Abstract
Background and Purpose
Therapeutic area guidelines (TAGs) published by the EMA and the FDA offer guidance in planning the launch of a trial in a certain indication. We assessed and compared the guidance on preclinical efficacy of all available TAGs from EMA and FDA.
Experimental Approach
EMA and FDA websites and databases were searched for all TAGs. A mixed deductive and inductive approach was applied to analyse and cluster content for preclinical efficacy.
Key Results
A total of 114 EMA and 120 FDA TAGs were identified, covering 126 indications. Our core finding is that 75% of EMA TAGs and 58% from the FDA TAGs do not offer any guidance on preclinical efficacy. TAGs varied widely on the extent, nature and detail of guidance.
Conclusions and Implications
Guidance on preclinical efficacy in a consistent, comprehensive and explicit way that still allows for justified deviations is an important but neglected aspect of transparency for drug development. This transparency would help sponsors in designing preclinical studies and in negotiating more efficiently with regulators.
Abbreviations
- EMA
European Medicines Agency
- FDA
U.S. Food and Drug Administration
- ICH
International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use
- IRBs
Institutional Review Boards
- STAIR
Stroke Therapy Academic Industry Roundtable
- TAGs
Therapeutic Area Guidelines
Introduction
Preclinical studies are intended to provide a key resource for risk–benefit assessment prior to early phase clinical trials. In general, the preclinical safety studies (mainly pharmacokinetics and toxicology) inform judgments about risk, and the preclinical efficacy studies (as part of the pharmacodynamics) inform judgments about benefit (‘clinical promise’). Those designing a particular early human study need to provide a set of preclinical studies (most often animal studies) that is sufficient to demonstrate a favourable balance of safety and clinical promise.
Whether a set of preclinical animal studies is ‘sufficient’ to demonstrate efficacy and safety or not depends on the questions these studies addressed as well as on their study design features that aim to reduce validity threats. Over the past 10 years, many commentators have raised concerns about the design and reporting of preclinical reports (Kilkenny et al., 2009; Prinz et al., 2011; Begley and Ellis, 2012; Howells et al., 2014), and some linked these concerns to the high attrition rates in clinical research (Prinz et al., 2011). Some have further argued that regulatory bodies do not routinely assess preclinical efficacy studies when authorizing early phase studies (Kimmelman and Federico, 2017). Recent analyses on how preclinical efficacy data are reported within investigator brochures for early phase studies intensified this concern (Wieschowski et al., 2018; Yasinski, 2018). One opportunity for probing this claim more deeply is to look at the recommendation on preclinical efficacy studies contained in regulatory guidelines.
There are no overarching regulatory guidelines that specify guidance on preclinical efficacy. For preclinical safety studies, in contrast, extensive regulatory guidance exists. The European Medicines Agency (EMA) provides over 50 guidelines for non‐clinical toxicology that “help medicine developers prepare marketing authorisation applications for human medicines” (European Medicines Agency, 2017a). Another five guidelines specify non‐clinical guidance on pharmacokinetics and toxicokinetics as well as safety pharmacology (European Medicines Agency, 2017a). Most of these safety guidelines have an overarching character and address non‐clinical guidance that applies to several drug classes or therapeutic areas.
This lack of ‘overarching’ efficacy guidelines, however, might not be surprising because judgements about whether a certain set of preclinical efficacy studies is sufficient for clinical translation is much more dependent on specific disease models, therapeutic areas or drug class. Therefore, regulatory guidelines for specific therapeutic areas could provide a more suitable approach to navigate the complexity of preclinical efficacy recommendations. Within the European Union, common market applications for marketing authorization are required to fully comply with the EMA ‘guidelines on clinical efficacy and safety’ and to justify any deviation (European Medicines Agency, 2009). These guidelines are ”intended to give guidance (…) in planning the overall pharmaceutical product development, as well as the non‐clinical and clinical tests and studies of a compound intended to be used as human or veterinary medicinal products” (European Medicines Agency, 2009). The U.S. Food and Drug Administration (FDA) also recommends compliance with similar guidelines, and deviations must be discussed with the agency (Food and Drug Administration, 2017b). We will refer to these EMA and FDA guidelines as Therapeutic Area Guidelines (TAGs).
This study assessed and compared the content for preclinical efficacy in all accessible TAGs from the EMA and the FDA. In addition, we compared the recommendations in TAGs with those presented in a systematically derived sample of experts guidelines for in vivo animal experiments (Henderson et al., 2013).
Methods
TAGs search
EMA and FDA websites were searched for TAGs in December 2016. The EMA lists all guidelines on ‘clinical efficacy and safety’ under a common heading (European Medicines Agency, 2017b). A subsequent search using the site‐wide search on the EMA's website was conducted to retrieve potentially relevant TAGs not listed under the common heading. Because the FDA does not group scientific guidelines under a common heading, a search was conducted using the database for FDA Guidance Documents (Food and Drug Administration, 2017a). All EMA and FDA guidelines were checked for relevance by two authors (W.C. and S.W.), and all documents relating to clinical development in specific therapeutic areas were identified and downloaded.
