Abstract
FtsZ is the main regulator of bacterial cell division. It has been implicated in acting as a scaffolding protein for other division proteins, a force generator during constriction, and more recently, as an active regulator of septal cell wall production. FtsZ assembles into a heterogeneous structure coined the Z-ring due to its resemblance to a ring confined by the midcell geometry. Here, to establish a framework for examining geometrical influences on proper Z-ring assembly and dynamics, we sculpted Escherichia coli cells into unnatural shapes using division- and cell wall-specific inhibitors in a micro-fabrication scheme. This approach allowed us to examine FtsZ behavior in engineered Z-squares and Z-hearts. We use stimulated emission depletion (STED) nanoscopy to show that FtsZ clusters in sculpted cells maintain the same dimensions as their wild-type counterparts. Based on our results, we propose that the underlying membrane geometry is not a deciding factor for FtsZ cluster maintenance and dynamics in vivo.
The FtsZ protein assembles into a structure known as ‘Z-ring’ at midcell for bacterial cell division. Here, Söderström et al. show that Z-ring assembly and dynamics in E. coli cells with unnatural shapes, such as squares and hearts, are generally similar to those observed in cells with normal shape.
Introduction
Most bacterial cells divide by binary fission, whereby one mother cell splits into two identical daughters1–3. Decades of study have led to a detailed understanding of how the cell division machinery, the divisome, carries out this task during the later stages of the cell cycle4,5. At the heart of this process is the eukaryotic tubulin homolog, FtsZ6 that, together with its membrane anchors FtsA and ZipA (in E. coli), forms an intermediate structure called the proto-ring (Fig. 1a)7. Functioning as a recruitment base, the proto-ring components then enlist the remaining essential division proteins to form a mature divisome5. As soon as it is fully assembled, the divisome starts to constrict the cell envelope by reshaping the septal geometry, ultimately leading to sequential closure of the inner and outer membranes8–10.
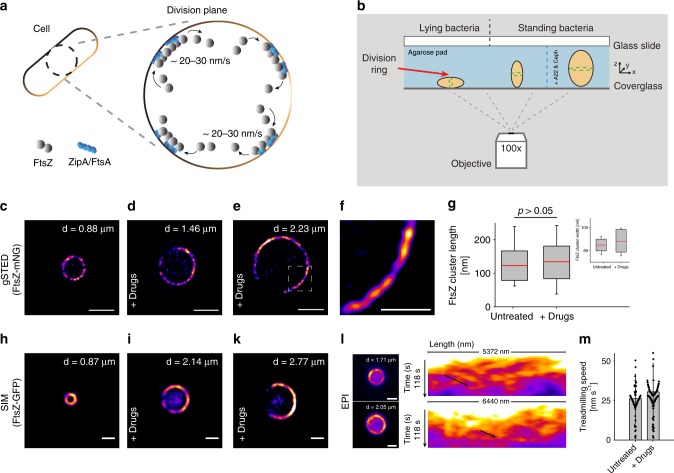
Fig. 1.
Midcell FtsZ-ring assembly is unaffected by increased cell diameter. a Simplified cartoon showing FtsZ treadmilling at the division plane of an E. coli cell. For clarity, only FtsZ (gray dots), it’s membrane tethers, FtsA and ZipA (blue dots), and the membrane (brown) are shown. b Schematic representation of cell placement for imaging. Green dotted ring in the cells represents the FtsZ-ring (red arrow). Standing cells were trapped in a vertical position in micron-sized holes in agarose pads created using micron-sized pillars. Conditions for proper division ring placement are met when width < length. The left and middle cells represent untreated cells. The cell on the right has increased dimensions due to drug exposure (A22 and cephalexin). c Time-gated STED (gSTED) image of a typical FtsZ-ring (FtsZ-mNeonGreen) in an untreated standing cell. Scale bar = 1 μm. d, e gSTED images of FtsZ-mNeonGreen rings in E. coli cells treated with drugs, showing increased ring diameter. Scale bar = 1 μm. Drugs refer to A22 and cephalexin. f Close-up of representative FtsZ clusters shown in e, from a cell with increased diameter. Scale bar = 0.5 μm. g Quantification of FtsZ cluster lengths in untreated and drug-treated cells. Mean ± S.D. was 122.8 ± 43.9 nm (n = 77) and 132.4 ± 48.7 nm (n = 172) for untreated and drug-treated cells, respectively. No statistically significant difference was measured, p > 0.05. Inset shows cluster widths in untreated (mean ± S.D. = 86.1 ± 6.3 nm (n = 77)) and drug-treated cells (mean ± S.D. = 88.4 ± 9.8 nm (n = 172)). Boxes represent S.D., with red lines indicating mean. Whiskers on the box plots encompass 95.5% of the distribution. h–k Structured illumination microscopy (SIM) images of FtsZ-GFP in E. coli cells (h) untreated or (i–k) treated with drugs. Scale bars = 1 μm. l Snapshots of epifluorescence (EPI) images from time-lapse series of FtsZ-GFP dynamics in drug-treated cells. Scale bars = 1 μm. Corresponding kymographs are shown adjacent to each image. Black arrows point to examples of FtsZ trajectories. m Average treadmilling speed of FtsZ-GFP in untreated (mean ± S.D. = 26 ± 15 nm s−1, n = 102) and drug-treated cells (mean ± S.D. = 30 ± 18 nm s−1, n = 102). Black dots represent individual data points, bars represent mean with error bars representing S.D. “d” in c–e, and h–l indicates cell diameter
In rod-shaped model bacteria such as Escherichia coli and Bacillus subtilis, FtsZ is believed to organize into short bundles of filaments, roughly 100 nm in length11,12, that treadmill at the septum with a circumferential velocity in the order of 20–30 nm s−113–15. The treadmilling filaments guide and regulate septal peptidoglycan (PG-) production and ingrowth, leading to septation16. This mode of action may be limited to rod-shaped bacteria that have two separate PG-machineries, as opposed to cocci, which have only one PG-machinery that is capable of finalizing division in cells with inhibited FtsZ dynamics17.
At a late stage of membrane constriction, but prior to inner membrane fusion, FtsZ disassembles from midcell, indicating the possible existence of an upper limit of ring curvature6,9. The actin homolog MreB is responsible for maintaining the rod shape of E. coli, and interacts directly with FtsZ, thus MreB could potentially play a role in FtsZ organization at the septum18–20. However, other purely geometrical constraints that might govern Z-ring maintenance and stability are currently unclear. In vitro data have shown that FtsZ can self-organize into swirling rings on supported bilayers when unconfined by geometrical constraints21, but we were curious as to whether geometrical changes to cell shape would influence Z-ring formation and dynamics, as this would result in a better understanding of FtsZ behavior in live cells. In this study, we systematically examine FtsZ formation, organization and behavior in E. coli cells that are sculpted into complex geometrical shapes in micron sized holes. We show that FtsZ formation and dynamics are independent of cell shape and membrane curvature.
Results
FtsZ structure and dynamics in Z-rings are not sensitive to increased ring size
As a reference for unmodified division rings, we imaged Z-rings in E. coli cells expressing FtsZ-mNeonGreen as the only source of FtsZ22. Under our experimental conditions, this strain produced normal-looking, sharp Z-rings (Supplementary Figure 1) and grew and divided similarly to wild-type (WT) E. coli (MC4100) (Supplementary Figure 2a-e). We then trapped the cells in a vertical position in micron-sized holes that were produced in agarose pads using silica micron pillar arrays14 (Fig. 1b, Supplementary Figure 3), and imaged the cells using super-resolution time-gated STimulated Emission Depletion (gSTED) nanoscopy. In these standing cells, a heterogeneous Z-ring with distinct FtsZ-mNeonGreen clusters was clearly seen traversing the circumference of the cell (Fig. 1c), similar to what has been observed before12,14.
