Abstract
Daily life stress is an omnipresent phenomenon in modern society. Research has linked prolonged activation of the hypothalamic-pituitary-adrenal axis to psychiatric and somatic diseases. Everyday stressors substantially contribute to these health risks. Despite the notion that the physiological stress response is highly dependent on concurrent psychological processes, investigations associating diurnal cortisol levels with subjective experience have primarily focused on affective states. The impact of everyday cognitive processes including thought content has been largely neglected. To investigate this link, moment-to-moment associations of psychological experience including subjective stress, thought content and affect, and cortisol levels were assessed throughout the daily routines of 289 healthy adult participants. We found that subjective stress interacted with current thought content and affect in predicting cortisol release: more negative and future-directed thoughts were associated with higher cortisol levels after experiencing subjective stress, suggesting an increase in negative future anticipation. Concurrent cortisol rises might reflect proactive coping to adequately prepare for upcoming demands. In the absence of subjective stress, more past-directed thoughts and negative affect were associated with higher cortisol levels. These findings provide evidence for a fundamental link between thought content and daily cortisol activation, and highlight the significant contribution of thought patterns to physiological stress levels.
Introduction
Everyday stress is omnipresent in modern society. Organisms dynamically adapt to perceived stressors through activation of the sympathetic nervous system and the hypothalamic-pituitary-adrenal (HPA) axis. Subjective-psychological processes accompany and interact with these changes in neuro-physiological systems. Importantly, chronic stress and elevated levels of the associated hormone cortisol have been linked to the development of psychiatric and somatic diseases1,2. Thus, a firm understanding of the factors influencing diurnal cortisol release will be critical in resolving the current epidemic of stress-related disease.
The continuous adjustment of physiological activation to appropriately meet anticipated environmental demands is referred to as allostasis3. While acute increases in the hormone cortisol are adaptive and indicate adequate functioning of the HPA axis in response to stressors4, allostatic load characterizes the ‘wear and tear’ on the body through accumulated regulatory efforts during repeated challenge5,6. Corroborating the strong evidence associating dysregulated stress systems with depression7,8, recent studies have focused on altered diurnal cortisol levels to prospectively predict the onset of depression9 and anxiety disorders10. Meta-analytic evidence further links changes in the circadian rhythm of cortisol to additional markers of adverse mental and physical health conditions11.
Substantial accumulation of allostatic load arises from daily-life stressors12–14 and the accompanying affective and cognitive processes15,16. In line with this notion, stress research has increasingly relied on the ambulatory assessment of cortisol17. Diurnal levels of cortisol collected in real-life environments have thus been linked to a variety of psychosocial variables ranging from emotional experience (e.g. self-reported stress and affect)18 to social contextual factors such as social support19, momentary loneliness and solitude20,21, and health-related factors including the duration and quality of sleep22. Regarding momentary subjective-emotional experience, a growing number of studies indicate a positive correlation of self-reported stress and cortisol in a naturalistic environment23–25; however some studies found no direct association (for a review see17). Mounting evidence for a link of negative affect and increased cortisol secretion26–29, and of positive affect and attenuated cortisol secretion30 illustrates the importance to account for concurrent subjective-emotional processes when investigating endocrine activation.
Relatively fewer studies have addressed how cognitive processes, such as particular thought patterns, impact cortisol release in daily life. A theoretical framework for such a link is provided by Brosschot and colleagues31. They propose repeated negative thoughts about past or future stressors to prolong the physiological stress response and thereby mediate the effects of stress on health. Rumination and worry are two prominent examples of dysfunctional thought patterns with potentially negative consequence for physical health32. While most work suggesting a link with cortisol was conducted in the laboratory33,34, only few studies directly targeting these dysfunctional cognitive styles have relied on ecological momentary assessments of cortisol and subjective experience in healthy subjects35–38. Among those, one study found daily averages of rumination to correlate with cortisol in a mixed sample of both depressed patients and healthy controls35 while another showed an association of work worries and cortisol in a sample of married couples38.
Moving away from pathological cognitive styles, a more recent line of research employs thought-sampling to focus on the content of an individual’s stream of thoughts. Studies of mindwandering, commonly defined as self-generated thoughts unrelated to the current task or external environment39, have measured thought content in three distinct dimensions targeting the temporal and social focus as well as the emotional valence of thoughts (the “cube of thought”40). Utilizing this thought-sampling approach, we identified moment-to-moment associations of self-generated thought content and cortisol release in a previous laboratory study: more negatively toned emotional thoughts and a pattern of more past-oriented, other-directed thoughts were associated with higher cortisol levels both at rest and after an acute laboratory stressor. A pattern of more future- and self-oriented thoughts, on the other hand, was associated with decreased overall cortisol levels41. These laboratory findings demonstrate a general association of specific thought content and cortisol levels.
In summary, an association between affective correlates, stress and cortisol in daily life is well established. Emerging evidence from laboratory studies also suggests a link between thought patterns and cortisol levels. However, evidence for such a link in a naturalistic environment is still lacking.
To fill this gap, the current study employed an experience sampling approach42 to simultaneously capture subjective experience and cortisol levels with a fine-grained temporal resolution throughout the daily routines of a large sample of healthy adult participants (N = 289, 172 women; mean ± SD: 40.6 ± 9.3 years; age range: 20–55 years). While various measures of subjective experience were captured, we placed a specific focus on mental content. By measuring participants’ subjective experience in a naturalistic environment, this approach offers high ecological validity17. To provide a more comprehensive investigation into daily life cortisol dynamics, we additionally explored sleep parameters and the momentary social context, both of which have previously been shown to relate to endocrine activation21,22.
Other than in our previous laboratory work41,43, thought content in the current study was not analysed in clusters determined by prior principal component analysis. Rather, each dimension of the cube of thought (valence, temporal, social) was considered separately. We employed a multi-level mixed effects model to associate cortisol levels during participants’ waking hours with momentary thought content, affect, arousal and subjective stress as markers of subjective experience. In line with prior research26,28, we expected to find more negative affect to be predictive of higher cortisol. We further hypothesized this association to be stronger when participants indicated that they had concurrently experienced subjective stress29. With respect to thought content, we hypothesized more negative and more past-oriented thoughts to be generally associated with higher levels of cortisol and, again, expected the experience of subjective stress to enhance this association. This hypothesis is in line with the preservative cognition hypothesis31, which suggests that dwelling on past stressors prolongs cortisol signalling. Accordingly, more future-directed thoughts were expected to predict relatively attenuated cortisol levels. Evaluative threat to the self is an especially salient aspect of psychosocial stress44. Therefore, regarding the social dimension of thought content, we expected more self-directed thoughts, especially after stress, to be associated with higher cortisol levels. Inversely, a less egocentric focus and thus more other-directed thoughts were expected to predict relatively attenuated cortisol levels.