Comparative content analysis of EMA and FDA guidelines
All included EMA and FDA TAGs were categorized under one of 12 therapeutic areas as proposed by the EMA (European Medicines Agency, n.y.). We then applied a mixed deductive and inductive approach to analyse the TAGs' recommendations on preclinical efficacy. First, for the deductive approach, a matrix of different preclinical efficacy study design elements was derived from the International Council for Harmonization (ICH) Guideline on Good Clinical Practice (International Conference on Harmonization Working Group, 1996). We referred to the ICH Guideline as it is recognized by the EMA and FDA, and the preclinical provisions outlined in this guideline apply to all clinical trials, though ICH does not itself provide any TAGs. Second, to increase sensitivity and specificity in our assessment, the matrix also allowed the (inductive) inclusion of ‘Other preclinical efficacy study design elements’ not addressed in the ICH guideline.
Two researchers (W.C. and S.W.) independently analysed all included TAGs. For each TAG, all relevant text passages that gave guidance on preclinical efficacy were extracted. Thematic text analysis guided by the assessment matrix was applied to all text passages to assess preclinical study design recommendations presented in each TAG. The researchers (W.C. and S.W.) compared their extractions in order to check for differences in rating and extracting. All differences were resolved by discussion. H.L. and D.S. further double‐checked the results of the thematic text analysis for validity and consistency, each taking a subsample of 20 guidelines.
Comparison with scientific expert guidelines for in vivo animal experiments
For the identification of scientific expert guidelines, we referred to a systematic review of guidelines addressing design and conduct of preclinical efficacy studies (Henderson et al., 2013). The 26 guidelines identified in this review cover a diverse range of therapeutic areas with a total of 18 distinct indications. For those expert guidelines covering an indication that matched with a TAG indication, we checked if the corresponding EMA and FDA TAGs contained guidance on preclinical efficacy.
As this study was literature based and did not involve human subjects, no ethical approval was necessary.
Results
We identified 114 unique TAGs from EMA, and 120 from FDA that met our eligibility criteria (see flow diagram in Figure 1). These 234 TAGs covered 126 distinct indications (Table 1; also Supporting Information Table S1), which fitted into 11 distinct therapeutic areas. Indications ranged from common (e.g. diabetes mellitus) to orphan diseases (e.g. Duchenne muscular dystrophy). For 34 indications, both the FDA and the EMA provided at least one guideline. For the remaining 92 indications, we found guidance from one agency only.
Figure 1.
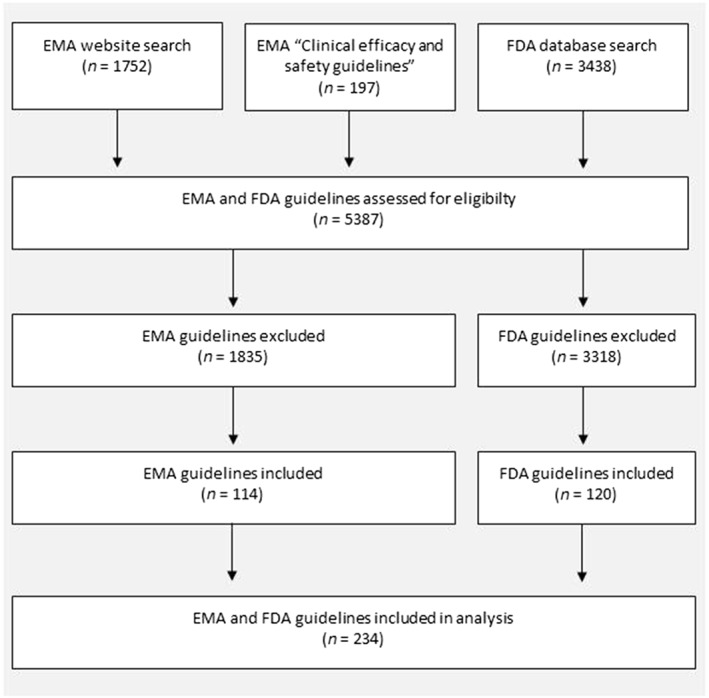
Flow chart: Search for EMA and FDA TAGs.
Table 1.
