Abstract
The P2X7 receptor has been widely studied for its ATP‐induced pro‐inflammatory effect, but in the absence of a ligand, P2X7 has a second function as a scavenger receptor, which is active in the development of the human brain. The scavenger activity of P2X7 is only evident in the absence of serum but is fully active in cerebrospinal fluid. P2X7 on the cell surface is present as a membrane complex, and an attachment to non‐muscle myosin of the cytoskeleton is required for particle engulfment. Selective antagonists of P2X7 pro‐inflammatory function have little effect on phagocytosis, but inheritance of a variant haplotype spanning the P2RX7 and P2RX4 genes has been associated with loss of P2X7‐mediated phagocytosis. Recent studies in mice suggest that the innate phagocytosis mediated by P2X7 receptors declines with ageing. Thus, defective P2X7‐mediated phagocytosis may contribute to age‐related neuro‐degenerative diseases including Alzheimer's disease, age‐related macular degeneration and primary progressive multiple sclerosis.
Abbreviations
- AD
Alzheimer's disease
- AMD
age‐related macular degeneration
- MS
multiple sclerosis
- NMMHC‐IIA
non‐muscle myosin heavy chain IIa
- PPMS
primary progressive multiple sclerosis
- RPE
retinal pigment epithelium
- YG
yellow‐green
Introduction
Innate phagocytosis is a vital function of multicellular organisms in which unwanted particles or cells (both self and non‐self) are engulfed and removed in the absence of adaptive immune mechanisms. This innate immune function can be carried out by a variety of cell types including cells of epithelial, endothelial, neuronal, lymphoid and macrophage lineages. In the brain, microglia and astrocytes express a variety of scavenger receptors specialized in the phagocytosis of different targets including pathogenic organisms, apoptotic cells and debris. While invasion by pathogenic microbes usually triggers an inflammatory response, phagocytosis of apoptotic cells has been associated with powerful anti‐inflammatory effects (Fadok et al., 1998; Cvetanovic et al., 2006). Thus, the scavenging of neurones in early neurogenesis by innate phagocytosis helps to maintain a non‐inflammatory milieu in the developing brain in early life (Kerr et al., 1972; Savill et al., 2002; Juncadella et al., 2013). Even before the morphological appearance of microglia, both neuroblasts and their precursors identified by doublecortin staining (DCX+) have been shown to clear apoptotic neighbouring cells (Lu et al., 2011). Thus, mice deficient in microglia can develop normally during embryonic neurogenesis and only develop lethal defects after birth (Ginhoux et al., 2010; Nandi et al., 2012).
Considerable interest has been focused on the role of scavenger receptors on phagocytic cells as well as defining the molecular events in the attachment and cytoskeletal rearrangement required for target engulfment. Among the scavenger receptors implicated in the phagocytosis of neurones destined for removal are milk fat globule EGF factor 8 (Hanayama et al., 2004), TAM receptor tyrosine kinases, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1837 and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1835 (Duncan et al., 2003), brain‐specific angiogenesis inhibitor 1 (http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=174 also known as ADGRB1) (Park et al., 2007) and low‐density lipoprotein receptor‐related protein (LRP‐1) (Hayashi et al., 2007). Emerging evidence, both functional and genetic, now points to an important scavenger role of an archaic purinergic receptor, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=484, within the CNS in which P2X7 was found expressed in brain macrophages, microglia, astrocytes, oligodendrocytes, new born rat and human embryonic brain neurones but not adult cortical neurones (Collo et al., 1997; Sim et al., 2004; Hamilton et al., 2009; Lovelace et al., 2015; Yamamoto et al., 2013). A recent study using wild‐type and P2X7−/− C57BL/6 mice demonstrated the expression of the P2X7 receptor on neural progenitor cells isolated from hippocampal subgranular and cerebral subventricular zones (Leeson et al., 2018). Most published studies of P2X7 receptors have focused on the pro‐inflammatory ‘pore’ function of this receptor, but the requirement for high concentrations (>100 μM, but usually 1–5 mM depending on ambient divalent cations) of extracellular http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1713 for pore opening would be rarely achieved in physiological conditions, suggesting that this receptor has an alternative non‐inflammatory function in the CNS. Only in pathological conditions, where the extracellular ATP level is elevated, as measured using in vivo bioluminescence technique, may it be high enough to trigger P2X7‐mediated pro‐inflammatory downstream effects (Di Virgilio et al., 2016; Amores‐Iniesta et al., 2017).
The P2X7 receptor is best known for its pro‐inflammatory function and effects on proliferation and apoptosis
The physiological role of this ubiquitous receptor varies in cells and tissues of different origin. The most characteristic downstream effect is the opening of a cation‐selective channel/pore permeable to ethidium or Yo‐PRO, which has been largely characterized in cells of immune or haemopoietic origin. In cells of monocytic lineage, the opening of the P2X7 channel/pore by ATP leads to K+ efflux, an effect that activates the assembly of the NALP3 inflammasome (also called http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1770) and secretion of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4973 and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4974 as well as http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4983 (Sluyter et al., 2004; Mariathasan et al., 2006; Di Virgilio, 2007; Pizzirani et al., 2007; Qu et al., 2007). Another important effect seen in many types of cells is blebbing and microvesiculation of the cell surface membrane within seconds to minutes following P2X7 activation (MacKenzie et al., 2001; Verhoef et al., 2003). Yet another downstream effect of P2X7 activation is the shedding of cell surface L‐selectin (CD62L) and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2935 from immune cells resulting from the stimulated proteolytic activity of http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1658 and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1662 (Gu et al., 1998; Pupovac and Sluyter, 2016). Other pro‐inflammatory effects include rapid secretion of http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1633 from monocytes (Gu and Wiley, 2006), activation of the MAP kinase pathway (Panenka et al., 2001) and activation of the key transcriptional factor NF‐κB complex (Ferrari et al., 1997; Korcok et al., 2004).
Another function of the P2X7 receptor is to increase the proliferation rate of lymphoid cells, although this effect requires the absence of serum or serum growth factors (Baricordi et al., 1999; Di Virgilio et al., 2009). In a serum‐free condition, increased proliferation of embryonic murine microglia was also found to be controlled by the P2X7 receptor (Rigato et al., 2012). In the presence of serum, the trophic effects of the P2X7 receptor pore has also been reported in cultures of rat microglia (Monif et al., 2009), although the same group later found that such trophic effects may be due to IL‐1β release, which also is mediated by the P2X7 pore formation (Monif et al., 2016). Thus, it is still unclear whether the observed increased proliferation is mediated directly by the P2X7 receptor is a result of its downstream effects, for example, cytokine release or altered transcription, especially during long‐term culture.
The great majority of cellular P2X7 receptors is present in intracellular membranes (Gu et al., 2000). However, little has been published on the function of intracellular P2X7 receptor except its established role in phagosome‐lysosome fusion (Fairbairn et al., 2001; Kusner and Barton, 2001). Considering the ATP‐rich intracellular environment, it is likely that the P2X7 receptor will be shown to have other organelle‐specific functions in the future, such as mitophagy and autophagy.
In contrast to the many studies of these pro‐inflammatory or proliferative effects resulting from P2X7 receptor activation or pore formation, less is known about a possible role of P2X7 receptors in the absence of its physiological agonist, ATP.
An alternate role for the P2X7 receptor in innate phagocytosis
Transfection experiments provided the first clue that P2X7 receptor expression confers a phagocytic ability on the cell. Assays using real‐time flow cytometry showed that kidney epithelial HEK293 cells which had been transfected transiently with P2X7 receptor constructs were able to engulf 3 μm yellow‐green (YG) fluorescent latex beads. Brisk uptake of YG beads occurred over 6 min at 37°C, and bead engulfment could be blocked by either cytochalasin D or by a monoclonal antibody against the extracellular domain of P2X7 (Gu et al., 2010). The intracellular location of the beads was shown by confocal microscopy, and the results were confirmed in fresh human macrophages in which a high surface expression of P2X7 receptors is associated with the rapid engulfment of unopsonized beads (Gu et al., 2010). Both P2X7‐transfected HEK293 cells and human macrophages, which express high levels of P2X7 receptors were shown to phagocytose apoptotic lymphocytes (Figure 1) or neuronal cells under serum‐free conditions, thus further confirming that the P2X7 receptor in its non‐activated state acts like a scavenger receptor (Gu et al., 2011). Furthermore, when the synthesis and surface expression of the P2X7 receptor was blocked by siRNA, the phagocytic function of human monocytic cells was much impaired (Gu et al., 2011).
Figure 1.
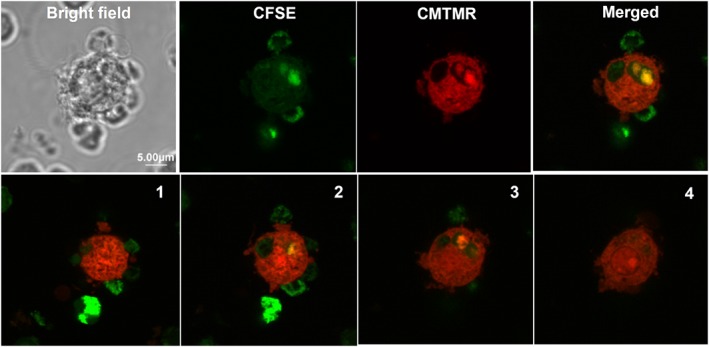
Phagocytosis of apoptotic lymphocytes by monocyte‐derived macrophages expressing P2X7 receptors. Bright field image and a series of Z‐stack confocal images depicting CMTMR‐labelled macrophage (red) which has engulfed three CFSE‐labelled lymphocytes (green, indicated with arrows) (from Gu et al., 2011, with permission).