Results
Compliance
Compliance with the sampling of saliva and subjective experience was generally satisfactory: 2672 (92.46%) of the potential 2890 cortisol probes were provided over the two sampling days, while 218 (7.54%) samples were missing or found to be empty. Among the available cortisol samples, concurrent subjective experience reports were missing or incomplete in 185 (8.79%) cases, resulting in a total of 1916 complete observations entering the final model.
Descriptive statistics
Figure 1 depicts the average raw cortisol values for all measurement time-points. Average waking cortisol level in our sample was 8.59 nmol/l. As expected, after the initial steep increase, representing the cortisol awakening response, the measures throughout the day showed a gradual decrease. The average time of wakeup was 7:07 a.m. and the remaining cortisol samples corresponding with measures of subjective experience were taken on average at 11:11 a.m., 1:12 p.m., 3:13 p.m., and 5:11 p.m.
Figure 1.
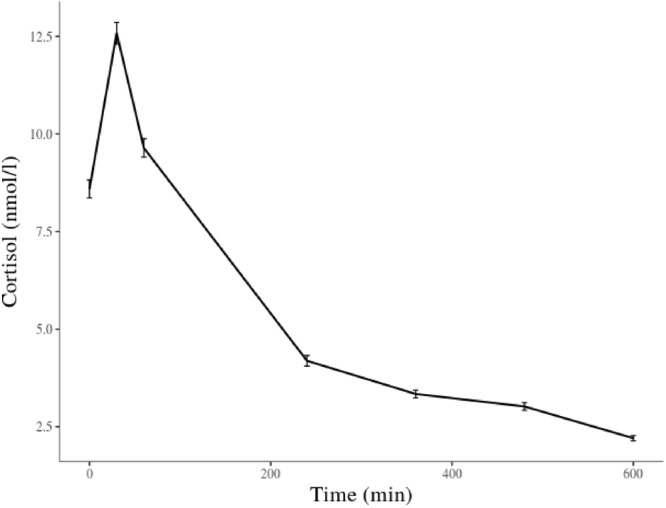
Mean observed cortisol (untransformed raw values) across the day. Time is depicted in minutes relative to awakening. Bars indicate standard errors.
Table 1 presents the means and standard errors of the subjective experience outcomes. On average, participants displayed a tendency towards more positively-toned thought content (mean ± SE = 13.41 ± 0.07), more future-directed thought content (13.37 ± 0.07) and more positive affect (6.30 ± 0.03). Participants reported a wide range of current activities throughout their daily routines (see Supplementary Materials for details). Stressful events occurred in 20.2% of all samples.
Table 1.
Descriptive statistics of experience sampling outcomes.
| Measure | N | Mean (SE) |
|---|---|---|
| Affect | 2912 | 6.30 (0.03) |
| Arousal | 2911 | 4.47 (0.03) |
| Thoughts Valence | 2910 | 13.41 (0.07) |
| Thoughts Temporal | 2910 | 13.37 (0.07) |
| Thoughts Social | 2910 | 10.65 (0.09) |
| Stress (no/yes) Stress magnitude Stress coping |
2324/588 587 587 |
11.59 (0.15) 12.77 (0.16) |
| Company (no/yes) Closeness |
1434/1478 1478 |
12.26 (0.15) |
Effect of subjective stress on concurrent subjective experience
Welch’s unequal variances t-tests revealed significant differences between stress and non-stress samples in several measures of subjective experience. When reporting subjective stress, participants showed a significant decrease in arousal (t(971.43) = −15.817, p < 0.001), more negative affect (t(766.61) = −17.143, p < 0.001), and more negative thoughts (t(829.43) = −13.641, p < 0.001). Temporal and social thought content did not significantly differ depending on concurrent subjective stress (both p values > 0.38).
Association of subjective experience and cortisol levels
Before assessing the effects of subjective experience on cortisol levels, we fit an empty multilevel model (i.e., without predictor variables) to extract variance components of model factors and to calculate the intra class coefficient (ICC). Results indicated that 9.75% of the total variance in cortisol levels was attributed to between-individual differences. Adding ‘sampling time’ to the empty model accounted for 67.21% of the total explained variance in momentary cortisol levels and 76.96% of variance explained by the fixed factors combined, in comparison to the final full model. The remaining covariates accounted for an additional 15.23% (sex and awakening time), 1.08% (thought content), and 1.74% (affective states) of variance explained by the fixed factors compared to the final model.
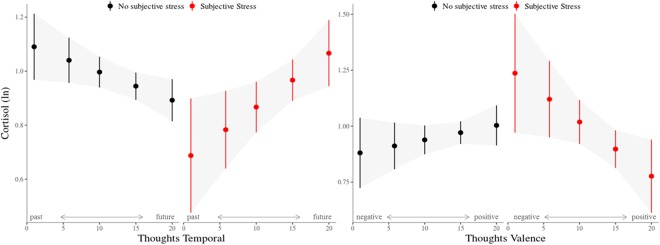
The association of cortisol levels with all available measures of subjective experience was analysed in a mixed-effects model. Table 2 shows the estimated values and margins of error (representing a 95% confidence interval) of the model parameters including all subjective experience measures, covariates (time, sex) and the contextual parameters sleep and company (i.e., momentarily being in company of someone vs. being alone). Sampling time was the strongest predictor of cortisol levels (β10 = −0.337, t = −17.27, p < 0.001) with longer time intervals since waking associated with lower cortisol levels. Female participants exhibited significantly lower cortisol levels than males (γ001 = −0.142, t = −3.09, p = 0.002). Interactions of subjective stress and affect predicted cortisol levels (π8 = 0.053, t = 2.02, p = 0.043, see Fig. 2). Simple effects analyses revealed an effect of negative affect and cortisol only in the no-stress samples (F = 6.36, p = 0.01), with more negative affect associated with higher cortisol levels. Affect and cortisol were unrelated in the samples reporting stressful events (F = 1.38, p = 0.24). Reports of subjective stress also interacted with the temporal (π11 = −0.031, t = −3.35, p < 0.001) and valence (π10 = −0.031, t = −2.51, p = 0.01) dimensions of thought content in predicting cortisol levels (Fig. 3). The negativity of thought was associated with cortisol only after stressful experiences (F = 6.72, p < 0.01) where more negative thoughts predicted higher cortisol levels. There was no significant association in the no-stress samples (F = 1.39, p = 0.238). Past-directed thoughts predicted cortisol in the no-stress samples (F = 4.075, p = 0.044) while future-directed thoughts were predictive of cortisol after stressful experiences (F = 7.86, p < 0.01). In both cases the stronger the thoughts were rooted in one of the temporal domains, the more cortisol was released. Subjective stress did not interact with the social dimension of thought (π12 = −0.003, t = −0.62, p > 0.5). Momentary cortisol levels were not associated with sleep quality or sleep duration after controlling for sampling time. However, wake time predicted cortisol levels (β03 = −0.074, t = −3.642, p < 0.001) with higher cortisol associated with earlier waking.
Table 2.
