Abstract
The Gal antigen is synthesized by glycoprotein galactosyltransferase alpha 1, 3 (GGTA1) or (and) isoglobotrihexosylceramide 3 synthase (iGb3S). However, whether iGb3S deletion changes Gal epitope expression and immunological properties in animals is still not clear. The objective of this study was to develop iGb3S deficient mice, and characterize their Gal epitope expression and Gal epitope-related immunological properties. iGb3S gene knockout mice were generated on the C57BL/6 background using the bacterial artificial chromosome homology region recombination technique. Gal epitope expression in the iGb3S deficient mice was determined by using a monoclonal anti-Gal antibody. Immunological properties were analyzed by enzyme linked immune sorbent assay. It was found that Gal epitope expression was decreased from 5.19% to 21.74% in the main organs of iGb3S deficient mice, compared with that of C57BL/6 wild type mice, suggesting that the iGb3S gene participated to Gal epitope expression. However, iGb3S deletion alone did not cause significant changes in the immunological properties of iGb3S deficient mice with or without exogenous Gal antigen (Rabbit Red Blood Cell) stimulation. The data from this study suggest that the iGb3S gene likely contributes to Gal epitope expression, but may have a very weak effect on immunological properties of the iGb3S deficient mice.
Introduction
Many studies have shown that the major antigen in pig tissue recognized by primate antibodies is a terminal galalpha1-3gal carbohydrate structure (Gal antigen) present on glycolipids and glycoproteins1–4. Furthermore, anti-Gal natural antibodies are responsible for hyperacute rejection in pig-to-primate xenotransplantation5–7. It is known that Gal antigen is synthesized by glycoprotein galactosyltransferase alpha 1, 3 (GGTA1) or (and) isoglobotrihexosylceramide 3 synthase (iGb3S), and that GGTA1 contributes to the glycoprotein type and iGb3S contributes to the glycolipidtype8–10. iGb3S mRNA was detected in mouse tissues and pig tissues9,10, but humans lack iGb3S expression except in the thymus and monocyte-derived dendritic cells11.
Several studies showed that the Gal epitope is expressed in GGTA1 deficient mice (splenic fibroblasts and tissues including the pancreas, spleen, kidney and liver), and in fetal-pig homozygous GGTA1 knockout (KO) fibroblasts when stained with anti-Gal alpha(1,3)Gal mAb or with sensitized human serum9,12,13. We also verified that the Gal epitope was expressed in GGTA1 KO mice developed in our laboratory, by a standardized Gal antigen quantitative detection method using a commercial specific anti-Gal antibody (M86, mAb) [unpublished data]. Christiansen et al.11 showed that purified normal human anti-Gal immunoglobulin G can bind to iGb3 lipid to mediate complement lysis of transfected human cells expressing iGb3, suggesting that iGb3 may represent an important obstacle in xenotransplantation11. The results from Milland et al.13 verified that GGTA1 KO mice have mRNA for iGb3S and induce an antibody response to Gal antigen synthesized by iGb3S12. Another study by Milland et al.9 showed that transfection of iGb3S cDNA resulted in high levels of cell surface Galalpha(1, 3)Gal synthesized via the isoglobo series pathway, thus demonstrating that mouse iGb3S is an additional enzyme capable of synthesizing the xenoreactive Galalpha(1, 3)Gal epitope. Anti-Gal antibody responses were induced in GGTA1 KO mice after immunization with GGTA1-positive cells or iGb3S-positive cells, indicating iGb3S mediates Gal antigen mediated immunologic toxicity9.
However, a study by Puga Yung et al.14 demonstrated that iGb3S mRNA was expressed in all pig tissues tested whether derived from wild-type (WT) or GGTA1 KO animals, but iGb3 was absent14. Another study showed that iGb3 or other isoglobo-series glycosphingolipids were not detected in pig organs, including the heart, liver, pancreas, and kidney, by ion-trap mass spectrometry15. Diswall et al.16 demonstrated that a complete lack of α-Gal glycolipid reactivity in the GGTA1 KO pig small intestine examined with different anti-Gal reagents such as mono and polyclonal Abs and lectins16.
Currently, information about the relevance of iGb3S with Gal epitope expression is controversial, and there is a lack of data to indicate whether iGb3S contributes to Gal epitope expression. Some studies of iGb3S KO mice only focused on iNKT cell function11,17,18, but did not show any information about the relevance of the iGb3S gene with Gal epitope expression. In this study, a C57BL/6 derived embryonic stem (ES) cell line was used to establish an iGb3S deficient mouse. The use of the C57BL/6 background for iGb3S deficient model provided a pure genetic background suitable for immunological study, unlike those made with ES cell lines derived from 129/Sv mice, which need several generations of backcrossing to C57BL/6 mice to obtain a uniform genetic background. Gal epitope expression profiling in the main organs and immunological properties, including total antibody and anti-Gal antibody activity (anti-Gal IgG, IgM, and IgA) in the iGb3S deficient mouse were examined. The results from this study provide basic information to help understand whether the iGb3S gene contributes to Gal epitope expression, and the xeno-species anti-Gal antibody-mediated immune response.