The crystal structure of the vertebrate P2X7 receptor has been published showing clear differences between the open and closed pore state of this transmembrane molecule (Karasawa and Kawate, 2016; Allsopp et al., 2017; Karasawa and Kawate, 2017). While the open state mediates the pro‐inflammatory downstream effects, it is likely that the closed state is responsible for P2X7 scavenger activity. These structural data have identified the inhibitory binding sites separate from previously identified ATP binding sites (Karasawa and Kawate, 2016; Allsopp et al., 2017; Karasawa and Kawate, 2017). Thus, selective P2X7 antagonists may block the open pore state without affecting P2X7 scavenger activity, although oxidized ATP is an exception as it blocks both pore and scavenger function (Ou et al., 2018). Although a dozen or more polymorphisms have been shown to affect the permeability of the open pore conformation, these polymorphisms have little or no effect on the scavenger activity of the P2X7 receptor. The only exception is the Ile568Asn variant, which affects trafficking of this receptor to the cell surface and confers a reduction in scavenger function (Gu et al., 2015a).
Effect of serum on P2X7‐mediated phagocytosis
The innate phagocytic capacity of human monocytes suspended in saline media is readily observed in the absence of serum (Cline and Lehrer, 1968), but only recently was it recognized that the innate engulfment of apoptotic cells or latex beads involving the P2X7 receptor could be abolished by inclusion of as little as 1–5% serum of either autologous or heterologous origin. The inhibitory effect of serum has been shown to reside in a protein fraction containing copper and/or zinc‐containing glycoproteins, including ceruloplasmin, serum amyloid P‐component and amyloid precursor protein (Gu et al., 2012). This inhibitory effect of serum suggests that phagocytosis mediated by P2X7 receptors does not play a physiological role in any of the vascular or extracellular spaces containing significant amounts of plasma proteins. In contrast, human adult CSF which contains little or no serum glycoprotein supported a robust phagocytosis of either beads or apoptotic cells by human monocytes and macrophages (Gu et al., 2012). This observation together with the high expression of P2X7 receptors on microglia and astrocytes is consistent with an alternate role of P2X7 in the removal of apoptotic debris from the CNS. It is worth noting that serum proteins do not exert inhibitory effects on P2X7 pore function or the associated pro‐inflammatory response.
Reduction of innate phagocytosis by ATP identifies a P2X7‐mediated component
Exposure of human monocytes to ATP or periodate oxidized ATP (ox‐ATP, an irreversible antagonist of P2X7 pore formation) inhibited the subsequent engulfment of particles or beads by these cells when incubated in serum‐free media (Gu et al., 2012). The basis of this effect is explained by the dissociation of P2X7 receptors from the underlying cytoskeleton following ATP or ox‐ATP treatment (Figure 2 and see below). Thus, ATP, an agonist for P2X7 pore formation, becomes the antagonist for innate phagocytosis mediated by the same receptor. This unusual example adds to evidence that question the ‘one receptor‐one function’ concept (Mueller and Nickel, 2012). Whether the ATP‐sensitive component of phagocytosis can be used to estimate the proportion of phagocytosis mediated by P2X7 receptors or whether P2X7 works in synergy with other scavenger receptors remains uncertain.
Figure 2.
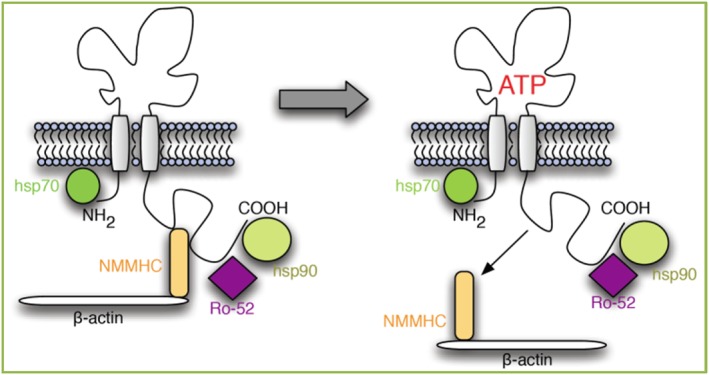
Change in the complex conformation of the P2X7 receptor upon ATP binding. In the absence of ATP (left panel), the P2X7 complex is anchored to actin cytoskeleton via non‐muscle myosin heavy chain (NMMHC) to mediate phagocytosis. ATP binding (right panel) dissociates non‐muscle myosin from the complex, leading to loss of phagocytic ability. Heat shock protein‐70 and ‐90 and Ro‐52 remain closely associated with P2X7 (from Gu et al., 2015b, with permission).
The P2X7 membrane complex and its attachment to the cytoskeleton
Engulfment of particles by a phagocyte involves membrane and cytoskeletal rearrangements, which is a highly energetic process requiring hydrolysis of ATP by non‐muscle myosin (Yildiz et al., 2003). It may be essential for a scavenger receptor to have a tight attachment to the cytoskeleton in order to engulf particles. The molecular events that underlie the inhibitory the effects of ATP on innate phagocytosis were revealed from analysis of the protein composition of the rat P2X7 membrane complex. Mass spectrometry of the P2X7 complex immunoprecipitated from P2X7‐transfected HEK293 cells showed that the P2X7 membrane complex contained actin, other cytoskeletal proteins and heat shock proteins (Kim et al., 2001; Adinolfi et al., 2003). The finding was confirmed for the human P2X7 membrane complex, with data showing that innate and transfected monocytic cells contained cytoskeletal proteins including actin and non‐muscle myosin heavy chain (NMMHC)‐IIA as well as heat shock proteins, signalling molecules and Ro‐52, an E3 ubiquitin ligase (Gu et al., 2009). Evidence for a close molecular interaction between the P2X7 receptor and NMMHC‐IIA was found using FRET (Gu et al., 2009). Following the binding of ATP, the physiological agonist of P2X7, the molecular interaction slowly disappeared consistent with a slow dissociation of non‐muscle myosin from the P2X7 complex (Figure 2). The time course of this change over tens of seconds corresponded to the slow loss of phagocytic ability over the same time frame, giving strong support to the concept that particle engulfment via P2X7 requires an attachment between the intracellular domain of P2X7 and the actin‐non‐muscle myosin cytoskeleton. Further evidence for an attachment of the P2X7 receptor to the cytoskeleton comes from the potent inhibition of P2X7‐mediated innate phagocytosis by either latrunculin A or cytochalasin D, agents that promote actin depolymerization. More support for this concept comes from the effect of S‐blebbistatin, a specific inhibitor of NMMHC II ATPase, which blocked P2X7‐mediated phagocytosis, while the inactive analogue R‐blebbistatin failed to do so (Gu et al., 2010). It is worth noting that in different types of cells, P2X7 may anchor to a different non‐muscle myosin partner, for example, in transfected epithelial HEK‐293 cells, non‐muscle myosin V was found in the P2X7 membrane complex (Gu et al., 2009).
In line with our findings, others have shown that MERTK (Mer), a TAM scavenger receptor, forms a complex with NMMHC‐IIA and participates in the daily ingestion of photoreceptor outer segment discs by retinal pigment epithelium (RPE), as RPE treated with blebbistatin or myosin II siRNA exhibited a significant phagocytic defect (Strick et al., 2009). Thus, the myosin/actin network plays a critical role in the engulfment step of phagocytosis, which under some conditions may be a rate‐limiting factor in phagocytosis. In this context, we found that increasing myosin IIA expression by transfection significantly enhanced the phagocytic rate of HEK‐293 cells in engulfing 1.0 μm YG beads (Gu et al., 2010).
A peptide motif in the P2X7 receptor contributes to target recognition
While engulfment of the target particle requires the interaction of P2X7 receptors with the cytoskeleton, the earlier recognition step requires the specific interaction of the P2X7 ectodomain with the surface of the target particle. To explore this interaction between P2X7 and either bead or apoptotic cells, our group studied a series of biotin‐labelled peptides mimicking short sequences of the extracellular domain of P2X7. The binding profile of these peptides to a range of targets including beads, bacteria and apoptotic cells was assessed with a chemiluminescence method. One peptide (residues 306–320 of P2X7) bound strongly and non‐selectively to all particles, but in contrast, three peptides (residues 115–128, 129–143 and 150–162) showed strong and selective binding only to apoptotic cells and not to beads or bacteria (Gu et al., 2011). Of note, each of these short peptides that bound to apoptotic cells contained a cysteine residue, suggesting a possible interaction involving thiol‐disulfide bond exchange. This possibility gains support from the effect of 5 mM dithiothreitol which completely abolished binding of all three peptides to the apoptotic cell surface. Substitution of alanine for cysteine in peptide 5 (residues 118–126) also abolished its binding to apoptotic cells. Other small thiol compounds have been shown to affect phagocytosis of apoptotic lymphocytes by human macrophages since pretreatment of macrophages with reduced glutathione or N‐acetyl‐L‐cysteine abolished their subsequent phagocytosis of apoptotic lymphocytes (Gu et al., 2011). All P2X receptors have 10 cysteines in their extracellular domains, forming five disulphide bridges. There is now evidence that P2X7 may have a structural and functional interaction with the http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=481 receptor based on co‐immunoprecipitation of native receptors from mouse macrophages as well as a functional interaction based on electrophysiology (Guo et al., 2007). It seems feasible that this interaction may be based on thiol‐disulphide bridge interactions between P2X7 and P2X4 receptors and this concept is supported by the observation that coexpression of the Tyr315Cys variant of the P2X4 receptor with the Gly150Arg variant of the P2X7 receptor is associated with total loss of P2X7 phagocytic function (Gu et al., 2013). These data suggest the possibility that P2X7‐P2X4 (trimer‐trimer) heteromeric receptors may have a specific role in the scavenger properties of this receptor.
