Abstract
We used the lesser prairie‐chicken (Tympanuchus pallidicinctus), an iconic grouse species that exhibits a boom–bust life history strategy, on the Southern High Plains, USA, as a bioindicator of main and interactive effects of severe drought and grazing. This region experienced the worst drought on record in 2011. We surveyed lesser prairie‐chicken leks (i.e., communal breeding grounds) across 12 years that represented 7 years before the 2011 drought (predrought) and 4 years during and following the 2011 drought (postdrought). Grazing was annually managed with the objective of achieving ≤50% utilization of aboveground vegetation biomass. We used lek (n = 49) count data and covariates of weather and managed grazing to: (a) estimate long‐term lesser prairie‐chicken abundance and compare abundance predrought and postdrought; (b) examine the influence of annual and seasonal drought (modified Palmer drought index), temperature, and precipitation on long‐term lesser prairie‐chicken survival and recruitment; and (c) assess and compare the influence of grazing on lesser prairie‐chicken population predrought and postdrought. Lesser prairie‐chicken abundance was nearly seven times greater predrought than postdrought, and population declines were attributed to decreased survival and recruitment. The number of days with temperature >90th percentile had the greatest effect, particularly on recruitment. The population exhibited a substantial bust during 2011 and 2012 without a boom to recover in four postdrought years. Adaptive grazing positively influenced the population predrought, but had no effects postdrought. Results suggest that the severe drought in 2011 may have been beyond the range of environmental conditions to which lesser prairie‐chickens, and likely other species, have adapted. Land management practices, such as grazing, should remain adaptive to ensure potential negative influences to all species are avoided. Increasing habitat quantity and quality by reducing habitat loss and fragmentation likely will increase resiliency of the ecosystem and individual species.
Keywords: climate change, drought, grazing, lesser prairie‐chicken, sand shinnery oak grasslands, Southern High Plains
1. INTRODUCTION
Anthropogenic‐induced climate change has altered the frequency and intensity of weather to the extent that these events are affecting biotic systems. Species’ responses to global climate change include shifts in distributions, changes in population sizes, and alterations in reproductive phenology (Charmantier & Gienapp, 2014; Thomas et al., 2004). In arid landscapes, where precipitation is a key limiting resource (Noy‐Meir, 1973; Schwinning & Sala, 2004), precipitation pulses (i.e., discrete, infrequent, and unpredictable precipitation events) are important drivers of ecosystem structure and function (Ehleringer, Schwinning, & Gebauer, 1999; Schwinning & Sala, 2004). Climate change‐induced alterations in the seasonality and variability of precipitation events likely will have cascading effects from soil moisture to plant and wildlife species (Weltzin et al., 2003). In fact, changes in precipitation frequency and magnitude have the potential to transform entire landscapes over the course of the next 50 years.
As species and ecological systems are affected by alterations in climate, their resilience (the ability of a system to return to an equilibrium state after a temporary disturbance) and resistance (a measure of the persistence of systems and their ability to absorb change and disturbance while maintaining the same relationships among populations or state variables (Chambers et al., 2017; Holling, 1973) will influence population persistence. In arid ecosystems, the boom–bust life history strategy is not uncommon (Arthington & Balcombe, 2011; Dickman, Greenville, Beh, Tamayo, & Wardle, 2010; Kingsford, Curtin, & Porter, 1999) and likely evolved in response to pulses in precipitation with increased abundance during wet years (booms) and decreased abundance during dry years (bust). However, species and systems may have low resistance and resilience to future climatic conditions if they are outside of the range to which they have adapted. Therefore, understanding and incorporating resistance and resilience of species and systems are becoming increasingly important to conservation planning and adaptive management strategies, particularly in regard to the management of wildlife and their habitats, to facilitate population persistence in a changing climate (Chambers et al., 2017; Hannah et al., 2002; Lawler, 2009; Mawdsley, O'Malley, & Ojima, 2009; Scheffer, Carpenter, Foley, Folke, & Walker, 2001).
