Abstract
Background:
Cancer is one of the major comorbidities in patients with sepsis, and conversely, bloodstream infections (BSI) may precede the diagnosis of colorectal malignancy, in particular when Streptococcus gallolyticus is isolated. We present the rare case of an Escherichia coli BSI preceding the diagnosis of rectal adenocarcinoma.
Case presentation:
A 56-year-old man with a history of ocular myasthenia gravis presented with fever and shaking chills, and was diagnosed with E. coli BSI of unknown origin. After a thorough history and examination, diagnostic workup revealed a rectal adenocarcinoma as portal of entry for E. coli BSI. The choice of definitive antibiotic treatment was complicated by the risk of myasthenia gravis exacerbation by several classes of antibiotics.
Conclusions:
In patients with E. coli BSI of unknown origin, clinicians need a high index of suspicion regarding underlying colorectal malignancies. This may permit earlier diagnosis in a potentially curable stage.
INTRODUCTION
The burden of bloodstream infections (BSI) with Gram-negative bacilli is significant in in- and outpatients. Portals of entry differ according to the setting with the urinary- and gastrointestinal tract representing the main entry sites in community-onset Gram-negative bacteremia in developed countries. Although the presence of cancer is reported as one of the major comorbidities in patients with sepsis, undiagnosed tumors are rarely identified as the primary source of BSI. Moreover, only certain bacterial species such as Streptococcus gallolyticus, Fusobacterium nucleatum and Clostridium septicum have been found to be associated with gastrointestinal carcinomas [1].
In this report, we present a case of Escherichia coli bacteremia as the consequence of a previously unknown, locally advanced rectal adenocarcinoma. To our knowledge, the present case is the second report of an Escherichia coli BSI preceding the diagnosis of rectal adenocarcinoma [2].
CASE REPORT
A 56-year-old man presented with sudden onset of fever and shaking chills. He had experienced a similar, self-limiting episode 1 month ago. His medical history included ocular myasthenia gravis treated with low-dose pyridostigmine. His family history was unremarkable.
In the emergency department, the patient was febrile (39.7°C), tachycardic and normotensive. Physical examination not including a rectal exam was unremarkable. Routine laboratory analyses revealed mild leukocytosis (12.3 × 109/L, norm: 3.5–10) with a neutrophil left shift, whereas renal and liver function tests, C-reactive protein (CRP), urine dipstick and a chest X-ray were unremarkable. The patient was admitted with sepsis of unknown origin and started on ceftriaxone. Blood cultures flagged positive for E. coli overnight, resistant to amoxicillin/clavulanic acid and cotrimoxazole.
On the ward, review of systems revealed intermittent episodes of diarrhea and pencil-shaped stools with bright-red blood streaks during the past month and a weight loss of 10 kg. In addition, a rectal exam was significant for a painless mass in the rectal vault with small quantities of fresh blood. An abdominal CT scan demonstrated thickening of the rectal wall with perirectal stranding and reactive lymphadenitis. A pelvic MRI for local staging revealed infiltration of the mesorectal fat tissue without involvement of adjacent structures or lymphatic dissemination (Fig. 1). Recto-sigmoidoscopy showed a partially obstructing rectal tumor (Fig. 2). Biopsy results demonstrated a tubular adenoma with high-grade dysplasia and focal transition into an invasive adenocarcinoma. After histopathologic examination and additional endosonography, the patient was diagnosed with a rectal adenocarcinoma (Stage IIA, cT3, N0, M0) and the E. coli bacteremia was judged as a consequence of the tissue invasive cancer.
Figure 1:
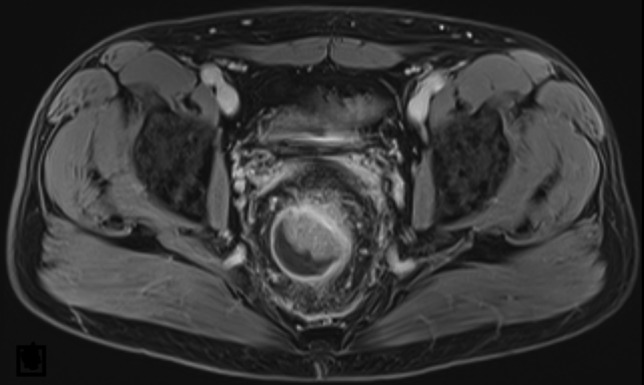
Initial MRI (t2) of the pelvis showing a rectal mass with luminal stenosis and infiltration of the mesorectal fat tissue without involvement of adjacent structures or lymphatic tissue.