Results
Generation of iGb3S KO mice
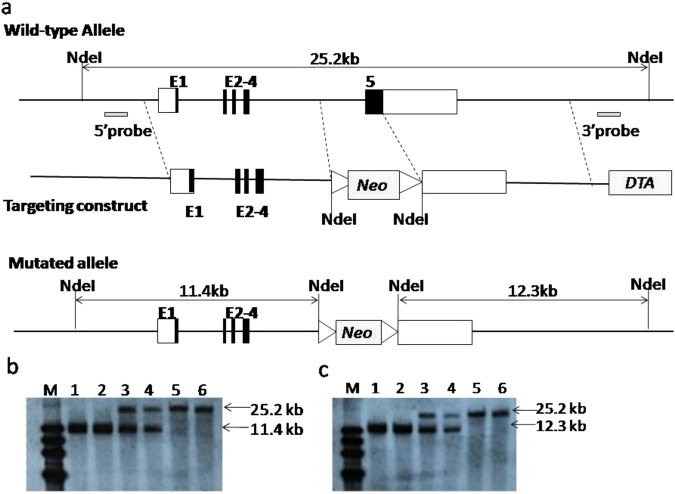
The coding sequence of the fifth exon of iGb3S responsible for enzymatic activity was replaced by a loxP-flanked neomycin resistance cassette (Fig. 1a). Homologous recombination at the iGb3S locus in C57BL/6 ES cells was confirmed by Southern blotting and 11 mutant ES cell clones were obtained (Figs S1 and S2). After microinjection of mutant ES cell clones into pseudo pregnant white mice, six chimeric iGb3S mice (white color with black-dot) were obtained. Heterozygous F1 progenies were obtained by breeding chimeric iGb3S mice with WT C57BL/6 mice and F1 progenies with pure black color were selected as parents for breeding and expansion. Homozygous iGb3S KO mice were obtained by intercrossing (Fig. S3). The homologous recombination at the iGb3S-locus and exon 5 deletion of iGb3S gene was confirmed over five generations of iGb3S KO mice by Southern blot analysis with 3′- and 5′-probes. As shown in Fig. 1b,c, the band of the 3′-probe 1-NdeI was 25.2 kb in WT mice and 11.4 kb in mutant mice, and the band of the 5′-probe 2-NdeI was 25.2 kb in WT mice and 12.3 kb in mutant mice. Bands 1 and 2 were from samples taken from homologous iGb3S KO mice. iGb3S KO mice reproduced normally and progeny were born at expected Mendelian ratios. They grew normally and exhibited no overt developmental or behavioral defects. Body and organ weights were not significantly different compared with WT littermates (Tables S1 and S2.). Histological examination of main organs did not reveal differences compared with WT littermates (Fig. S4). The fifth or later generations of iGb3S KO mice were used to detect Gal epitope expression and for the immunological properties assay.
Figure 1.
Targeting vector construction strategies. (a) The top graph shows the wild-type allele with exon 1 to 5 in the murine iGb3S WT locus, together with relevant enzyme restriction sites and probes for Southern blot analysis. The middle graph shows the targeting vector constructed by replacing a 1.5-kb fragment encoding the iGb3S exon 5 with a loxP-flanked neomycin-resistance gene cassette (neo), and a diphtheria toxin A (DTA). The lower graph shows the mutated allele with an11.4 kb Nde1-fragment at the 5′-arm immediately upstream of the neo cassette and 12.3 kb of the Nde1-fragment at the 3′-arm immediately downstream of the neo cassette. Coding and non-coding regions of exon 1–5 are marked by filled and non-filled boxes, respectively. Homology arms are depicted as bold lines. (b,c) show Southern blotting results. (b) Genomic DNA was digested with NdeI in the 5′-probe and resulted in 25.2 kb and 11.4 kb fragments. (c) Genomic DNA was digested with NdeI in the 3′-probe and resulted in 25.2 kb and 12.3 kb fragments. M, DNA marker; lanes 1 and 2 are mut/mut, lanes 3 and 4 are mut/wt; lanes 5 and 6 are wt/wt.
mRNA expression of iGb3S and GGTA1 in iGb3S KO mice
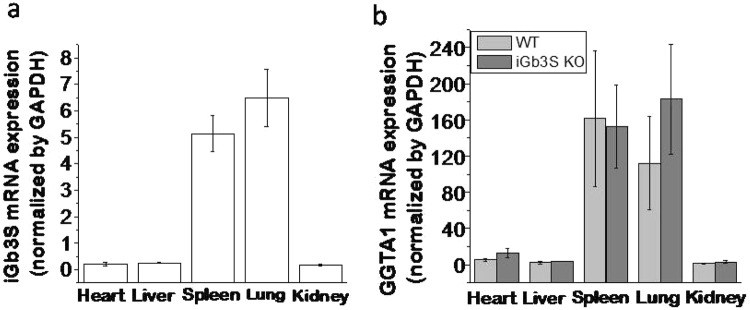
iGb3S deficiency was confirmed at the mRNA level in selected organs of iGb3S KO mice by real-time polymerase chain reaction (RT-PCR). As expected, iGb3S mRNA expression disappeared in iGb3S KO mice (data not shown). To obtain more information regarding the relevance of the GGTA1 and iGb3S genes in Gal antigen expression, GGTA1 mRNA from WT (n = 5) and iGb3S KO mice (n = 8) was investigated. Relative GGTA1 and iGb3S mRNA expressions in WT mice were strongly expressed in the spleen and lung, and weakly expressed in the heart, liver, and kidney as shown in Fig. 2a,b. No significant differences in the GGTA1 mRNA expression in iGb3S KO mice were observed when compared with WT littermates (Fig. 2b).
Figure 2.
The mRNA expression level determined by RT-PCR. (a) iGb3S mRNA expression level in WT mice; (b) GGTA1 mRNA expression level in WT and iGb3S KO mice. The relative mRNA expression level is shown as 2−ΔCt, and is normalized to the GAPDH gene. There is no significant difference at GGTA1 mRNA expression level in iGb3S KO mice compared to WT mice.