In addition to ongoing effects of climate change, shifts in the primary ecological drivers that historically maintained landscapes have affected the structuring of plant and animal communities. A loss of ecological drivers is especially true for grasslands, which represent one of the most altered ecosystems in North America (Samson & Knopf, 1994). Within the Southern High Plains, a semiarid subregion at the southwestern extent of the Great Plains, traditional ecological drivers of drought, fire, and grazing have often changed as native prairies have been converted to other land use types (Milchunas, Lauenroth, Chapman, & Kazempour, 1989; Milchunas, Sala, & Lauenroth, 1988; Samson & Knopf, 1994, 1996; Savage, 1937). Combined, climate change‐driven alterations in temperature and extreme drought occurrence and alterations in other ecological drivers may influence wildlife on the Southern High Plains directly and through changes in vegetation composition and structure (reviewed in Grisham, Godar, Boal, & Haukos, 2016).
Understanding the roles of ecological drivers on ecosystem structure and function is a vital issue in conservation (Knopf & Samson, 1997), particularly in grassland ecosystems where biota are adapted to the periodic and intermittent effects of drivers. One important ecological driver in grassland structure and function is grazer population densities and species (McNaughton, Ruess, & Seagle, 1988). In history, several species, including American bison (Bison bison), pronghorn (Antilocapra americana), elk (Cervus canadensis), and mule deer (Odocoileus hemionus), grazed the Southern High Plains (Peterson & Boyd, 1998); however, humans have influenced grazing patterns by replacing nomadic American bison and elk with domestic cattle, which have different foraging ecologies (Plumb & Dodd, 1993). These changes in grazing regimes can act independently or with other drivers to alter the vegetation structure and function over vast landscapes, thereby affecting wildlife populations.
Species that are range‐restricted, isolated, and at the periphery of their range may be at greater risk of extinction or extirpation than those with wide distributions (Gibson, Van der Marel, & Starzomski, 2009; Thomas et al., 2004). As such, the sensitivity of species at the edge of their range makes them ideal bioindicators to assess the influences of climate change and other alterations occurring within their habitats. The lesser prairie‐chicken (Tympanuchus pallidicinctus), an iconic grouse species of the Great Plains, is declining, in part because of habitat alterations and changes in disturbance regimes that have historically maintained the species’ habitat (Bailey & Painter, 1994; Dhillion, McGinley, Friese, & Zak, 1994; Grisham et al., 2013; Hagen & Giesen, 2005; Hagen, Jamison, Giesen, & Riley, 2004). Within Sand Shinnery Oak Prairies on the Southern High Plains, which represents both the southernmost and westernmost extent of the lesser prairie‐chicken range, populations exhibit a boom–bust reproductive strategy, a life history adaptation where adult females invest more in their own survival when weather conditions are suboptimal and allocate reproductive efforts during relatively cool, wet breeding seasons (Grisham et al., 2013; Patten, Wolfe, Shochat, & Sherrod, 2005b). Although lesser prairie‐chickens evolved with recurring drought within the Southern High Plains, increases in drought magnitude and frequency may hinder population recovery during nondrought years (Chen & Newman, 1998; Peterson & Boyd, 1998; Wester et al., 2007) due to mismatch between phenology of rainfall events, life span, and reproductive efforts.
Landscapes within the Southern High Plains and their species composition have been drastically reshaped by interannual variability in precipitation and recurring drought, which, historically, occurs at 5‐ to 10‐year intervals (Chen & Newman, 1998; Peterson & Boyd, 1998; Wester et al., 2007). Hence, drought is one of the main historical drivers of plant and wildlife populations on the Southern High Plains (Grisham, Godar & Griffin, 2016; Haukos, 2011; Samson, Knopf, & Ostlie, 2004). However, multiscenario climate models identify the Southern High Plains as a hot spot of extreme impacts of climate change (Diffenbaugh, Giorgi, & Pal, 2008). Extreme impacts include decreases in precipitation and increases in temperature, drought frequency, and drought intensity (Cook, Ault, & Smerdon, 2015; Karl et al., 2010; Oliver, Brereton, & Roy, 2013; Peterson, Stott, & Herring, 2012). The number of days >37.8°C is expected to quadruple to approximately 26–28 days under high emission scenarios by mid‐century, and summers in the region are expected to have less rainfall and longer periods without rainfall (Shafer et al., 2014). The region experienced the worst drought on record in 2011 with >100 days >37.8°C and set new records for the hottest summer since documentation began in 1895 (Nielsen‐Gammon, 2012). Cumulative rainfall during the first 10 months of 2011 was nearly six times lower than the 30‐year average of 43.94 cm at the West Texas Mesonet Site (Lubbock, TX, USA). Substantial increases in year‐to‐year variability in rainfall patterns and more severe storms also are expected (Christian, Christian, & Basara, 2015; Cook et al., 2015).