Previous work has shown that FtsZ clusters generally maintain the same length throughout envelope constriction12,14. We wanted to see if this was also true for unnaturally large cells, i.e., would FtsZ clusters maintain the same dimensions in Z-rings of cells with increased diameter at midcell? In order to increase cell diameter, we treated E. coli cells with A22 and cephalexin (hereafter collectively referred to as “drugs”), in a way similar to what has previously proven successful for cell shape manipulations23. A22 disrupts MreB dynamics and therefore perturbs the characteristic rod-shape of E. coli cells19,24, while cephalexin blocks cell division by inhibiting the transpeptidase activity of FtsI25. The net effect of this dual drug treatment is the growth of cells into shapeable blebs that are unable to divide (Supplementary Figure 4a).
We hypothesized that as long as cell width remains less than cell length, FtsZ molecules should be directed to midcell by the Min system23 and other FtsZ placements systems26, such that a ring-like structure may be observed in the xy-plane of vertically-oriented, standing cells (Fig. 1b). To test this, we exposed E. coli cells expressing FtsZ-mNeonGreen to drugs, and then trapped the cells vertically in holes with a diameter of up to 3.5 μm and a depth of 4.5–6 μm. It has been shown that reshaped E. coli cells longer than 6 μm may revert from pole-to-pole oscillations to high mode oscillations23. Depending on the size of the holes, cells were incubated between 120 and 240 min prior to imaging; over-incubation resulted in cells that outgrew the holes (Supplementary Figure 4b. Allowing cells to grow for longer times (>10 h) produced giant blobs with internalized FtsZ-mNeonGreen chains, see Supplementary Note 1, Supplementary Figures 5 and 6).
The resulting Z-rings in drug-treated cells spanned the midcell circumference for all cell diameters that were imaged (~ 1–3 μm) (Fig. 1d, e). Fluorescence intensity increased as ring size increased (Supplementary Figure 7), possibly indicating an upregulation of cellular FtsZ expression, assuming similar ratios of ring to non-ring associated FtsZ molecules in all cell sizes (~30% of FtsZ molecules are in the Z-ring27). Importantly, confocal Z-stacks showed that each cell contained only one Z-ring (Supplementary Figure 8 and Supplementary Movie 1). Close inspection of STED images revealed that the Z-rings in larger cells were composed of fluorescent clusters (Fig. 1f) with average lengths and radial widths of 132 ± 48 nm and 88 ± 9 nm (mean ± S.D., n = 172), respectively, which were similar to Z-ring clusters in untreated cells (p > 0.05) (Fig. 1g).
After we had established that large Z-rings can form in cells with increased diameter, we proceeded to calculate FtsZ dynamics in these larger rings. However, strains expressing FtsZ-FP (fluorescent protein (FP), e.g., mNeonGreen) as the only source of FtsZ have been shown to have a phenotype similar to that of FtsZ mutants deficient in GTPase activity, with severely impaired treadmilling speed13. Therefore, we chose to image cells that expressed FtsZ-GFP from an ectopic locus on the chromosome, in addition to native FtsZ28. Earlier studies showed that FtsZ-GFP, when expressed at levels below 50% of total cellular FtsZ levels, caused no observable phenotypic changes9,12,28,29. In our experimental setup, FtsZ-GFP was expressed at ~ 30% of total FtsZ levels (Supplementary Figure 2).
SIM of drug-treated E. coli cells expressing FtsZ-GFP showed large heterogeneous rings that were similar to those of FtsZ-mNeonGreen (Fig. 1h–k). Time-lapse imaging revealed that FtsZ clusters moved around the midcell circumference, even in Z-rings with a diameter up to three times larger than that of a WT cell (Supplementary Movie 2). There was no difference in the speed of individual clusters in the rings of untreated cells compared to those in sculpted cells that had a diameter 50–200% larger than WT (26 ± 15 nm s−1 (mean ± S.D., n = 102) and 30 ± 18 nm s−1 (mean ± S.D., n = 102), respectively) (Fig. 1l–m), suggesting that cluster treadmilling speed is not influenced by the length of the cell circumference. ZipA-GFP, an FtsZ membrane anchor, also moved at essentially the same speed in both normal-sized and large-sized rings (26 ± 8 nm s−1, mean ± S.D., n = 10) (Supplementary Figure 9 and Supplementary Movie 3), which is comparable to previously reported speeds14.
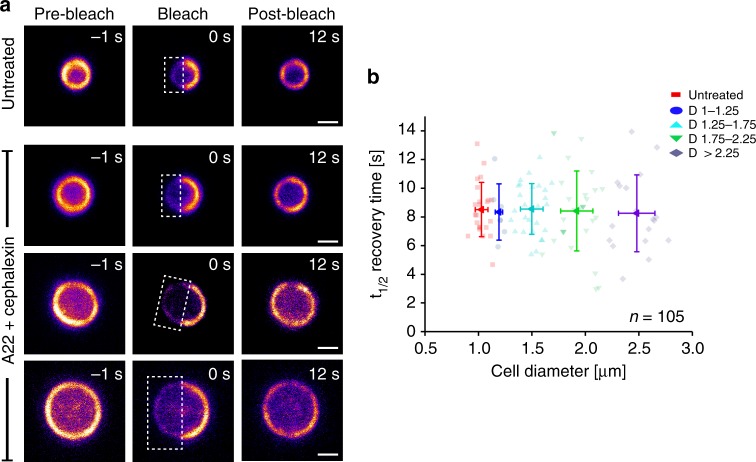
Since treadmilling behavior of FtsZ in large cells was very similar to that in WT cells, we were curious to see whether FtsZ subunit exchange in the rings would also be similar. To assess this, we performed Fluorescence Recovery After Photobleaching (FRAP) experiments on both untreated and drug-treated cells. We bleached half of the FtsZ-GFP molecules in the rings of standing cells and monitored fluorescence recovery over time (Fig. 2a). Z-rings in untreated cells had a mean t1/2 recovery time of 8.4 ± 1.9 s (mean ± S.D., n = 23) (Fig. 2b), consistent with previous studies14,30. We found that the average t1/2 recovery time was the same for Z-rings with a wide range of diameters (Fig. 2b), indicating that the rate of FtsZ subunit exchange is independent of cell diameter. This further suggests that cell size, and hence membrane curvature, might not be a factor in determining Z-ring dynamics.
Fig. 2.
Cell size independent recovery of fluorescence in FtsZ-rings. FRAP measurements on FtsZ-GFP rings in E. coli cells trapped standing in a vertical position. a Representative cells of different diameter, untreated or treated with drugs. White boxes indicate bleached areas. Scale bars = 1 μm. b Quantification of FRAP data from untreated cells (red), and drug-treated cells (other colors), showing that fluorescence recovery time is independent of cell diameter (in the range investigated, i.e., ~ 1–3 μm). ntot = 105. D = cell diameter range. Mean t1/2 recovery time WT FtsZ-rings was 8.4 ± 1.9 s (mean ± S.D., n = 23, red squares), while for large FtsZ-rings, recovery times were 8.2 ± 1.9 s (n = 7, dark blue circles), 8.4 ± 1.7 s (n = 27, light blue triangles), 8.3 ± 2.8 s (n = 30, green triangles) and 8.2 ± 2.6 s (n = 18, purple diamond) for cells with diameters in the ranges of 1–1.25 μm, 1.25–1.75 μm, 1.75–2.25 μm and > 2.25 μm, respectively. Error bars represent S.D. Note that FRAP measurements on fixed cells indicated that trapped cells were stationary, and not rotating in the holes (Supplementary Figure 10)
The FtsZ-square
Next, we wanted to know if drug-treated cells placed in deep (5 μm) rectangular volumes would adapt to these shapes and effectively form Z-rectangles or Z-squares instead of Z-rings. Previous work has shown that cells can adapt to rectangular shapes in shallow wells, approximately 1 μm deep23. Here, we produced quadrilateral patterns in agarose pads using silica micron pillar arrays similar to those previously described14, with the exception that the pillars were rectangular and 5.5 ± 0.5 μm in height. Sides of the micron chambers were up to 3.5 μm in length (Supplementary Figure 11), resulting in well volumes up to 80 μm3, roughly 50-fold larger than the volume of a WT cell (assuming a WT cell size of 2 μm in length and 1 μm in width) (Supplementary Figure 12).
