA‐ and AdA‐induced release of kynurenic acid, xanthurenic acid, cinnabarinic acid and quinolinic acid (Figure 8A–D). LEV also inhibited the stimulatory effects of IFNγ on AdA‐induced release of all kynurenine‐pathway metabolites (Figure 8A–D).
Discussion
The occurrence of the kynurenine pathway in microglia of the CNS was confirmed in previous studies (Guillemin et al., 2003), although it is only present in some astrocytes due to the lack of http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2886 (Guillemin et al., 2001). Thus, the major product of the kynurenine pathway in astrocytes is considered to be kynurenic acid, but astrocytes cannot synthesize quinolinic acid (Guillemin et al., 2001). The present study also demonstrated that, under non‐treatment conditions, astroglial release of kynurenine and kynurenic acid was detectable, whereas that of xanthurenic acid, cinnabarinic acid and quinolinic acid, which are kynurenine‐pathway metabolites downstream from kynurenine 3‐monooxygenase, could not be detected. However, upon incubation in fDMEM containing 1 μM 3OH‐kynurenine, which is synthesized by kynurenine 3‐monooxygenase from kynurenine, astroglial release of xanthurenic acid, cinnabarinic acid and quinolinic acid became detectable. These results suggest that the kynurenine pathway in astrocytes comprises two sub‐branches, namely, the ‘kynurenic acid branch’ and ‘quinolinic acid branch’ (Figure 1). Under physiological conditions, the kynurenic acid branch is the major astroglial metabolic kynurenine pathway, which can mainly synthesize kynurenic acid but not 3OH‐kynurenine (Guillemin et al., 2001). The other quinolinic acid branch, which can synthesize the downstream kynurenine‐pathway metabolites from 3OH‐kynurenine, is dormant under physiological conditions (Guillemin et al., 2001). In other words, if astrocytes are supplied with 3OH‐kynurenine from neighbouring microglia or is transported across the blood–brain barrier from the circulation (Fukui et al., 1991), they can synthesize kynurenine‐pathway metabolites in the quinolinic acid branch, including xanthurenic acid, cinnabarinic acid and quinolinic acid (Guillemin et al., 2001).
Effects of IFNγ on astroglial release of kynurenine‐pathway metabolites
IFNγ enhances kynurenine‐pathway turnover in astrocytes through the up‐regulated expression of http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2829 (Guillemin et al., 2007; Asp et al., 2011); however, kynurenine 3‐monooxygenase is decreased by IFNγ (Asp et al., 2011). In the present study, the chronic administration of IFNγ (for 7 days) concentration‐dependently increased the astroglial release of both kynurenine and kynurenic acid; however, the KAT activity index (Kocki et al., 2006; Myint et al., 2007) and extracellular xanthurenic acid level were reduced by IFNγ in a concentration‐dependent manner. Indeed, after incubation in fDMEM containing 1 μM kynurenine (for 7 days), the chronic administration of IFNγ decreased extracellular kynurenic acid levels. These results suggest that IFNγ probably reduces KAT activity (Asp et al., 2011) but at the same time enhances the production of kynurenine (kynurenic acid precursor) resulting in an increased kynurenic acid level.
After incubation in fDMEM containing 1 μM 3OH‐kynurenine, chronic IFNγ administration decreased the astroglial release of xanthurenic acid, which is produced by KAT from 3OH‐kynurenine. Consistent with the findings for xanthurenic acid, chronic IFNγ administration increased the astroglial release of cinnabarinic acid and quinolinic acid. Furthermore, an increase in 3OH‐kynirenine in the culture medium (higher than 1 μM) reduced the astroglial release of kynurenine and kynurenic acid (Fukuyama et al., 2014). These results suggest that, if epileptic reactive astrocytes are supplied with 3OH‐kynurenine, chronic exposure to IFNγ probably induces a reduction in anti‐convulsive metabolites (kynurenic acid and xanthurenic acid) and an enhancement of pro‐absence (cinnabarinic acid) and pro‐convulsive (quinolinic acid) metabolites.