Synergistically, drought and grazing can alter vegetation communities differently than each of the drivers independently, which may magnify their combined influence on wildlife populations (Loeser, Sisk, & Crews, 2007). Moreover, humans have exacerbated the rate and intensity of drought impacts by influencing the process of desertification through unmanaged grazing (Grover & Musick, 1990). Locally, grazing can shift the structure and composition of vegetation and result in woody shrub encroachment (e.g., Grisham, Borsdorf, Boal, & Boydston, 2014; Haukos & Smith, 1989; Peterson & Boyd, 1998; Riley, Davis, Ortiz, & Wisdom, 1992). Vegetation transitions occur in part because grazing intensity directly affects the time needed for vegetation to recover postdrought, which can take ≥20 years if overgrazed during a drought, and likely is exacerbated in semiarid or precipitation‐limited ecosystems (Albertson, Tomanek, & Riegel, 1957). Although the effects of drought (Grisham et al., 2013; Merchant, 1982; Peterson & Silvy, 1994; Ross, Haukos, Hagen, & Pitman, 2016a) and grazing (Fritts et al., 2016; Jackson & DeArment, 1963; Silvy, Peterson, & Lopez, 2004) on prairie grouse have been surmised, there are considerable knowledge gaps regarding potential interactive effects of these two important ecological drivers, particularly at the core habitat complex scale (i.e., ≤10,200 ha) (Bidwell et al., 2003).
To assess the main and interactive effects of drought and grazing on lesser prairie‐chicken populations, we used grazing data from 7 years before the 2011 drought “predrought” (2004–2010) and 5 years during and after the 2011 drought “postdrought” (2011–2015). In this study, grazing was adaptively managed annually based on vegetation biomass; therefore, we examined lesser prairie‐chicken response to alterations in grazing pressure because of drought‐induced decreases in vegetation biomass. In particular, we had three objectives: (a) to estimate long‐term lesser prairie‐chicken abundance and compare abundance predrought and postdrought; (b) to examine the influence of annual and seasonal drought (modified Palmer drought index), temperature, and precipitation on long‐term lesser prairie‐chicken survival and recruitment; and (c) to assess and compare the influence of grazing on lesser prairie‐chicken population predrought and postdrought. Lesser prairie‐chicken abundance declines following severe drought (Jackson & DeArment, 1963; Ross et al., 2016a; Sullivan, Hughes, & Lionberger, 2001), and we hypothesized abundance estimates would severely decline following the 2011 drought due to declines in both survival and recruitment (Grisham et al., 2013, 2014; Patten, Wolfe, Shochat, & Sherrod, 2005a; Patten et al., 2005b). Because appropriate grazing pressure can stimulate biomass production that can be used for nesting and thermoregulatory cover, we hypothesized that lesser prairie‐chicken abundance would be positively correlated with grazing predrought (Fritts et al., 2016; Hagen, Grisham, Boal, & Haukos, 2013). At last, because survival is dependent on microhabitat and microclimate availability potentially because temperature and wind affect metabolic rates (Patten et al., 2005a; Sherfy & Pekins, 1995), we hypothesized that lesser prairie‐chicken abundance, survival, and recruitment would be negatively related to grazing during/postdrought.
2. MATERIALS AND METHODS
2.1. Study site
Milnesand Prairie Preserve (hereafter “Preserve,” 33 69°N, 103 38°W) was a 75‐km2 , privately owned rangeland in Roosevelt County, New Mexico, USA, on the western edge of the Southern High Plains. The preserve was primarily used for livestock production and management of lesser prairie‐chicken habitat. Strahan (2008) provides a floristic survey of Milnesand Prairie Preserve.
The conservation strategy of Milnesand Prairie Preserve was to maintain and improve habitat for lesser prairie‐chickens through adaptive grazing management, while maintaining the producer's financial viability. The grazing rotation and animal unit months had a goal of 50% utilization of available forage (actual utilization across pastures and years of study (mean ± SD) was 51% ± 23%). Although considerations of stocking rate depend largely on precipitation, moderate rates often are aimed at achieving 35%–50% utilization (Benson, Zhu, Farmer, & Villalobos, 2011; Holechek, Thomas, Molinar, & Galt, 1999). During this study, the grazing system was a cow–calf operation split into two herds. Calving occurred in January, with the annual calf‐crop sold between October and November. Pastures were 731 ± 250 ha (mean ± SD) in size and were grazed throughout the duration of the study, and herds were rotated through most pastures at least once each year with periods of rest with the goal of achieving a 50% utilization rate (Table 1). In addition, cattle were removed from some pastures from late April–mid‐May because tannins in sand shinnery oak (Quercus havardii) catkins can cause decreased recruitment in cattle if ingested in large quantities (Bausch & Carson, 1981). We calculated the number of cow days/ha as the number of cattle in the pasture multiplied by the number of days the cows were in a specific pasture divided by the pasture size (ha). Cow days/ha and percent utilization (Table 1) were not related (Pearson's R correlation coefficient = −0.17).
