Abstract
Cystic brain necrosis (CBN) is a rare form of BN. It typically occurs as a very late complication, and no standard treatment has been established. We report a case of a 59-year-old man who developed CBN 10 years after radiation therapy for metastatic brain tumors. The therapy consisted of whole brain radiotherapy followed by linac-based stereotactic radiosurgery as a boost. Initially, the CBN continued to expand despite treatment with corticosteroids and bevacizumab. Therefore, we resected the tumor and implanted an Ommaya reservoir, which successfully stabilized the lesion. Although the prognosis of patients with brain metastases is generally poor, some patients, like the one reported here, achieve long survival. Therefore, we should follow such cases carefully, considering the possibility of developing CBN as a late complication.
INTRODUCTION
Recently, a unique type of radiation-induced brain necrosis (BN), which accompanies cyst formation, has been reported mainly in patients treated with Gamma knife (GK)-based stereotactic radiosurgery (SRS) for brain arteriovenous malformations (AVMs) [1–3]. This cystic BN (CBN) has also been previously reported as a late complication of conventional radiation therapy (RT) for head and neck cancers, for example, in nasopharyngeal carcinoma [4, 5]. CBN presents a completely different clinical course from regular BN without cyst formation (non-cystic BN). Non-cystic BN typically develops within a few years after RT and is mostly asymptomatic or controllable with conservative therapy [4]. In comparison, CBN tends to occur with longer latency and is usually symptomatic and difficult to manage with conservative therapy [1–3]. Although there have been several reports of CBN following SRS, its long-term clinical features are poorly documented, and reports related to SRS for brain metastasis (BM) are extremely limited [6, 7].
Here, we report a case of CBN that developed 10 years after SRS and whole brain RT (WBRT) for BM; the cyst was successfully stabilized with surgical resection.
CASE REPORT
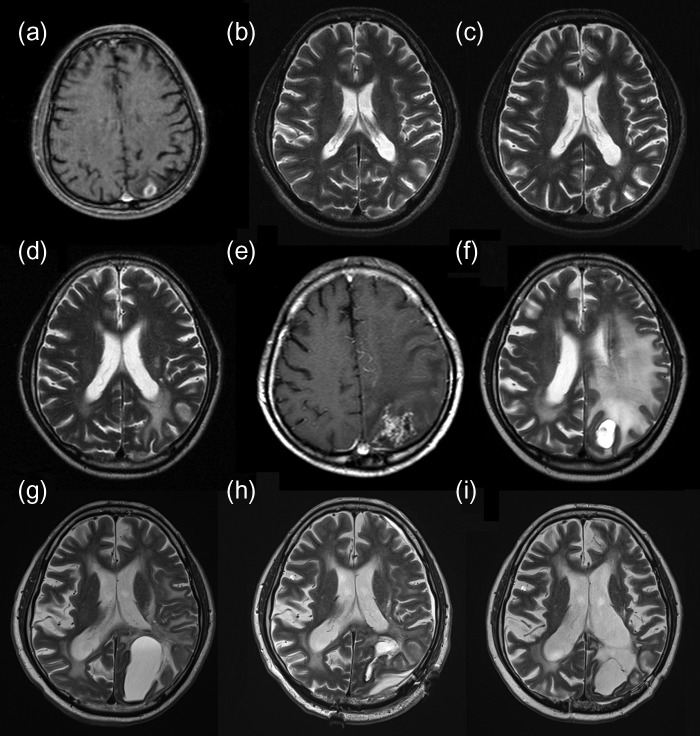
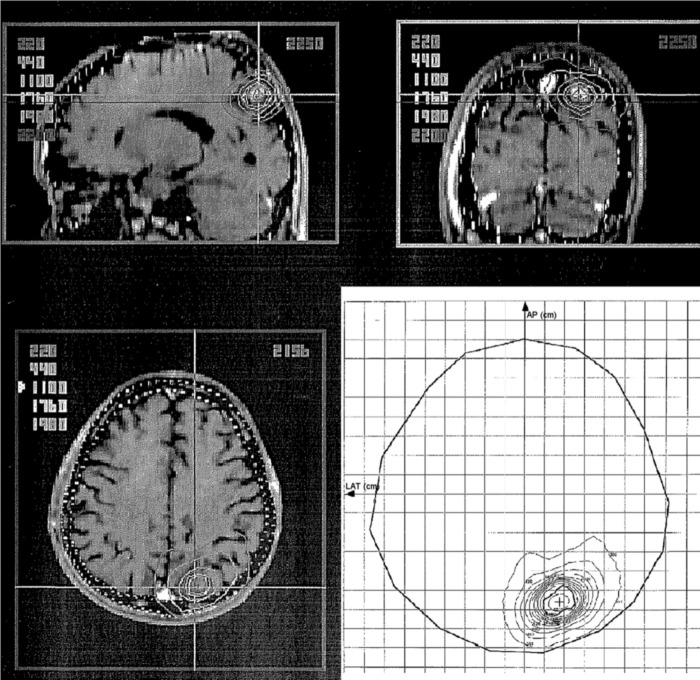
A 47-year-old man underwent surgical resection of a mucoepidermoid carcinoma of a left submandibular gland in 1999 and received RT (60 Gy in 30 fractions) for a solitary lung metastasis in May 2000. In July 2000, two BMs were detected on follow-up magnetic resonance imaging (MRI): the larger lesion in the left parietal lobe was 30 mm in diameter. The patient underwent WBRT (40 Gy in 16 fractions) and was then referred to our hospital for further treatment of the remaining left parietal lobe metastases (Fig. 1a). An SRS boost of 22.5 Gy in single fraction at the isocenter [18 Gy at the periphery of the planning target volume (PTV)] was performed using a linac-based SRS system, X-knife system (Radionics, Burlington, MA, USA) with a 6-MeV X-ray beam. The longest diameter of the residual tumor was 11 mm, and the volume of the PTV was 1.54 ml (Fig. 2). The patient remained recurrence- and complication-free for 8 years, except for the occurrence of WBRT-induced leukoencephalopathy, but a small high-signal area on T2-weighted images appeared on the SRS-treated area in April 2008 (Fig. 1d). In June 2010, at the age of 59 years, the patient developed right-sided partial paralysis, and an enhanced lesion with perifocal edema was apparent (Fig. 1e), which was clinically diagnosed as a radiation-induced BN. A steroid was prescribed to prevent its progression; however, cyst formation appeared in December 2010 (Fig. 1f). In November 2011, the patient was hospitalized again for CBN treatment. Steroid and bevacizumab were included in the treatment; however, the lesion continued to expand (Fig. 1g). In April 2013, resection of the cyst and Ommaya reservoir placement were performed, which finally controlled the CBN. Histological examination revealed hemosiderin deposits and necrotic brain tissue, and showed no evidence of recurrent tumor. A follow-up MRI on November 2016 (43 months post resection) showed only passive expansion of the removal cavity because of communication to the left lateral ventricle (Fig 1i). Fluid-attenuated inversion recovery image revealed disappearance of abnormal high-signal of cyst contents (Fig. 3). No evidence of CBN recurrence was observed.
Figure 1:
(a) Enhanced T1WI before SRS revealing a tumor in the left parietal lobe. (b) T2WI (12 months after SRS) without evidence of recurrence or complications. (c) T2WI (35 months after SRS) without evidence of recurrence or complications. (d) T2WI (92 months after SRS) showing a high-intensity area suspected of being a change secondary to irradiation, and no evidence of tumor recurrence. (e) T1WI (118 months after SRS) showing an enhanced lesion with perifocal edema. (f) T2WI (123 months after SRS) showing cyst with an edematous-appearing area. (g) Preoperative T2WI (150 months after SRS) showing expansion of the cyst despite conservative therapy. (h) Postoperative T2WI (151 months after SRS, 1 week after resection) showing cyst shrinkage. (i) Postoperative T2WI (193 months after SRS, 43 months after resection) showing only the removal cavity. T1WI, T1-weighted imaging; SRS, stereotactic radiosurgery; T2WI, T2-weighted imaging.
Figure 2:
Computed isodose distribution for the SRS. For the isocenter, 22.5 Gy was prescribed, and the PTV was covered by the 80% isodose line. SRS, stereotactic radiosurgery; PTV, planning target volume.
Figure 3:
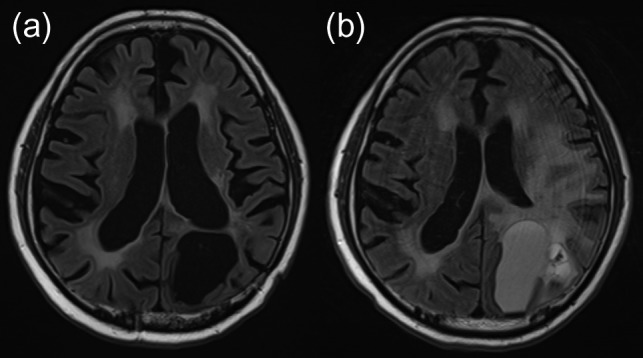
Fluid-attenuated inversion recovery image obtained 193 months after SRS, 43 months after resection, revealed disappearance of abnormal high-signal of cyst contents (a), which was observed before resection (2 days before resection) (b). SRS, stereotactic radiosurgery.
DISCUSSION
CBN after SRS has been reported mainly as a late complication after GK-based SRS for benign non-neoplastic diseases, like AVMs [1] (reported incidence: 3%). However, cases related to BMs after SRS have been scarcely reported [6, 7]. Our patient developed CBN 10 years after linac-based SRS for BM and was successfully treated with surgical resection, even though conservative therapies failed to stabilize. In previous reports, several terminologies have been used to describe this complication: CBN, delayed cyst formation, BN with cystic change and so on. Here, we defined the term ‘CBN’ as BN with secondary cyst formation after RT without tumor recurrence.