Drug-exposed cells expressing FtsZ-mNeonGreen were placed in rectangular micron holes and incubated at room temperature for 300–420 min (longer incubation times were needed due to increased well size). The cells adapted to their new shapes and formed rectangular cuboids with only one Z-square per cell (Fig. 3a, Supplementary Movie 4). Notably, FtsZ clusters were observed both in the sharp corners and along the sides of the rectangles (Fig. 3b, Supplementary Figure 13). Quantification of the FtsZ-mNeonGreen clusters showed that they had similar dimensions to those in untreated cells, with an average length of 105.4 ± 39.6 nm and radial width of 79.6 ± 18.2 nm (n = 147) (Fig. 3c). This suggests that FtsZ cluster dimensions in vivo are insensitive to membrane curvature (or lack thereof).
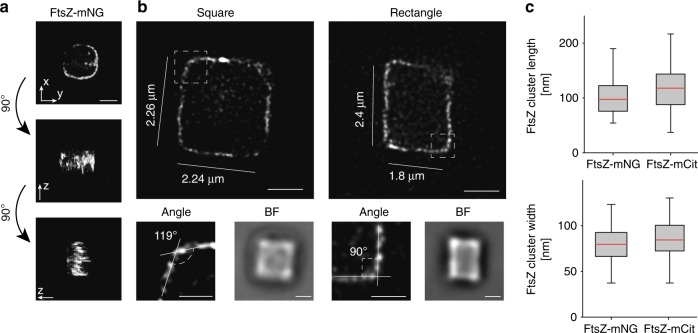
Fig. 3.
FtsZ-squares and FtsZ-rectangles in shaped cells. Drug-treated (A22 and cephalexin) E. coli cells expressing FtsZ-mNeonGreen were sculpted into rectangular shapes and imaged using super-resolution STED nanoscopy. a 3D rendering of a confocal Z-stack of an FtsZ-mNeonGreen square, showing only one band of FtsZ. Note that information along the z-axis is elongated. b Representative STED images of FtsZ-mNeonGreen in square (left) and rectangular (right) cells with perimeters ranging from 8.4 to 11.52 μm (compared to WT ~ 3 μm). Additional examples are provided in Supplementary Figure 13. Close-up images show representative corner angles. BF, brightfield image of corresponding cells. c Quantification of FtsZ cluster dimensions, showing little difference between FtsZ-mNeonGreen (105.4 ± 39.6 nm, 79.6 ± 18.2 nm; mean ± S.D., length and width, respectively. n = 147) and FtsZ-mCitrine (118.3 ± 41.3 nm, 86.3 ± 22.5 nm; length and width, respectively. n = 162. Example images of FtsZ-mCitrine squares are shown in Supplementary Figure 14). Scale bars = 1 μm. Boxes represent S.D., with red lines indicating mean. Whiskers on the box plots encompass 95.5% of the distribution
To generate a fluorescent FtsZ fusion protein that could be used for both super-resolution STED imaging and examination of cluster dynamics when grown in rich media at 37 °C, we constructed a plasmid-expressed FtsZ-mCitrine fusion. FtsZ-mCitrine was expressed from an IPTG-inducible, medium copy-number plasmid, pTrc99a, at a level approximately equal to 30% of total cellular FtsZ. Under these conditions, FtsZ-mCitrine formed normal-looking, sharp Z-rings (Supplementary Figures 1 and 2). Cells expressing FtsZ-mCitrine were then exposed to drugs, trapped in rectangular micron-sized holes, and incubated for 180–280 min at room temperature before gSTED imaging. We found that FtsZ-mCitrine formed clusters that were 118.3 ± 41.3 nm long and 86.3 ± 22.5 nm wide radially (n = 162), similar to FtsZ-mNeonGreen cluster dimensions (Fig. 3c, Supplementary Figure 14), indicating that fluorophore choice did not influence cluster dimensions in the rings. For consistency, we also imaged rectangular cells expressing FtsZ-GFP from the chromosome using SIM (Supplementary Figure 14). All three strains tested adapted to the rectangular shape, producing sharp-cornered Z-rectangles.
FtsZ dynamics in rectangular-shaped cells
In order to examine the dynamics of FtsZ in rectangular cells, we performed time-lapse imaging on cells expressing either FtsZ-mCitrine or FtsZ-GFP. Although a few fluorescence spots were abnormally bright and immobile (~ 1 spot/5 cells, with a maximum of 2 spots in one cell) (Fig. 4b, Supplementary Movie 7, red arrow), the majority of FtsZ clusters were highly dynamic (Fig. 4a, b, Supplementary Movies 5–6). Note that the bright, immobile spots were excluded from treadmilling analyses. Close inspection of time-lapse sequences suggested that FtsZ clusters in rectangular-shaped cells could treadmill continuously around the perimeter of the cells (Fig. 4a), and importantly, even across sharp corners, without an apparent change in speed (Fig. 4b, c, Supplementary Movie 8). The average treadmilling speed of FtsZ-mCitrine clusters in rectangular cells with perimeter lengths up to 13 μm (more than four times the circumference of a WT cell) was 27.6 ± 12.5 nm s−1 (n = 109), which was consistent with the measured treadmilling speed of FtsZ-GFP in rectangular cells (25.3 ± 11.3 nm s−1, n = 122) (Fig. 4d), large cylindrical cells (30 ± 18 nm s−1, Fig. 1m) and untreated cells (~25 nm s−1)13,14.
Fig. 4.