Comparison of EMA and FDA TAGs
| Therapeutic area | Guideline topic | EMA guidelines | FDA guidelines |
|---|---|---|---|
| Alimentary tract & metabolism | Weight control | 2 | 1 |
| Irritable bowel syndrome | 1 | 1 | |
| Diabetes mellitus | 2 | 2 | |
| Nausea and vomiting | 1 | – | |
| Crohn's disease | 1 | – | |
| Gastroesophageal reflux disease | 1 | – | |
| Chronic constipation | 1 | – | |
| Ulcerative colitis | 1 | 1 | |
| Exocrine pancreatic insufficiency | – | 2 | |
| Gastroparesis | – | 1 | |
| Duodenal ulcer disease | – | 1 | |
| Enzyme replacement therapy | – | 1 | |
| Endocrine‐related drug toxicity | – | 1 | |
| Growth | 2 | 1 | |
| Liver injury | – | 1 | |
| Allergy & immunology | Allergic rhino‐conjunctivitis | 1 | 2 |
| Transplantation | 1 | 1 | |
| Allergic diseases | 1 | – | |
| Disorders affecting the haematopoietic system | – | 2 | |
| Primary humoral immunodeficiency | – | 1 | |
| Anti‐infectives for systemic use | Infectious disease | 2 | 11 |
| HIV | 2 | 10 | |
| Fungal disease | 1 | 1 | |
| Hepatitis C | 1 | 2 | |
| Hepatitis B | 1 | 1 | |
| Sepsis | 1 | – | |
| Acute bacterial otitis media | – | 1 | |
| Acute bacterial sinusitis | – | 2 | |
| Influenza | 2 | 4 | |
| Clostridium difficile infection | – | 1 | |
| Gingivitis | – | 3 | |
| Sexually transmitted diseases | – | 2 | |
| Recurrent herpes labialis | – | 1 | |
| Sinusitis | – | 1 | |
| Smallpox | 1 | 1 | |
| Intra‐abdominal infection | – | 1 | |
| Head lice infestation | – | 1 | |
| Antineoplastic & immune‐modulating agents | Cancer | 5 | 7 |
| Chronic primary immune thrombocytopenia | 1 | – | |
| Infection | 1 | – | |
| Chronic lymphocytic leukaemia | 1 | – | |
| Non‐small cell lung cancer | – | 2 | |
| Breast cancer | – | 1 | |
| Colon and rectal cancer | – | 1 | |
| Blood product & biotech | Hepatitis B | 1 | – |
| Haemostasis | 1 | 1 | |
| Haemophilia A | 1 | – | |
| Haemophilia B | 1 | – | |
| Immuno‐deficiency syndromes | 2 | – | |
| Bleeding disorder | 3 | – | |
| Blood | 3 | 3 | |
| Cardiovascular system | Hypertension | 3 | 1 |
| Lipid disorders | 2 | – | |
| Antiarrhythmics | 1 | – | |
| Restenosis | 1 | 2 | |
| Pulmonary arterial hypertension | 1 | – | |
| Stroke and systemic embolic events | 1 | – | |
| Venous thromboembolism | 3 | – | |
| Acute heart failure | 2 | – | |
| Chronic heart failure | 1 | – | |
| Angina pectoris | 1 | – | |
| Acute myocardial infarction | 1 | – | |
| Acute coronary syndrome | 1 | – | |
| Peripheral‐arterial occlusive disease | 1 | – | |
| Cardiovascular disease | 2 | 1 | |
| Dermatologicals | Skin conditions | 1 | 1 |
| Psoriasis | 1 | – | |
| Acne | – | 1 | |
| Acute bacterial skin and skin structure infection | – | 1 | |
| Chronic cutaneous ulcer and burn wounds | – | 1 | |
| Upper facial lines | – | 1 | |
| Genito‐urinary system & sex hormones | Postmenopausal & vasomotor symptoms and vulvar and vaginal atrophy symptoms | 1 | 2 |
| Urinary incontinence | 1 | – | |
| Contraceptives | 1 | 1 | |
| Chronic renal insufficiency | 1 | – | |
| Autosomal dominant polycystic kidney disease | – | 1 | |
| Bacterial vaginosis | – | 1 | |
| Urinary tract infection | – | 1 | |
| Vulvovaginal candidiasis | – | 1 | |
| Nervous system | Anxiety disorder | 2 | 1 |
| Epileptic disorders | 1 | 1 | |
| Duchenne and Becker muscular dystrophy | 1 | 1 | |
| Alzheimer's disease and other dementias | 1 | 1 | |
| Pain | 1 | – | |
| Alcoholism | 1 | 1 | |
| Migraine | 1 | 1 | |
| Depression | 1 | 1 | |
| Premenstrual dysphoric disorder | 1 | – | |
| Attention‐deficit/hyperactivity disorder | 1 | – | |
| Insomnia | 1 | – | |
| Nociceptive pain | 1 | – | |
| Post‐traumatic stress disorder | 1 | – | |
| Panic disorder | 1 | – | |
| Obsessive compulsive disorder | 1 | – | |
| Smoking | 1 | – | |
| Acute stroke | 1 | – | |
| Bipolar disorder | 1 | – | |
| Schizophrenia | 3 | – | |
| Multiple sclerosis | 2 | – | |
| Neuropathic pain | 1 | – | |
| Amyotrophic lateral sclerosis | 1 | – | |
| Autism spectrum disorder | 1 | – | |
| Parkinson's disease | 1 | – | |
| Chronic fatigue syndrome/myalgic encephalomyelitis | – | 1 | |
| Hypnotic | – | 1 | |
| Psychoactive | – | 1 | |
| Respiratory system | Asthma and chronic obstructive pulmonary disease | 2 | – |
| Chronic obstructive pulmonary disease | 1 | 4 | |
| Acute respiratory distress syndrome | 1 | – | |
| Cystic fibrosis | 1 | – | |
| Asthma | 1 | – | |
| Severe acute respiratory syndrome | – | 1 | |
| Pneumonia | – | 2 | |
| Tuberculosis | – | 1 | |
| Bronchitis | – | 1 | |
| Rheumatology | Osteoporosis | 1 | 1 |