Model estimates for fixed effects on cortisol levels.
| Fixed Effects | B (SE) | CI | p |
|---|---|---|---|
| (Intercept) | 1.29 (0.159) | 0.98–1.61 | <0.001 |
| Stress | −0.36 (0.175) | −0.70–−0.01 | 0.041 |
| Affect | −0.04 (0.014) | −0.06–−0.01 | 0.010 |
| Arousal | −0.02 (0.008) | −0.03–−0.00 | 0.046 |
| Thoughts Valence | 0.01 (0.006) | −0.01–0.02 | 0.285 |
| Thoughts Temporal | −0.01 (0.004) | −0.02–−0.00 | 0.026 |
| Thoughts Social | −0.00 (0.003) | −0.01–0.01 | 0.936 |
| Sleep duration | −0.01 (0.017) | −0.04–0.03 | 0.648 |
| Sleep quality | 0.00 (0.004) | −0.01–0.01 | 0.830 |
| Company | 0.01 (0.028) | −0.04–0.07 | 0.661 |
| Sex | 0.14 (0.044) | 0.04–0.23 | 0.002 |
| Time | −0.34 (0.019) | −0.38–−0.30 | <0.001 |
| Awakening time | −0.07 (0.020) | −0.11–−0.03 | <0.001 |
| Stress*Valence | −0.03 (0.012) | −0.05–−0.01 | 0.012 |
| Stress*Temporal | 0.03 (0.009) | 0.01–0.05 | <0.001 |
| Stress*Social | −0.00 (0.006) | −0.02–0.01 | 0.532 |
| Stress*Affect | 0.05 (0.026) | 0.00–0.11 | 0.043 |
| Stress*Arousal | 0.01 (0.018) | −0.03–0.05 | 0.604 |
| Variance | SD | ||
| Random Effects | |||
| Individual (Intercept) | 0.094 | 0.30 | |
| Day (Intercept) | 0.028 | 0.17 | |
| Residual | 0.248 | 0.49 | |
Figure 2.
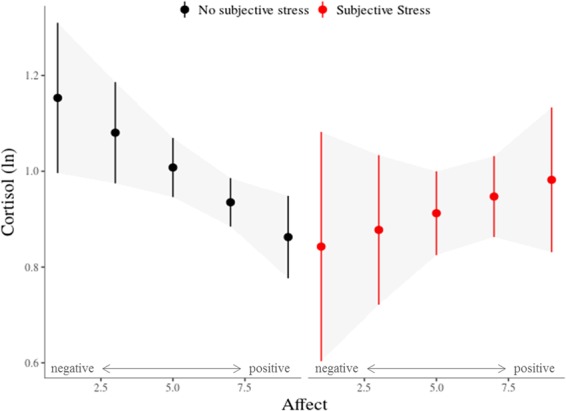
Association of cortisol (ln) and state affect (negative to positive) for samples with- and without concurrent reports of subjective stress. Bars indicate standard errors.
Figure 3.
Association of cortisol (ln) and thought dimensions ‘valence’ (negative to positive) and ‘temporal’ (past-directed to future-directed) for samples with- and without concurrent reports of subjective stress. Bars indicate standard errors.
Given that the focus of the present study was on momentary associations of cortisol with measures of subjective experience, analyses of individual differences in the diurnal cortisol rhythm will be reported elsewhere.
Discussion
Research relating psychological concomitants of daily life to stress-related cortisol release has predominantly focused on affective states. The impact of thought content on HPA axis activation has received far less attention. In the present study, we investigated the association of diurnal cortisol levels with concurrent subjective experience in daily life, using an experience-sampling approach. In addition to relating measures of affect and subjective stress experience to diurnal cortisol levels, we sought to explore if, and how, the content of thought predicts cortisol release. We show that experiencing daily life stress increases both negative affect and the negativity of thought. However, their relation to cortisol differed depending on whether participants reported having experienced stress or not. In more detail, negative affect was associated with cortisol release only when participants did not experience a concurrent stressful event. Conversely, the negativity of thought predicted cortisol release only when subjective stress was reported. The relation of the temporal orientation of thought and cortisol was also dependent on the presence or absence of subjective stress experience: Thoughts more firmly rooted in the past were associated with higher cortisol levels in the absence of subjective stress, while more future-directed thoughts were associated with higher cortisol levels in the presence of subjective stress.
Regarding affect, we found the expected positive association between negative affect and cortisol levels, as has been shown in most prior research in ambulatory settings25,27. Contrary to our hypothesis however, subjective stress did not amplify this relation. Rather, no significant relation was found between momentary affect and cortisol levels after stress experience, which may in part be attributable to comparably lower statistical power in testing this association in stress-samples, which occurred much less frequently than non-stress samples. While previous studies have even proposed negative affect to mediate the effects of stress on cortisol release29,45, our finding suggests a general association of momentary affect and cortisol, in the absence of stress, which at the very least seems not to be enhanced after subjective stress. The existing inconsistencies among ambulatory findings might partly be due to diverging sampling designs (ranging from momentary assessments to retrospective diary methods) and different operationalization of subjective stress and affect17. Our data, by dissecting subjective stress, concurrent affect, and several dimensions of thought content, point towards a relation of negative affect and cortisol levels, independent of subjective stress.
With respect to cognition, we found that subjective stress modulated the interaction of thought content and cortisol regulation. Interestingly, and contrary to negative affect, the hypothesized association of cortisol and negative thought content was only observed in samples with concurrent subjective stress. Our previous laboratory findings41 suggested this association applied to both stress- and non-stress environments. Two main differences between our former and the present study may account for the observed inconsistencies.
First, the context and nature of the stressors fundamentally differed. The former study applied the Trier Social Stress Test (TSST46), a highly standardized socio-evaluative laboratory stress protocol, whereas the present study focused on daily-life experiences. While most daily life stressors are likely less pronounced than the TSST, they may be far more frequent, persistent or reoccurring, making the time of stress exposure during event sampling highly volatile14. Second, the character of the captured thought content differed between studies. While thought content in the former study was sampled in short intervals and during a low-demand task specifically designed to disengage attention and thus induce mindwandering, the present study sampled thought content throughout the day, over long periods of time and in various contexts. Compared to the mindwandering episodes, these daily thoughts were likely more heterogeneous, reflecting the participant’s current environment, momentary activities or social interactions. Moreover, the mindwandering pattern associated with increased baseline cortisol in our previous study comprised negatively toned emotional thoughts. Thus, it might rather reflect a combination of the two domains, affect and cognition, which we aimed to disentangle here. In fact, depending on the presence of stress, the current results suggest differential relations of affect and thought content with cortisol levels: While greater negative affect was generally associated with higher cortisol in the absence of subjective stress, increasingly negative thoughts were associated with higher cortisol only after stress experience. Although the measures overlap to some degree, their complementary relation to cortisol suggests the influence of distinct underlying processes, which may have different temporal dynamics. Along these lines, research has shown negative affect to be characterized by substantial short-term fluctuation47. The valence of thought content may be more stable over time—particularly when thoughts are still reflective of coping with the consequences of a stressor32 —and may thus more accurately reflect the time-point of stress exposure. Overall, the finding that negative thoughts after a stressful experience, rather than negative affect, are related to higher cortisol levels supports Brosschot’s perseverative cognition hypothesis31, which argues that the activation of stress systems exceeds the mere duration of a stressor and is maintained by cognitive representations of the latter.