Table 1.
The number of birds counted on a lek (mean ± SD) per observation, the maximum number of birds seen on a lek, the number of cow days/ha (cow days), percent utilization (% Util), annual precipitation (cm) average annual modified Palmer drought index (Avg PMDI), minimum PMDI (min PMDI), annual maximum temperature (°C; max temp), and the number of hot days (days above the 90th percentile) in pastures containing 49 lesser prairie‐chicken (Tympanuchus pallidicinctus) leks in Milnesand Prairie Preserve, New Mexico, 2004–2015. Blank spaces indicate missing data. Mean ± SD are reported when values differed by pasture
| Covariate | Predrought | Postdrought | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | |
| Avg birds | 8 ± 9 | 11 ± 9 | 15 ± 13 | 8 ± 8 | 9 ± 12 | 5 ± 7 | 3 ± 3 | 6 ± 10 | 3 ± 5 | 2 ± 4 | 1 ± 2 | 1 ± 2 |
| Max birds | 35 | 36 | 67 | 44 | 65 | 28 | 10 | 51 | 14 | 21 | 5 | 9 |
| Cow days | 2.0 ± 0.8 | 1.0 ± 0.5 | 1.2 ± 0.5 | 0.9 ± 0.6 | 1.4 ± 0.9 | 1.0 ± 0.7 | 0.4 ± 0.3 | 0.2 ± 0.1 | 0.2 ± 0.2 | 0.3 ± 0.2 | ||
| % Util | 61 ± 24 | 50 ± 27 | 46 ± 18 | 52 ± 27 | 59 ± 23 | |||||||
| Precipitation | 69.55 | 33.7 | 47.78 | 45.57 | 31.88 | 43.1 | 43.36 | 20.47 | 21.11 | 46.46 | 31.678 | 66.09 |
| Avg PMDI | 0.53 | 3.36 | −1.55 | 0.09 | −1.36 | −2.22 | 0.85 | −3.74 | −4.56 | −3.25 | −2.62 | 2.72 |
| Min PMDI | −3.85 | −0.06 | −3.92 | −2.59 | −3.31 | −3.55 | −1.09 | −6.18 | −5.86 | −5.01 | −3.4 | −0.09 |
| Max temp | 38.33 | 38.89 | 39.44 | 40.56 | 40.00 | 39.44 | 39.44 | 42.78 | 41.11 | 40.00 | 41.11 | 40.56 |
| Hot days | 27 | 22 | 41 | 12 | 30 | 37 | 36 | 84 | 59 | 35 | 27 | 44 |
Weather data were collected approximately 60 km from the study site (Portales, New Mexico (latitude = 34.17417, longitude = −103.352). From 1932 to 2015, the annual precipitation was (mean ± SD) 40.5 ± 13.7 cm (National Oceanic and Atmospheric Administration [NOAA], 2017a,b). The majority of precipitation occurs in July and August on our study site (NOAA, 2017a,b). The 25th percentile of annual precipitation from 1950 to 2015 was 32 cm and 75th percentile was 46 cm (NOAA, 2017a,b). Although 2011 received only slightly less rain than 2012, all but 1.3% occurred after the breeding/brood rearing season (during/after June). Maximum annual temperature was 1.7°C hotter in 2011 than any other year during the study. From 2000 to 2014, the 90th percentile temperature of daily maximum temperature was 35°C. The number of days with a temperature >90th percentile was nearly 1.5 times greater in 2011 than any other year of study.
2.2. Lesser prairie‐chicken surveys
We counted lesser prairie‐chickens on known active leks (n = 49) from March 17 to April 27 in 2004–2015. We defined leks as an area having ≥3 actively displaying males. To find leks, we conducted roadside surveys throughout the study site. We stopped every 0.6 km to perform visual and auditory searches for lesser prairie‐chickens. Once we located a lek, we returned to the lek 1–5 times per season to flush lekking individuals to get an accurate count. We surveyed from 30 min before sunrise to 4 hrs after sunrise.