Figure 2:
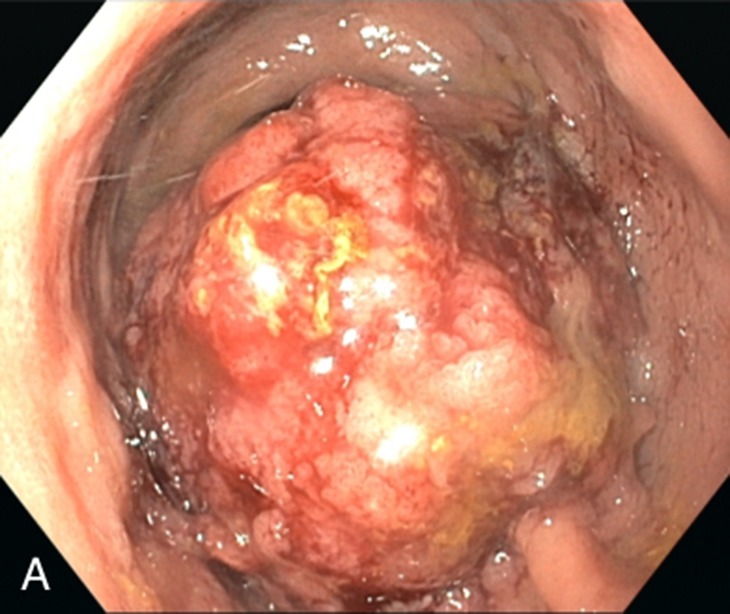
Sigmoidoscopy demonstrating a large (11 cm), circumferential, obstructing rectal mass with exophytic growth and located 2 cm from the anal verge.
Due to resistance to cotrimoxazole, low bioavailability of oral cephalosporins and the neuromuscular-blocking activity of ciprofloxacin, ceftriaxone i.v. was continued for a total of 7 days. The patient was afebrile and clinically stable after 1 day of treatment (peak CRP 40.8 mg/L). Neo-adjuvant combined radio-chemotherapy followed by total mesorectal excision were planned in a curative intent after discussion at the local tumor board.
DISCUSSION
The patient described in this report presented with an episode of fever secondary to an E. coli bacteremia. A thorough history and clinical examination lead us to the hypothesis of an underlying colorectal malignancy as cause of the E. coli BSI, which was confirmed in the subsequent work up. With only superficial patient history and physical examination, the source of this patient’s BSI could have been missed. This may have delayed the diagnosis of rectal cancer.
Interestingly, the clinical presentation of the patient’s Gram-negative BSI was quite oligosymptomatic and likely included a previous self-limiting episode 1 month prior to his consultation. We concluded that—in contrast to other infective sources such as pyelonephritis—a colorectal carcinoma serving as port of entry may lead to repetitive invasions of the bloodstream followed by immediate clearance in some cases.
There is a well-known link between colorectal cancer and certain bacterial pathogens such as Streptococcus gallolyticus, Clostridium septicum and Fusobacterium nucleatum, with S. gallolyticus BSI being a red flag for underlying colorectal cancer in particular [1]. Escherichia coli BSI (without previous or subsequent bacteremic episodes caused by other pathogens) was previously reported with underlying, undetected colon cancer in only seven patients (rectum (1/7), sigmoid (3/7), right (2/7) and left (1/7) colon) [2–5]. Mechanisms that render solid tumor patients prone to BSI such as progressive catabolic state, malnutrition, ulcerating lesions, obstructive processes, and immune suppression due to chemotherapy, radiation and/or the malignancy itself have been described. Interestingly, functional studies have identified specific bacterial species playing a causal role in carcinogenesis of the gastrointestinal tract [6]. With regards to colorectal cancer, abnormal local colonization with E. coli has been reported [7] in addition to mucosal translocation and intracellular persistence in human macrophages [8], which ultimately may lead to invasion of the bloodstream. In addition, localized pericolic abscess formation due to microperforation may be responsible for episodes of E. coli bacteremia [9]. However, more research is needed regarding repetitive oligosymptomatic bacteremia and microbial dysbiosis in colorectal cancer patients.