The exact mechanism underlying cyst formation remains unclear. Ishikawa et al. [6] hypothesized that CBN after GK-based SRS for BM is essentially the same as or very similar to the lesions seen in patients with AVMs and are not the result of BM progression, and the breakdown of the blood–brain barrier and the increased permeability of injured vessel walls play important roles in cyst formation. In their latest report, they histologically observed degenerated tissue mainly comprising fibrous tissue and various stage of hemorrhage and speculated that this tissue remains for many years and reparative processes cause an expanding mass lesion [7]. In our patient, hemosiderin deposits and necrotic tissue were histologically observed, and no evidence of tumor recurrence was detected.
In non-cystic BN, conservative therapies, such as hyperbaric oxygen therapy, corticosteroids or bevacizumab, are the standard of care [4, 8, 9], and surgical procedures are seldom required. Conversely, CBN tends to be resistant to these conservative therapies and readily recurs, and no standard therapy has been established yet; surgical resection or continuous cyst drainage using a cystoperitoneal shunt or Ommaya reservoir placement is usually required [1–3, 6, 7]. According to previous reports, 32.8–88% of CBNs were symptomatic and 32.8–63% required surgery [1, 6]. In our patient, conservative therapies (the administration of corticosteroids and bevacizumab) failed, and surgical resection with continuous drainage by the placement of an Ommaya reservoir was necessary to stabilize the lesion.
The unique point of this complication is its extremely long latency (≥4–7 years after SRS) [1–3, 6, 7]. Considering its latency, the typical follow-up period for BMs was insufficient to detect this complication. Our patient developed symptomatic CBN long after SRS, although RT initially appeared successful; no visible abnormality on MRI for 8 years and no symptomatic complications for 10 years were observed. Longer follow-up with special attention to this complication should be considered in long-term survivors, given the improved favorable prognosis of patients with BMs because of the recent advances in systemic therapy [10].
CBN is a rare radiation-induced BN with a long latency. CBN is generally intractable, and there are no established treatment. We should be aware of the risk of CBN development in long-term BM survivors after SRS.
ACKNOWLEDGMENTS
None.
FUNDING
This work was funded in part by the Bayer research subsides from Japan Radiological Society.
CONFLICT OF INTEREST
No conflicts of interest.
ETHICAL APPROVAL
The present study adhered to the 1964 Helsinki declaration, and our Institutional Ethics Review Board approved the research (approval number E2277).
CONSENT
We obtained a signed informed consent from the patient referred to in this study.
GUARANTOR
Takashi Mizowaki (corresponding author).
REFERENCES
- 1. Ilyas A, Chen CJ, Ding D, Mastorakos P, Taylor DG, Pomeraniec IJ, et al. Cyst formation after stereotactic radiosurgery for brain arteriovenous malformations: a systematic review. J Neurosurg 2018;128:1354–63. [DOI] [PubMed] [Google Scholar]
- 2. Pollock BE, Gorman DA, Coffey RJ. Patient outcomes after arteriovenous malformation radiosurgical management: results based on a 5- to 14-year follow-up study. Neurosurgery 2003;52:1291–6. [DOI] [PubMed] [Google Scholar]
- 3. Shuto T, Matsunaga S, Suenaga J. Surgical treatment for late complications following gamma knife surgery for arteriovenous malformations. Stereotact Funct Neurosurg 2011;89:96–102. [DOI] [PubMed] [Google Scholar]
- 4. Na A, Haghigi N, Drummond KJ. Cerebral radiation necrosis. Asia Pac J Clin Oncol 2014;10:11–21. [DOI] [PubMed] [Google Scholar]
- 5. Fang W, Gu B, Jing X, Xiao S, Fan S, Liao W, et al. Late-onset cystic brain necrosis after radiotherapy for nasopharyngeal carcinoma. Jpn J Clin Oncol 2017;47:499–504. [DOI] [PubMed] [Google Scholar]
- 6. Ishikawa E, Yamamoto M, Saito A, Kujiraoka Y, Iijima T, Akutsu H, et al. Delayed cyst formation after gamma knife radiosurgery for brain metastases. Neurosurgery 2009;65:689–94. [DOI] [PubMed] [Google Scholar]
- 7. Yamamoto M, Kawabe T, Higuchi Y, Sato Y, Nariai T, Barfod BE, et al. Delayed complications in patients surviving at least 3 years after stereotactic radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys 2013;85:53–60. [DOI] [PubMed] [Google Scholar]
- 8. Bui QC, Lieber M, Withers HR, Corson K, van Rijnsoever M, Elsaleh H. The efficacy of hyperbaric oxygen therapy in the treatment of radiation-induced late side effects. Int J Radiat Oncol Biol Phys 2004;60:871–8. [DOI] [PubMed] [Google Scholar]
- 9. Gonzalez J, Kumar AJ, Conrad CA, Levin VA. Effect of bevacizumab on radiation necrosis of the brain. Int J Radiat Oncol Biol Phys 2007;67:323–6. [DOI] [PubMed] [Google Scholar]
- 10. Sperduto PW, Kased N, Roberge D, Xu Z, Shanley R, Luo X, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol 2012;30:419–25. [DOI] [PMC free article] [PubMed] [Google Scholar]