As hypothesized, we found that the extent of past-directed thoughts predicted higher cortisol levels in the absence of stress. When reporting subjective stress, however, we found that increasingly future-oriented thoughts were associated with more pronounced cortisol levels. This later result again stands in contrast to our previous laboratory study showing future-directed mindwandering to attenuate cortisol levels, independent of prior stress exposure41. In addition to the explanation invoked above, the different measurement designs of stressors captured in a laboratory procedure versus an event-sampling paradigm likely played a big role in the inconsistency. While in the laboratory, mindwandering was assessed immediately following acute stress exposure, the experience sampling procedure allowed for an extended timeframe between stressor and thought sample. Experience sampling studies predicting the duration of emotions in daily life found that most emotional episodes ended within an hour of the eliciting stimulus48. Accordingly, the more time that passes between a stressor and the sampling of the experience, the less likely it should be that thoughts are occupied with past stressors. Indeed, our data do not show an increase in past thinking after stress experience, but rather a predominance of future thoughts irrespective of subjective stress. A second fundamental difference between laboratory-induced and experience-sampled stress was also mentioned earlier: most real-life stressors are likely to be minor12, characterised by less discrete onset and termination, and potentially persisting or recurring. Analyses included in our supplementary materials support this notion, showing that there was an increased likelihood of (re-)experiencing subjective stress when already having reported stress in the previous sample. It could thus be argued that the association of future thought and cortisol levels is a consequence of stress anticipation. In other words, after having experienced stress, future thought might target another stressful event yet to occur. Alternatively, the association of future thought and cortisol after experienced stress may be explained by proactive coping. Proactive coping is characterised by efforts to prevent or modify a potentially stressful event49. It takes place prior to anticipatory coping, and while proactive coping involves efforts to detect, prevent and proactively manage potentially occurring stressful future events, anticipatory coping targets the stressful consequences of an event that is certain to happen50. Stages of proactive coping involve both future simulation in order to identify potentially stressful events and initial coping efforts to prevent the likelihood of such stressors51. The increased release of cortisol after stress experience may thus provide the necessary energy for such proactive problem-solving activities. Potentially supporting this reasoning, a recent experience-sampling study investigating emotion regulation strategies showed that more frequent engagement in problem solving was associated with increased cortisol awakening responses52. Momentary problem solving, on the other hand, was associated with transient increases in cortisol levels in a subsample of participants with current internalizing disorders52.
Regarding health implications of the present findings, proactive coping efforts (and associated future-thinking) are primarily viewed as adaptive and have been linked to beneficial mental and physical health outcomes50. Several recent articles have argued against an overly simplistic view of high or rising cortisol levels as inherently adverse. Rather, they emphasize the adaptive nature and context dependency in assessing costs and benefits of acute cortisol elevation53,54. Along these lines, momentarily increased cortisol levels associated with future thoughts, in the present study, may well reflect adaptive coping processes. Increased cortisol may supply the ‘energetic boost’ needed to pre-empt future or terminate ongoing stressors. However, needlessly triggered or upheld stress responses—as in the case of anticipated, but non-actualised stressors—bear the risk of unnecessarily prolonging or failing to inhibit activated stress systems and may thus contribute to negative health consequences31,55.
Evaluative threat to the self is an especially salient aspect of psychosocial stress44 and it would have been conceivable that orienting thoughts away from the self, and towards others, would help buffer the stress response. However, we found no association of cortisol and the social dimension of thought; neither during stress nor in its absence. With respect to the social context, cortisol levels were unrelated to whether participants reported being alone or in company of others. While previous investigations have reported a link between momentary solitude and increased cortisol levels, in one study this association was partly moderated by the affective states correlated with being alone21. In another study, the association of cortisol and being alone significantly declined with age28, while a prior study by the same author found no association in an adult population56. Based on these findings suggestive of an age-dependent effect, it may have been unlikely to detect an influence of solitude on cortisol given the considerable age range of our sample (20–55 years).
Our study has some limitations. For practical reasons, we assessed cortisol and subjective experience simultaneously. However, some studies have argued for a staggered assessment to account for the dynamics of cortisol release27. The current study also would have benefitted from an objective verification of sampling times. Hence, the respective data have to be treated with some caution as the possibility of non-adherence-related confounding cannot be excluded57. Also, it would have been advantageous to increase the minimal required time interval between any oral intake and sampling as has been proposed in other studies (e.g.18).
Thought content in the present study was operationalized quite broadly involving three bipolar dimensions. This precluded more complex analyses that have been done in the past, such as PCA40,41, and limits the precision with which we were able to draw conclusions about the specific nature of thought. It would be interesting to know whether past thoughts are predominantly ruminative in quality or whether future thoughts imply worry. Not critical to our results, but certainly of interest, is to what extent our thought samples reflected mindwandering episodes (i.e., were mainly task-unrelated and self-generated), or if they were predominantly driven by the external environment. Considering that mindwandering episodes occur frequently in daily life, occupying an estimated 25% to 50% of our waking time58,59, certainly a fair share of the thoughts we captured here overlapped with the mindwandering characteristics of being unrelated to current task or environment. Future studies may wish to examine how thought content in a naturalistic environment translates to the frequency and content of mindwandering in the laboratory. This would be particularly fruitful considering the ongoing debate about the costs and benefits of the wandering mind60.
The major objective of our study was to explore the role of thought content and its interaction with experienced stress in predicting HPA axis activity in daily life. Building upon previous laboratory studies41,61, we examined thought-body correlations in an ecologically valid setting. We show that over a wide range of activities and contexts, throughout daily life, the content of thought is associated with cortisol release. When exploring everyday subjective experience, not only affective but also cognitive measures should be considered to further our understanding of how psychological processes shape our physiological stress levels. As stressful episodes are an inevitable part of modern life, learning to be aware and more actively control our daily thoughts may be an important first step in regulating excessive activation of the HPA axis. As a next step, within the scope of the ReSource Project, we will investigate whether distinct mental training modules targeting the plasticity of mental content indeed have the capacity to change our thought-body correlations towards the aim of reducing stress and fostering well-being.