The choice and duration of antibiotic therapy warrants further discussion. In our patient, intravenous to oral switch would have been desirable given his rapid clinical stabilization and lack of drainable abscess. However, several antibiotics are known to exacerbate symptoms in patients with myasthenia gravis, in particular aminoglycosides. Furthermore, fluoroquinolones—usually a good oral option—had to be dismissed due to their detrimental effect on neuromuscular transmission and myasthenia gravis, which lead to a Boxed Warning in the USA in 2011 [10]. Given the availability of a suitable alternative agent (ceftriaxone) we chose not to administer ciprofloxacin with the risk of exacerbating myasthenia, although the disease of our patient was mild and careful monitoring after exposure to ciprofloxacin would have been possible.
Given the patient’s rapid clinical response and the fact that he may have had self-limiting bacteremia in the past, a treatment duration of 7 days was chosen in line with recent data demonstrating equal outcomes of short (median 8 d) vs. long (median 15 d) courses of antibiotic therapy in BSI with Enterobacteriaceae [11].
CONCLUSIONS
Clinicians treating patients with Gram-negative BSI without a readily identifiable source after a thorough work-up including a careful clinical examination may consider searching for an underlying colorectal cancer in order to facilitate the diagnosis in an earlier, potentially curable stage. In patients with myasthenia gravis, clinicians need to consider the risk of symptom-exacerbation associated with certain antibiotics.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.
ETHICAL APPROVAL
No ethical approval was required.
CONSENT
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
REFERENCES
- 1. Kwong TNY, Wang X, Nakatsu G, Chow TC, Tipoe T, Dai RZW, et al. . Association between bacteremia from specific microbes and subsequent diagnosis of colorectal cancer. Gastroenterology 2018. 10.1053/j.gastro.2018.04.028. [DOI] [PubMed] [Google Scholar]
- 2. Kelleher JP, Sales JEL. Pyrexia of unknown origin and colorectal carcinoma. Br Med J (Clin Res Ed) 1986;293:1475–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Belhassen-Garcia M, Velasco-Tirado V, Lopez-Bernus A, Alonso-Sardon M, Carpio-Pérez A, Fuentes-Pardo L, et al. . Fever of unknown origin as the first manifestation of colonic pathology. Clin Med (Lond) 2013;13:141–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lecoules S, Carmoi T, Klotz C, Rapp C, Perrot G, Galeano C, et al. . Fever as the presenting manifestation of colon cancer: a case series of 11 patients. Rev Med Interne 2013;34:136–40. [DOI] [PubMed] [Google Scholar]
- 5. Patel HG, Tabassum S, Shaikh S. E. coli sepsis: red flag for colon carcinoma—a case report and review of the literature. Case Rep Gastrointest Med 2017;2017:2570524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wu S, Rhee K-J, Albesiano E, Rabizadeh S, Wu X, Yen HR, et al. . A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med 2009;15:1016–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Buc E, Dubois D, Sauvanet P, Raisch J, Delmas J, Darfeuille-Michaud A, et al. . High prevalence of mucosa-associated E. coli producing cyclomodulin and genotoxin in colon cancer. PLoS One 2013;8:e56964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Raisch J, Rolhion N, Dubois A, Darfeuille-Michaud A, Bringer A. Intracellular colon cancer-associated Escherichia coli promote protumoral activities of human macrophages by inducing sustained COX-2 expression. Lab Invest 2015;95:296–307. [DOI] [PubMed] [Google Scholar]
- 9. Agmon-Levin N, Ziv-Sokolovsky N, Shull P, Sthoeger ZM. Carcinoma of colon presenting as fever of unknown origin. Am J Med Sci 2005;329:322–6. [DOI] [PubMed] [Google Scholar]
- 10. Jones SC, Sorbello A, Boucher RM. Fluoroquinolone-associated myasthenia gravis exacerbation: evaluation of postmarketing reports from the US FDA adverse event reporting system and a literature review. Drug Saf 2011;34:839–47. [DOI] [PubMed] [Google Scholar]
- 11. Chotiprasitsakul D, Han JH, Conley AT, Cosgrove SE, Harris AD, Lautenbach E, et al. . Comparing the outcomes of adults with enterobacteriaceae bacteremia receiving short-course versus prolonged-course antibiotic therapy in a multicenter, propensity Score-matched cohort. Clin Infect Dis 2017;767:1093. [DOI] [PMC free article] [PubMed] [Google Scholar]