| Juvenile idiopathic arthritis | 1 | – | |
| Osteoarthritis | 1 | 1 | |
| Ankylosing spondylitis | 1 | – | |
| Psoriatic arthritis | 1 | – | |
| Rheumatoid arthritis | – | 2 | |
| Systemic lupus erythematosus and lupus nephritis | 1 | 1 | |
| Chronic disorders | 1 | – | |
| Others | Anaesthesiology | – | 2 |
| Life‐threatening diseases | – | 1 | |
| Rare disease | – | 2 | |
| Total | 114 | 120 |
In total, 25% (n = 29) of all 114 EMA and 42% (n = 50) of all 120 FDA TAGs included at least one recommendation on a particular aspect of preclinical efficacy studies or at least one remark on the need to test efficacy in preclinical studies (see Table 2). More specifically, 9% (n = 10) of all EMA and 25% (n = 30) of all FDA TAGs described a suitable preclinical efficacy model. Efficacy characteristics such as the desired nature, intensity or duration of effects were mentioned in 12% (n = 14) of all EMA and 16% (n = 19) of all FDA TAGs.
Table 2.
Guidance on preclinical efficacy in EMA and FDA TAGs: distribution and specification
| Therapeutic area guidelines (TAGs) | EMA (N = 114) | FDA (N = 120) |
|---|---|---|
| Any guidance on preclinical efficacy | 29 (25%) | 50 (42%) |
| Types of preclinical efficacy study design elements | No. of TAGs | Rating of guidance | No. of TAGs | Rating of guidance | ||
|---|---|---|---|---|---|---|
| Generala | Specifica | Generala | Specifica | |||
| Efficacy models | 10 (9%) | 3 (30%) | 7 (70%) | 30 (25%) | 12 (40%) | 18 (60%) |
| Effect characteristicsb | 14 (12%) | 9 (64%) | 5 (36%) | 19 (16%) | 13 (68%) | 6 (32%) |
| Dosing characteristics AND/OR administrationc | 12 (11%) | 5 (42%) | 7 (58%) | 29 (24%) | 15 (52%) | 14 (48%) |
| Receptor binding and specificity | 0 (0%) | 0 (0%) | 0 (0%) | 5 (4%) | 2 (40%) | 3 (60%) |
| Otherd | 6 (5%) | 2 (33%) | 4 (67%) | 15 (13%) | 5 (33%) | 10 (67%) |
Supporting Information Table S2 gives examples for how guidelines' guidance on preclinical efficacy was rated as general or specific.
For example, nature, frequency, intensity of pharmacological effects, time to onset or duration of effects.
For example, dose duration, dose interval, administration route, dose response or comparison of nontoxic dose findings.
Other preclinical efficacy studies, for example, special studies to assess pharmacological actions other than the intended therapeutic effects.
We further differentiated guidance on preclinical efficacy based on whether it addressed general versus specific study design elements, with the underlying assumption that general guidance is less helpful for practical needs in risk–benefit assessment (see Table 2). Guidance on choice of animal models tended to be specific (in 7 of 10 EMA TAGs and in 18 of the 30 FDA TAGs).
An example for specific guidance on efficacy models was found in the EMA TAG on ‘Non‐clinical and clinical development of similar biological medicinal products containing low‐molecular‐weight heparins (LMWH)’:
“(…) in vivo pharmacodynamic activity of the similar and the reference LMWH should be quantitatively compared in an appropriate in vivo pharmacodynamic model, which takes into account state of the art knowledge about clinically relevant pharmacodynamic effects of LMWH and includes, at least, an evaluation of anti‐FXa, and anti‐FIIa activity and of release of tissue factor pathway inhibitor. (European Medicines Agency, 2016)”
An example of a general guidance for efficacy models can be found in the EMA TAG ‘Evaluation of anticancer medicinal products in man – addendum on paediatric oncology’:
“Sponsors should include a comprehensive overview on any testing of the agent for activity against pre‐clinical model systems of paediatric tumours. (European Medicines Agency, 2003)”
Other guidance, such as on outcome measurements, was more often of general nature (in 9 of the 14 EMA TAGs and in 13 of the 19 FDA TAGs). Please refer to Supporting Information Table S2 for further text examples of general and specific guidance. None of the 234 TAGs addressed general internal validity issues like randomization, blinding of outcome assessment or sample size calculation.
Of all 234 TAGs, 18 addressed indications that were also addressed by scientific expert guidelines on preclinical efficacy issues reviewed in the Henderson et al. (2013) study. While these 18 TAGs could have drawn on the already existing set of preclinical recommendations, half of them (n = 9) did not address any of these preclinical efficacy recommendations (see Table 3 for details). Only one TAG explicitly referred to the existing expert guidelines.