FtsZ dynamics in rectangular-shaped cells. The dynamics of FtsZ in rectangular shapes were assessed by time-lapse imaging and FRAP measurements on E. coli cells expressing FtsZ-mCitrine (FtsZ-mCit) or FtsZ-GFP. a–c Snapshot images from time-lapse series of FtsZ-mCitrine or FtsZ-GFP in rectangular shaped cells. Corresponding kymographs are shown next to each image. a Kymographs were taken around the entire perimeter (starting at the upper left corner, moving counter-clockwise, indicated by the yellow arrowheads). b Kymographs were taken along the white line (left kymograph), or over the bright spot indicated by the red arrow (right kymograph). FtsZ can clearly be seen treadmilling continuously across the sharp corner (indicated by the red arrowhead). The red arrows and arrowheads in the images correspond to the arrows and arrowheads on the kymographs. The black dashed line in b indicates the upper left corner of the cell. c Kymograph taken between the yellow arrowheads (top to bottom is left to right in the kymograph). d Average treadmilling speed of FtsZ-mCitrine and FtsZ-GFP in rectangles was 27.6 ± 12.5 nm s−1 (n = 97) and 25.3 ± 11.3 nm s−1 (n = 122), respectively. e Typical FRAP measurement of FtsZ-GFP in a rectangular E. coli cell. Half of the rectangle was bleached. f Average recovery times for FtsZ-mCitrine (dark, ntot = 24) and FtsZ-GFP (light, ntot = 22) in FtsZ-rectangles of various perimeter lengths. FRAP recovery times for rectangular cells with different perimeters: FtsZ-mCitrine t1/2 recovery times: 9 ± 2.9 s (Circ. 8 ± 1 μm, n = 6), 10 ± 3.2 s (Circ. 10 ± 1 μm, n = 7), 10.4 ± 2.3 s (Circ. 12 ± 1 μm, n = 7), 9.9 ± 1.9 s (Circ. 14 ± 1 μm, n = 4). FtsZ-GFP t1/2 recovery times: 8.1 ± 1.9 s (Circ. 8 ± 1 μm, n = 5), 8.8 ± 2.6 s (Circ. 10 ± 1 μm, n = 5), 10.1 ± 2.5 s (Circ. 12 ± 1 μm, n = 8), 9.7 ± 3.2 s (Circ. 14 ± 1 μm, n = 4). Circ. = Cell circumference. Values represent mean ± S.D. Dots represent individual data points, bars represent mean with error bars representing S.D. Scale bars = 1 μm
To determine whether the dynamics of FtsZ subunit exchange are affected by changes to circumferential length and shape, we collected FRAP measurements on FtsZ bundles in rectangular-shaped cells (Fig. 4e, Supplementary Movie 9). The recovery times of half-bleached rectangles of varying sizes matched those of rings, with mean t1/2 recovery times of 9.85 ± 2.58 s (n = 24) and 9.15 ± 2.55 s (n = 22) for FtsZ-mCitrine and FtsZ-GFP, respectively (Fig. 4f). This suggests that subunit exchange from the cytoplasmic FtsZ pool is independent of circumference length and membrane curvature. The data thus far indicate that the maintenance and dynamics of FtsZ clusters are preserved in both large Z-rings and Z-rectangles of varying size.
FtsZ dimensions and dynamics in heart-shaped cells
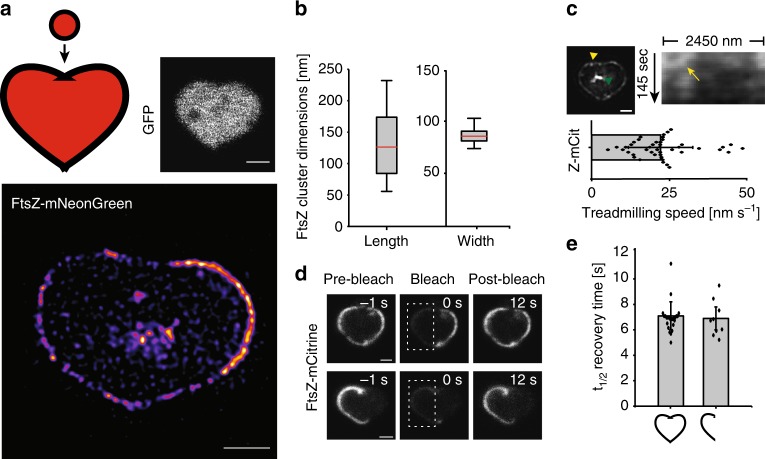
To examine whether FtsZ could literally be (at) the heart of cell division, we engineered micron pillar arrays that were heart-shaped (Supplementary Figure 15). Heart shapes were chosen because they would sculpt cells in such a way that highly curved, straight, and angled membrane segments would be present within a single cell. Drug-treated E. coli cells expressing cytoplasmic GFP, FtsZ-mNeonGreen or FtsZ-mCitrine were sculpted into hearts as described above (Fig. 5a). Perhaps not surprisingly, quantification of 155 individual FtsZ clusters from the heart-shaped cells revealed dimensions similar to those in round and rectangular cells (129 ± 44 nm long and 84 ± 9 nm wide) (Fig. 5b). We also found that the average treadmilling speed of FtsZ-mCitrine in heart-shaped cells (22 ± 10 nm s−1, n = 44) was essentially the same as that in untreated cells (Fig. 5c, Supplementary Movie 10).
Fig. 5.
FtsZ cluster dimensions and dynamics in heart-shaped cells. FtsZ behavior in E. coli cells sculpted into heart shapes. a Upper left, Cartoon representation of a WT E. coli cell and a heart shape (both colored red for visualization), highlighting the large and complex structural changes of a cell-to-heart transition, approximately to scale. Upper right, Drug-treated cell expressing cytoplasmic GFP, shaped as a heart. Lower, STED image of an FtsZ-heart (FtsZ-mNeonGreen) in a drug-treated E. coli cell. b Lengths and widths of 155 individual FtsZ-mNeonGreen fluorescence clusters in cells shaped as hearts. Average length = 129 ± 44 nm and width = 84 ± 9 nm. Boxes represent S.D., with red lines indicating mean. Whiskers on the box plots encompass 95.5% of the distribution. c Upper row, SIM image from a time-lapse series (epi-fluorescence) of a heart-shaped cell expressing FtsZ-mCitrine. Green arrowhead indicates internal FtsZ clustering. Corresponding kymograph is shown adjacent to the image, and was generated starting at the yellow arrowhead in the SIM image, moving counter-clockwise for the indicated length. The yellow arrow points to an FtsZ trajectory. Lower, average treadmilling speed of FtsZ-mCitrine (Z-mCit) clusters in hearts (22.6 ± 10.4 nm s−1, n = 44). d FRAP measurements of FtsZ-mCitrine in heart-shaped cells. Top row, bleaching of half the FtsZ-mCitrine molecules in a full heart. Bottom row, bleaching of a half-full heart. No difference in recovery times was observed. e Histogram of average t1/2 recovery times calculated from FRAP measurements. Recovery in full hearts: 7.1 ± 1.1 s (n = 24), recovery in half-hearts: 6.9 ± 0.9 s (n = 9). Scale bars = 1 μm. Dots represent individual data points, bars represent mean with error bars representing S.D
For about one-third of the heart-shaped cells, we noticed bright spots of internalized FtsZ-FP signal that accumulated close to the cell center (Fig. 5c, green arrowhead). Although we couldn’t distinguish whether these were true FtsZ clusters or aggregated protein, cytoplasmic clustering of FtsZ in WT cells have previously been reported12. Furthermore, although most hearts had FtsZ-FP signal spanning the full perimeter of the cell, approximately 20% were only half-full (Fig. 5d, lower left). We do not fully understand the underlying reason for this, however it is unlikely due to image focus or cell tilt issues, as every cell was scanned in the z-direction prior to imaging. Nevertheless, when we subjected the heart-shaped cells to FRAP, fluorescence recovery rates were equal for both full and half-full hearts (Fig. 5d), with mean t1/2 recovery times of 7.1 ± 1.1 s (n = 24) and 6.9 ± 0.9 s (n = 9), respectively (Fig. 5e).