Antagonists of pro‐inflammatory P2X7 pore formation
There are now a number of selective P2X7 antagonists of different chemical classes which at nanomolar concentrations inhibit pro‐inflammatory P2X7 pore formation (Bhattacharya and Biber, 2016). The effect of three P2X7 selective antagonists AF27139 (a kind gift from Lundbeck Corporation, Copenhagen), http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=9021 and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4118 on phagocytosis and pore formation was studied in fresh human monocytes from subjects of different P2RX7 genotypes (wild type or variants at residues 496 or 348). All antagonists gave complete or near complete inhibition of P2X7 pore function (Figure 3). However, none of the above three inhibitors reduced phagocytosis of YG beads by monocytes at inhibitor concentrations of 1 and 10 μM. In contrast, the earlier and less selective P2X7 inhibitor, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4235, reduced both YG bead uptake and ATP‐induced pore formation, perhaps as a results of its known inhibitory effects on Ca2+/calmodulin‐dependent kinase type II (http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1554) (Ou et al., 2018). These data show that the selective and potent P2X7 antagonists currently available are effective at blocking P2X7 pore formation but do not affect P2X7 phagocytosis in monocytes expressing wild‐type P2X7 receptors or the two common P2X7 functional variants. This divergence of features between pore formation and phagocytosis is consistent with recent data showing antagonists such as AZ10606120 bind to an allosteric site, which is separate from the ATP‐binding site that controls pore opening (Karasawa and Kawate, 2016; Allsopp et al., 2017).
Figure 3.
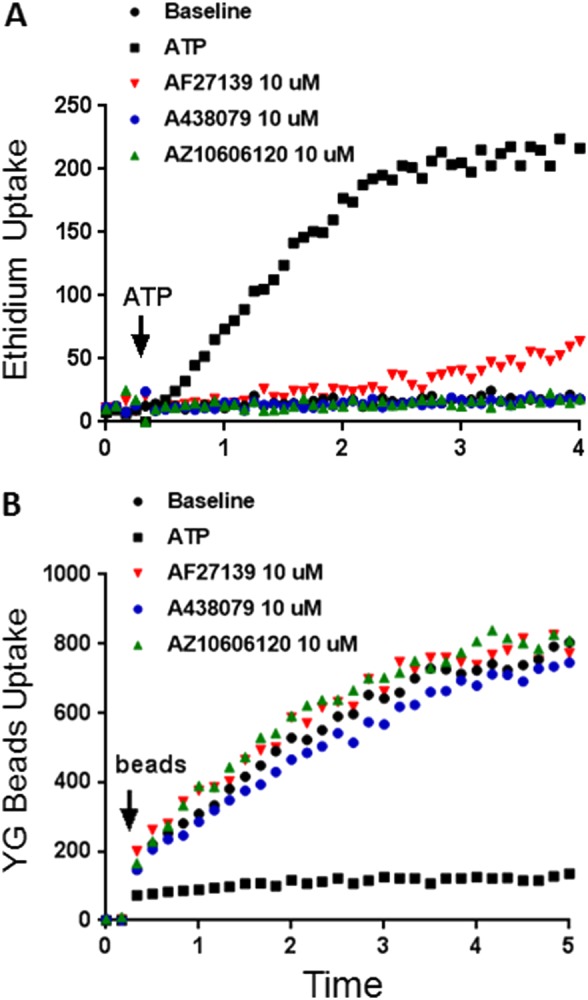
Three potent P2X7 antagonists abolish ATP‐induced ethidium uptake but not phagocytosis. (A) Pore function detected by ethidium uptake was measured in fresh human peripheral blood mononuclear cells (PBMCs), prelabelled with FITC‐CD14 antibody and incubated with AF27139, A438079, AZ10606120 and KN62 for 10 min. (B) Fresh human PBMCs prelabelled with APC‐CD14 antibody were incubated with YG fluorescent beads and bead uptake into CD14+ cells measured.
A P2X7‐P2X4 haplotype is associated with loss of P2X7‐mediated phagocytosis
Genetic studies have identified a rare polymorphic haplotype spanning the P2RX4 and P2RX7 gene loci which is over‐represented (odds ratio of 4.05) in patients with age‐related macular degeneration (AMD), a neurodegenerative disease in which impaired innate phagocytosis is a major contributor to the pathogenesis of the visual impairment. The inhibitory haplotype contained two variants, Tyr315Cys in the P2RX4 gene and Gly150Arg in the P2RX7 gene, which when expressed together in doubly transfected HEK293 cells totally abolished the phagocytosis of YG beads (Gu et al., 2013). Because the haplotype is rare in the human population, functional studies of fresh tissue have only been possible in fresh human monocytes harbouring the P2X7 Gly150Arg–P2X4 Tyr315Cys haplotype in a heterozygous dose. These showed that fresh monocytes harbouring the haplotype showed impaired phagocytosis with a 50% reduction in particle phagocytosis suggesting that this haplotype may be a marker of reduced P2X7 scavenger function in other diseases. We therefore examined large combined Australasian and European cohorts of MS cases and controls (Gu et al., 2015a) for relative frequency of the P2X7 Gly150Arg–P2X4 Tyr315Cys haplotype. In 777 patients with primary progressive multiple sclerosis (PPMS), this haplotype, which inhibits P2X7‐mediated phagocytosis (Gu et al., 2013), was associated as a risk factor for the PPMS subtype with an odds ratio of 1.82 (P = 0.039) (Table 1). This association was not found in other subtypes of MS. PPMS often affects the spinal cord (Bieniek et al., 2006); postmortem examinations have found that in the spinal cord of PPMS patients, microglia/macrophage accumulate around demyelinated lesions, which exhibited poor phagocytic activity towards myelin debris (Lieury et al., 2014).
Table 1.
Inheritance of P2X7‐P2X4 rare variant haplotype is associated with susceptibility to primary progressive MS (PPMS)
| Variant | Patients with PPMS, n = 777 | Control subjects, n = 3289 | Odds Ratio | P value |
|---|---|---|---|---|
| PSX7 315‐Cys (n) | 27 | 79 | – | – |
| P2X7 150‐Arg (n) | 34 | 119 | – | – |
| P2RX4 and P2RX7 coinheritance 315‐Cys with 150‐Arg (n) | 16 | 37 | 1.84 | 0.0389 |
| Minor haplotype frequency (5) | 1.03 | 0.56 | – | – |
| Subjects affected (% of total) | 2.06 | 1.15 | – | – |
Odds ratio (OR) and P values were calculated using PLINK.
Although multi‐incident families are uncommon in multiple sclerosis (MS), a second unique P2X7‐P2X4 haplotype containing three rare missense mutations has been shown to segregate with the disease (logarithm of odds of 3.07) in six members of a Canadian pedigree. This rare haplotype comprises Thr205Met and Asn361Ser in P2RX7 plus Gly135Ser in P2RX4. Transfection of plasmids with this haplotype in HEK293 cells gave impaired P2X7 surface expression with values only 5% of wild type, which in turn almost abolished P2X7‐mediated phagocytosis (Sadovnick et al., 2017). Although the Thr205Met mutation is extremely rare in the population, these data suggest that a major reduction in the surface expression of P2X7 receptors may lead to impairment of P2X7‐mediated innate phagocytosis in the brain. In contrast, common polymorphisms in P2X7 receptors, which cause either gain or loss of pore function have little effect on P2X7‐mediated phagocytosis, with the exception of homozygosity of the Ala348Thr gain‐of‐function variant, which modestly increases monocyte phagocytic capacity (Ou et al., 2018). Further examination of the relationship between surface expression of P2X7 receptors and phagocytic function of the cell is needed.
P2X7‐mediated phagocytosis in early human neurogenesis
The scavenger function of the P2X7 receptor is evident in early human neurogenesis, which is characterized by an overproduction of neuroblasts and neurones accompanied by much apoptosis and cellular removal by innate phagocytosis (Blaschke et al., 1996). There is strong evidence that inhibition of innate phagocytosis has adverse effects on neuronal differentiation and survival and that blocking phagocytic removal of effete cells impairs neurogenesis, such as in the ‘engulfment and cell motility 1’ (ELMO1, an intracellular engulfment protein) null mice (Lu et al., 2011). In human fetal tissue at gestational ages of 16–19 weeks, the P2X7 receptor is highly expressed in both neuroblasts and their neuronal precursor cells (Lovelace et al., 2015) with neuronal identity of the tissue shown by strong doublecortin staining (Lu et al., 2011; Walker et al., 2007). These neuroblasts of neuronal origin in primary culture were shown to phagocytose targets as diverse as beads, apoptotic ReN cells (a human neural progenitor cell line) and autologous apoptotic neuroblasts (Gu et al., 2015b; Lovelace et al., 2015). Pretreatment of the embryonic neuroblasts with ATP, ox‐ATP or siRNA against P2X7 inhibited phagocytosis following each pretreatment suggesting that P2X7 acts as a major scavenger receptor in early human neurogenesis (Lovelace et al., 2015). This important phagocytic function requires a high surface expression of P2X7 receptors and complete absence of ambient ATP both of which are conditions present during CNS development early in pregnancy. While the P2X7 receptor is clearly important, it is likely that other scavenger receptors participate in early CNS development and are able to compensate for genetic ablation of the P2RX7 gene in mouse models.