Gal epitope expression in iGb3S KO mice
Gal epitope expression in WT C57BL/6 mice was higher in the spleen and lung compared with the heart, liver, and kidney (Table 1). This distribution tendency of Gal epitope expression in different tissues was in accord with the tendency of GGTA1 and iGb3S mRNA expression, implying the GGTA1 and iGb3S genes correlated with Gal epitope expression. In the selected tissues of iGb3S KO mice (n = 8), Gal epitope expression was significantly decreased by about 16.2% in the spleen, 10.1% in the lung, and 21.74% in the liver, and was slightly decreased in the kidney (5.19%) and heart (8.31%) compared with WT C57BL/6 mice (Table 2, one-way analysis of variance, p < 0.05). These results indicated that the iGb3S gene participated in Gal epitope expression in mice.
Table 1.
Gal epitope expression in the main organs of iGb3S KO mice and WT mice.
| (n × 1011 Gal epitopes/mg wet tissue) | |||||
|---|---|---|---|---|---|
| Spleen | Heart | Liver | Kidney | Lung | |
| WT type mice | 327.70 ± 20.74 | 81.70 ± 5.91 | 21.47 ± 3.95 | 48.18 ± 0.67 | 360.34 ± 18.37 |
| iGb3S KO mice | 274.61 ± 0.14# | 74.91 ± 7.61 | 12.03 ± 1.38# | 45.68 ± 0.81 | 323.95 ± 10.22# |
| Decrease rate (%)* | 16.20 | 8.31 | 21.74 | 5.19 | 10.10 |
*Decreased percentage of Gal epitope expression in the main organs of iGb3S KO mice (n = 8) compared with WT C57BL/6 mice (n = 5); #p < 0.05 compared to WT mice.
Table 2.
Primers used in target vector construction in this study.
| Primer | Sequence | Restriction Enzyme | Product size | Tm |
|---|---|---|---|---|
| iGb3S-A-F | cgatGGTACCGATATCACAGAATCTTTCTCTGTTTTC | KpnI/EcoRV | 547 bp | 53 |
| iGb3S-A-R | cgatGAATTCCATATGCAGGGTTTCTCTGTGTAGCC | EcoRI/NdeI | 56 | |
| iGb3S-B-F | cgatGGATCCCATATGCAGCCCTTCCCTGGCCAAGC | BamHI/NdeI | 486 bp | 64 |
| iGb3S-B-R | cgatGCGGCCGCGATATCCTGGTCACAGGAATGGCTTCA | NotI/EcoRV | 64 | |
| iGb3S-C-F | cgatCTCGAGGTGGATGTCTCAGTGTGCGAA | XhoI | 586 bp | 58 |
| iGb3S-C-R(in) | TACCGCAGACGGTGGATATCATTGACAGTCACTGAGCAA | EcoRV | 56 | |
| iGb3S-C-F(in) | TTGCTCAGTGACTGTCAATGATATCCACCGTCTGCGGTA | EcoRV | 585 bp | 56 |
| iGb3S-C-R | cgatGCGGCCGCCTATCGAGTGGTTATTCTCAGGG | NotI | 56 | |
| iGb3S-Atest-F | TAGAATACACACCTAATATTGATTAGCA | 673 bp | 54 | |
| iGb3S-Atest-R | TGGACGTAAACTCCTCTTCAG | 55 | ||
| iGb3S-Btest-F | Neo-F | 734 bp | 56 | |
| iGb3S-Btest-R | GGAGAGCTGAGGCTGAAGTC | 58 | ||
| iGb3S-C1test-F | M13R | 730 bp | 56 | |
| iGb3S-C1test-R | GGCTAAATGCACCTGTCATG | 56 | ||
| iGb3S-C2test-F | ACACAAGGACTTGACCATGG | 667 bp | 56 | |
| iGb3S-C2test-R | C2testR | 56 |
The immunological properties of iGb3S KO mice
To investigate the immunological properties of iGb3S KO mice, immunological factors were measured. Serum total IgG, IgM, and IgA expression levels are shown in Fig. 3a,b. There was no significant difference in total Ig levels between iGb3S KO mice with or without rabbit blood red cell (RRBC) immunization and WT mice. But, IgG2b expression was significantly increased after RRBC immunized treatment in iGb3S KO and WT mice (one-way analysis of variance, p < 0.05) compared with no-treatment control mice, respectively. IgG1, IgG2a, and Ig G3 expressions were not significantly different between iGb3S KO mice with or without RRBC immunization and WT mice.
Figure 3.