Table 3.
Comparison of EMA and FDA TAGs with scientific guidelines for in vivo animal experiments
| Guideline topic | Guidelines/authors | EMA | FDA | ||
|---|---|---|---|---|---|
| No. of TAGs on topic | Preclinical guidance contained? | No. of TAGs on topic | Preclinical guidance contained? | ||
| Acute renal failure | Bellomo et al. (2004) | 0 | n.a. | 0 | n.a. |
| ALS | Ludolph et al. (2010) and Scott et al. (2008) | 0 | n.a. | 0 | n.a. |
| Alzheimer | Shineman et al. (2011) | 1 | n.a. | 1 | n.a. |
| Analgesic drugs | Rice et al. (2008) | 1 | n.a. | 0 | n.a. |
| Antiarthritic molecules | Bolon et al. (2010) | 2 | General | 2 | Specifica |
| Arrhythmia | Curtis et al. (2013) | 1 | Specific | 0 | n.a. |
| Brain injury | Margulies and Hicks (2009) | 0 | n.a. | 0 | n.a. |
| Chemoprevention | Verhagen et al. (2003) and Kelloff et al. (1994) | 0 | n.a. | 0 | n.a. |
| Drug‐eluting stents | Schwartz et al. (2008) | 1 | Specific | 2 | Specific |
| Endometriosis | Pullen et al. (2011) | 0 | n.a. | 0 | n.a. |
| Morbus Duchenne | Willmann et al. (2012) and Grounds et al. (2008) | 1 | Specific | 1 | General |
| Multiple sclerosis | Moreno et al. (2012) | 1 | n.a. | 0 | n.a. |
| Myocardial infarction | Bolli et al. (2004) | 1 | n.a. | 0 | n.a. |
| Rett syndrome | Katz et al. (2012) | 0 | n.a. | 0 | n.a. |
| Sepsis | Piper et al. (1996) | 1 | n.a. | 0 | n.a. |
| Stroke | Stroke Therapy Academic Industry Roundtable (STAIR) (1999), Macleod et al. (2009), Liu et al. (2009), Garcia‐Bonilla et al. (2011), Savitz et al. (2011) and US National Institutes of Health National Institute of Neurological Disorders and Stroke (2011) | 1 | n.a. | 0 | n.a. |
| TBC | Kamath et al. (2005) | 0 | n.a. | 1 | General |
| Total | 11 |
General: 2 Specific: 3 |
7 |
General: 2 Specific: 2 |
|
Only one of the two FDA TAG on antiarthritic molecules gives specific guidance.
Discussion
In this study, we reviewed the full sample of TAGs (Therapeutic Area Guidelines) from EMA and FDA and assessed to what extent they provide advice on preferred design measures or other characteristics of preclinical efficacy studies. A core finding is that most TAGs (75% of EMA and 58% of FDA TAGs) do not include any remarks on preclinical efficacy. Among those TAGs that do contain at least some advice, most recommendations are of general nature, addressing neither specific design measures nor specific characteristics of animal models.
Whereas regulatory agencies provide extensive guidance on the design and objectives of preclinical safety studies, they do not do so for preclinical efficacy studies. However, regulatory agencies might have good reason for more recommendations on preclinical study design. Though the majority of new investigational drugs survive the phase I trials evaluating safety, 70% of phase II trials fail to demonstrate efficacy (Hay et al., 2014). Such failures are costly and expose patients to ineffective therapeutic regimens. Many commentators have questioned design and reporting standards for preclinical efficacy studies (Prinz et al., 2011; Begley and Ellis, 2012; Kimmelman and Federico, 2017; Mattina et al., 2017). Better regulatory guidance on the design of preclinical efficacy studies might help reduce the burdens and costs associated with attrition during drug development. Just recently, the need for more robust study designs in preclinical efficacy studies was highlighted (Mattina et al., 2016). Regulatory guidelines could strongly support these activities (Begley and Ellis, 2012).
The limited guidance on the design of preclinical efficacy studies in TAGs contrasts with the abundance of recommendations in the scientific literature (Henderson et al., 2013). For instance, the Stroke Therapy Academic Industry Roundtable (STAIR) publishes and updates recommendations for preclinical standards in the development of drugs for acute ischaemic stroke (Fisher et al., 2009). STAIR provides a section with ‘Recommendations to Clinicians on the Evaluation of Preclinical Data With Neuroprotective Drugs’. These recommendations include, for example, (i) demonstrating dose response, (ii) the window of opportunity or therapeutic time window, (iii) physiological monitoring performed in randomized and blinded studies, (iv) outcome measures including infarct volume and functional assessment in acute as well as long‐term phase studies and (v) initial studies performed in rodents, with subsequent studies in larger species, such as cats. However, the EMA TAG for stroke does not refer to any of these preclinical recommendations (European Medicines Agency, 2001) and the FDA does not provide a stroke‐specific TAG. Indeed, we found only one intervention – drug eluting stent – where regulatory guidelines appeared to draw on state‐of‐the‐art thinking about design of preclinical efficacy studies (Schwartz et al., 2008).