A clinical study demonstrated that the peripheral 3OH‐kynurenine level of patients with uncontrolled epilepsy was higher than that of controlled patients (Dolina et al., 2012). Thus, epileptic reactive astrocytes possibly take 3OH‐kynurenine from the peripheral circulation by crossing the blood–brain barrier (Fukui et al., 1991). Additionally, the expression of quinolinic acid in astrocytes in patients with epilepsy has not been studied; however, quinolinic acid was detected in astrocytes in cases of Alzheimer's disease (Guillemin et al., 2005). Reactive microglia contribute to acute seizures, degeneration and aberrant neurogenesis in epileptic models. Therefore, reactive astrocytes can probably take in 3OH‐kynurenine from neighbouring microglia or the peripheral circulation. Upon 3OH‐kynurenine exposure, the activation of IFNγ in the CNS can induce a reduction in anti‐convulsive metabolites (kynurenic acid and xanthurenic acid) and enhancement of pro‐absence (cinnabarinic acid) and pro‐convulsive (quinolinic acid) metabolites. To clarify our hypothesis, we will explored the epileptogenetic microglia‐induced 3OH‐kynurenine production (Eyo et al., 2017) in the CNS of patients with epilepsy.
Chronic exposure to IFNγ increased the expression of IP3 receptors without affecting that of AMPA receptors (Sahu et al., 2007; Park et al., 2009). The present study also demonstrated that chronic IFNγ administration enhanced AdA‐induced astroglial release without affecting AMPA‐induced release. Hyperactivation of the IP3 receptor contributes to seizure maintenance and seizure‐related neuronal damage (Pal et al., 2001; Fukuyama et al., 2012). Therefore, the present results suggest that the process of epileptogenesis induced by chronic activation of IFNγ probably includes the combined increase in the release of cinnabarinic acid and quinolinic acid, decrease in the release of kynurenic acid and xanthurenic acid and activation of the IP3 receptor in astrocytes (Getts et al., 2007; Somera‐Molina et al., 2007).
Effects of LEV on astroglial release of kynurenine‐pathway metabolites
Several conventional anti‐convulsants, such as carbamazepine, phenytoin, phenobarbital, felbamate and lamotrigine, enhance the production of kynurenic acid (Kocki et al., 2004; Kocki et al., 2006). We recently demonstrated that ONO‐2506, which inhibits absence seizure of Cacna1a conditional knockdown mice but does not affect traditional convulsion models, also increased astroglial kynurenic acid release (Yamamura et al., 2013). Unfortunately, the effects of conventional anti‐convulsants on other kynurenine‐pathway metabolites have not been demonstrated. Similar to conventional anti‐convulsants, chronic LEV administration increased the astroglial release of kynurenic acid. Furthermore, chronic LEV administration increased the astroglial release of anti‐convulsive metabolites, kynurenic acid and xanthurenic acid, without affecting pro‐absence cinnabarinic acid and pro‐convulsive quinolinic acid. Surprisingly, during chronic IFNγ exposure, chronic LEV administration increased the astroglial release of kynurenic acid and xanthurenic acid but decreased that of cinnabarinic acid and quinolinic acid. Therefore, LEV appears to antagonize the pharmacological pro‐convulsive and pro‐absence actions of IFNγ. In other words, the inhibitory effects of LEV on the anti‐convulsive and pro‐absence cinnabarinic acid appear to be well correlated with the anti‐absence but lack of anti‐convulsive profile of LEV (De Smedt et al., 2007b; Privitter and Cavitt, 2007). Recent preclinical studies suggested that IFNγ‐induced negative feedback on anti‐inflammatory and repair functional processes associated with M2 microglia or A2 astrocytes probably plays an important role in the development of epileptogenesis (Sinha et al., 2008; Li et al., 2017). Therefore, the suppression of IFNγ‐induced astroglial responses by LEV also possibly contributes to the anti‐convulsive and anti‐absence actions of LEV.