FtsZ-rings form in complex cell shapes
To explore if cell geometry plays a role in Z-ring formation, we set out to remodel cells into other complex shapes. Even though highly complex-shaped bacteria occur in nature, such as star-shaped bacteria31, we wanted to test whether rod-shaped E. coli cells would allow themselves to be drastically remodeled. Using micron pillars of various shapes, we produced holes in agarose pads such that drug-exposed cells could be sculpted into complex shapes, such as pentagons, half-moons, stars, triangles and crosses (Fig. 6a, middle row. Supplementary Figure 15). The cells conformed remarkably well to these shapes, forming sharp boundary angles < 70° (Fig. 6a, star). After we confirmed that cells could adapt to these complex shapes, we placed cells expressing FtsZ-mCitrine into the micron holes, allowed for reshaping to occur, and then imaged the cells using STED nanoscopy. Cells of all tested shapes produced FtsZ-shapes at midcell (Fig. 6a, bottom row). Subsequent analysis of the lengths and widths of the FtsZ clusters revealed little difference in dimensions between the different shapes, suggesting a minimal role of cell shape in determining FtsZ cluster dimensions in vivo (Fig. 6b). Additionally, time-lapse imaging of cells expressing FtsZ-mCitrine in various shapes showed similar dynamics to those measured in untreated cells. Specifically, FtsZ clusters treadmilled continuously over sharp corners and severe angles with an average speed of 28.7 ± 11.1 nm s−1 (Supplementary Figure 16 and Supplementary Movie 10), which is similar to the treadmilling speed in both square-shaped and WT cells.
Fig. 6.
FtsZ bundle dimensions in complex shapes. a Cells expressing cytosolic GFP or FtsZ-mCitrine were remodeled into various shapes. Top row, schematic representation of the cell shapes (colored green for visualization). Middle row, representative cells expressing cytosolic GFP, and sculpted in the corresponding shapes. Bottom row, an FtsZ-pentagon, FtsZ-half-moon, FtsZ-star, FtsZ-triangle and FtsZ-cross in sculpted cells. Scale bars = 1 μm. b Quantification of FtsZ cluster lengths and widths in shaped cells. Cluster dimensions in pentagons (l = 131.4 ± 52 nm, w = 74.6 ± 9.5 nm, n = 121), half-moons (l = 129 ± 45 nm, w = 79.9 ± 9.6 nm, n = 98), stars (l = 139.6 ± 67 nm, w = 75.5 ± 15.5 nm, n = 113), triangles (l = 110 ± 35 nm, w = 78 ± 10.7 nm, n = 81) and crosses (l = 118.9 ± 21 nm, w = 71.1 ± 17.8 nm, n = 45). l, length, w, width. Boxes represent S.D., with red lines indicating mean. Whiskers on the box plots encompass 95.5% of the distribution
Discussion
Cells, both bacterial and eukaryotic, have the ability to adapt to their local environments32–36, reverting to their original shapes after stress37,38 and dividing with striking midcell accuracy even when remodeled into irregular cell shapes32,35. In bacteria, the tubulin homolog FtsZ assembles into a ring-like structure at midcell and is responsible for overall maintenance of the cell division machinery5,6. The general dynamics and organization of the FtsZ-ring have been shown to be quite similar across many bacterial species11,13–15,17,39–42. Common to these species is confinement of the FtsZ-ring to a circular geometry at midcell. Strikingly, when purified FtsZ is placed on supported lipid bilayers, it assembles into a dynamic, swirling ring-like assembly with a diameter resembling that of wild-type E. coli cells (approximately 1 μm). This phenomenon is observed when FtsZ is anchored to the lipid bilayer, either by FtsA or a membrane targeting sequence, hinting at an intrinsically preferred FtsZ-ring curvature6,21,43.
In this study, we characterized FtsZ midcell accumulation and dynamics in cell shape-determining environments by ‘looking through the Z-ring’ along the long-axis of cells. We observed normal-looking FtsZ-rings in cells with diameters three times the size found in WT cells. However, this might not be surprising, as the total intensity fluorescence increased in large cells (Supplementary Figure 7) and considering only ~30% of the pool of FtsZ molecules are in the ring of WT cells at any given point in time27. Quantification of FtsZ cluster dimensions revealed little variation between different cell shapes, such as squares, pentagons, triangles and stars (on average 123 × 80 nm, length × width, respectively, and summarized in Table 1), suggesting that local membrane geometry has minimal influence on FtsZ cluster dimensions. Compared to untreated cells, rectangular and heart-shaped cells with perimeter lengths more than four times that of a WT cell exhibited similar FtsZ cluster dynamics, as FtsZ-FP clusters treadmilled continuously at the same average velocity throughout the perimeter of the shaped cells (including over sharp corners and regions with severe angles), and FtsZ subunit exchange occurred at similar rates, independent of cell shape and size (Table 2).
Table 1.
Summary of FtsZ cluster dimensions at midcell in various cell shapes
| Cell shape | FP | Drugsa | Length (nm) | Width (nm) |
|---|---|---|---|---|
| FtsZ cluster dimensions | ||||
| Circle (WT) | mNG | − | 123 ± 44 | 80 ± 2 |
| Circle (large) | mNG | + | 132 ± 48 | 88 ± 9 |
| Square | mNG | + | 105 ± 40 | 80 ± 18 |
| Square | mCit | + | 118 ± 41 | 86 ± 22 |
| Heart | mNG | + | 129 ± 44 | 84 ± 9 |
| Pentagon | mNG | + | 131 ± 52 | 74 ± 9 |
| Half-moon | mNG | + | 129 ± 45 | 80 ± 10 |
| Star | mNG | + | 140 ± 67 | 76 ± 16 |
| Triangle | mNG | + | 110 ± 35 | 78 ± 11 |
| Cross | mNG | + | 119 ± 21 | 71 ± 18 |
In all cell shapes, the average measured cluster lengths were within 17% of WT, while average widths were within 13%. Numbers represent mean ± S.D. Note that values have been rounded to the nearest integer
FP fluorescent protein, mNG mNeonGreen, mCit mCitrine
aDrugs; A22 [16 μm] and Cephalexin [20 μm]
Table 2.
Summary of FtsZ dynamics in various cell shapes
| Cell shape | FP | Drugsa | Treadmilling speed (nm s-1) | t1/2 recovery (s) |
|---|---|---|---|---|
| FtsZ dynamics | ||||
| Circle (WT) | GFP | − | 26 ± 15 | 8 ± 2 |
| Circle (large) | GFP | + | 30 ± 18 | 8 ± 2 |
| Square | mCit | + | 28 ± 13 | 10 ± 3 |
| Square | GFP | + | 25 ± 11 | 9 ± 3 |
| Heart | mCit | + | 23 ± 10 | 7 ± 1 |
Numbers represent mean ± S.D. Note that values have been rounded to the nearest integer
FP fluorescent protein, mCit mCitrine
aDrugs; A22 [16 μm] and Cephalexin [20 μm]
In summary, our results from different shaped cells show that Z-ring formation and dynamics are not limited to cells of a certain shape or size. This agrees with previous findings, which show that internal cellular structures are maintained in cells that have been reshaped into unnatural forms23. Our observation that FtsZ clusters conform to the geometric shape of the membrane at midcell suggests that FtsZ-ring formation is not affected by changes in membrane curvature. Indeed, cell shape and size are important for proper cellular functions44, however, with the many naturally-occurring shape variations of bacteria31,45, it is perhaps not surprising that FtsZ can adapt to changing environments without compromising its own ability to maintain fundamental functionality. Although our data do not explicitly show that sculpted cells can divide (since downstream division proteins were inhibited), the fact that the dynamic properties of FtsZ were conserved in these cells shows that the Z-ring can be decoupled from the constriction process. Presently, we have shown in vivo that E. coli FtsZ-ring formation and dynamics are conserved, irrespective of cell shape and size.
Methods
Bacterial growth
All experiments were performed in E. coli strain MC4100, unless otherwise stated. Pre-cultures were grown overnight in 20 ml of rich media (LB) at 37 °C or M9 minimal media supplemented with 1 μg ml−1 thiamine, 0.2% (w/v) glucose and 0.1% (w/v) casamino acids. The following morning, cultures were back-diluted 1:50 in either LB or M9 (with supplements) and antibiotics (ampicillin 25 μg ml−1) when needed, and incubated at 30 or 37 °C.