Opponents of more explicit guidance on preclinical design standards in TAGs might argue that the determination of the sufficient set of preclinical efficacy studies to inform phase I/II trials is too case specific. Vestergaard et al. claim that for innovative medicines with high complexity, classical regulatory approaches based on guidelines are too inflexible and thus insufficient (Vestergaard et al., 2013; Narayanan et al., 2014). Instead, they argue that regulators should establish preclinical study recommendations on a case‐by‐case approach. Their conclusion is based on an analysis of the scientific advice provided by the Committee for Medicinal Products for Human Use at the EMA and is thus limited to Advanced Therapy Medicinal Products. However, the authors further argue that such tailored approaches might also be suitable for other medicinal products with high levels of specificity (Vestergaard et al., 2013).
Nevertheless, tailored approaches without any underlying (specific) standards have drawbacks. First, decisions on preclinical efficacy studies that are based solely on expert judgments made in individual negotiations between sponsors and regulatory agencies will lack transparency. Second, it seems highly inefficient to ‘tailor’ negotiations for preclinical efficacy study design if there are recurring features; made‐to‐measure clothes also start with at least basic models and body plans. The more transparent and specific regulatory standards for preclinical studies are, the easier it is to justify deviations from such standards.
Making guidance on preclinical efficacy studies for drug development more explicit in TAGs would also provide a measure of transparency on how regulatory agencies assess risk–benefit in early phase trials. It would also help Institutional Review Boards (IRBs), Data and Safety Monitoring Boards or investigators to clarify and judge the risk–benefit profile for early human studies (Kimmelman and Federico, 2017). Furthermore, explicit guidance on preclinical efficacy studies might help to reduce the number of animals used in research, especially when accompanied by other means to contribute to this reduction, such as animal study registries (Wieschowski et al., 2016).
We recognise that our analysis has several limitations. We only reviewed TAGs from the EMA and FDA. TAGs from other regulatory agencies might complement a sample for future studies. Moreover, further research is needed to better understand the rationales for why regulatory TAGs from FDA and EMA do not include recommendation on how to design preclinical efficacy studies more often and in more detail.
The EMA, FDA and other regulatory bodies are accountable for scientific oversight in drug development including risk–benefit assessment for clinical trials. TAGs provide a basis to support structure and transparency in judgements on whether the evidence for preclinical efficacy is appropriate for starting early phase clinical trials on new investigational drugs. Our findings indicate, however, that this basis could in many cases be improved and extended by giving more explicit guidance on how to appropriately design preclinical efficacy studies. Further research, expert discussion and pilot tests are needed to determine the characteristics of efficient, but at the same time sufficient, guidance on preclinical efficacy studies that should be included in TAGs.
Author contributions
The research study was conceived and designed by D.S., J.K., H.L. and C.F. The data were acquired by W.C., S.W. and H.L. The data were analysed by W.C., S.W., H.L. and D.S. H.L., D.S., C.F. and J.K. wrote the manuscript
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This http://onlinelibrary.wiley.com/doi/10.1111/bph.13405/abstract acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Supporting information
Table S1 Comparison and matching of EMA and FDA therapeutic area guidelines (TAG) – Full list. All TAG offering any guidance on preclinical efficacy (specific or general) underlined.
Table S2 Rating of preclinical guidance in EMA and FDA TAGs: text examples illustrating general versus specific guidance.
Acknowledgement
This study was funded by the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG).
Langhof, H. , Chin, W. W. L. , Wieschowski, S. , Federico, C. , Kimmelman, J. , and Strech, D. (2018) Preclinical efficacy in therapeutic area guidelines from the U.S. Food and Drug Administration and the European Medicines Agency: a cross‐sectional study. British Journal of Pharmacology, 175: 4229–4238. 10.1111/bph.14485.
References
- Begley CG, Ellis LM (2012). Drug development: raise standards for preclinical cancer research. Nature 483: 531–533. [DOI] [PubMed] [Google Scholar]
- Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P (2004). Acute renal failure – definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 8: R204–R212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolli R, Becker L, Gross G, Mentzer R Jr, Balshaw D, Lathrop DA (2004). Myocardial protection at a crossroads: the need for translation into clinical therapy. Circ Res 95: 125–134. [DOI] [PubMed] [Google Scholar]
- Bolon B, Stolina M, King C, Middleton S, Gasser J, Zack D et al (2010). Rodent preclinical models for developing novel antiarthritic molecules: comparative biology and preferred methods for evaluating efficacy. Biomed Res Int 2011; 2011: 569068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MJ, Hancox JC, Farkas A, Wainwright CL, Stables CL, Saint DA et al (2013). The Lambeth Conventions (II): guidelines for the study of animal and human ventricular and supraventricular arrhythmias. Pharmacol Ther 139: 213–248. [DOI] [PubMed] [Google Scholar]
- European Medicines Agency (2001). Clinical investigation of medicinal products for the treatment of acute stroke.