Phagocytic function of P2X7 receptor decreases with ageing
Emerging evidence has shown that defective phagocytosis could be one common pathogenic mechanism in neurological disorders, particularly in age‐related neurodegenerative diseases. Many studies have shown that phagocytic ability is decreased with ageing: a recent study on wild‐type C57BL/6J mice confirmed the reduced phagocytic function in monocyte/macrophages from aged mice (18 months) compared with young mice (4 months) (Vessey et al., 2017). Old mice showed impaired clearance of apoptotic cells by peritoneal macrophages in vivo (Aprahamian et al., 2008), while bone marrow‐derived macrophages and bone marrow monocytes retained their phagocytic function (Linehan et al., 2014). These data suggest that innate phagocytosis is impaired by ageing, but extrinsic factors such as microenvironments may influence this functional decline. Besides apoptotic cells, protein clearance in the brain is also impaired by 40% in old mice, accompanied by a decline in CSF‐interstitial fluid exchange (Kress et al., 2014), which could directly lead to an accumulation of damaged and misfolded proteins, impairing cell function and tissue homeostasis (Vilchez et al., 2014). An age‐related increase of protein degradation, for example, myelin degradation, could further burden the clearance function (Safaiyan et al., 2016) and cause an age‐related decline in remyelination (Natrajan et al., 2015). Thus, the correlation between ageing and phagocytic function clearly underpins an important role of impaired phagocytosis in age‐related neurodegenerative diseases.
Defective P2X7‐mediated phagocytosis in age‐related neurodegenerative diseases and Alzheimer's disease
Although each neurodegenerative disease has distinctive pathogenetic mechanisms, some common features support the concept that an impairment of innate phagocytosis is a key feature of AMD, Alzheimer's disease (AD) and a progressive subtype of MS. In each affected tissue (eye and brain), the extracellular environment is largely serum free; thus, clearance of apoptotic cells and debris is dependent on innate phagocytosis. Furthermore, sporadic cases are usually age‐related, suggesting an age‐related decline in innate phagocytosis, as a major contributor to disease.
Genome‐wide association studies of sporadic AD have identified a cluster of AD risk genes belonging to core innate immune pathways, including CD33, CR1, MS4A6A, MS4A4E, ABCA7 and TREM2 (Hollingworth et al., 2011; Jonsson et al., 2013). Variants of these genes, especially TREM2 and CD33, are associated with compromised phagocytic function of monocytes/macrophages and amyloid Aβ accumulation in AD brains (Bradshaw et al., 2013; Jay et al., 2015). Thus, besides excessive neuroinflammation, systemically defective phagocytosis, involving both the CNS and peripheral tissues, could be the major cause of Aβ accumulation resulting from failure of clearance in AD. Neuroinflammation often accompanies AD especially in its advanced stages, and this has been attributed to amyloid Aβ1–42 stimulation of microglia, which in turn increases IL‐Iβ secretion (Halle et al., 2008; Heneka et al., 2013). Agonists for P2X7 receptors are known to activate a pathway to NLRP3, and Sanz and others have shown that the presence of P2X7 on microglia is required for the activation of microglia by Aβ (Rampe et al., 2004; Sanz et al., 2009). Certainly, the P2X7 receptor has a role in the neuroinflammation associated with AD, and an up‐regulation of microglial P2X7 has been shown in the region of Aβ plaques in the brain in patients with AD (McLarnon et al., 2006). A recent human study has examined the phagocytic function of P2X7 receptors in AD, in peripheral blood monocyte subsets (Gu et al., 2016), a cell type that shares many functional characteristics with microglia. A significant decrease of P2X7 expression in AD patients and in subjects with higher Aβ‐amyloid burden was found as compared with healthy normal participants. However, it was noted that the P2X7‐mediated proportion of total phagocytosis in peripheral monocytes from AD was correlated with neocortical amyloid burden, suggesting some functional role of P2X7 in the progression of AD (Gu et al., 2016). A possible explanation is an alteration of cytoskeletal characteristics, which prevents the dissociation og the P2X7 receptor from the P2X7/NMMHC‐IIA complex (Gu et al., 2016). While these data implicate a major role for P2X7 receptors in the neuroinflammation associated with AD, the altered scavenger function of this receptor may also play a part (Gu et al., 2013; Gu et al., 2009; Gu et al., 2010; Gu et al., 2011; Lovelace et al., 2015; Yamamoto et al., 2013).
Age‐related macular degeneration
AMD is another well‐studied neurodegenerative disease where build‐up of debris (‘drusen’) is implicated as a major contributor to the pathogenesis. The drusen accumulate beneath the retinal pigment epithelium (RPE), between the retina and its blood supply in the choroid (Lim et al., 2012). The retina is probably the tissue with the greatest phagocytic activity in the body: RPE cells engulf and digest shed photoreceptor outer segment discs on a daily basis, with help from retinal macrophages and microglia, which are thought to have the ability to migrate towards the accumulated insoluble photo‐oxidized material found in drusen.
Compared with the phagocytic role of P2X7, the pro‐inflammatory role of this receptor has been studied more extensively in AMD. Ambati's group has shown that the P2X7 receptor plays a critical role in Alu RNA transcripts‐induced NLRP3 inflammasome activation in both mouse models of geographical atrophy (GA, also known as the ‘dry’ type of advanced AMD) and choroidal neovascularization (CNV, also known as the ‘wet’ type of late AMD) (Fowler et al., 2014; Kerur et al., 2013; Mizutani et al., 2015). Both nucleoside reverse transcriptase inhibitors and P2X7 antagonists suppressed the clinical symptoms of GA and CNV in animals (Kerur et al., 2013; Mizutani et al., 2015). P2X7‐mediated retinal cell apoptosis can also be stimulated by ligation of CD40 (Portillo et al., 2016) and amyloid β peptides (Wakx et al., 2016), for which P2X7 antagonists have shown beneficial effects. It is worth noting that although complete loss of P2X7 receptors in P2X7−/− mice protected the animals from developing GA (Carver et al., 2017; Kerur et al., 2013), it failed to protect the animals from developing laser‐induced CNV, a more severe form of late AMD (Mizutani et al., 2015). Since P2X7−/− mice not only lose the pro‐inflammatory role of P2X7 but also lack the beneficial scavenger role of this receptor, these observations suggest that the phagocytic function of P2X7 may become more critical in the advanced AMD associated with retinal haemorrhage and serum extravasation.
Multiple sclerosis
MS is an autoimmune relapsing disorder of the CNS in which both genetic and environmental susceptibility factors lead to chronic neuroinflammation, demyelination and oligodendrocyte and neuronal cell death. Recurring episodes of neuroinflammation underlie the common relapsing remitting form of disease but some 15% of MS patients from onset follow a clinical course without remission (termed PPMS). In actively demyelinating lesions (plaques), both myelin and neuroaxonal debris are engulfed and degraded by microglia/macrophages (Huizinga et al., 2012), and defective phagocytosis may be one of the mechanisms contributing to the progression of MS. A number of scavenger receptors, principally P2X7 but including CD68, CXCL16 and SR‐AI/II, were found to be up‐regulated in microglia/macrophages present around MS lesions (Hendrickx et al., 2013; Yiangou et al., 2006). A large cohort genetic study identified a polymorphism Arg307Gln, which largely abolishes the inflammatory response mediated by P2X7 receptors (Gu et al., 2004) while retaining all its scavenger and phagocytic function, and this variant conferred a 1.8‐fold protective effect on MS risk (Gu et al., 2015a).
Prospects for a P2X7‐targeted therapeutic strategy in neurodegenerative diseases
The balance of pro‐inflammatory (M1) and anti‐inflammatory (M2) phenotypes of microglia is critical to maintain homeostasis of CNS (Tang and Le, 2015; Parisi et al., 2016). These two opposite phenotypes may be associated with the two exclusive functional modes of P2X7: a pro‐inflammatory receptor following ATP activation or a scavenger receptor in a quiescent environment, although the M1/M2 concept is still controversial (Ransohoff, 2016). However, a new insight has been given to this concept by the finding that activated microglia can secrete cytokines, which induce A1 astrocytes, a phenotype that induces the death of neurons and oligodendrocytes, and loses the phagocytic capacity, which normally characterizes cells of astrocytic lineage (Liddelow et al., 2017; Yamamoto et al., 2013). Neuroinflammation is a frequent accompaniment of many neurodegenerative diseases; thus, inhibition of P2X7‐mediated pro‐inflammatory response by antagonists has been proven beneficial in a number of neurodegenerative diseases including AD (Ryu and McLarnon, 2008; Diaz‐Hernandez et al., 2012; Chen et al., 2014b), MS (Matute et al., 2007), ischaemic stroke (Arbeloa et al., 2012; Bindra et al., 2014; Chen et al., 2014a; Cisneros‐Mejorado et al., 2015; Yan et al., 2015), spinal cord injury (Peng et al., 2009; Wang et al., 2004), traumatic brain injury (Wang et al., 2015; Zhao et al., 2016), amyotrophic lateral sclerosis (ALS) (Apolloni et al., 2014; Bartlett et al., 2017; Cervetto et al., 2013; Parisi et al., 2016) and Parkinson's disease (Carmo et al., 2014; Ferrazoli et al., 2017; Kumar et al., 2017; Marcellino et al., 2010; Wang et al., 2017). These abundant animal studies clearly demonstrated a neuroprotective effect in various neurodegenerative diseases following blocking of P2X7 pore formation by its antagonists. If stimulation of the pro‐inflammatory response is the only function of P2X7, one would expect that complete ablation of the P2X7 receptor gives even better neuroprotection. However, P2X7−/− mice did not show protection against experimental autoimmune encephalomyelitis (EAE), a mouse model of MS, but rather had an exacerbated or similar disease course to their wild‐type controls (Chen and Brosnan, 2006). This discrepancy is in line with the finding that P2X7−/− mice have a fourfold reduced incidence of EAE compared to P2X7+/+ mice, despite identical immunization protocols using MOG35‐55 peptide (Sharp et al., 2008). P2X7−/− mice also failed to show neuroprotection in an MPTP (a toxin)‐induced animal model of Parkinson's disease (Hracsko et al., 2011). In the SOD1‐G93A microglia model of ALS, the clinical onset was significantly anticipated and the disease progression worsened in both male and female P2X7−/−/SOD1‐G93A mice (Apolloni et al., 2013). This in vivo work with P2X7−/− mice strongly suggests the dual function of P2X7 receptors (Apolloni et al., 2013) in the CNS. A lack of effective phagocytic function would therefore contribute to the progress of neurodegenerative diseases and may even override the beneficial effect of removal of P2X7‐mediated pro‐inflammation. Thus, ideally, blocking P2X7 pro‐inflammatory role while retaining or promoting its scavenger function should be considered as a principle when designing a P2X7‐targeted therapeutic strategy for neurodegenerative diseases.