In vitro experiments have also shown that LEV prevents the Ca2+ influx accompanying seizure generation, reducing the fluctuations in membrane excitability occurring during epileptic discharges (Pisani et al., 2004) via inhibition of the IP3 receptor (Nagarkatti et al., 2008). LEV also decreases kainate‐ and AMPA‐induced currents in cultured cortical neurons, as demonstrated in a whole‐cell patch clamp study (Carunchio et al., 2007). Our microdialysis study demonstrated that LEV inhibited AdA‐evoked releases of frontal monoamine, L‐glutamate and GABA (Fukuyama et al., 2012). The present study indicates that both acute and chronic administration of LEV inhibited hyperexcitable astroglial release induced by AMPA and IP3 receptor activation.
Using hippocampal slices, 30 min exposure to LEV suppressed the later field potential of a burst, whereas such suppression of the relative size of later field potential required more than 60 min of exposure to LEV (Yang et al., 2007; Yang and Rothman, 2009). In the present study, acute LEV administration inhibited both AMPA‐ and AdA‐induced astroglial release of all kynurenine‐pathway metabolites, such as kynurenic acid, xanthurenic acid, cinnabarinic acid and quinolinic acid. In particular, acute LEV‐induced suppression of AMPA‐ and AdA‐induced astroglial release of kynurenine‐pathway metabolites required exposure to LEV for longer than 2 h.
Our results provide a mechanism for the anti‐convulsive, anti‐absence and neuroprotective actions of LEV but do not explain the mechanisms behind the contradictory actions of LEV in epileptic patients and animal models of convulsion and absence epilepsy. Differences in astroglial functions have been demonstrated between humans and rodents. In the present study, chronic IFNγ exposure reduced KAT activity. IFNγ is also known to reduce KAT expression in fibroblasts (Asp et al., 2011). However, chronic IFNγ administration increased the kynurenic acid level in human astrocytes via an increase in the expression of KAT (Guillemin et al., 2007). Additionally, recent studies have demonstrated that the transplantation of human astrocytes in rodent brain enhanced synapse plasticity and learning (Han et al., 2013). Therefore, human astrocytes are an important player in the patho‐mechanisms of neuropsychiatric diseases, neurodevelopmental disorders and epilepsy. To clarify the different effects of LEV in patients with epilepsy and animal models of convulsion and epilepsy, we need to study the effects of LEV on the astroglial release of metabolites of the kynurenine pathway using cultured human astrocytes.
Conclusion
The present paper describes the extremely complex mechanisms of action of LEV on the astroglial release of kynurenine‐pathway metabolites. (i) Under physiological conditions, LEV increased the basal astroglial release of anti‐convulsive and anti‐absence metabolites, kynurenic acid and xanthurenic acid, without affecting the release of cinnabarinic acid and quinolinic acid. (ii) In contrast, under chronic exposure to IFNγ, which can modulate seizure susceptibility (Getts et al., 2007), LEV enhanced the IFNγ‐induced astroglial release of kynurenic acid and xanthurenic acid but inhibited the anti‐convulsive and pro‐absence metabolite cinnabarinic acid, and the pro‐convulsive metabolite quinolinic acid. (iii) LEV inhibited the enhanced astroglial release of all kynurenine‐pathway metabolites induced by hyperactivation of IP3 and AMPA receptors. (iv) Chronic IFNγ exposure increased the AdA‐induced release of all kynurenine‐pathway metabolites, whereas chronic LEV administration inhibited the stimulatory effects of IFNγ on IP3 receptor‐associated astroglial release. These four effects of LEV provide insights into the mechanisms behind the anti‐epileptic action of LEV. In particular, the inhibitory effects of LEV on the enhanced release of cinnabarinic acid induced by IFNγ at least partly contribute to the anti‐absence but lack of anti‐convulsive profile of LEV.
Author contributions
M.O. designed the research. K.F. and M.O. performed experiments. Both authors have given their final approval for the manuscript.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This http://onlinelibrary.wiley.com/doi/10.1111/bph.13405/abstract acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Acknowledgements
This study was supported by Japan Society for the Promotion of Science (15H04892) and Japan Agency for Medical Research and development, AMED (JP17ek0109120).
Fukuyama, K. , and Okada, M. (2018) Effects of levetiracetam on astroglial release of kynurenine‐pathway metabolites. British Journal of Pharmacology, 175: 4253–4265. 10.1111/bph.14491.