- European Medicines Agency (2003). Evaluation of anticancer medicinal products in man – addendum on paediatric oncology.
- European Medicines Agency (2009). Procedure for European Union guidelines and related documents within the pharmaceutical legislative framework.
- European Medicines Agency (2016). Guideline on non‐clinical and clinical development of similar biological medicinal products containing low‐molecular‐weight‐heparins.
- European Medicines Agency (2017a). Non‐clinical guidelines. Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/general/general_content_000083.jsp&mid=WC0b01ac0580027548 (accessed 3/15/2017)
- European Medicines Agency (2017b). Clinical efficacy and safety guidelines. [Online] Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/general/general_content_000085.jsp&mid=WC0b01ac0580027549 (accessed 3/15/2017).
- Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI et al (2009). Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke 40: 2244–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Drug Administration (2017a). Search for FDA Guidance Documents (database). [Online] Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/general/general_content_000085.jsp&mid=WC0b01ac0580027549 (accessed 3/15/2017).
- Food and Drug Administration (2017b). Guidance for Industry. [Online] Available at: https://www.fda.gov/RegulatoryInformation/Guidances/default.htm (accessed 3/15/2017).
- Garcia‐Bonilla L, Rosell A, Torregrosa G, Salom JB, Alborch E, Gutierrez M et al (2011). Recommendations guide for experimental animal models in stroke research. Neurologia 26: 105–110. [DOI] [PubMed] [Google Scholar]
- Grounds MD, Radley HG, Lynch GS, Nagaraju K, De Luca A (2008). Towards developing standard operating procedures for pre‐clinical testing in the mdx mouse model of Duchenne muscular dystrophy. Neurobiol Dis 31: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay M, Thomas DW, Craighead JL, Economides C, Rosenthal J (2014). Clinical development success rates for investigational drugs. Nat Biotechnol 32: 40–51. [DOI] [PubMed] [Google Scholar]
- Henderson VC, Kimmelman J, Fergusson D, Grimshaw JM, Hackam DG (2013). Threats to validity in the design and conduct of preclinical efficacy studies: a systematic review of guidelines for in vivo animal experiments. PLoS Med 10: e1001489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howells DW, Sena ES, Macleod MR (2014). Bringing rigour to translational medicine. Nat Rev Neurol 10: 37–43. [DOI] [PubMed] [Google Scholar]
- International Conference on Harmonisation Working Group (1996). ICH harmonised tripartite guideline: guideline for good clinical practice E6 (R1) 1996. ICH: Geneva. [Google Scholar]
- Kamath AT, Fruth U, Brennan MJ, Dobbelaer R, Hubrechts P, Ho MM et al (2005). New live mycobacterial vaccines: the Geneva consensus on essential steps towards clinical development. Vaccine 23: 3753–3761. [DOI] [PubMed] [Google Scholar]
- Katz DM, Berger‐Sweeney JE, Eubanks JH, Justice MJ, Neul JL, Pozzo‐Miller L et al (2012). Preclinical research in Rett syndrome: setting the foundation for translational success. Dis Model Mech 5: 733–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelloff GJ, Johnson JR, Crowell JA, Boone CW, DeGeorge JJ, Steele VE et al (1994). Guidance for development of chemopreventive agents. J Cell Biochem Suppl 20: 25–31. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Parsons N, Kadyszewski E, Festing MF, Cuthill IC, Fry D et al (2009). Survey of the quality of experimental design, statistical analysis and reporting of research using animals. PLoS One 4: e7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmelman J, Federico C (2017). Consider drug efficacy before first‐in‐human trials. Nature 542: 25–27. [DOI] [PubMed] [Google Scholar]
- Liu S, Zhen G, Meloni BP, Campbell K, Winn HR (2009). Rodent stroke model guidelines for preclinical stroke trials (1st edition). J Exp Stroke Transl Med 2: 2–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludolph AC, Bendotti C, Blaugrund E, Chio A, Greensmith L, Loeffler JP et al (2010). Guidelines for preclinical animal research in ALS/MND: a consensus meeting. Amyotroph Lateral Scler 11: 38–45. [DOI] [PubMed] [Google Scholar]
- Macleod MR, Fisher M, O'Collins V, Sena ES, Dirnagl U, Bath PM et al (2009). Good laboratory practice: preventing introduction of bias at the bench. Stroke 40: e50–e52. [DOI] [PubMed] [Google Scholar]