Methods
Participants
Participants attended the baseline measurement of the ReSource Project, a large-scale, longitudinal, mental training study conducted in Leipzig and Berlin62. Volunteers were recruited in winter 2012/2013 and underwent a comprehensive mental health diagnostic interview with a trained clinical psychologist. The interview included a computer-assisted German version of the structured Clinical Interview for DSM-IV Axis-I disorders, the SCID-I DIA-X64, and a personal interview for Axis-II disorders, the SCID-II64,65. Volunteers were excluded if they fulfilled criteria for an Axis-I disorder within the past two years, or for schizophrenia, psychotic disorder, bipolar disorder, substance dependency or an Axis-II disorder at any time in their life. Volunteers taking medication influencing the HPA axis were also excluded. Details of the multistep recruitment procedure, the complete list of inclusion/exclusion criteria and the final sample description of the ReSource Project can be found in Singer et al.62. Of the total 332 participants enrolled in the ReSource Project, 289 (172 women, mean ± SD = 40.6 ± 9.3 years, 20–55 years, all Caucasian) provided data for the present study, which was conducted at the baseline testing time-point before any mental training had taken place. The missing 43 participants either dropped out of the study (N = 22, 12 women), were excluded from the study (N = 18, 12 women), were repeatedly unavailable or provided insufficient data for the experience sampling (N = 3, 1 woman). Reasons for drop-out were time constraints (N = 17, 8 women) and discomfort with the study or experiments (N = 5, 4 women). Reasons for exclusion were medical (N = 12, 8 women) or prior mental training experience (N = 6, 4 woman). A higher education degree (e.g. university) was held by 65.4% of participants, whereas 24.7% held a higher education entrance qualification (e.g., high-school diploma) and 9.89% received lower secondary education. Participants’ median annual household net income was €30,000 (mean ± SD = 35,832 ± 20,493). The ReSource Project was registered with the Protocol Registration System of ClinicalTrial.gov on April 16th, 2013 under the title “Plasticity of the Compassionate Brain” (Identifier NCT01833104). It was approved by the Research Ethics Boards of Leipzig University (ethic number: 376/12-ff) and Humboldt University Berlin (ethic numbers: 2013-20, 2013-29, 2014-10). All methods were performed in accordance with the protocols mentioned above. Participants gave written informed consent, could withdraw from the study at any time and were financially compensated.
Procedure
Participants were advised to choose two regular consecutive weekdays, representative of their daily life routines, for saliva- and experience sampling (e.g. Mondays/Tuesdays, Wednesdays/Thursdays or Thursdays/Fridays, depending on their availability). All experience sampling data was collected in a standardized manner using mobile devices equipped with a customized software application that reminded participants to take each sample at the designated time of day. Before data collection, participants received an introductory training to ensure proficiency in handling the mobile device and self-administering saliva samples.
In detail, sampling times for saliva were immediately upon free awakening (while still in bed), and 30, 60, 240, 360, 480 and 600 minutes thereafter. Experience sampling questionnaires were answered upon waking and in intervals of 120 minutes until 12 hours after wake up (i.e., 120, 240, 360, 480, 600 and 720 minutes, see Fig. 4). The awakening probe, as well as probes at 240, 360, 480 and 600 minutes overlapped with the cortisol sampling schedule. Because we focus only on concurrent samples of subjective experience and cortisol, the samples taken at 30 and 60 minutes after waking (which capture the cortisol awakening response) were not included in the current analysis. Sampling times during the day had a margin of fluctuation (+/−15 min of the fixed interval) to avoid complete predictability.
Figure 4.
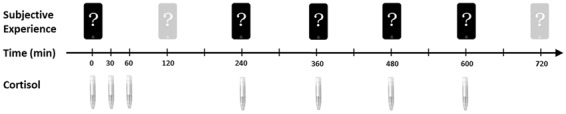
Time schedules of salivary cortisol and experience samples relative to wakeup.
Measures
Salivary cortisol
Saliva was sampled with Salivette collection devices (Sarstedt, Nümbrecht, Germany). Participants were instructed to place the collection swabs in their mouths and to refrain from chewing for 2 min. They were asked to take nothing by mouth other than water, to refrain from brushing their teeth for 10 minutes, and from smoking for 30 minutes prior to sampling. If deviating from this guideline, they were asked to thoroughly rinse their mouth with water before taking a sample. Participants otherwise followed their normal daily routine. To control for confounding effects of female hormonal status on cortisol66, hormonal status was assessed via self-report prior to the sampling days (i.e., no menstrual cycle because of menopause or polycystic ovary syndrome, hormonal contraceptives, natural menstrual cycle, male). Salivettes were stored in participants’ freezers until returned to the laboratory where they were stored at −30 °C until assay (Department of Biological and Clinical Psychology, University of Trier, Germany). Cortisol levels (expressed in nmol/l) were determined using a time-resolved fluorescence immunoassay with intra-/interassay variabilities of <10%/12%67.
Subjective experience
Throughout the day, when probed, participants provided information on their momentary subjective experience including thought content, affect and arousal, and the occurrence of subjective stress. Further contextual information included the current activity and whether participants were alone or in the company of others when sampling.
Thought content: Participants indicated current thought content along the three dimensions of the cube of thought40. Unlike previous studies, which used PCA to extract patterns of thought content based on covariance of several single content items40,41, in the current study we operationalized thought content along only three dimensions with opposing poles (e.g., negative to positive), precluding the previously used approach. The thought dimensions were represented by three visual analogue scales (valence, temporal and social), each ranging from 1–20 with written anchors at each pole (“Please categorize your current thoughts”). The valence scale was anchored by ‘negative’ and ‘positive’, the temporal scale by ‘past-oriented’ and ‘future-oriented’ and the social scale by ‘self-oriented’ and ‘other-oriented’.
Affect and arousal: Measures of momentary affect and arousal were obtained using the Affect Grid, a single item scale assessing the dimensions pleasure-displeasure and arousal-sleepiness (both on a scale from 1–9). The Affect Grid has adequate reliability, convergent and discriminant validity68.
Subjective stress: At each sampling point, participants reported on the occurrence of subjective stress in a yes/no format. If they had experienced a stressful event since taking their last sample, they additionally indicated how stressed they felt and how well they were able to cope with the stressor (both on visual analog scales ranging from 1–20). Since these stress characteristics were contingent on reporting a subjective stress experience, they were only available in a subsample and were thus analysed separately (see Supplementary Materials).
Company: Participants reported on whether they were currently alone or in company of others when completing a probe. If with others, they additionally indicated how close they felt to their company (on a visual analog scale ranging from 1–20).
Sleep: Duration (in hours) and quality of sleep (on a visual analog scale ranging from 1–20) was reported immediately upon awakening in parallel to taking the first Salivette of the day.
Statistical analysis
To assess the moment-to-moment associations of cortisol and self-report data, a multi-level linear mixed effects model was fitted to the hierarchical data structure: Samples (level 1) within days (level 2) were nested within individuals (level 3). The model integrates all available parallel measures of cortisol and subjective experience (at 240, 360, 480 and 600 minutes) and includes day- and person-specific fixed and random factors.