2.3. Influence of Annual Weather and Grazing on Demographic rates
We estimated abundance, apparent survival (deaths and emigrations), and recruitment (births and immigrations) and assessed the relationships between both weather and grazing covariates and survival and recruitment using multiseason open N‐mixture models with function pcountOpen in package unmarked (Fiske & Chandler, 2011) in R version 3.0.3 (R Core Team, 2014). N‐mixture models can provide robust population trends for lekking species when data are sparse (McCaffery, Nowak, & Lukacs, 2016). Open population N‐mixture models fit the model of Dail and Madsen (2011), which is a generalized form of the Royle (2004) N‐mixture model. These models were specifically designed to use count data from unmarked individuals to estimate: γ, the recruitment rate (births and immigration, the finite rate of increase, or the maximum instantaneous rate of increase); and ω, the apparent survival rate (deaths and emigrations; Fiske et al., 2017).
For all N‐mixture models, we followed a four‐step process. First, we determined the appropriate distribution of the response variable (count data) by comparing null models with zero‐inflated Poisson, Poisson, and negative binomial distributions with AIC (Akaike, 1998). Second, we identified significant predictors of detection by assessing the global detection model with daily rainfall, daily maximum temperature, and effort (the number of times a lek was visited) on the detection process. We omitted variables that had a beta value with a 95% confidence interval that overlapped 0. In the same way, we examined whether the number of individuals counted on each lek the year prior was a predictor of initial abundance. At last, we included covariates of grazing or weather on the survival or recruitment process to assess their influence on lesser prairie‐chicken populations. For recruitment models, we used autoregressive population dynamics, which models recruitment as gamma*N[i, t‐1]. For survival models, we used constant population dynamics, which indicates no relationship between survival and recruitment. Both autoregressive and constant population dynamics use a Markovian process to model the latent abundance state following the initial sampling period in which survivors are modeled as S it ~ Binomial(N it‐1 , w it) and recruits follow G it ~ Poisson(γit).
To assess the influence of annual weather on lesser prairie‐chicken populations, we combined 12 years of lesser prairie‐chicken lek counts. We included 10 variables; annual precipitation (NOAA 2017a), annual average of the modified Palmer drought index (PMDI; NOAA 2017b), and the annual minimum PMDI, annual maximum temperature, and annual number of hot days (the number of days over the 90th percentile in temperature; Table 1) on either the survival or recruitment process. In addition, we included the same five variables with a 1‐year time lag in separate models. We used one of the 10 weather covariates (each of the five variables measured during the same year as the lesser prairie‐chicken population estimates and each with a 1‐year time lag) as a continuous covariate on either recruitment or survival for a total of 20 weather models. We modeled only one covariate on either survival or recruitment because pcountOpen is particularly limited (e.g., slow and has difficulty converging) when including covariates on gamma or omega (Fiske et al., 2017). We estimated annual abundance using the AIC best model and compared between predrought and during/postdrought using an analysis of variance. Using the same methods as above, we assessed the influence of seasonal weather on lesser prairie‐chicken demographics. We used the same response variable (count data from 2004 to 2015) and seasonal (winter: December– February, breeding: March–May, summer: June–August, and fall: September–November) total rainfall and average PMDI during the same year and with a 1‐year time lag as continuous independent variables. We did not assess maximum temperature, minimum PMDI, or hot days seasonally because nearly all were in the summer; thus, those were assessed using the annual covariates. We also did not assess weather influences from the winter or spring the year before because we assumed the weather from >9 months before did not affect the population.
To assess the influence of grazing predrought and postdrought, we analyzed data similar to assessing weather covariates, except we divided data into two time periods: predrought (2004–2010) and during/postdrought (2011–2015). We separated these time periods because we hypothesized that the influence of grazing would be different predrought compared to postdrought. We used the number of cow days/ha as a continuous explanatory covariate on either the recruitment or survival process. Land managers did not record the number of cows in the herd in 2012; therefore, this year was omitted from the grazing assessment. In addition, utilization was recorded intermittently and was not used as a covariate.