- Margulies S, Hicks R (2009). Combination therapies for traumatic brain injury: prospective considerations. J Neurotrauma 26: 925–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattina J, Carlisle B, Hachem Y, Fergusson D, Kimmelman J (2017). Inefficiencies and patient burdens in the development of the targeted cancer drug sorafenib: a systematic review. PLoS Biol 15: e2000487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattina J, MacKinnon N, Henderson VC, Fergusson D, Kimmelman J (2016). Design and reporting of targeted anticancer preclinical studies: a meta‐analysis of animal studies investigating sorafenib antitumor efficacy. Cancer Res 76: 4627–4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno B, Espejo C, Mestre L, Suardiaz M, Clemente D, de Castro F et al (2012). Guidelines on the appropriate use of animal models for developing therapies in multiple sclerosis. Rev Neurol 54: 114–124. [PubMed] [Google Scholar]
- Narayanan G, Cossu G, Galli MC, Flory E, Ovelgonne H, Salmikangas P et al (2014). Clinical development of gene therapy needs a tailored approach: a regulatory perspective from the European Union. Hum Gene Ther Clin Dev 25: 1–6. [DOI] [PubMed] [Google Scholar]
- Piper RD, Cook DJ, Bone RC, Sibbald WJ (1996). Introducing critical appraisal to studies of animal models investigating novel therapies in sepsis. Crit Care Med 24: 2059–2070. [DOI] [PubMed] [Google Scholar]
- Prinz F, Schlange T, Asadullah K (2011). Believe it or not: how much can we rely on published data on potential drug targets? Nat Rev Drug Discov 10: 712. [DOI] [PubMed] [Google Scholar]
- Pullen N, Birch CL, Douglas GJ, Hussain Q, Pruimboom‐Brees I, Walley RJ (2011). The translational challenge in the development of new and effective therapies for endometriosis: a review of confidence from published preclinical efficacy studies. Hum Reprod Update 17: 791–802. [DOI] [PubMed] [Google Scholar]
- Rice AS, Cimino‐Brown D, Eisenach JC, Kontinen VK, Lacroix‐Fralish ML, Machin I et al (2008). Animal models and the prediction of efficacy in clinical trials of analgesic drugs: a critical appraisal and call for uniform reporting standards. Pain 139: 243–247. [DOI] [PubMed] [Google Scholar]
- Savitz SI, Chopp M, Deans R, Carmichael T, Phinney D, Wechsler L (2011). Stem cell therapy as an emerging paradigm for stroke (STEPS) II. Stroke 42: 825–829. [DOI] [PubMed] [Google Scholar]
- Schwartz RS, Edelman E, Virmani R, Carter A, Granada JF, Kaluza GL et al (2008). Drug‐eluting stents in preclinical studies: updated consensus recommendations for preclinical evaluation. Circ Cardiovasc Interv 1: 143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott S, Kranz JE, Cole J, Lincecum JM, Thompson K, Kelly N et al (2008). Design, power, and interpretation of studies in the standard murine model of ALS. Amyotroph Lateral Scler 9: 4–15. [DOI] [PubMed] [Google Scholar]
- Shineman DW, Basi GS, Bizon JL, Colton CA, Greenberg BD, Hollister BA et al (2011). Accelerating drug discovery for Alzheimer's disease: best practices for preclinical animal studies. Alzheimers Res Ther 3: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroke Therapy Academic Industry Roundtable (STAIR) (1999). Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke 30: 2752–2758. [DOI] [PubMed] [Google Scholar]
- US National Institutes of Health National Institute of Neurological Disorders and Stroke (2011) Improving the quality of NINDS‐supported preclinical and clinical research through rigorous study design and transparent reporting. US National Institutes of Health National Institute of Neurological Disorders and Stroke: Bethesda (Maryland).
- Verhagen H, Aruoma OI, van Delft JH, Dragsted LO, Ferguson LR, Knasmuller S et al (2003). The 10 basic requirements for a scientific paper reporting antioxidant, antimutagenic or anticarcinogenic potential of test substances in in vitro experiments and animal studies in vivo. Food Chem Toxicol 41: 603–610. [DOI] [PubMed] [Google Scholar]
- Vestergaard HT, Apote LD, Schneider CK, Herberts C (2013). The evolution of nonclinical regulatory science: advanced therapy medicinal products as a paradigm. Mol Ther 21: 1294–1296. [DOI] [PubMed] [Google Scholar]
- Wieschowski S, Chin WWL, Federico C, Sievers S, Kimmelman J, Strech D (2018). Preclinical efficacy studies in investigator brochures: do they enable risk–benefit assessment? PLoS Biol 16: e2004879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieschowski S, Silva DS, Strech D (2016). Animal study registries: results from a stakeholder analysis on potential strengths, weaknesses, facilitators, and barriers. PLoS Biol 14: e2000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmann R, De Luca A, Benatar M, Grounds M, Dubach J, Raymackers JM et al (2012). Enhancing translation: guidelines for standard pre‐clinical experiments in mdx mice. Neuromuscul Disord 22: 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasinski E (2018). Study questions animal efficacy data behind trials. Science (New York, NY) 360: 142. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Comparison and matching of EMA and FDA therapeutic area guidelines (TAG) – Full list. All TAG offering any guidance on preclinical efficacy (specific or general) underlined.
Table S2 Rating of preclinical guidance in EMA and FDA TAGs: text examples illustrating general versus specific guidance.