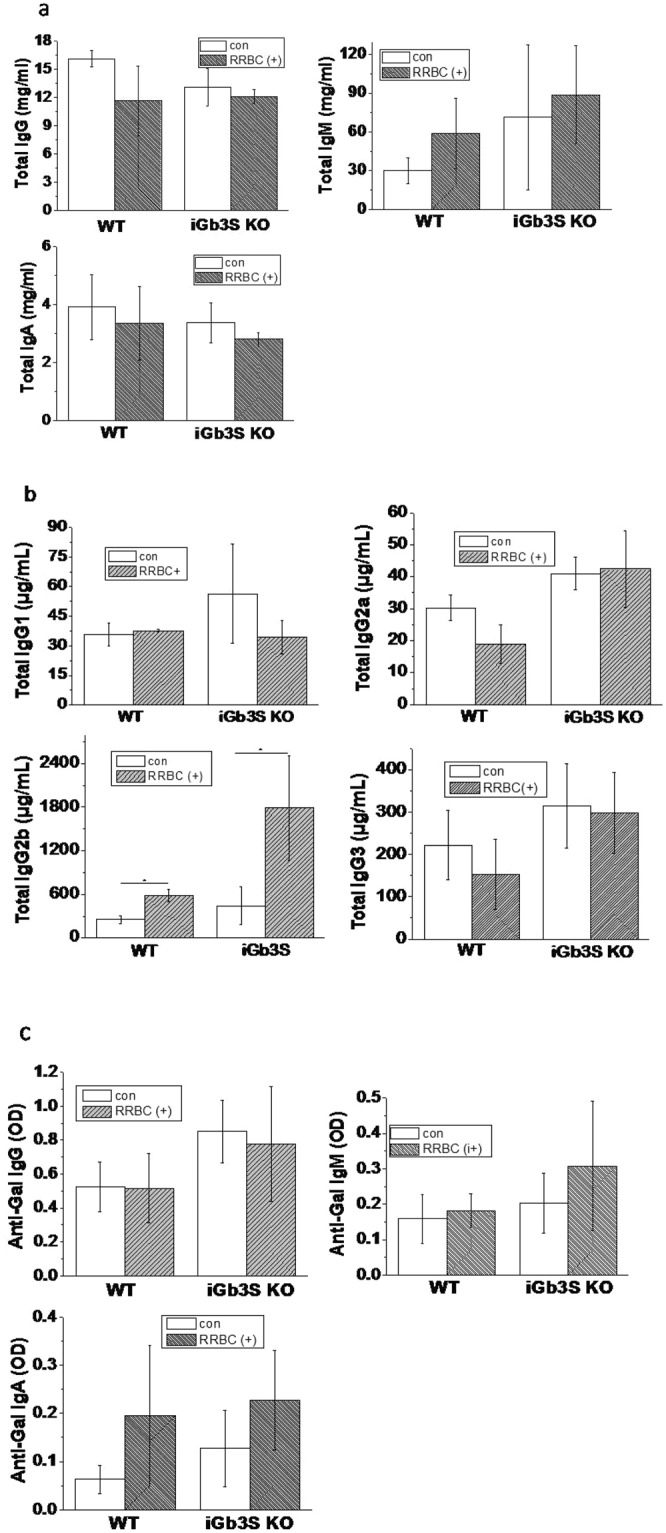
The level of immunological factors in WT and iGb3S KO mice with or without RRBC immunization. (a) Total immunoglobulin levels (IgG, IgM, and IgA); (b) Sub-group IgG levels; c. Anti-Gal antibody levels (anti-Gal IgG, anti-Gal IgM, and anti-Gal IgA). *p < 0.05, in mice with RRBC immunization compared to mice without RRBC immunization. Indication: Origin 8 graphics program was used to create the artwork, and save them as tiff.
To determine whether iGb3S KO mice would respond to specific Gal epitope immunization, anti-Gal IgG, anti-Gal IgM and anti-Gal IgA were assessed in iGb3S KO mice (Fig. 3c). Only background optical density (OD) values, similar to OD values in WT mice, were detected in iGb3S KO mice, and no significant differences in the OD values of anti-Gal IgG and anti-Gal IgM after RRBC immunization of iGb3S KO mice was observed compared with the non-treatment control group and WT mice. The OD value of IgA expression in iGb3S KO mice was slightly higher than that in WT mice with and without RRBC treatment, but this was not statistically significant.
Discussion
To understand whether iGb3S is involved in Gal antigen expression and anti-Gal antibody responses, we generated iGb3S KO mice. First, it was confirmed that WT C57BL/6 mice strongly expressed iGb3S mRNA in the spleen and lung, and weakly in the heart, liver, and kidney (Fig. 2a). This distribution pattern of iGb3S mRNA in the main organs is similar to GGTA1 mRNA expression (Fig. 2b), albeit at a lower level than GGTA1 mRNA. iGb3S mRNA in iGb3S KO mice completely disappeared as expected, but there were no changes in GGTA1 mRNA compared with that in WT mice (Fig. 2b). It was found that Gal epitope expression in iGb3S KO mice was decreased by about 5.19% to 21.74% in the main organs (Table 2), indicating that the iGb3S gene is likely contributes to Gal epitope expression. Previously, studies of the iGb3S gene have focused on the influence of the iGb3 protein on the development and function of invariant natural killer cells11,17,18. Here, the data showed that deletion of the iGb3S gene decreased Gal epitope expression, even though the data was not significance in the heart and kidney, that possibly because the Gal epitope expression was very low (near the detection limit) in these tissues, as well as large difference between individuals. Our unpublished data and the data from several previous studies demonstrated that the Gal epitope was still expressed in GGTA1 KO mice9,10,12,13. Milland et al.9 showed that transfection of iGb3S cDNA resulted in high levels of cell surface Gal synthesized via the isoglobo series pathway, demonstrating that mouse iGb3S can synthesize the xenoreactive Gal epitope. Our data support that the iGb3S gene is another source for the synthesis of Gal antigen in mice.
A recent study demonstrated that silencing the porcine iGb3S gene did not affect Gal levels. However, they used IB4 staining for α-GAL/iGb3 measurements. It is known that the specificity of IB4 binding to different number of sugars in the Gal antigen is varied. A previous study showed that IB4 lectin is insufficient for the detection of relatively small numbers of Gal epitopes, because of the low binding affinity of the monomeric interaction of the lectin molecule with more than one of the four combining sites19. The length of the sugar chains influenced the lectin-carbohydrate interactions and the terminal and subterminal sugars affected lectin binding20. Several studies showed that the Gal epitope was still expressed in GGTA1 deficient mice and pigs when stained with anti-Gal alpha(1, 3)Gal mAb or sensitized human serum9,12,13, but not IB4 lectin.