Three different P2X7 antagonists have entered human studies with three randomized placebo‐controlled Phase 2 trials in rheumatoid arthritis and one in active Crohn's disease (Table 2). All three trials in rheumatoid arthritis showed that the study drug lacked efficacy based on objective ACR20 criteria or improvement in blood markers such as C‐reactive protein. Moreover, a randomized placebo‐controlled, double‐blind study of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7826 in active Crohn's disease reported by Eser et al. (2015) showed no decrease in C‐reactive protein, although a statistical improvement in abdominal pain and Mental Component Score was noted in the analysis (Table 2). It is noteworthy that both AZD9056 and CE224535 were well‐tolerated with no serious adverse events reported. Nevertheless, these data for two different human inflammatory conditions suggest that other pathways leading to the production of pro‐inflammatory cytokines operate in these diseases and that diseases affecting the pro‐inflammatory function of the P2X7 receptor in the brain may be more relevant to investigate. Whether P2X7 antagonists will have a role in chronic inflammatory pain is also uncertain (Bhattacharya and Biber, 2016), although there are some data that a loss of function polymorphism in the P2X7 receptor is associated with a reduced risk of developing clinically relevant pain (Sorge et al., 2012). To date, no P2X7 antagonist has been trialled in a neurodegenerative disease.
Table 2.
Randomized studies of oral antagonists targeting the human P2X7 receptor
| Antagonist to human P2X7 | Trial | Numbers randomized | Adverse events | Drug dosage | Drug efficacy | Markers of inflammation | Ref |
|---|---|---|---|---|---|---|---|
| AZD9056 | Phase 2a study in rheumatoid arthritis | n = 71 | Mild nausea, vomiting, diarrhoea | 100 and 400 mg daily for 4 weeks | Improvement in swollen, tender joint counts | No improvement | Keystone et al. (2012) |
| AZD9056 | Phase 2b study in rheumatoid arthritis | n = 385 | Nausea, vomiting, diarrhoea | 50, 100, 200 and 400 mg daily for 24 weeks | No improvement in ACR20 criteria | No Improvement | Keystone et al. (2012) |
| AZD9056 | Phase 2a study in active Crohn's disease | n = 34 | Nausea, diarrhoea | 200 mg daily for 4 weeks | Decrease in disease pain | No Improvement | Eser et al. (2015) |
| CE‐224535 | Phase 2a study in rheumatoid arthritis | n = 153 | Nausea, diarrhoea | 500 mg BID for 12 weeks | No efficacy in ACR20 criteria | No improvement | Stock et al. (2012) |
| GSK1482160 | Randomized placebo‐controlled in healthy humans | n = 24 | Headache, arrhythmia in one subject | Escalating, single doses of up to 1 g on four occasions | No progression to clinical trials | No in vivo markers measured | Ali et al. (2013) |
Our recent study showed that three P2X7 antagonists, AZ10606120, A438079 and AF27139 completely abolished P2X7 pore formation at 1–10 μM without affecting the overall phagocytic function of monocytes, which were studied as surrogates for microglia (Ou et al., 2018). Our data support the use of P2X7 antagonists for neurodegenerative diseases, in which the neuropathological features are accompanied by an inflammatory response (Heppner et al., 2015). To date, there is no therapy that specifically targets P2X7‐mediated innate phagocytosis or innate phagocytosis in general. One FDA‐approved disease‐modifying drug for MS, glatiramer acetate (trade name Copaxone®), was found to promote the phagocytic function of classical monocytes (CD14+CD16−) but not intermediate monocytes, which have already developed higher phagocytic activity (Gu et al., 2016). Glatiramer acetate is a randomly synthesized polymer of four major residues of myelin basic protein: glutamic acid, lysine, alanine and tyrosine. High concentrations (>50 μg·mL−1) are required to show its effectiveness on phagocytosis, but other short peptides may be effective at a lower dosage. Thus, considering the broad impact of defects in innate phagocytosis in age‐related neurodegenerative diseases, it is a worthwhile objective to find more potent compounds that promote phagocytosis. Such compounds can then be used together with existing P2X7 antagonists. This novel strategy of blocking P2X7‐mediated neuroinflammation, but promoting innate phagocytosis could bring progress to the treatment of neurodegenerative diseases.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017a,b,c,d).
Conflict of interest
The authors declare no conflicts of interest.
Gu, B. J. , and Wiley, J. S. (2018) P2X7 as a scavenger receptor for innate phagocytosis in the brain. British Journal of Pharmacology, 175: 4195–4208. 10.1111/bph.14470.
References
- Adinolfi E, Kim M, Young MT, Di Virgilio F, Surprenant A (2003). Tyrosine phosphorylation of HSP90 within the P2X7 receptor complex negatively regulates P2X7 receptors. J Biol Chem 278: 37344–37351. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Christopoulos A, Davenport AP, Kelly E, Marrion NV, Peters JA et al (2017a). The Concise Guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. Br J Pharmacol 174: S17–S129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017b). The Concise Guide to PHARMACOLOGY 2017/18: Catalytic receptors. Br J Pharmacol 174: S225–S271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017c). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. Br J Pharmacol 174: S272–S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Peters JA, Kelly E, Marrion NV, Faccenda E, Harding SD et al (2017d). The Concise Guide to PHARMACOLOGY 2017/18: Ligand‐gated ion channels. Br J Pharmacol 174: S130–S159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali Z, Laurijssens B, Ostenfeld T, McHugh S, Stylianou A, Scott‐Stevens P et al (2013). Pharmacokinetic and pharmacodynamic profiling of P2X7 receptor allosteric modulator GSK1482160 in healthy human subjects. Br J Clin Pharmacol 75: 197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allsopp RC, Dayl S, Schmid R, Evans RJ (2017). Unique residues in the ATP gated human P2X7 receptor define a novel allosteric binding pocket for the selective antagonist AZ10606120. Sci Rep 7: 725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amores‐Iniesta J, Barbera‐Cremades M, Martinez CM, Pons JA, Revilla‐Nuin B, Martinez‐Alarcon L et al (2017). Extracellular ATP activates the NLRP3 inflammasome and is an early danger signal of skin allograft rejection. Cell Rep 21: 3414–3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apolloni S, Amadio S, Montilli C, Volonte C, D'Ambrosi N (2013). Ablation of P2X7 receptor exacerbates gliosis and motoneuron death in the SOD1‐G93A mouse model of amyotrophic lateral sclerosis. Hum Mol Genet 22: 4102–4116. [DOI] [PubMed] [Google Scholar]
- Apolloni S, Amadio S, Parisi C, Matteucci A, Potenza RL, Armida M et al (2014). Spinal cord pathology is ameliorated by P2X7 antagonism in a SOD1‐mutant mouse model of amyotrophic lateral sclerosis. Dis Model Mech 7: 1101–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aprahamian T, Takemura Y, Goukassian D, Walsh K (2008). Ageing is associated with diminished apoptotic cell clearance in vivo . Clin Exp Immunol 152: 448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbeloa J, Perez‐Samartin A, Gottlieb M, Matute C (2012). P2X7 receptor blockade prevents ATP excitotoxicity in neurons and reduces brain damage after ischemia. Neurobiol Dis 45: 954–961. [DOI] [PubMed] [Google Scholar]
- Baricordi OR, Melchiorri L, Adinolfi E, Falzoni S, Chiozzi P, Buell G et al (1999). Increased proliferation rate of lymphoid cells transfected with the P2X(7) ATP receptor. J Biol Chem 274: 33206–33208. [DOI] [PubMed] [Google Scholar]
- Bartlett R, Sluyter V, Watson D, Sluyter R, Yerbury JJ (2017). P2X7 antagonism using Brilliant Blue G reduces body weight loss and prolongs survival in female SOD1G93A amyotrophic lateral sclerosis mice. PeerJ 5: e3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya A, Biber K (2016). The microglial ATP‐gated ion channel P2X7 as a CNS drug target. Glia 64: 1772–1787. [DOI] [PubMed] [Google Scholar]
- Bieniek M, Altmann DR, Davies GR, Ingle GT, Rashid W, Sastre‐Garriga J et al (2006). Cord atrophy separates early primary progressive and relapsing remitting multiple sclerosis. J Neurol Neurosurg Psychiatry 77: 1036–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindra CS, Jaggi AS, Singh N (2014). Role of P2X7 purinoceptors in neuroprotective mechanism of ischemic postconditioning in mice. Mol Cell Biochem 390: 161–173. [DOI] [PubMed] [Google Scholar]
- Blaschke AJ, Staley K, Chun J (1996). Widespread programmed cell death in proliferative and postmitotic regions of the fetal cerebral cortex. Development 122: 1165–1174. [DOI] [PubMed] [Google Scholar]