References
- Alexander SPH, Christopoulos A, Davenport AP, Kelly E, Marrion NV, Peters JA et al (2017a). The Concise Guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. Br J Pharmacol 174: S17–S129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017b). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. Br J Pharmacol 174: S272–S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion NV, Peters JA, Faccenda E, Harding SD et al (2017c). The Concise Guide to PHARMACOLOGY 2017/18: Transporters. Br J Pharmacol 174: S360–S446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Peters JA, Kelly E, Marrion NV, Faccenda E, Harding SD et al (2017d). The Concise Guide to PHARMACOLOGY 2017/18: Ligand‐gated ion channels. Br J Pharmacol 174: S130–S159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Striessnig J, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017e). The Concise Guide To PHARMACOLOGY 2017/18: Voltage‐gated ion channels. Br J Pharmacol 174: S160–S194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asp L, Johansson AS, Mann A, Owe‐Larsson B, Urbanska EM, Kocki T et al (2011). Effects of pro‐inflammatory cytokines on expression of kynurenine pathway enzymes in human dermal fibroblasts. J Inflamm 8: 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcastro V, Pierguidi L, Tambasco N (2011). Levetiracetam in brain ischemia: clinical implications in neuroprotection and prevention of post‐stroke epilepsy. Brain Dev 33: 289–293. [DOI] [PubMed] [Google Scholar]
- Berkovic SF, Knowlton RC, Leroy RF, Schiemann J, Falter U (2007). Placebo‐controlled study of levetiracetam in idiopathic generalized epilepsy. Neurology 69: 1751–1760. [DOI] [PubMed] [Google Scholar]
- Carunchio I, Pieri M, Ciotti MT, Albo F, Zona C (2007). Modulation of AMPA receptors in cultured cortical neurons induced by the antiepileptic drug levetiracetam. Epilepsia 48: 654–662. [DOI] [PubMed] [Google Scholar]
- Christensen KV, Leffers H, Watson WP, Sanchez C, Kallunki P, Egebjerg J (2010). Levetiracetam attenuates hippocampal expression of synaptic plasticity‐related immediate early and late response genes in amygdala‐kindled rats. BMC Neurosci 11: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland CS, Neale SA, Salt TE (2013). Actions of xanthurenic acid, a putative endogenous Group II metabotropic glutamate receptor agonist, on sensory transmission in the thalamus. Neuropharmacology 66: 133–142. [DOI] [PubMed] [Google Scholar]
- Curtis MJ, Alexander S, Cirino G, Docherty JR, George CH, Giembycz MA et al (2018). Experimental design and analysis and their reporting II: updated and simplified guidance for authors and peer reviewers. Br J Pharmacol 175: 987–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Smedt T, Raedt R, Vonck K, Boon P (2007a). Levetiracetam: part II, the clinical profile of a novel anticonvulsant drug. CNS Drug Rev 13: 57–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Smedt T, Raedt R, Vonck K, Boon P (2007b). Levetiracetam: the profile of a novel anticonvulsant drug‐part I: preclinical data. CNS Drug Rev 13: 43–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolina S, Margalit D, Malitsky S, Pressman E, Rabinkov A (2012). Epilepsy as a pyridoxine‐dependent condition: quantified urinary biomarkers for status evaluation and monitoring antiepileptic treatment. Med Hypotheses 79: 157–164. [DOI] [PubMed] [Google Scholar]
- Eftekhari S, Mehrabi S, Soleimani M, Hassanzadeh G, Shahrokhi A, Mostafavi H et al (2014). BDNF modifies hippocampal KCC2 and NKCC1 expression in a temporal lobe epilepsy model. Acta Neurobiol Exp (Wars) 74: 276–287. [DOI] [PubMed] [Google Scholar]