To determine whether iGb3S deletion affects the immunological properties of iGb3S KO mice, antibody titers in iGb3S KO mice were analyzed. We did not find any significant differences in total IgG, IgM, and IgA titers in iGb3S KO mice when compared with WT mice. Furthermore, RRBC immunization did not induce significant changes in total IgG, IgM, and IgA in iGb3S KO mice. However, IgG2b was significantly increased after RRBC immunization in iGb3S KO and WT mice, and the increase was greater in iGb3S KO mice compared with WT mice. IgG2 is the major IgG form specific for the Gal epitope21, and an in vitro study showed that the most abundant anti-SIS (a commercial regenerative medical product) antibody subtype that bound to SIS following exposure to human plasma was IgG222. In the present study, higher levels of IgG2 were observed in iGb3S KO mice compared with WT mice after RRBC immunization, might suggesting iGb3S KO mice are more sensitive to RRBC stimulation compared with WT mice.
Anti-Gal specific antibodies did not be detected in iGb3S KO mice (Fig. 3c), even after exogenous immune challenge with RRBC membrane injected intraperitoneally. An explanation might be that because GGTA1 is the major gene for synthesizing Gal epitope and iGb3S gene KO did not affect GGTA1 mRNA expression (Fig. 3b), therefore iGb3S KO mice still express large Gal epitope (only decreased about 5.19% to 21.74% in the main organs), so that the iGb3S KO mice could not produce anti-Gal antibodies. These results suggest that the iGb3S gene might have a weak effect on the immunological properties of mice. A previous study demonstrated that silencing the porcine iGb3S gene did not affect measures of anticipated pig-to-human and pig-to-primate acute rejection, suggesting iGb3S is not a contributor to antibody-mediated rejection in pig-to-primate or pig-to-human xenotransplantation23. But, they confirmed that iGb3S gene silencing significantly changed the renal glycosphingolipid profile. Therefore, whether iGb3S deficiency causes other changes to the glycosphingolipid profile and what effects it has on the biological functions of animals requires further investigation.
In conclusion, we generated an iGb3S KO mouse model. Our data suggest that the iGb3S gene likely contributes to Gal epitope expression, but that iGb3S deletion alone did not significantly change immunological properties, implying iGb3S deletion alone might have a weak effect on the immunological properties in mice.
Our further study have verified that the Gal antigen expression was decreased by 97.5–99.6% in the main organs of GGTA1 KO mice (the manuscript is under-reviewing by Journal of Applied Genetics), and GGTA1 and iGb3S double KO in mice generated a completely disappearance in Gal antigen expression (the manuscript is under-drafting). The data from our study suggested that both GGTA1 and iGb3S are the regulators for Gal antigen expression. The detail relationship between GGTA1 and iGb3S for the regulation of Gal antigen expression is investigating in our laboratory.
Materials and Methods
All animals used in this study were housed and handled in accordance with the guidelines set by the Association for the Assessment and Accreditation of Laboratory Animal Care. The protocol of this study was approved by the National Institutes for Food and Drug Control (NIFDC) Institutional Animal Care and Use Committee. All methods were performed in accordance with the relevant guidelines and regulations of Good Laboratory Practice in NIFDC quality system.
Animals
C57BL/6 mice (black) and Kunming (KM) mice (white) were purchased from the Institute for Laboratory Animal Resources of NIFDC, and together with iGb3S KO mice were maintained in a specific pathogen-free facility at a temperature of 23 ± 1 °C, relative humidity of 30–70%, and a 12-h light/12-h dark cycle.
Generation of iGb3S deficient mice
The iGb3S deficient mouse model was generated with support from Beijing Biocytogen (Beijing, China). Briefly, homology regions covering 1.4-kb upstream of exon 1 and 7.5-kb downstream of exon 5 of the iGb3S gene were subcloned from a bacterial artificial chromosome (BAC) clone (RP23-241C3; Invitrogen) from the C57BL/6 J mouse genomic RPCI-23 BAC Library.
The targeting vector was constructed by replacing a 1.5-kb fragment encoding the iGb3S exon 5 with a loxP-flanked neomycin-resistance gene cassette (neo), and a Diphtheria toxin A (DTA) gene driven by the herpes simplex virus thymidine kinase (TK) promoter inserted into the genomic fragment for negative selection. The two homologous arms, 8.3-kb 5′-arm immediately upstream of the neo cassette and the 9.1-kb 3′-arm immediately downstream of the neo cassette are shown in Fig. 1. The primers used in target vector construction are shown in Table 2.
After linearization, the targeting vector was electroporated into E14 ES cells, and 200 G418-resistant clones were picked, expanded, and characterized by Southern blot analysis using the 5′-external probe and 3′-external probe (data not shown). Of eleven positive ES cell clones, five were microinjected into C57BL/6 blastocysts. Chimeric offspring were backcrossed to C57BL/6 mice and germline transmission was confirmed by PCR of tail genomic DNA (data not shown). Heterozygous F1 progenies were intercrossed to obtain iGb3S KO homozygous mice. The mutant mice were kept under specific pathogen-free conditions.