- Bradshaw EM, Chibnik LB, Keenan BT, Ottoboni L, Raj T, Tang A et al (2013). CD33 Alzheimer's disease locus: altered monocyte function and amyloid biology. Nat Neurosci 16: 848–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmo MR, Menezes AP, Nunes AC, Pliassova A, Rolo AP, Palmeira CM et al (2014). The P2X7 receptor antagonist Brilliant Blue G attenuates contralateral rotations in a rat model of Parkinsonism through a combined control of synaptotoxicity, neurotoxicity and gliosis. Neuropharmacology 81: 142–152. [DOI] [PubMed] [Google Scholar]
- Carver KA, Lin CM, Bowes Rickman C, Yang D (2017). Lack of the P2X7 receptor protects against AMD‐like defects and microparticle accumulation in a chronic oxidative stress‐induced mouse model of AMD. Biochem Biophys Res Commun 482: 81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervetto C, Frattaroli D, Maura G, Marcoli M (2013). Motor neuron dysfunction in a mouse model of ALS: gender‐dependent effect of P2X7 antagonism. Toxicology 311: 69–77. [DOI] [PubMed] [Google Scholar]
- Chen L, Brosnan CF (2006). Exacerbation of experimental autoimmune encephalomyelitis in P2X7R−/− mice: evidence for loss of apoptotic activity in lymphocytes. J Immunol 176: 3115–3126. [DOI] [PubMed] [Google Scholar]
- Chen S, Zhu Z, Klebe D, Bian H, Krafft PR, Tang J et al (2014a). Role of P2X purinoceptor 7 in neurogenic pulmonary edema after subarachnoid hemorrhage in rats. Plos One 9: e89042–e89042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Hu J, Jiang L, Xu S, Zheng B, Wang C et al (2014b). Brilliant Blue G improves cognition in an animal model of Alzheimer's disease and inhibits amyloid‐β‐induced loss of filopodia and dendrite spines in hippocampal neurons. Neuroscience 279: 94–101. [DOI] [PubMed] [Google Scholar]
- Cisneros‐Mejorado A, Gottlieb M, Cavaliere F, Magnus T, Koch‐Nolte F, Scemes E et al (2015). Blockade of P2X7 receptors or pannexin‐1 channels similarly attenuates postischemic damage. J Cereb Blood Flow Metab 35: 843–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline MJ, Lehrer RI (1968). Phagocytosis by human monocytes. Blood 32: 423–435. [PubMed] [Google Scholar]
- Collo G, Neidhart S, Kawashima E, Kosco‐Vilbois M, North RA, Buell G (1997). Tissue distribution of the P2X7 receptor. Neuropharmacology 36: 1277–1283. [DOI] [PubMed] [Google Scholar]
- Cvetanovic M, Mitchell JE, Patel V, Avner BS, Su Y, van der Saag PT et al (2006). Specific recognition of apoptotic cells reveals a ubiquitous and unconventional innate immunity. J Biol Chem 281: 20055–20067. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F (2007). Liaisons dangereuses: P2X7 and the inflammasome. Trends Pharmacol Sci 28: 465–472. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F, Ferrari D, Adinolfi E (2009). P2X(7): a growth‐promoting receptor‐implications for cancer. Purinergic Signal 5: 251–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio F, Pinton P, Falzoni S (2016). Assessing extracellular ATP as danger signal in vivo: the pmeLuc system. Methods Mol Biol 1417: 115–129. [DOI] [PubMed] [Google Scholar]
- Diaz‐Hernandez JI, Gomez‐Villafuertes R, Leon‐Otegui M, Hontecillas‐Prieto L, Del Puerto A, Trejo JL et al (2012). In vivo P2X7 inhibition reduces amyloid plaques in Alzheimer's disease through GSK3beta and secretases. Neurobiol Aging 33: 1816–1828. [DOI] [PubMed] [Google Scholar]
- Duncan JL, LaVail MM, Yasumura D, Matthes MT, Yang H, Trautmann N et al (2003). An RCS‐like retinal dystrophy phenotype in mer knockout mice. Invest Ophthalmol Vis Sci 44: 826–838. [DOI] [PubMed] [Google Scholar]
- Eser A, Colombel JF, Rutgeerts P, Vermeire S, Vogelsang H, Braddock M et al (2015). Safety and efficacy of an oral inhibitor of the purinergic receptor P2X7 in adult patients with moderately to severely active Crohn's disease: a randomized placebo‐controlled, double‐blind, phase IIa study. Inflamm Bowel Dis 21: 2247–2253. [DOI] [PubMed] [Google Scholar]
- Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM (1998). Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF‐beta, PGE2, and PAF. J Clin Invest 101: 890–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbairn IP, Stober CB, Kumararatne DS, Lammas DA (2001). ATP‐mediated killing of intracellular mycobacteria by macrophages is a P2X(7)‐dependent process inducing bacterial death by phagosome‐lysosome fusion. J Immunol 167: 3300–3307. [DOI] [PubMed] [Google Scholar]
- Ferrari D, Wesselborg S, Bauer MK, Schulze‐Osthoff K (1997). Extracellular ATP activates transcription factor NF‐kappaB through the P2Z purinoreceptor by selectively targeting NF‐kappaB p65. J Cell Biol 139: 1635–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrazoli EG, de Souza HD, Nascimento IC, Oliveira‐Giacomelli A, Schwindt TT, Britto LR et al (2017). Brilliant Blue G, but not fenofibrate, treatment reverts hemiparkinsonian behavior and restores dopamine levels in an animal model of Parkinson's disease. Cell Transplant 26: 669–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler BJ, Gelfand BD, Kim Y, Kerur N, Tarallo V, Hirano Y et al (2014). Nucleoside reverse transcriptase inhibitors possess intrinsic anti‐inflammatory activity. Science 346: 1000–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S et al (2010). Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330: 841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu B, Bendall LJ, Wiley JS (1998). Adenosine triphosphate‐induced shedding of CD23 and L‐selectin (CD62L) from lymphocytes is mediated by the same receptor but different metalloproteases. Blood 92: 946–951. [PubMed] [Google Scholar]
- Gu BJ, Baird PN, Vessey KA, Skarratt KK, Fletcher EL, Fuller SJ et al (2013). A rare functional haplotype of the P2RX4 and P2RX7 genes leads to loss of innate phagocytosis and confers increased risk of age‐related macular degeneration. FASEB J 27: 1479–1487. [DOI] [PubMed] [Google Scholar]
- Gu BJ, Duce JA, Valova VA, Wong B, Bush AI, Petrou S et al (2012). P2X7 receptor‐mediated scavenger activity of mononuclear phagocytes toward non‐opsonized particles and apoptotic cells is inhibited by serum glycoproteins but remains active in cerebrospinal fluid. J Biol Chem 287: 17318–17330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu BJ, Field J, Dutertre S, Ou A, Kilpatrick TJ, Lechner‐Scott J et al (2015a). A rare P2X7 variant Arg307Gln with absent pore formation function protects against neuroinflammation in multiple sclerosis. Hum Mol Genet 24: 5644–5654. [DOI] [PubMed] [Google Scholar]
- Gu BJ, Huang X, Ou A, Rembach A, Fowler C, Avula PK et al (2016). Innate phagocytosis by peripheral blood monocytes is altered in Alzheimer's disease. Acta Neuropathol 132: 377–389. [DOI] [PubMed] [Google Scholar]
- Gu BJ, Lovelace MD, Weible MW II, Allen DG, Eamagdool SS, Chan‐Ling T et al (2015b). P2X7 is an archaic scavenger receptor recognizing apoptotic neuroblasts in early human neurogenesis. Recept Clin Invest 2: e699. [Google Scholar]
- Gu BJ, Rathsam C, Stokes L, McGeachie AB, Wiley JS (2009). Extracellular ATP dissociates nonmuscle myosin from P2X(7) complex: this dissociation regulates P2X(7) pore formation. Am J Physiol Cell Physiol 297: C430–C439. [DOI] [PubMed] [Google Scholar]
- Gu BJ, Saunders BM, Jursik C, Wiley JS (2010). The P2X7‐nonmuscle myosin membrane complex regulates phagocytosis of nonopsonized particles and bacteria by a pathway attenuated by extracellular ATP. Blood 115: 1621–1631. [DOI] [PubMed] [Google Scholar]
- Gu BJ, Saunders BM, Petrou S, Wiley JS (2011). P2X(7) is a scavenger receptor for apoptotic cells in the absence of its ligand, extracellular ATP. J Immunol 187: 2365–2375. [DOI] [PubMed] [Google Scholar]
- Gu BJ, Sluyter R, Skarratt KK, Shemon AN, Dao‐Ung LP, Fuller SJ et al (2004). An Arg307 to Gln polymorphism within the ATP‐binding site causes loss of function of the human P2X7 receptor. J Biol Chem 279: 31287–31295. [DOI] [PubMed] [Google Scholar]
- Gu BJ, Wiley JS (2006). Rapid ATP‐induced release of matrix metalloproteinase 9 is mediated by the P2X7 receptor. Blood 107: 4946–4953. [DOI] [PubMed] [Google Scholar]
- Gu BJ, Zhang WY, Bendall LJ, Chessell IP, Buell GN, Wiley JS (2000). Expression of P2X7 purinoceptors on human lymphocytes and monocytes: evidence for nonfunctional P2X7 receptors. Am J Phys Cell Phys 279: C1189–C1197. [DOI] [PubMed] [Google Scholar]
- Guo C, Masin M, Qureshi OS, Murrell‐Lagnado RD (2007). Evidence for functional P2X4/P2X7 heteromeric receptors. Mol Pharmacol 72: 1447–1456. [DOI] [PubMed] [Google Scholar]
- Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T et al (2008). The NALP3 inflammasome is involved in the innate immune response to amyloid‐beta. Nat Immunol 9: 857–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton N, Vayro S, Wigley R, Butt AM (2009). Axons and astrocytes release ATP and glutamate to evoke calcium signals in NG2‐glia. Glia 58: 66–79. [DOI] [PubMed] [Google Scholar]