- Eyo UB, Murugan M, Wu LJ (2017). Microglia‐neuron communication in epilepsy. Glia 65: 5–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazio F, Lionetto L, Curto M, Iacovelli L, Copeland CS, Neale SA et al (2017). Cinnabarinic acid and xanthurenic acid: two kynurenine metabolites that interact with metabotropic glutamate receptors. Neuropharmacology 112 (Pt B): 365–372. [DOI] [PubMed] [Google Scholar]
- Fazio F, Lionetto L, Molinaro G, Bertrand HO, Acher F, Ngomba RT et al (2012). Cinnabarinic acid, an endogenous metabolite of the kynurenine pathway, activates type 4 metabotropic glutamate receptors. Mol Pharmacol 81: 643–656. [DOI] [PubMed] [Google Scholar]
- Folbergrova J, Haugvicova R, Mares P (2001). Attenuation of seizures induced by homocysteic acid in immature rats by metabotropic glutamate group II and group III receptor agonists. Brain Res 908: 120–129. [DOI] [PubMed] [Google Scholar]
- Folbergrova J, Haugvicova R, Mares P (2003). Seizures induced by homocysteic acid in immature rats are prevented by group III metabotropic glutamate receptoragonist (R,S)‐4‐phosphonophenylglycine. Exp Neurol 180: 46–54. [DOI] [PubMed] [Google Scholar]
- Fukui S, Schwarcz R, Rapoport SI, Takada Y, Smith QR (1991). Blood‐brain barrier transport of kynurenines: implications for brain synthesis and metabolism. J Neurochem 56: 2007–2017. [DOI] [PubMed] [Google Scholar]
- Fukuyama K, Tanahashi S, Hoshikawa M, Shinagawa R, Okada M (2014). Zonisamide regulates basal ganglia transmission via astroglial kynurenine pathway. Neuropharmacology 76 (Pt A): 137–145. [DOI] [PubMed] [Google Scholar]
- Fukuyama K, Tanahashi S, Nakagawa M, Yamamura S, Motomura E, Shiroyama T et al (2012). Levetiracetam inhibits neurotransmitter release associated with CICR. Neurosci Lett 518: 69–74. [DOI] [PubMed] [Google Scholar]
- Getts DR, Matsumoto I, Muller M, Getts MT, Radford J, Shrestha B et al (2007). Role of IFN‐gamma in an experimental murine model of West Nile virus‐induced seizures. J Neurochem 103: 1019–1030. [DOI] [PubMed] [Google Scholar]
- Guenther S, Bauer S, Hagge M, Knake S, Olmes DG, Tackenberg B et al (2014). Chronic valproate or levetiracetam treatment does not influence cytokine levels in humans. Seizure 23: 666–669. [DOI] [PubMed] [Google Scholar]
- Guillemin GJ, Brew BJ, Noonan CE, Takikawa O, Cullen KM (2005). Indoleamine 2,3 dioxygenase and quinolinic acid immunoreactivity in Alzheimer's disease hippocampus. Neuropathol Appl Neurobiol 31: 395–404. [DOI] [PubMed] [Google Scholar]
- Guillemin GJ, Cullen KM, Lim CK, Smythe GA, Garner B, Kapoor V et al (2007). Characterization of the kynurenine pathway in human neurons. J Neurosci 27: 12884–12892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemin GJ, Kerr SJ, Smythe GA, Smith DG, Kapoor V, Armati PJ et al (2001). Kynurenine pathway metabolism in human astrocytes: a paradox for neuronal protection. J Neurochem 78: 842–853. [DOI] [PubMed] [Google Scholar]
- Guillemin GJ, Smith DG, Smythe GA, Armati PJ, Brew BJ (2003). Expression of the kynurenine pathway enzymes in human microglia and macrophages. Adv Exp Med Biol 527: 105–112. [DOI] [PubMed] [Google Scholar]
- Haghikia A, Ladage K, Hinkerohe D, Vollmar P, Heupel K, Dermietzel R et al (2008). Implications of antiinflammatory properties of the anticonvulsant drug levetiracetam in astrocytes. J Neurosci Res 86: 1781–1788. [DOI] [PubMed] [Google Scholar]
- Hamilton NB, Attwell D (2010). Do astrocytes really exocytose neurotransmitters? Nat Rev Neurosci 11: 227–238. [DOI] [PubMed] [Google Scholar]