Initially, an empty multi-level model including only a random intercept for subject was fitted to the data in order to assess the intra class coefficient (ICC). For the final model, predictors entering on the sample level (level 1) were the cortisol sampling time relative to awakening (time), the occurrence of subjective stress (stress), affect and arousal, the respective thought content dimensions (valence, temporal, social), whether participants were currently with someone or alone (company) and the interaction terms of subjective stress with affect, arousal and thought content (stress*affect, stress*arousal, stress*valence, stress*temporal, stress*social). Predictors corresponding to each day (level 2) were sleep duration, sleep quality, and awakening time, nested within individuals (level 3) with a fixed factor for sex. A random intercept for subjects and days nested into subjects as well as random slopes for stress and time were included to increase model fit. The final model including all fixed and random effects is specified below in Raudenbush and Bryk69 notation:
| 1 |
| 2 |
| 3 |
Cortisol levels for a sample s on day d for subject i (Level 1) were predicted by the intercept (π0di), the time of the sample relative to waking up (π1di), the occurrence of subjective stress (π2di), company (π13di) and the affective and cognitive measures of subjective experience (π3di − π7di) as well as their respective interactions with subjective stress (π8di − π12di).
On level 2, β01i − β03i describe the day-specific factors entering the model (sleep duration, sleep quality, awakening time), and u0di, u1di, u2di, are the respective random effects of intercept, time and stress, which account for variation within individuals across days.
On level 3, γ001 reflects the individuals’ sex, while r00i, r10i and r20i are the random factors accounting for individual differences in the intercept, time and stress.
The final model included only a linear time term. Adding a quadratic time term to the model yielded only very minimal increases in explained variance, particularly in comparison to the linear term, did not change the pattern of results and was thus not included. Likewise, the additional person-specific covariates age and hormonal status were not included in the final model since they had no significant effect on cortisol and did not improve the relative model quality.
Raw cortisol values were ln-transformed before analysis to account for positive skew (however analyses with raw values yielded negligible differences with respect to the estimated effects). The model’s residuals displayed satisfactory approximation to normal distribution and calculation of variance inflation factors indicated moderate, but tenable levels of multicollinearity of the model factors70. Estimates reported are restricted maximum likelihood marginal estimates using an unstructured covariance structure.
Several additional analyses were conducted for conditional subsamples. The respective models were derived from the main model specified above and fit to assess a) the effects of stressor characteristics on cortisol levels in the samples reporting subjective stress, and b) the effects of closeness of one’s company for the samples in which participants reported being in company of others (see Supplementary Materials for details).
Finally, to examine whether thought content differed in samples with reported subjective stress, as compared to non-stress samples, t-tests were calculated for measures of subjective experience. We used Welch’s t-tests for these independent samples with unequal variances and unequal sample sizes71. Variance components explained by the fixed and random factors were examined by stepwise addition of factors of interest to an empty model only containing random factors and subsequent comparison to the final model72. All analyses were performed using R version 3.3.2 (R Core Team, 2016) and the packages lme4, car and Mumin were used to fit models and obtain p-values and measures of explained variance. Significance was set to a level of p < 0.05.
Electronic supplementary material
Acknowledgements
We are thankful to the members of the Social Neuroscience Department involved in the ReSource Project over many years. M. Bolz, and S. Zurborg for managing the large-scale study; E. Murzik, N. Otto, S. Tydecks, and K. Träger for help with recruiting; H. Grunert for technical assistance; H. Niederhausen and T. Kästner for data management; and J. Grant for proofreading the manuscript. We also thank the research assistants and students, especially L. Nix for their help in carrying out the testing. T. S. as the principal investigator received funding for the ReSource Project from the European Research Council (ERC) under the European Community’s Seventh Framework Programme (FP7/2007–2013) ERC grant agreement number 205557 and the Max Planck Society.
Author Contributions
T.S. and V.E. developed the study concept. T.S., V.E. and R.L. contributed to the study design. Testing, data collection and analyses were performed by R.L. and V.E. The manuscript was drafted by R.L., and V.E. and T.S. provided critical revisions. All authors approved the final version of the manuscript for submission.
Data Availability.
The datasets generated and/or analysed during the current study are not publicly available due to ongoing analyses in the context of the large-scale ReSource Project. The data are available on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
T. Singer and V. Engert jointly supervised this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-33708-0.
References
- 1.Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5:374–381. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- 2.Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. JAMA. 2007;298:1685–1687. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- 3.Sterling P. Allostasis: a model of predictive regulation. Physiology & behavior. 2012;106:5–15. doi: 10.1016/j.physbeh.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 4.McEwen BS. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. European journal of pharmacology. 2008;583:174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Juster R-P, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neuroscience & Biobehavioral Reviews. 2010;35:2–16. doi: 10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 6.McEwen BS, Stellar E. Stress and the individual. Mechanisms leading to disease. Arch Intern Med. 1993;153:2093–2101. doi: 10.1001/archinte.1993.00410180039004. [DOI] [PubMed] [Google Scholar]
- 7.Gold P. The organization of the stress system and its dysregulation in depressive illness. Molecular psychiatry. 2015;20:32–47. doi: 10.1038/mp.2014.163. [DOI] [PubMed] [Google Scholar]
- 8.Cowen P. Not fade away: the HPA axis and depression. Psychological medicine. 2010;40:1–4. doi: 10.1017/S0033291709005558. [DOI] [PubMed] [Google Scholar]