Fluorescent protein production
Chromosomally-encoded FtsZ-mNeonGreen was integrated at the native ftsZ locus and did not require any inducer22. Chromosomally-encoded FtsZ-GFP (strain BS001), GFPCYTO (strain BS008) and ZipA-GFP were induced with 2.5 μM, 5 μM and 50 μM IPTG, respectively9.
The plasmid pHC054 (ftsZ-mCitrine) was constructed using Gibson assembly46 to generate an IPTG-inducible FtsZ-mCitrine fusion expressed from pTrc99a47. PCR was performed using Q5 High-Fidelity DNA polymerase (New England Biolabs). A DNA fragment containing ftsZ was amplified from E. coli MC4100 genomic DNA using primers FtsZ(F) (5′-caatttcacacaggaaacagaccatggatgtttgaaccaatggaac-3′) and FtsZ(R) (5′-gcccttgctcaccatctgcaggttgttgttatcagcttgcttacgcagg-3′). mCitrine was amplified from mCitrine-N1 plasmid DNA using primers mCitrine(F) (5′-cgtaagcaagctgataacaacaacctgcagatggtgagcaagggcgaggag-3′) and mCitrine(R) (5′-ccgccaaaacagccaagcttttacttgtacagctcgtccatgc-3). pTrc99a plasmid DNA was amplified using primers pTrc99a(F) (5′-ccatggtctgtttcctgtgtg-3′) and pTrc99a(R) (5′-aagcttggctgttttggcgg-3′). The ftsZ and mCitrine coding regions are separated by a short linker encoding NNNLQ. The plasmid sequence was verified by DNA sequencing (Fasmac, Japan). FtsZ-mCitrine expression was induced with 2.5 μM IPTG. All FtsZ levels were quantified using Western blotting.
Western blot analysis
Cell extracts from a volume corresponding to 0.1 OD600 units were collected for each strain to be analyzed. The extracts were suspended in loading buffer and resolved by SDS-PAGE gel electrophoresis. Proteins were transferred to nitrocellulose membranes using a semi-dry Transfer-Blot apparatus (Bio-Rad). The membranes were blocked with 5% (w/v) milk and probed with antisera to FtsZ (Agrisera, Sweden) and detected using standard methods.
Nanofabrication of micro arrays
Micron pillars were engineered using two different, but related, approaches. The first approach was used for round and square/rectangular micron pillars, and was adapted from the refs. 14,15. Using a multi-step process similar to that described previously48, micron-scale pillars were fabricated on a silicon (Si) substrate by reactive ion etching. A pattern of hard-baked photoresist was created on a Si surface using UV lithography, to work as a mask for etching. Subsequent etching was performed using an Oxford Plasmalab100 ICP180 CVD/Etch system, with a mixture of SF6 and O2 plasma as an etchant. For our process, a SF6:O2 ratio of 1:1 was optimal. After etching, the remaining photoresist was removed by O2 plasma treatment. Pillar arrays (1 × 1 cm or 2 × 2 cm) with round pillars were engineered to contain one micron-sized pillar every 5 μm, with dimensions between 0.9 and 3.5 μm wide and 5.25 ± 0.75 μm high (Supplementary Figure 3). Pillar arrays (1 × 1 cm) with square pillars contained micron-sized pillars approximately every 5 μm, with side lengths varying between 1.8 and 3.5 μm, and heights of 5.5 ± 0.5 μm (Supplementary Figure 11).
To create more complex shapes, a second approach, based on electron beam lithography was used. For this, the micron-scale structures were fabricated on a Si substrate by a multi-step process, which was a combination of electron beam lithography and reactive ion etching techniques. Similar approaches to silicon patterning have been successfully used in a number of earlier works48–51. First, a pattern of e-beam resist was created on a Si surface using e-beam lithography. A 50 nm-thick Ti layer was then deposited, and a lift-off process was used to create a metal mask for etching. The use of a metal mask, instead of a baked e-beam resist mask, was necessary due to the high selectivity ratio required for generating structures only a few microns in height. Finally, the etching process was performed as described above, using an Oxford Plasmalab100 ICP180 CVD/Etch system and a mixture of SF6 and O2 plasma as an etchant. For our process, a SF6:O2 flow ratio of 3:2 produced the best results, with a Si:Ti etching selectivity ratio of approximately 100:1. Increased concentration of O2 in the mixture has two effects: (i) it improves etching anisotropy, which is essential for avoiding shape distortion from the undercut effect, and (ii) it reduces the selectivity ratio, as the Si etch rate gets slower. After etching, the structures were characterized using a Dektak surface profiler and SEM imaging. The micron structure arrays, which contained various shapes (hearts, triangles, pentagons, half-moons and crosses), were fabricated on 1 × 1 cm Si chips with inter-structure distances of approximately 5 μm, and structure heights of 5.5 ± 0.5 μm (Supplementary Figure 15).
Micron-sized chamber production and cell growth
Liquefied agarose (5% w/v) in M9 minimal media (supplemented with 0.2%(w/v) glucose, 0.1%(w/v) casamino acids, 2 μg ml−1 thiamine, 40 μM A22 and 20 μg ml−1 cephalexin) was dispersed on glass slides and the silica mold (pillar facing downwards) was placed on top. The molds contained either round or rectangular pillars, or various geometrical shapes, as described above. Once the agarose solidified, the mold was removed and ~ 5 μl of live cell culture at OD600 0.4–0.55 (pre-treated with 16 μM A22 for 10–15 min) was applied on top. To allow the cells to adapt to the different shapes, slides were incubated at RT or 30 °C in a parafilm-sealed petri dish together with a wet tissue to prevent drying. After incubation, cells were covered with a pre-cleaned cover glass (♯1.5) for live cell imaging. For STED imaging, cells were first fixed with ice-cold methanol for 5 min and carefully rinsed with PBS prior to cover glass application.
Microscopy
Gated STED (gSTED) images were acquired on a Leica TCS SP8 STED 3× system, using a HC PL Apo 100x oil immersion objective with NA 1.40. Fluorophores were excited using a white excitation laser operated at 488 nm for mNeonGreen and 509 nm for mCitrine. A STED depletion laser line was operated at 592 nm, using a detection time-delay of 0.8–1.6 ns for both fluorophores. The total depletion laser intensity was in the order of 20–40 MW cm−2 for all STED imaging. The final pixel size was 13 nm and scanning speed was either 400 or 600 Hz. The pinhole size was set to 0.9 AU.
Epi-fluorescence and confocal images were acquired on either a Zeiss LSM780 or Zeiss ELYRA PS1 (both equipped with a 100 × 1.46 NA plan Apo oil immersion objective) with acquisition times between 0.3 and 2 s. Time-lapse series for generating kymographs were recorded at 2 s intervals for a time period of at least 118 s.
SIM images were acquired using a Zeiss ELYRA PS1 equipped with a pco.edge sCMOS camera. The final pixel size in SIM images was 24 nm. Individual images were acquired using an acquisition time of 200 ms per image (a total of 15 images were acquired per SIM image reconstruction) and subsequently reconstructed from the raw data using ZEN2012 software. SIM time-lapse movies (containing at least 14 frames) were recorded without time delays between image stacks.
Confocal Z-stacks (focal plane ± ~3.5 μm) were acquired on a Leica TCS SP8 STED 3× system (operated in confocal mode) using predetermined optimal system settings (Leica, LAS X), with 0.22 μm steps (resulting in 30–32 images per stack), and pinhole size 1 AU. All imaging was performed at RT (~ 23–24 °C). In order to confirm that the cells were immobile, and that no visible cell movements occurred in the wells, each cell was initially monitored using brightfield and epi/confocal fluorescence illumination14. In this way, we could eliminate the contribution of motion blurring to any observed movements captured during image acquisition.