- Han X, Chen M, Wang F, Windrem M, Wang S, Shanz S et al (2013). Forebrain engraftment by human glial progenitor cells enhances synaptic plasticity and learning in adult mice. Cell Stem Cell 12: 342–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S et al (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Res 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilmas C, Pereira EF, Alkondon M, Rassoulpour A, Schwarcz R, Albuquerque EX (2001). The brain metabolite kynurenic acid inhibits alpha7 nicotinic receptor activity and increases non‐alpha7 nicotinic receptor expression: physiopathological implications. J Neurosci 21: 7463–7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski RM, Zielinska E, Dekundy A, van Luijtelaar G, Turski W (2003). Deficit of endogenous kynurenic acid in the frontal cortex of rats with a genetic form of absence epilepsy. Pol J Pharmacol 55: 741–746. [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JE, Choi HC, Song HK, Jo SM, Kim DS, Choi SY et al (2010). Levetiracetam inhibits interleukin‐1 beta inflammatory responses in the hippocampus and piriform cortex of epileptic rats. Neurosci Lett 471: 94–99. [DOI] [PubMed] [Google Scholar]
- Kocki T, Kocki J, Wielosz M, Turski WA, Urbanska EM (2004). Carbamazepine enhances brain production of kynurenic acid in vitro. Eur J Pharmacol 498: 325–326. [DOI] [PubMed] [Google Scholar]
- Kocki T, Wielosz M, Turski WA, Urbanska EM (2006). Enhancement of brain kynurenic acid production by anticonvulsants – novel mechanism of antiepileptic activity? Eur J Pharmacol 541: 147–151. [DOI] [PubMed] [Google Scholar]
- Lapin IP (1978). Stimulant and convulsive effects of kynurenines injected into brain ventricles in mice. J Neural Transm 42: 37–43. [DOI] [PubMed] [Google Scholar]
- Li T, Zhai X, Jiang J, Song X, Han W, Ma J et al (2017). Intraperitoneal injection of IL‐4/IFN‐gamma modulates the proportions of microglial phenotypes and improves epilepsy outcomes in a pilocarpine model of acquired epilepsy. Brain Res 1657: 120–129. [DOI] [PubMed] [Google Scholar]
- Liddelow SA, Barres BA (2017). Reactive astrocytes: production, function, and therapeutic potential. Immunity 46: 957–967. [DOI] [PubMed] [Google Scholar]
- Lynch BA, Lambeng N, Nocka K, Kensel‐Hammes P, Bajjalieh SM, Matagne A et al (2004). The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetam. Proc Natl Acad Sci U S A 101: 9861–9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldrich RX, Jeffrey M, Talebi A, Beart PM, Chapman AG, Meldrum BS (2001). Anti‐epileptic activity of group II metabotropic glutamate receptor agonists (−)‐2‐oxa‐4‐aminobicyclo[3.1.0]hexane‐4,6‐dicarboxylate (LY379268) and (−)‐2‐thia‐4‐aminobicyclo[3.1.0]hexane‐4,6‐dicarboxylate (LY389795). Neuropharmacology 41: 8–18. [DOI] [PubMed] [Google Scholar]
- Myint AM (2012). Kynurenines: from the perspective of major psychiatric disorders. FEBS J 279: 1375–1385. [DOI] [PubMed] [Google Scholar]
- Myint AM, Kim YK, Verkerk R, Scharpe S, Steinbusch H, Leonard B (2007). Kynurenine pathway in major depression: evidence of impaired neuroprotection. J Affect Disord 98: 143–151. [DOI] [PubMed] [Google Scholar]
- Nagarkatti N, Deshpande LS, DeLorenzo RJ (2008). Levetiracetam inhibits both ryanodine and IP3 receptor activated calcium induced calcium release in hippocampal neurons in culture. Neurosci Lett 436: 289–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngomba RT, Ferraguti F, Badura A, Citraro R, Santolini I, Battaglia G et al (2008). Positive allosteric modulation of metabotropic glutamate 4 (mGlu4) receptors enhances spontaneous and evoked absence seizures. Neuropharmacology 54: 344–354. [DOI] [PubMed] [Google Scholar]