- 9.Adam EK, et al. Prospective prediction of major depressive disorder from cortisol awakening responses in adolescence. Psychoneuroendocrinology. 2010;35:921–931. doi: 10.1016/j.psyneuen.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adam EK, et al. Prospective associations between the cortisol awakening response and first onsets of anxiety disorders over a six-year follow-up–2013 Curt Richter Award Winner. Psychoneuroendocrinology. 2014;44:47–59. doi: 10.1016/j.psyneuen.2014.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adam EK, et al. Diurnal cortisol slopes and mental and physical health outcomes: A systematic review and meta-analysis. Psychoneuroendocrinology. 2017;83:25–41. doi: 10.1016/j.psyneuen.2017.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Almeida DM. Resilience and vulnerability to daily stressors assessed via diary methods. Curr Dir Psychol Sci. 2005;14:64–68. doi: 10.1111/j.0963-7214.2005.00336.x. [DOI] [Google Scholar]
- 13.DeLongis A, Coyne JC, Dakof G, Folkman S, Lazarus RS. Relationship of daily hassles, uplifts, and major life events to health status. Health Psychology. 1982;1:119–136. doi: 10.1037/0278-6133.1.2.119. [DOI] [Google Scholar]
- 14.Lazarus RS. Puzzles in the study of daily hassles. J Behav Med. 1984;7:375–389. doi: 10.1007/BF00845271. [DOI] [PubMed] [Google Scholar]
- 15.Charles ST, Piazza JR, Mogle J, Sliwinski MJ, Almeida DM. The wear and tear of daily stressors on mental health. Psychol Sci. 2013;24:733–741. doi: 10.1177/0956797612462222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Denson TF, Spanovic M, Miller N. Cognitive appraisals and emotions predict cortisol and immune responses: a meta-analysis of acute laboratory social stressors and emotion inductions. Psychol Bull. 2009;135:823–853. doi: 10.1037/a0016909. [DOI] [PubMed] [Google Scholar]
- 17.Kudielka BM, Gierens A, Hellhammer DH, Wust S, Schlotz W. Salivary cortisol in ambulatory assessment–some dos, some don’ts, and some open questions. Psychosom Med. 2012;74:418–431. doi: 10.1097/PSY.0b013e31825434c7. [DOI] [PubMed] [Google Scholar]
- 18.Adam EK, Hawkley LC, Kudielka BM, Cacioppo JT. Day-to-day dynamics of experience–cortisol associations in a population-based sample of older adults. Proc Natl Acad Sci USA. 2006;103:17058–17063. doi: 10.1073/pnas.0605053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol Psychiatry. 2003;54:1389–1398. doi: 10.1016/S0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- 20.Doane LD, Adam EK. Loneliness and cortisol: momentary, day-to-day, and trait associations. Psychoneuroendocrinology. 2010;35:430–441. doi: 10.1016/j.psyneuen.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matias GP, Nicolson NA, Freire T. Solitude and cortisol: associations with state and trait affect in daily life. Biol Psychol. 2011;86:314–319. doi: 10.1016/j.biopsycho.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 22.Kumari M, et al. Self-reported sleep duration and sleep disturbance are independently associated with cortisol secretion in the Whitehall II study. J Clin Endocrinol Metab. 2009;94:4801–4809. doi: 10.1210/jc.2009-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ockenfels MC, et al. Effect of Chronic Stress Associated with Unemployment on Salivary Coltisol - Overall Cortisol-Levels, Diurnal Rhythm, and Acute Stress Reactivity. Psychosomatic Medicine. 1995;57:460–467. doi: 10.1097/00006842-199509000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Stawski RS, Cichy KE, Piazza JR, Almeida DM. Associations among daily stressors and salivary cortisol: findings from the National Study of Daily Experiences. Psychoneuroendocrinology. 2013;38:2654–2665. doi: 10.1016/j.psyneuen.2013.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.vanEck M, Berkhof H, Nicolson N, Sulon J. The effects of perceived stress, traits, mood states, and stressful daily events on salivary cortisol. Psychosomatic Medicine. 1996;58:447–458. doi: 10.1097/00006842-199609000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Hanson EKS, Maas CJM, Meijman TF, Godaert GLR. Cortisol secretion throughout the day, perceptions of the work environment, and negative affect. Ann Behav Med. 2000;22:316–324. doi: 10.1007/Bf02895668. [DOI] [PubMed] [Google Scholar]
- 27.Smyth J, et al. Stressors and mood measured on a momentary basis are associated with salivary cortisol secretion. Psychoneuroendocrinology. 1998;23:353–370. doi: 10.1016/S0306-4530(98)00008-0. [DOI] [PubMed] [Google Scholar]
- 28.Adam EK. Transactions among adolescent trait and state emotion and diurnal and momentary cortisol activity in naturalistic settings. Psychoneuroendocrinology. 2006;31:664–679. doi: 10.1016/j.psyneuen.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 29.Jacobs N, et al. A momentary assessment study of the relationship between affective and adrenocortical stress responses in daily life. Biol Psychol. 2007;74:60–66. doi: 10.1016/j.biopsycho.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 30.Steptoe A, Gibson EL, Hamer M, Wardle J. Neuroendocrine and cardiovascular correlates of positive affect measured by ecological momentary assessment and by questionnaire. Psychoneuroendocrinology. 2007;32:56–64. doi: 10.1016/j.psyneuen.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 31.Brosschot JF, Gerin W, Thayer JF. The perseverative cognition hypothesis: a review of worry, prolonged stress-related physiological activation, and health. J Psychosom Res. 2006;60:113–124. doi: 10.1016/j.jpsychores.2005.06.074. [DOI] [PubMed] [Google Scholar]
- 32.Watkins ER. Constructive and unconstructive repetitive thought. Psychol Bull. 2008;134:163–206. doi: 10.1037/0033-2909.134.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ottaviani C, et al. Physiological concomitants of perseverative cognition: A systematic review and meta-analysis. Psychol Bull. 2016;142:231–259. doi: 10.1037/bul0000036. [DOI] [PubMed] [Google Scholar]
- 34.Zoccola PM, Dickerson SS. Assessing the relationship between rumination and cortisol: a review. J Psychosom Res. 2012;73:1–9. doi: 10.1016/j.jpsychores.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 35.Huffziger S, et al. Effects of mood and rumination on cortisol levels in daily life: an ambulatory assessment study in remitted depressed patients and healthy controls. Psychoneuroendocrinology. 2013;38:2258–2267. doi: 10.1016/j.psyneuen.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 36.McCullough ME, Orsulak P, Brandon A, Akers L. Rumination, fear, and cortisol: An in vivo study of interpersonal transgressions. Health Psychology. 2007;26:126. doi: 10.1037/0278-6133.26.1.126. [DOI] [PubMed] [Google Scholar]
- 37.Rydstedt LW, Cropley M, Devereux J. Long-term impact of role stress and cognitive rumination upon morning and evening saliva cortisol secretion. Ergonomics. 2011;54:430–435. doi: 10.1080/00140139.2011.558639. [DOI] [PubMed] [Google Scholar]
- 38.Slatcher RB, Robles TF, Repetti RL, Fellows MD. Momentary work worries, marital disclosure, and salivary cortisol among parents of young children. Psychosom Med. 2010;72:887–896. doi: 10.1097/PSY.0b013e3181f60fcc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smallwood J, Schooler JW. The science of mind wandering: empirically navigating the stream of consciousness. Annu Rev Psychol. 2015;66:487–518. doi: 10.1146/annurev-psych-010814-015331. [DOI] [PubMed] [Google Scholar]