- Hanayama R, Tanaka M, Miyasaka K, Aozasa K, Koike M, Uchiyama Y et al (2004). Autoimmune disease and impaired uptake of apoptotic cells in MFG‐E8‐deficient mice. Science 304: 1147–1150. [DOI] [PubMed] [Google Scholar]
- Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S et al (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi H, Campenot RB, Vance DE, Vance JE (2007). Apolipoprotein E‐containing lipoproteins protect neurons from apoptosis via a signaling pathway involving low‐density lipoprotein receptor‐related protein‐1. J Neurosci 27: 1933–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickx DAE, Koning N, Schuurman KG, van Strien ME, van Eden CG, Hamann J et al (2013). Selective upregulation of scavenger receptors in and around demyelinating areas in multiple sclerosis. J Neuropathol Exp Neurol 72: 106–118. [DOI] [PubMed] [Google Scholar]
- Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira‐Saecker A et al (2013). NLRP3 is activated in Alzheimer's disease and contributes to pathology in APP/PS1 mice. Nature 493: 674–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppner FL, Ransohoff RM, Becher B (2015). Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci 16: 358–372. [DOI] [PubMed] [Google Scholar]
- Hollingworth P, Harold D, Sims R, Gerrish A, Lambert JC, Carrasquillo MM et al (2011). Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer's disease. Nat Genet 43: 429–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hracsko Z, Baranyi M, Csolle C, Goloncser F, Madarasz E, Kittel A et al (2011). Lack of neuroprotection in the absence of P2X7 receptors in toxin‐induced animal models of Parkinson's disease. Mol Neurodegener 6: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizinga R, van der Star BJ, Kipp M, Jong R, Gerritsen W, Clarner T et al (2012). Phagocytosis of neuronal debris by microglia is associated with neuronal damage in multiple sclerosis. Glia 60: 422–431. [DOI] [PubMed] [Google Scholar]
- Jay TR, Miller CM, Cheng PJ, Graham LC, Bemiller S, Broihier ML et al (2015). TREM2 deficiency eliminates TREM2+ inflammatory macrophages and ameliorates pathology in Alzheimer's disease mouse models. J Exp Med 212: 287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson T, Stefansson H, Steinberg S, Jonsdottir I, Jonsson PV, Snaedal J et al (2013). Variant of TREM2 associated with the risk of Alzheimer's disease. N Engl J Med 368: 107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juncadella IJ, Kadl A, Sharma AK, Shim YM, Hochreiter‐Hufford A, Borish L et al (2013). Apoptotic cell clearance by bronchial epithelial cells critically influences airway inflammation. Nature 493: 547–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasawa A, Kawate T (2016). Structural basis for subtype‐specific inhibition of the P2X7 receptor. Elife 5: e22153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasawa A, Kawate T (2017). Expression and purification of a mammalian P2X7 receptor from Sf9 insect cells. Bio Protoc 7: e2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JF, Wyllie AH, Currie AR (1972). Apoptosis: a basic biological phenomenon with wide‐ranging implications in tissue kinetics. Br J Cancer 26: 239–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerur N, Hirano Y, Tarallo V, Fowler BJ, Bastos‐Carvalho A, Yasuma T et al (2013). TLR‐independent and P2X7‐dependent signaling mediate Alu RNA‐induced NLRP3 inflammasome activation in geographic atrophy. Invest Ophthalmol Vis Sci 54: 7395–7401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keystone EC, Wang MM, Layton M, Hollis S, McInnes IB (2012). Clinical evaluation of the efficacy of the P2X7 purinergic receptor antagonist AZD9056 on the signs and symptoms of rheumatoid arthritis in patients with active disease despite treatment with methotrexate or sulphasalazine. Ann Rheum Dis 71: 1630–1635. [DOI] [PubMed] [Google Scholar]
- Kim M, Jiang LH, Wilson HL, North RA, Surprenant A (2001). Proteomic and functional evidence for a P2X7 receptor signalling complex. EMBO J 20: 6347–6358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korcok J, Raimundo LN, Ke HZ, Sims SM, Dixon SJ (2004). Extracellular nucleotides act through P2X7 receptors to activate NF‐kappaB in osteoclasts. J Bone Miner Res 19: 642–651. [DOI] [PubMed] [Google Scholar]
- Kress BT, Iliff JJ, Xia M, Wang M, Wei HS, Zeppenfeld D et al (2014). Impairment of paravascular clearance pathways in the aging brain. Ann Neurol 76: 845–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Mishra A, Krishnamurthy S (2017). Purinergic antagonism prevents mitochondrial dysfunction and behavioral deficits associated with dopaminergic toxicity induced by 6‐OHDA in rats. Neurochem Res 42: 3414–3430. [DOI] [PubMed] [Google Scholar]
- Kusner DJ, Barton JA (2001). ATP stimulates human macrophages to kill intracellular virulent Mycobacterium tuberculosis via calcium‐dependent phagosome‐lysosome fusion. J Immunol 167: 3308–3315. [DOI] [PubMed] [Google Scholar]
- Leeson HC, Kasherman MA, Chan‐Ling T, Lovelace MD, Brownlie JC, Toppinen KM et al (2018). P2X7 receptors regulate phagocytosis and proliferation in adult hippocampal and SVZ neural progenitor cells: implications for inflammation in neurogenesis. Stem Cells. 10.1002/stem.2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L et al (2017). Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541: 481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieury A, Chanal M, Androdias G, Reynolds R, Cavagna S, Giraudon P et al (2014). Tissue remodeling in periplaque regions of multiple sclerosis spinal cord lesions. Glia 62: 1645–1658. [DOI] [PubMed] [Google Scholar]
- Lim LS, Mitchell P, Seddon JM, Holz FG, Wong TY (2012). Age‐related macular degeneration. Lancet 379: 1728–1738. [DOI] [PubMed] [Google Scholar]
- Linehan E, Dombrowski Y, Snoddy R, Fallon PG, Kissenpfennig A, Fitzgerald DC (2014). Aging impairs peritoneal but not bone marrow‐derived macrophage phagocytosis. Aging Cell 13: 699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovelace MD, Gu BJ, Eamegdool SS, Weible MW, Wiley JS, Allen DG et al (2015). P2X7 receptors mediate innate phagocytosis by human neural precursor cells and neuroblasts. Stem Cells 33: 526–541. [DOI] [PubMed] [Google Scholar]
- Lu Z, Elliott MR, Chen Y, Walsh JT, Klibanov AL, Ravichandran KS et al (2011). Phagocytic activity of neuronal progenitors regulates adult neurogenesis. Nat Cell Biol 13: 1076–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie A, Wilson HL, Kiss‐Toth E, Dower SK, North RA, Surprenant A (2001). Rapid secretion of interleukin‐1beta by microvesicle shedding. Immunity 15: 825–835. [DOI] [PubMed] [Google Scholar]
- Marcellino D, Suarez‐Boomgaard D, Sanchez‐Reina MD, Aguirre JA, Yoshitake T, Yoshitake S et al (2010). On the role of P2X7 receptors in dopamine nerve cell degeneration in a rat model of Parkinson's disease: studies with the P2X7 receptor antagonist A‐438079. J Neural Transm 117: 681–687. [DOI] [PubMed] [Google Scholar]
- Mariathasan S, Weiss DS, Newton K, McBride J, O'Rourke K, Roose‐Girma M et al (2006). Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440: 228–232. [DOI] [PubMed] [Google Scholar]
- Matute C, Torre I, Perez‐Cerda F, Perez‐Samartin A, Alberdi E, Etxebarria E et al (2007). P2X7 receptor blockade prevents ATP excitotoxicity in oligodendrocytes and ameliorates experimental autoimmune encephalomyelitis. J Neurosci 27: 9525–9533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLarnon JG, Ryu JK, Walker DG, Choi HB (2006). Upregulated expression of purinergic P2X(7) receptor in Alzheimer disease and amyloid‐beta peptide‐treated microglia and in peptide‐injected rat hippocampus. J Neuropathol Exp Neurol 65: 1090–1097. [DOI] [PubMed] [Google Scholar]
- Mizutani T, Fowler BJ, Kim Y, Yasuma R, Krueger LA, Gelfand BD et al (2015). Nucleoside reverse transcriptase inhibitors suppress laser‐induced choroidal neovascularization in mice. Invest Ophthalmol Vis Sci 56: 7122–7129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monif M, Reid CA, Powell KL, Drummond KJ, O'Brien TJ, Williams DA (2016). Interleukin‐1beta has trophic effects in microglia and its release is mediated by P2X7R pore. J Neuroinflammation 13: 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monif M, Reid CA, Powell KL, Smart ML, Williams DA (2009). The P2X7 receptor drives microglial activation and proliferation: a trophic role for P2X7R pore. J Neurosci 29: 3781–3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller TD, Nickel J (2012). Promiscuity and specificity in BMP receptor activation. FEBS Lett 586: 1846–1859. [DOI] [PubMed] [Google Scholar]