- Pal S, Sun D, Limbrick D, Rafiq A, DeLorenzo RJ (2001). Epileptogenesis induces long‐term alterations in intracellular calcium release and sequestration mechanisms in the hippocampal neuronal culture model of epilepsy. Cell Calcium 30: 285–296. [DOI] [PubMed] [Google Scholar]
- Park KM, Yule DI, Bowers WJ (2009). Tumor necrosis factor‐alpha‐mediated regulation of the inositol 1,4,5‐trisphosphate receptor promoter. J Biol Chem 284: 27557–27566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters BW, Ramakers GM, Vossen JM, Coenen AM (1994). The WAG/Rij rat model for nonconvulsive absence epilepsy: involvement of nonNMDA receptors. Brain Res Bull 33: 709–713. [DOI] [PubMed] [Google Scholar]
- Pisani A, Bonsi P, Martella G, De Persis C, Costa C, Pisani F et al (2004). Intracellular calcium increase in epileptiform activity: modulation by levetiracetam and lamotrigine. Epilepsia 45: 719–728. [DOI] [PubMed] [Google Scholar]
- Privitter M, Cavitt J (2007). Levetiracetam In: Engel J, Jr, Pedley TA. (eds). Epilepsy: A Comprehensive Text Book, Vol. 2 Lippincott Williams & Wilkins: Philadelphia, pp. 1583–1591. [Google Scholar]
- Rogawski M (2002). Principles of antiepileptic drug action In: Mattson RH, Meldrum B, Perucca E. (eds). Antiepileptic Drugs, 5th edn. Lippincott Williams & Wilkins: Philadelphia, pp. 3–22. [Google Scholar]
- Sahu SK, Gummadi SN, Manoj N, Aradhyam GK (2007). Phospholipid scramblases: an overview. Arch Biochem Biophys 462: 103–114. [DOI] [PubMed] [Google Scholar]
- Sinha S, Patil SA, Jayalekshmy V, Satishchandra P (2008). Do cytokines have any role in epilepsy? Epilepsy Res 82: 171–176. [DOI] [PubMed] [Google Scholar]
- Somera‐Molina KC, Robin B, Somera CA, Anderson C, Stine C, Koh S et al (2007). Glial activation links early‐life seizures and long‐term neurologic dysfunction: evidence using a small molecule inhibitor of proinflammatory cytokine upregulation. Epilepsia 48: 1785–1800. [DOI] [PubMed] [Google Scholar]
- Stienen MN, Haghikia A, Dambach H, Thone J, Wiemann M, Gold R et al (2011). Anti‐inflammatory effects of the anticonvulsant drug levetiracetam on electrophysiological properties of astroglia are mediated via TGFbeta1 regulation. Br J Pharmacol 162: 491–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone TW (2001). Kynurenines in the CNS: from endogenous obscurity to therapeutic importance. Prog Neurobiol 64: 185–218. [DOI] [PubMed] [Google Scholar]
- Tanahashi S, Yamamura S, Nakagawa M, Motomura E, Okada M (2012). Clozapine, but not haloperidol, enhances glial D‐serine and L‐glutamate release in rat frontal cortex and primary cultured astrocytes. Br J Pharmacol 165: 1543–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateishi N, Shimoda T, Manako J, Katsumata S, Shinagawa R, Ohno H (2006). Relevance of astrocytic activation to reductions of astrocytic GABAA receptors. Brain Res 1089: 79–91. [DOI] [PubMed] [Google Scholar]
- Yamamura S, Hoshikawa M, Dai K, Saito H, Suzuki N, Niwa O et al (2013). ONO‐2506 inhibits spike‐wave discharges in a genetic animal model without affecting traditional convulsive tests via gliotransmission regulation. Br J Pharmacol 168: 1088–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XF, Rothman SM (2009). Levetiracetam has a time‐ and stimulation‐dependent effect on synaptic transmission. Seizure 18: 615–619. [DOI] [PubMed] [Google Scholar]
- Yang XF, Weisenfeld A, Rothman SM (2007). Prolonged exposure to levetiracetam reveals a presynaptic effect on neurotransmission. Epilepsia 48: 1861–1869. [DOI] [PubMed] [Google Scholar]