Southern blot analysis
Genomic DNA isolated from the mutant offspring were digested with NdeI (NEB), separated on a 1% agarose gel, and transferred to a positively charged nylon membrane (Hybond N+; Amersham International plc, Little Chalfont, Buckinghamshire, UK). The filter was hybridized with a digoxigenin (DIG) labeled probe. Hybridizing bands corresponding to the iGb3S gene were detected using the DIG Luminescent Detection Kit (Roche Applied Science Inc. Indianapolis, IN, USA). For probe labeling, 5′-external and 3′-external DIG-labeled probes were prepared by PCR using Taq DNA polymerase and incorporating DIG-11-dUTP according to the manufacturer’s instructions (Roche Applied Science Inc.). The following primers were used to amplify the 5′ external (479 bp) probe:5′-CATTCTGATACTCTGTCAATCATTTC-3′ (forward primer), and 3′-GGATGGACCACCAGAAACTA-5′ (reverse primer). For the 3′-external (521 bp) probe, the following primers were used: 5′-GTATTACTCGTGAACACTCTTGGC-3′ (forward primer) and 3′-GTAGAGTCAGCGCCTCACAG-5′ (reverse primer).
RNA isolation and RT-PCR
RNA was extracted from the heart, liver, spleen, lung, and kidney of mice (WT and iGb3S KO) using the RNAiso plus Kit (TaKaRa, Tokyo, Japan). Then, 0.4 μg of total RNA was reverse-transcribed in 20 μL total volume using a PrimeScript™ RT reagent Kit with gDNA Eraser (Perfect Real Time, TaKaRa), according to the manufacturer’s instructions. RT-PCR was performed with 2 μL of cDNA. GAPDH was used as a housekeeping gene for the normalization of the detected gene. The primers used were as follows: GAPDH (182 bp, gene accession number: NC_000072.6): 5′-GTTGTCTCCTGCGACTTCA-3′(forward primer) and 5′-TGGTCCAGGGTTTCTTACTC-3′ (reverse primer); iGb3S (177 bp, gene accession number: NM_001009819.2): 5′-CACTTTCGACCCTCATGTAGC-3′ (forward primer) and 5′-GGGCGATCCGTAAACACATAGT-3′ (reverse primer); and GGTA1 (102 bp, gene accession number: NM_010283.3): 5′-ACCGCCCGGATGTTTTGAC-3′(forward primer) and 5′-TGACGTAAAATATGACCCGATGG-3′ (reverse primer).
Gal antigen determination
Gal antigen expression in the heart, liver, spleen, lung, and kidney tissues in experimental mice (WT and iGb3S KO) was determined using a commercial monoclonal anti-Gal mouse antibody (M86, ALX-801-090-1, α-Gal Epitope, mAb. Enzo Life Science, NY, USA) and a Gal antigen quantitative detection kit (70101, Meitan, Beijing San Yao Science & Technology Development Co., Beijing, China) following the manufacturer’s instructions and previous reports19,24–27. Briefly, the solid-phase antigen was coated with 100 μL/well of Galα(1,3)Gal-BSA (Gal-BSA) solution (1–2 μg/mL, NGP0203, Dextra Laboratories, READING, UK) in 96-well plates (Nunc, Rochester, NY, USA), and blocked with 200 μL/well of 1% human serum albumin (A8230, Solarbio, Beijing, China). A calibration curve was produced by using the Gal-BSA/Gal-free matrix (Gal antigen-negative biomaterial reference material provided by the National Institutes for Food and Drug Control, China) as a Gal antigen reference material. Briefly, serial of dilutions of Gal-BSA in 2 mg of Gal-free matrix lysate solution were produced. A Gal antigen-positive biomaterial reference material (provided by National Institutes for Food and Drug Control, China) was used as a positive control to monitor the sensitivity of the test system, and a Gal antigen-negative biomaterial reference material was used as a negative control to monitor the specificity of the test system. All test samples (200 μL/sample) were incubated with the primary monoclonal antibody M86 (200 μL, 20-times dilution according to the kit instructions) for 2 h at 37 °C with gentle shaking and then at 4 °C overnight. Before use, the reaction mixtures were centrifuged at 14,000 × g for 30 min at 4 °C, and 100 μL/well of the supernatant containing residual M86 antibody was loaded onto a Gal-BSA pre-coated 96-well plate, incubated for 1 h with gentle shaking at 37 °C in the dark. After washing, 100 μL of the secondary horseradish peroxidase (HRP)-conjugated antibody (goat anti-mouse IgM-HRP, sc-2064, Santa Cruz, CA, USA) diluted 1:2000 in PBS was loaded and incubated for 30 min. Finally, following washing, 100 μL of HRP color development solution (Trimethylbenzene, TMB, SE1005, Biokorad, Beijing, China) was added to each well for 15–30 min at room temperature in the dark and the reaction was stopped by 50–100 μL of 10% H2SO4. The absorbance was measured by a microplate reader (Spectramax M5, Molecular Devices, CA, USA) at 450 nm.
Our preliminary experiments confirmed that the mass weight of Galα1-3gal-BSA (Gal-BSA) was approximately equal to the BSA molecule by measuring protein content (data not shown). The relative molecular mass of BSA is 66.33 kD and the number of molecules is 9.08 × 1018/g. According to the product information, each Gal-BSA molecule contains about 20 Gal epitopes; therefore the number of Gal epitopes in Gal-BSA is about 1.82 × 1020/g. Gal epitopes of test sample were calculated relative to the Gal antigen reference material sample (Gal-BSA) as previously described27.
Immunization treatment
Twenty eight-week-old WT (C57BL/6, body weights are about 26–30 g) and thirty-week-old iGb3S KO mice (body weights are about 30–36 g) were immunized with RRBC membrane (1 × 108 RRBC) containing sufficient alpha(1,3)Gal epitopes, by intraperitoneal injection (2 week interval for a total of three times). Littermates without treatment were used as comparisons.