- Nandi S, Gokhan S, Dai XM, Wei S, Enikolopov G, Lin H et al (2012). The CSF‐1 receptor ligands IL‐34 and CSF‐1 exhibit distinct developmental brain expression patterns and regulate neural progenitor cell maintenance and maturation. Dev Biol 367: 100–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natrajan MS, de la Fuente AG, Crawford AH, Linehan E, Nunez V, Johnson KR et al (2015). Retinoid X receptor activation reverses age‐related deficiencies in myelin debris phagocytosis and remyelination. Brain 138: 3581–3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou A, Gu BJ, Wiley JS (2018). The scavenger activity of the human P2X7 receptor differs from P2X7 pore function by insensitivity to antagonists, genetic variation and sodium concentration: relevance to inflammatory brain diseases. Biochim Biophys Acta 1864: 1051–1059. [DOI] [PubMed] [Google Scholar]
- Panenka W, Jijon H, Herx LM, Armstrong JN, Feighan D, Wei T et al (2001). P2X7‐like receptor activation in astrocytes increases chemokine monocyte chemoattractant protein‐1 expression via mitogen‐activated protein kinase. J Neurosci 21: 7135–7142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi C, Napoli G, Pelegrin P, Volonte C (2016). M1 and M2 functional imprinting of primary microglia: role of P2X7 activation and miR‐125b. Mediators Inflamm 2016: 2989548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D, Tosello‐Trampont AC, Elliott MR, Lu M, Haney LB, Ma Z et al (2007). BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature 450: 430–434. [DOI] [PubMed] [Google Scholar]
- Peng W, Cotrina ML, Han X, Yu H, Bekar L, Blum L et al (2009). Systemic administration of an antagonist of the ATP‐sensitive receptor P2X7 improves recovery after spinal cord injury. Proc Natl Acad Sci U S A 106: 12489–12493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzirani C, Ferrari D, Chiozzi P, Adinolfi E, Sandona D, Savaglio E et al (2007). Stimulation of P2 receptors causes release of IL‐1beta‐loaded microvesicles from human dendritic cells. Blood 109: 3856–3864. [DOI] [PubMed] [Google Scholar]
- Portillo JC, Lopez Corcino Y, Dubyak GR, Kern TS, Matsuyama S, Subauste CS (2016). Ligation of CD40 in human muller cells induces P2X7 receptor‐dependent death of retinal endothelial cells. Invest Ophthalmol Vis Sci 57: 6278–6286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pupovac A, Sluyter R (2016). Roles of Extracellular Nucleotides and P2 Receptors in Ectodomain Shedding. Cell Mol Life Sci; CMLS 73: 4159–4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Y, Franchi L, Nunez G, Dubyak GR (2007). Nonclassical IL‐1β secretion stimulated by P2X7 receptors is dependent on inflammasome activation and correlated with exosome release in murine macrophages. J Immunol 179: 1913–1925. [DOI] [PubMed] [Google Scholar]
- Rampe D, Wang L, Ringheim GE (2004). P2X7 receptor modulation of β‐amyloid‐ and LPS‐induced cytokine secretion from human macrophages and microglia. J Neuroimmunol 147: 56–61. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM (2016). A polarizing question: do M1 and M2 microglia exist? Nat Neurosci 19: 987–991. [DOI] [PubMed] [Google Scholar]
- Rigato C, Swinnen N, Buckinx R, Couillin I, Mangin JM, Rigo JM et al (2012). Microglia proliferation is controlled by P2X7 receptors in a Pannexin‐1‐independent manner during early embryonic spinal cord invasion. J Neurosci 32: 11559–11573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu JK, McLarnon JG (2008). Block of purinergic P2X(7) receptor is neuroprotective in an animal model of Alzheimer's disease. Neuroreport 19: 1715–1719. [DOI] [PubMed] [Google Scholar]
- Sadovnick AD, Gu BJ, Traboulsee AL, Bernales CQ, Encarnacion M, Yee IM et al (2017). Purinergic receptors P2RX4 and P2RX7 in familial multiple sclerosis. Hum Mutat 38: 736–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safaiyan S, Kannaiyan N, Snaidero N, Brioschi S, Biber K, Yona S et al (2016). Age‐related myelin degradation burdens the clearance function of microglia during aging. Nat Neurosci 19: 995–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz JM, Chiozzi P, Ferrari D, Colaianna M, Idzko M, Falzoni S et al (2009). Activation of microglia by amyloid β requires P2X7 receptor expression. J Immunol 182: 4378–4385. [DOI] [PubMed] [Google Scholar]
- Savill J, Dransfield I, Gregory C, Haslett C (2002). A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol 2: 965–975. [DOI] [PubMed] [Google Scholar]
- Sharp AJ, Polak PE, Simonini V, Lin SX, Richardson JC, Bongarzone ER et al (2008). P2X7 deficiency suppresses development of experimental autoimmune encephalomyelitis. J Neuroinflammation 5: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim JA, Young MT, Sung H‐Y, North RA, Surprenant A (2004). Reanalysis of P2X7 receptor expression in rodent brain. J Neurosci 24: 6307–6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluyter R, Dalitz JG, Wiley JS (2004). P2X7 receptor polymorphism impairs extracellular adenosine 5′‐triphosphate‐induced interleukin‐18 release from human monocytes. Genes Immun 5: 588–591. [DOI] [PubMed] [Google Scholar]
- Sorge RE, Trang T, Dorfman R, Smith SB, Beggs S, Ritchie J et al (2012). Genetically determined P2X7 receptor pore formation regulates variability in chronic pain sensitivity. Nat Med 18: 595–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock TC, Bloom BJ, Wei N, Ishaq S, Park W, Wang X et al (2012). Efficacy and safety of CE‐224,535, an antagonist of P2X7 receptor, in treatment of patients with rheumatoid arthritis inadequately controlled by methotrexate. J Rheumatol 39: 720–727. [DOI] [PubMed] [Google Scholar]
- Strick DJ, Feng W, Vollrath D (2009). Mertk drives myosin II redistribution during retinal pigment epithelial phagocytosis. Invest Ophthalmol Vis Sci 50: 2427–2435. [DOI] [PubMed] [Google Scholar]
- Tang Y, Le W (2015). Differential roles of M1 and M2 microglia in neurodegenerative diseases. Mol Neurobiol 53: 1181–1194. [DOI] [PubMed] [Google Scholar]
- Verhoef PA, Estacion M, Schilling W, Dubyak GR (2003). P2X7 receptor‐dependent blebbing and the activation of rho‐effector kinases, caspases, and IL‐1β release. J Immunol 170: 5728–5738. [DOI] [PubMed] [Google Scholar]
- Vessey KA, Gu BJ, Jobling AI, Phipps JA, Greferath U, Tran MX et al (2017). Loss of function of P2X7 receptor scavenger activity in aging mice: a novel model for investigating the early pathogenesis of age‐related macular degeneration. Am J Pathol 187: 1670–1685. [DOI] [PubMed] [Google Scholar]
- Vilchez D, Saez I, Dillin A (2014). The role of protein clearance mechanisms in organismal ageing and age‐related diseases. Nat Commun 5: 5659. [DOI] [PubMed] [Google Scholar]
- Wakx A, Dutot M, Massicot F, Mascarelli F, Limb GA, Rat P (2016). Amyloid beta peptide induces apoptosis through P2X7 cell death receptor in retinal cells: modulation by marine omega‐3 fatty acid DHA and EPA. Appl Biochem Biotechnol 178: 368–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker TL, Yasuda T, Adams DJ, Bartlett PF (2007). The doublecortin‐expressing population in the developing and adult brain contains multipotential precursors in addition to neuronal‐lineage cells. J Neurosci 27: 3734–3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Arcuino G, Takano T, Lin J, Peng WG, Wan P et al (2004). P2X7 receptor inhibition improves recovery after spinal cord injury. Nat Med 10: 821–827. [DOI] [PubMed] [Google Scholar]
- Wang XH, Xie X, Luo XG, Shang H, He ZY (2017). Inhibiting purinergic P2X7 receptors with the antagonist brilliant blue G is neuroprotective in an intranigral lipopolysaccharide animal model of Parkinson's disease. Mol Med Rep 15: 768–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YC, Cui Y, Cui JZ, Sun LQ, Cui CM, Zhang HA et al (2015). Neuroprotective effects of brilliant blue G on the brain following traumatic brain injury in rats. Mol Med Rep 12: 2149–2154. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Kamatsuka Y, Ohishi A, Nishida K, Nagasawa K (2013). P2X7 receptors regulate engulfing activity of non‐stimulated resting astrocytes. Biochem Biophys Res Commun 439: 90–95. [DOI] [PubMed] [Google Scholar]
- Yan Y, Bai J, Zhou X, Tang J, Jiang C, Tolbert E et al (2015). P2X7 receptor inhibition protects against ischemic acute kidney injury in mice. Am J Physiol 308: C463–C472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiangou Y, Facer P, Durrenberger P, Chessell IP, Naylor A, Bountra C et al (2006). COX‐2, CB2 and P2X7‐immunoreactivities are increased in activated microglial cells/macrophages of multiple sclerosis and amyotrophic lateral sclerosis spinal cord. BMC Neurol 6: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildiz A, Forkey JN, McKinney SA, Ha T, Goldman YE, Selvin PR (2003). Myosin V walks hand‐over‐hand: single fluorophore imaging with 1.5‐nm localization. Science 300: 2061–2065. [DOI] [PubMed] [Google Scholar]
- Zhao H, Zhang X, Dai Z, Feng Y, Li Q, Zhang JH et al (2016). P2X7 receptor suppression preserves blood‐brain barrier through inhibiting rhoa activation after experimental intracerebral hemorrhage in rats. Sci Rep 6: 23286. [DOI] [PMC free article] [PubMed] [Google Scholar]