FRAP measurements
Confocal FRAP measurements were performed on a Zeiss LSM780 system using a 100 × 1.4 NA plan Apo oil immersion objective and pinhole size 60 μm14. Bleaching was performed for 0.5–0.7 s using 100% laser power applied over the region of interest. Data were collected in time intervals of 1–2 s until steady state was reached. Following background correction, and to account for overall successive bleaching, the fluorescence intensity (F) of the bleached region (half a ring) was normalized to the average ring fluorescence of an unbleached area of the same size, for each time point (t); FNORM(t) = FBLEACHED(t) * (FBLEACHED(t) + UNBLEACHED(t))−1. All data were exported to Origin9 Pro and data points were fitted to the single exponential function F(t) = Fend – (Fend—Fstart) * e−kt, where F(t) is the fluorescence intensity at time t, Fend is the fluorescence intensity at maximum recovery, Fstart is the fluorescence recovery momentarily after bleaching (at t = 0), and k is a free parameter. The recovery half-time was then extracted from t1/2 = ln 2 * k−1. Importantly, all cells were scanned from top to bottom in order to find the division plane (in which the rings reside).
Image analysis
Image analysis was performed using Fiji. When necessary, images were background-corrected using a rolling ball with radius 36. Image stacks were motion-corrected using the plug-in StackReg. Kymographs were generated from time-lapse images using the KymoResliceWide plugin (line width 5), from which treadmilling speeds were calculated using the slope of the fluorescence trace21.
STED images were deconvolved using Huygens Professional deconvolution software (SVI, the Netherlands). FtsZ-ring diameters were extracted from the average values of the Gaussian fitted fluorescence profiles drawn from 12 to 6 o’clock and 3 to 9 o’clock. Side lengths of shaped cells were determined by applying line profiles in ImageJ. The lengths and widths of individual FtsZ clusters were obtained using line scans (line size 4) over at least five randomly selected individual fluorescence spots from each deconvolved cell image, whereby a Gaussian was fitted to the intensity profiles in order to extract the full width at half maximum (FWHM). Note that the long and short axes of each individual FtsZ cluster were assigned as length and width, respectively, regardless of orientation relative to the membrane. FtsZ cluster dimensions are given in mean ± S.D. n indicates number of cells, unless explicitly specified.
Statistical analysis
For statistical analyses, two-tailed Student’s t-tests were performed using Origin Pro 9. A p-value of <0.05 was considered as statistically significant.
Electronic supplementary material
Description of Additional Supplementary Files
Acknowledgements
The authors would like to thank Prof. Harold Erickson (Duke Uni.) for sharing the FtsZ-mNeonGreen strain. Prof. Daniel Daley (Stockholm Uni.) is acknowledged for valuable suggestions to improve the manuscript. BS is supported by JSPS KAKENHI (grant number JP17K15694). Work in the SCB unit at OIST is funded by core subsidy from Okinawa Institute of Science and Technology Graduate University.
Author contributions
B.S. conceived the study and performed the experiments. A.B. and B.S. designed and engineered the micron pillar arrays. H.C. contributed reagents. B.S. and U.S. analyzed the data. B.S. wrote the manuscript with input from all authors. All authors approved the final version of the manuscript.
Data availability
The data supporting the findings of the study are available in this article and its Supplementary Information files, or from the corresponding author upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41467-018-06887-7.
References
- 1.de Boer PA. Advances in understanding E. coli cell fission. Curr. Opin. Microbiol. 2010;13:730–737. doi: 10.1016/j.mib.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lutkenhaus J, Pichoff S, Du S. Bacterial cytokinesis: from Z ring to divisome. Cytoskeleton. 2012;69:778–790. doi: 10.1002/cm.21054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meier EL, Goley ED. Form and function of the bacterial cytokinetic ring. Curr. Opin. Cell Biol. 2014;26:19–27. doi: 10.1016/j.ceb.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Haeusser DP, Margolin W. Splitsville: structural and functional insights into the dynamic bacterial Z ring. Nat. Rev. Microbiol. 2016;14:305–319. doi: 10.1038/nrmicro.2016.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.den Blaauwen T, Hamoen LW, Levin PA. The divisome at 25: the road ahead. Curr. Opin. Microbiol. 2017;36:85–94. doi: 10.1016/j.mib.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erickson HP, Anderson DE, Osawa M. FtsZ in bacterial cytokinesis: cytoskeleton and force generator all in one. Microbiol. Mol. Biol. Rev. 2010;74:504–528. doi: 10.1128/MMBR.00021-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ortiz C, Natale P, Cueto L, Vicente M. The keepers of the ring: regulators of FtsZ assembly. FEMS Microbiol. Rev. 2016;40:57–67. doi: 10.1093/femsre/fuv040. [DOI] [PubMed] [Google Scholar]
- 8.Skoog K, Söderström B, Widengren J, von Heijne G, Daley DO. Sequential closure of the cytoplasm and then the periplasm during cell division in Escherichia coli. J. Bacteriol. 2012;194:584–586. doi: 10.1128/JB.06091-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Söderström B, et al. Disassembly of the divisome in Escherichia coli: evidence that FtsZ dissociates before compartmentalization. Mol. Microbiol. 2014;92:1–9. doi: 10.1111/mmi.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egan AJF. Bacterial outer membrane constriction. Mol. Microbiol. 2018;107:676–687. doi: 10.1111/mmi.13908. [DOI] [PubMed] [Google Scholar]
- 11.Fu G, et al. In vivo structure of the E. coli FtsZ-ring revealed by photoactivated localization microscopy (PALM) PLoS One. 2010;5:e12682. doi: 10.1371/journal.pone.0012682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coltharp C, Buss J, Plumer TM, Xiao J. Defining the rate-limiting processes of bacterial cytokinesis. Proc. Natl Acad. Sci. USA. 2016;113:E1044–E1053. doi: 10.1073/pnas.1514296113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang X, et al. GTPase activity-coupled treadmilling of the bacterial tubulin FtsZ organizes septal cell wall synthesis. Science. 2017;355:744–747. doi: 10.1126/science.aak9995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Söderström B, Chan H, Shilling PJ, Skoglund U, Daley DO. Spatial separation of FtsZ and FtsN during cell division. Mol. Microbiol. 2018;107:387–401. doi: 10.1111/mmi.13888. [DOI] [PubMed] [Google Scholar]
- 15.Bisson-Filho AW, et al. Treadmilling by FtsZ filaments drives peptidoglycan synthesis and bacterial cell division. Science. 2017;355:739–743. doi: 10.1126/science.aak9973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schoenemann KM, Margolin W. Bacterial division: FtsZ treadmills to build a beautiful wall. Curr. Biol. 2017;27:R301–R303. doi: 10.1016/j.cub.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 17.Monteiro JM, et al. Peptidoglycan synthesis drives an FtsZ-treadmilling-independent step of cytokinesis. Nature. 2018;554:528–532. doi: 10.1038/nature25506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fenton AK, Gerdes K. Direct interaction of FtsZ and MreB is required for septum synthesis and cell division in Escherichia coli. EMBO J. 2013;32:1953–1965. doi: 10.1038/emboj.2013.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garner EC, et al. Coupled, circumferential motions of the cell wall synthesis machinery and MreB filaments in B. subtilis. Science. 2011;333:222–225. doi: 10.1126/science.1203285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Errington J. Bacterial morphogenesis and the enigmatic MreB helix. Nat. Rev. Microbiol. 2015;13:241–248. doi: 10.1038/nrmicro3398. [DOI] [PubMed] [Google Scholar]