Determination of immunological factors
The serum of experimental animals was collected and tested for the presence of immunological factors. The total IgG was determined by mouse IgG enzyme-linked immunosorbent assay (ELISA) kit (EMC116, Neobioscience, Beijing, China), and the serum was diluted 1:500,000. Total IgM was determined by mouse IgM ELISA kit (88-50470-22, EBioscience, CA, USA) and the serum was diluted 1:150,000. Total IgA was determined by mouse IgA ELISA kit (88-50450-22, EBioscience) and the serum was diluted 1:10,000. Levels of IgG sub-group, include IgG1, IgG2a, IgG2b, and IgG3 were determined by a seven-immunofactor assay kit (EPX070-20815-901, Beijing, China) and the serum samples were diluted 1:10,000.
Anti-Gal antibody detection was performed by ELISA using Gal-BSA as a solid phase antigen. Briefly, 100 μL/well of Gal-BSA (1.0–2.0 μg/mL in carbonate buffer, pH 9.5) was loaded into a 96-well plate for overnight coating, and then washed with PBS-0.05% Tween-20. It was then blocked with 1% human serum albumin (A8230, Solarbio) for 2 h at 37 °C. Following a wash step, serial dilutions of each serum sample were made in phosphate-buffered saline (PBS)-1% human serum albumin (HSA) and added to the pre-coated plates. The plates were incubated for 2 h at 37 °C and then washed three times with PBS-0.05% Tween-20. Then, 100 μL of goat anti-mouse IgG (1:32,000) (IgG-HRP, sc-2005, Santa Cruz, CA, USA), IgM (1:16,000) (IgM-HRP, sc-2064, Santa Cruz) and IgA (1:1000) (IgA–HRP, sc-3793, Santa Cruz) were added into each well for 1 h at 37 °C. After washing, TMB (SE1005, Biokorad) was added and the colorization was stopped using 10% H2SO4. The OD was read at 450 nm.
Statistical analysis
Data are expressed as the mean ± SD. Differences were considered significant when p < 0.05.The significance in different groups was determined by one-way analysis of variance.
Electronic supplementary material
Acknowledgements
This work was supported by funds from The National Key Research and Development Program of China (2016YFC1103200, 2016YFC1103203) and National Engineering Laboratory for Regenerative Medical Devices Principle Investigator (PI) Project (2012 NELRMD002).
Author Contributions
Liming Xu contributes for experimental design and manuscript preparation. Anliang Shao and Yan Lu contribute for experiment and manuscript preparation. Xi Wu, Susu Liu and Changfa Fan contribute for generating iGb3S definite mice.
Data Availability
The all of data are available.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Anliang Shao, Email: shaoanliang@nifdc.org.cn.
Liming Xu, Email: xuliming@nifdc.org.cn.
Changfa Fan, Email: fanchangfa@nifdc.org.cn.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-33032-7.
References
- 1.Galili U, Shohet SB, Kobrin E, Stults LM, Macher BA. Man, apes, and old world monkeys differ from other mammals in the expression of alpha-galactosyl epitopes on nucleated cells. J. Biol. Chem. 1988;263(3):17755–17762. [PubMed] [Google Scholar]
- 2.Sandrin MS, Mckenzie IF. Gal alpha (1,3)Gal, the major xenoantigen(s) recognised in pigs by human natural antibodies. Immunol. Rev. 1994;141(10):169–190. doi: 10.1111/j.1600-065X.1994.tb00877.x. [DOI] [PubMed] [Google Scholar]
- 3.Joziasse DH, Oriol R. Xenotransplantation: the importance of the Gal α 1,3Gal epitope in hyperacute vascular rejection. Biochim. Biophys. Acta. 1999;1455(23):403–418. doi: 10.1016/S0925-4439(99)00056-3. [DOI] [PubMed] [Google Scholar]
- 4.Badylak SF, Gilbert TW. Immune response to biologic scaffold materials. Semin. Immunol. 2008;20(2):109–116. doi: 10.1016/j.smim.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galili U, Macher BA, Buehler J, Shohet SB. Human natural anti alpha-galactosyl IgG. II. The specific recognition of α(1, 3)-linked galactose residues. Exp. Med. 1985;162(2):573–582. doi: 10.1084/jem.162.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Posekany KJ, Pittman HK, Bradfield JF, Haisch CE, Verbanac KM. Induction of cytolytic anti-Gal antibodies in α-1, 3-galactosyltransferase gene knockout mice by oral inoculation with escherichia coli O86:B7 Bacteria. Infect. Immun. 2002;70(11):6215–6222. doi: 10.1128/IAI.70.11.6215-6222.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teranishi K, Manez R, Awwad M, Cooper DKC. Anti-Gal α1–3Gal IgM and IgG antibody levels in sera of humans and old world non-human primates. Xenotransplantation. 2002;9(2):148–154. doi: 10.1034/j.1399-3089.2002.1o058.x. [DOI] [PubMed] [Google Scholar]
- 8.Taylor SG, McKenzie IF, Sandrin MS. Characterization of the rat alpha(1,3) galactosyltransferase: evidence for two independent genes encoding glycosyltransferases that synthesize Galalpha(1, 3)Gal by two separate glycosylation pathways. Glycobiology. 2003;13(5):327–37. doi: 10.1093/glycob/cwg030. [DOI] [PubMed] [Google Scholar]