- 40.Ruby FJ, Smallwood J, Engen H, Singer T. How self-generated thought shapes mood–the relation between mind-wandering and mood depends on the socio-temporal content of thoughts. PLoS One. 2013;8:e77554. doi: 10.1371/journal.pone.0077554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Engert V, Smallwood J, Singer T. Mind your thoughts: associations between self-generated thoughts and stress-induced and baseline levels of cortisol and alpha-amylase. Biol Psychol. 2014;103:283–291. doi: 10.1016/j.biopsycho.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 42.Csikszentmihalyi M, Larson R. Validity and reliability of the Experience-Sampling Method. J Nerv Ment Dis. 1987;175:526–536. doi: 10.1097/00005053-198709000-00004. [DOI] [PubMed] [Google Scholar]
- 43.Ruby, F. J., Smallwood, J., Sackur, J. & Singer, T. Is self-generated thought a means of social problem solving? Frontiers in psychology4 (2013). [DOI] [PMC free article] [PubMed]
- 44.Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- 45.Peeters F, Nicholson NA, Berkhof J. Cortisol responses to daily events in major depressive disorder. Psychosomatic medicine. 2003;65:836–841. doi: 10.1097/01.PSY.0000088594.17747.2E. [DOI] [PubMed] [Google Scholar]
- 46.Kirschbaum C, Pirke KM, Hellhammer DH. The Trier Social Stress Test - a Tool for Investigating Psychobiological Stress Responses in a Laboratory Setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- 47.Kuppens P, Oravecz Z, Tuerlinckx F. Feelings change: accounting for individual differences in the temporal dynamics of affect. Journal of personality and social psychology. 2010;99:1042. doi: 10.1037/a0020962. [DOI] [PubMed] [Google Scholar]
- 48.Verduyn P, Delvaux E, Van Coillie H, Tuerlinckx F, Van Mechelen I. Predicting the duration of emotional experience: two experience sampling studies. Emotion. 2009;9:83. doi: 10.1037/a0014610. [DOI] [PubMed] [Google Scholar]
- 49.Aspinwall LG, Taylor SE. A stitch in time: self-regulation and proactive coping. Psychological bulletin. 1997;121:417. doi: 10.1037/0033-2909.121.3.417. [DOI] [PubMed] [Google Scholar]
- 50.Aspinwall LG. The psychology of future-oriented thinking: From achievement to proactive coping, adaptation, and aging. Motivation and Emotion. 2005;29:203–235. doi: 10.1007/s11031-006-9013-1. [DOI] [Google Scholar]
- 51.Taylor SE, Pham LB, Rivkin ID, Armor DA. Harnessing the imagination: Mental simulation, self-regulation, and coping. American psychologist. 1998;53:429. doi: 10.1037/0003-066X.53.4.429. [DOI] [PubMed] [Google Scholar]
- 52.Gilbert K, Mineka S, Zinbarg RE, Craske MG, Adam EK. Emotion regulation regulates more than emotion: Associations of momentary emotion regulation with diurnal cortisol in current and past depression and anxiety. Clinical Psychological Science. 2017;5:37–51. doi: 10.1177/2167702616654437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shirtcliff EA, Peres JC, Dismukes AR, Lee Y, Phan JM. Hormones: Commentary: Riding the physiological roller coaster: Adaptive significance of cortisol stress reactivity to social contexts. Journal of personality disorders. 2014;28:40–51. doi: 10.1521/pedi.2014.28.1.40. [DOI] [PubMed] [Google Scholar]
- 54.Hoyt LT, Zeiders KH, Ehrlich KB, Adam EK. Positive upshots of cortisol in everyday life. Emotion. 2016;16:431. doi: 10.1037/emo0000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brosschot JF, Verkuil B, Thayer JF. Exposed to events that never happen: Generalized unsafety, the default stress response, and prolonged autonomic activity. Neuroscience & Biobehavioral Reviews. 2017;74:287–296. doi: 10.1016/j.neubiorev.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 56.Adam, E. Being together, working apart: Dual career families and the work-life balance. (2005).
- 57.Stadler T, et al. Assessment of the cortisol awakening response: Expert consensus. Psychoneuroendrocrinology. 2016;63:414–432. doi: 10.1016/j.psyneuen.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 58.Kane MJ, et al. For whom the mind wanders, and when: An experience-sampling study of working memory and executive control in daily life. Psychological science. 2007;18:614–621. doi: 10.1111/j.1467-9280.2007.01948.x. [DOI] [PubMed] [Google Scholar]
- 59.Killingsworth MA, Gilbert DT. A wandering mind is an unhappy mind. Science. 2010;330:932–932. doi: 10.1126/science.1192439. [DOI] [PubMed] [Google Scholar]
- 60.Mooneyham BW, Schooler JW. The costs and benefits of mind-wandering: a review. Canadian Journal of Experimental Psychology/Revue canadienne de psychologie expérimentale. 2013;67:11. doi: 10.1037/a0031569. [DOI] [PubMed] [Google Scholar]
- 61.Hoffmann F, Banzhaf C, Kanske P, Bermpohl F, Singer T. Where the depressed mind wanders: Self-generated thought patterns as assessed through experience sampling as a state marker of depression. Journal of affective disorders. 2016;198:127–134. doi: 10.1016/j.jad.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 62.Singer, T. et alThe ReSource Project: Background, design, samples, and measurements (Max Planck Institute for Human Cognitive and Brain Sciences 2016).
- 63.Wittchen, H.-U. P. H. DIA-X-Interviews: Manual für Screening-Verfahren und Interview. (Swets & Zeitlinger, 1997).
- 64.First, M., Gibbon, M., Spitzer, R. L., Williams, J. B. W. & Benjamin, L. S. Structured Clinical Interview for DSM-IV Axis II Personality Disorders, (SCID-II). (American Psychiatric Press, Inc., 1997).
- 65.Wittchen, H. U., Zaudig, M. & Fydrich, T. SKID. Strukturiertes Klinisches Interview fuer DSM-IV. Achse I und II. Handanweisung. (Hogrefe, 1997).
- 66.Kajantie E, Phillips DI. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology. 2006;31:151–178. doi: 10.1016/j.psyneuen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 67.Dressendörfer R, Kirschbaum C, Rohde W, Stahl F, Strasburger C. Synthesis of a cortisol-biotin conjugate and evaluation as a tracer in an immunoassay for salivary cortisol measurement. The Journal of steroid biochemistry and molecular biology. 1992;43:683–692. doi: 10.1016/0960-0760(92)90294-S. [DOI] [PubMed] [Google Scholar]
- 68.Russel JA, Weiss A, Mendelsohn GA. Affect grid: A single-item scale of pleasure and arousal. Journal of Personality and Social Psychology. 1989;57:493–502. doi: 10.1037/0022-3514.57.3.493. [DOI] [Google Scholar]
- 69.Raudenbush, S. W. & Bryk, A. S. Hierarchical Linear Models: Applications and Data Analysis Methods. Second edn, (Sage Publications, 2002).
- 70.Hair, J. F., Black, W. C., Babin, B. J., Anderson, R. E. & Tatham, R. L. Multivariate data analysis. Vol. 5 (Prentice Hall Upper Saddle River, NJ, 1998).
- 71.Welch BL. The generalization ofstudent’s’ problem when several different population variances are involved. Biometrika. 1947;34:28–35. doi: 10.1093/biomet/34.1-2.28. [DOI] [PubMed] [Google Scholar]
- 72.Nakagawa S, Schielzeth H. A general and simple method for obtaining R2 from generalized linear mixed‐effects models. Methods in Ecology and Evolution. 2013;4:133–142. doi: 10.1111/j.2041-210x.2012.00261.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analysed during the current study are not publicly available due to ongoing analyses in the context of the large-scale ReSource Project. The data are available on reasonable request.