- 21.Loose M, Mitchison TJ. The bacterial cell division proteins FtsA and FtsZ self-organize into dynamic cytoskeletal patterns. Nat. Cell Biol. 2014;16:38–46. doi: 10.1038/ncb2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore DA, Whatley ZN, Joshi CP, Osawa M, Erickson HP. Probing for binding regions of the FtsZ protein surface through site-directed insertions: discovery of fully functional FtsZ-fluorescent proteins. J. Bacteriol. 2017;199:e00553–16. doi: 10.1128/JB.00553-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu F, van Schie BG, Keymer JE, Dekker C. Symmetry and scale orient Min protein patterns in shaped bacterial sculptures. Nat. Nanotechnol. 2015;10:719–726. doi: 10.1038/nnano.2015.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hussain, S. et al. MreB filaments align along greatest principal membrane curvature to orient cell wall synthesis. eLife, 10.7554/eLife.32471 (2018). [DOI] [PMC free article] [PubMed]
- 25.Weiss DS, Chen JC, Ghigo JM, Boyd D, Beckwith J. Localization of FtsI (PBP3) to the septal ring requires its membrane anchor, the Z ring, FtsA, FtsQ, and FtsL. J. Bacteriol. 1999;181:508–520. doi: 10.1128/jb.181.2.508-520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rothfield L, Taghbalout A, Shih YL. Spatial control of bacterial division-site placement. Nat. Rev. Microbiol. 2005;3:959–968. doi: 10.1038/nrmicro1290. [DOI] [PubMed] [Google Scholar]
- 27.Stricker J, Maddox P, Salmon ED, Erickson HP. Rapid assembly dynamics of the Escherichia coli FtsZ-ring demonstrated by fluorescence recovery after photobleaching. Proc. Natl Acad. Sci. USA. 2002;99:3171–3175. doi: 10.1073/pnas.052595099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Söderström B, et al. Coordinated disassembly of the divisome complex in Escherichia coli. Mol. Microbiol. 2016;101:425–438. doi: 10.1111/mmi.13400. [DOI] [PubMed] [Google Scholar]
- 29.Rowlett VW, Margolin W. 3D-SIM super-resolution of FtsZ and its membrane tethers in Escherichia coli cells. Biophys. J. 2014;107:L17–L20. doi: 10.1016/j.bpj.2014.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson DE, Gueiros-Filho FJ, Erickson HP. Assembly dynamics of FtsZ rings in Bacillus subtilis and Escherichia coli and effects of FtsZ-regulating proteins. J. Bacteriol. 2004;186:5775–5781. doi: 10.1128/JB.186.17.5775-5781.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Young KD. The selective value of bacterial shape. Microbiol. Mol. Biol. Rev. 2006;70:660–703. doi: 10.1128/MMBR.00001-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mannik J, et al. Robustness and accuracy of cell division in Escherichia coli in diverse cell shapes. Proc. Natl Acad. Sci. USA. 2012;109:6957–6962. doi: 10.1073/pnas.1120854109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mannik J, Driessen R, Galajda P, Keymer JE, Dekker C. Bacterial growth and motility in sub-micron constrictions. Proc. Natl Acad. Sci. USA. 2009;106:14861–14866. doi: 10.1073/pnas.0907542106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou Z, et al. The contractile ring coordinates curvature-dependent septum assembly during fission yeast cytokinesis. Mol. Biol. Cell. 2015;26:78–90. doi: 10.1091/mbc.e14-10-1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Minc N, Burgess D, Chang F. Influence of cell geometry on division-plane positioning. Cell. 2011;144:414–426. doi: 10.1016/j.cell.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takeuchi S, DiLuzio WR, Weibel DB, Whitesides GM. Controlling the shape of filamentous cells of Escherichia coli. Nano Lett. 2005;5:1819–1823. doi: 10.1021/nl0507360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong F, et al. Mechanical strain sensing implicated in cell shape recovery in Escherichia coli. Nat. Microbiol. 2017;2:17115. doi: 10.1038/nmicrobiol.2017.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Monahan LG, et al. Rapid conversion of Pseudomonas aeruginosa to a spherical cell morphotype facilitates tolerance to carbapenems and penicillins but increases susceptibility to antimicrobial peptides. Antimicrob. Agents Chemother. 2014;58:1956–1962. doi: 10.1128/AAC.01901-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yao Q, et al. Short FtsZ filaments can drive asymmetric cell envelope constriction at the onset of bacterial cytokinesis. EMBO J. 2017 doi: 10.15252/embj.201696235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strauss MP, et al. 3D-SIM super resolution microscopy reveals a bead-like arrangement for FtsZ and the division machinery: implications for triggering cytokinesis. PLoS Biol. 2012;10:e1001389. doi: 10.1371/journal.pbio.1001389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holden SJ, et al. High throughput 3D super-resolution microscopy reveals Caulobacter crescentus in vivo Z-ring organization. Proc. Natl. Acad. Sci. USA. 2014;111:4566–4571. doi: 10.1073/pnas.1313368111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jacq, M. et al. Remodeling of the Z-ring nanostructure during the Streptococcus pneumoniae cell cycle revealed by photoactivated localization microscopy. mBio, 10.1128/mBio.01108-15 (2015). [DOI] [PMC free article] [PubMed]
- 43.Ramirez-Diaz DA, et al. Treadmilling analysis reveals new insights into dynamic FtsZ ring architecture. PLoS Biol. 2018;16:e2004845. doi: 10.1371/journal.pbio.2004845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Surovtsev IV, Jacobs-Wagner C. Subcellular organization: a critical feature of bacterial cell replication. Cell. 2018;172:1271–1293. doi: 10.1016/j.cell.2018.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Caccamo PD, Brun YV. The molecular basis of noncanonical bacterial morphology. Trends Microbiol. 2018;26:191–208. doi: 10.1016/j.tim.2017.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gibson DG, et al. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods. 2009;6:343–U341. doi: 10.1038/nmeth.1318. [DOI] [PubMed] [Google Scholar]
- 47.Amann E, Ochs B, Abel KJ. Tightly regulated tac promoter vectors useful for the expression of unfused and fused proteins in Escherichia coli. Gene. 1988;69:301–315. doi: 10.1016/0378-1119(88)90440-4. [DOI] [PubMed] [Google Scholar]
- 48.Antonov PV, Zuiddam MR, Frenken JWM. Fabrication of high-aspect ratio silicon nanopillars for tribological experiments. J. Micro-Nanolith Mem. 2015;14:044506. doi: 10.1117/1.JMM.14.4.044506. [DOI] [Google Scholar]
- 49.Ayari-Kanoun A, Aydinoglu F, Cui B, Saffih F. Silicon nanostructures with very large negatively tapered profile by inductively coupled plasma-RIE. J. Vac. Sci. Technol. B. 2016;34:06KD01. doi: 10.1116/1.4964402. [DOI] [Google Scholar]
- 50.Martin M, et al. Fabrication of high-density Si and SixGe1-x nanowire arrays based on the single step plasma etching process. J. Vac. Sci. Technol. B. 2013;31:041806. doi: 10.1116/1.4812792. [DOI] [Google Scholar]
- 51.Engstrom DS, Soh YA. Controlling the silicon nanowire tapering angle in dense arrays of silicon nanowires using deep reactive ion etching. J. Vac. Sci. Technol. B. 2013;31:021806. doi: 10.1116/1.4793500. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The data supporting the findings of the study are available in this article and its Supplementary Information files, or from the corresponding author upon request.