- 9.Milland J, et al. The molecular basis for galalpha(1,3)gal expression in animals with a deletion of thealpha1,3galactosyltransferase gene. J. Immunol. 2006;176(4):2448–54. doi: 10.4049/jimmunol.176.4.2448. [DOI] [PubMed] [Google Scholar]
- 10.keusch JJ, Manzella SM, Nyame KA, Cummings RD, Baenziger JU. Expression cloning of a new member of the ABO blood group glycosyltransferases, iGb3 synthase, that directs the synthesis of isoglobo-glycosphingolipids. J. Biol. Chem. 2000;275(33):25308–14. doi: 10.1074/jbc.M002629200. [DOI] [PubMed] [Google Scholar]
- 11. Christiansen, D. et al. Human lack iGb3 due to absence of functional iGb3-synthase: implications for NKT cell development and transplantation. PLoS Biol. 6(7), e172. doi: 10.1371 (2008). [DOI] [PMC free article] [PubMed]
- 12.Sharma A, et al. Pig cells that lack the gene for alpha1-3 galactosyltransferase express low levels of the gal antigen. Transplantation. 2003;75(4):430–6. doi: 10.1097/01.TP.0000053615.98201.77. [DOI] [PubMed] [Google Scholar]
- 13.Milland J, Christiansen D, Sandrin MS. Alpha1,3-galactosyltransferase knockout pigs are available for xenotransplantation: are glycosyltransferases still relevant? Immunol. Cell Biol. 2005;83(6):687–93. doi: 10.1111/j.1440-1711.2005.01398.x. [DOI] [PubMed] [Google Scholar]
- 14.Puga Yung GL, et al. Complete absence of the α-Gal xenoantigen and isoglobotrihexosylceramide in α1, 3 galactosyltransferase knockout pigs. Xenotransplantation. 2012;19(3):196–206. doi: 10.1111/j.1399-3089.2012.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tahiri F, et al. Lack of iGb3 and Isoglobo-Series Glycosphingolipids in Pig Organs Used for Xenotransplantation: Implications for Natural Killer T-Cell Biology. J. Carbohydr. Chem. 2013;32(1):44–67. doi: 10.1080/07328303.2012.741637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diswall M, Gustafsson A, Holgersson J, Sandrin MS, Breimer ME. Antigen-binding specificity of anti-αGal reagents determined by solid-phase glycolipid-binding assays. A complete lack of αGal glycolipid reactivity in α1, 3GalT-KO pig small intestine. Xenotransplantation. 2011;18(1):28–39. doi: 10.1111/j.1399-3089.2011.00623.x. [DOI] [PubMed] [Google Scholar]
- 17.Porubsky S, et al. Normal development and function of invariant natural killer T cells in mice with isoglobotrihexosylceramide (iGb3) deficiency. Proc. Natl. Acad. Sci. USA. 2007;104(14):5977–82. doi: 10.1073/pnas.0611139104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanderson JP, et al. CD1d protein structure determines species-selective antigenicity of isoglobotrihexosylceramide (iGb3) to invariant NKT cells. Eur. J. Immunol. 2013;43(3):815–25. doi: 10.1002/eji.201242952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galili U, Latemple DC, Radic MZ. A sensitive assay for measuring alpha-Gal epitope expression on cells by a monoclonal anti-Gal antibody. Transplantation. 1998;65(8):1129–1132. doi: 10.1097/00007890-199804270-00020. [DOI] [PubMed] [Google Scholar]
- 20.Kirkeby S, Moe D. Binding of Griffonia simplicifolian 1 isolectin B4 (GS1 B4) to α-galactose antigens. Immonol. and Cell Biol. 2001;79:121–127. doi: 10.1046/j.1440-1711.2001.00992.x. [DOI] [PubMed] [Google Scholar]
- 21.Yu PB, Holzknecht ZE, Bruno D, Parker W, Platt JL. Modulation of natural IgM binding and complement activation by natural IgG antibodies: a role for IgG anti-Gal alpha1-3Gal antibodies. J. Immunol. 1996;157(11):5163–8. [PubMed] [Google Scholar]
- 22.Mcpherson TB, Liang H, Record RD, Badylak SF. Galalpha(1,3)Gal epitope in porcine small intestinal submucosa. Tssue Eng. 2000;6:233–9. doi: 10.1089/10763270050044416. [DOI] [PubMed] [Google Scholar]
- 23.Butler JR, et al. Silencing the porcine iGb3S gene does not affect Galα3Gal levels or measures of anticipated pig-to-human and pig-to-primate acute rejection. Xenotransplantation. 2016;23(2):106–16. doi: 10.1111/xen.12217. [DOI] [PubMed] [Google Scholar]
- 24.Naso F, Gandaglia A, Iop l, Spina M, Gerosa G. First quantitative assay of alpha-Gal in soft tissues: presence and distribution of the epitope before and after cell removal from xenogeneic heart valves. Acta. Biomater. 2011;7(4):1728–1734. doi: 10.1016/j.actbio.2010.11.030. [DOI] [PubMed] [Google Scholar]
- 25.Shan Y, et al. Assessment method of remnant α-1, 3-Galactosyle epitopes in animal tissue-derived biomaterials. J. Biomed Eng. 2015;32(2):680–687. [PubMed] [Google Scholar]
- 26.Lu Y, Shan Y, Shao A, Zeng B, Xu L. Assessment of α1, 3-Gal antigen in animal tissues by ELISA inhibition method. Chin. J. Pharm. Anal. 2015;35(10):40–46. [Google Scholar]
- 27.Wu Y, et al. Development of α-Gal antigen quantitative detective Kit. Chin. J. Pharm. Anal. 2017;37(10):1940–1949. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The all of data are available.