3. RESULTS
Lesser prairie‐chicken abundance estimates were nearly seven times greater predrought than postdrought (F 1,10 = 16.46; p < 0.01) and failed to rebound following the 2011 drought (Figure 1). Decreases in abundance can be attributed to reductions in mostly recruitment, but survival may have decreased as well. Hot days on the recruitment process was the AIC best model and accounted for 100% of model cumulative weight (Table 2). Recruitment significantly decreased once the number of hot days was above 20 days (log‐scale β = −1.14, SE = 0.24, 95% CI = −1.61 to −0.67; Figure 2). The AIC best model of survival included average PMDI and accounted for 67% of weights of models that included survival (Table 2). As PMDI increased to above −2, survival increased rapidly (Figure 3). Spring precipitation accounted for 32% of the survival model weights (Table 2). Survival was approximately 50% when spring precipitation was approximately 3 cm and maximized when spring precipitation was slightly over 4 cm (Figure 4).
Figure 1.
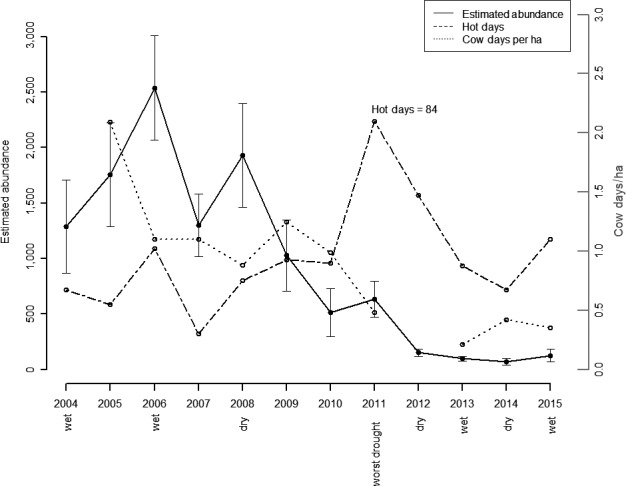
Average estimated abundance of lesser prairie‐chickens (Tympanuchus pallidicinctus) in 49 leks, the number of cow days/ha in pastures containing leks, and annual precipitation in Sand Shinnery Oak Prairie Ecoregion of eastern New Mexico. Wet years are above the 75th percentile of annual precipitation totals since 1950, whereas dry years are below the 25th percentile of annual precipitation totals since 1950. Bars represent 95% confidence intervals on abundance estimates
Table 2.
Open population N‐mixture models to assess the influence of annual precipitation, annual average of modified Palmer drought index (PMDI), annual minimum PMDI, seasonal precipitation, and seasonal average PMDI during the same year each with a 1‐year time lag on lesser prairie‐chicken (Tympanuchus pallidicinctus) survival and recruitment over 49 leks in Milnesand Prairie Preserve, New Mexico, USA, in 2005–2015. Model selection based on Akiake's information criteria (AIC) model fit, number of parameters (K), the difference AICc from the best fit model (ΔAICc), and model weights (w i). Beta values of covariates and 95% confidence intervals are listed
| Demographic | Covariate | Time period | ΔAICc | w i | β | LCL | UCL |
|---|---|---|---|---|---|---|---|
| Recruitment | Hot days | Same year | 0.00 | 1.00 | 1.14 | −1.61 | −0.67 |
| Recruitment | Rainfall spring | Same year | 0.00 | 0.97 | 0.41 | 0.23 | 0.60 |
| Survival | Average PMDI | Same year | 17.77 | 1.00 | 5.41 | 2.42 | 8.39 |
| Survival | Hot days | Same year | 19.34 | 1.00 | −0.13 | −0.21 | −0.05 |
| Survival | Average PMDI summer | Same year | 9.24 | 0.98 | 4.12 | 1.08 | 7.16 |
| Recruitment | Average PMDI winter | Same year | 10.02 | 0.99 | 1.22 | 0.39 | 2.05 |
| Recruitment | Precipitation | Same year | 22.35 | 1.00 | 0.75 | 0.19 | 1.03 |
| Survival | Rainfall spring | Same year | 10.67 | 0.99 | 5.03 | 1.33 | 8.72 |
| Recruitment | Average PMDI | Same year | 22.77 | 1.00 | 0.75 | 0.20 | 1.30 |
| Recruitment | Average PMDI summer | Same year | 11.05 | 1.00 | 0.53 | 0.14 | 0.93 |
| Recruitment | Rainfall fall | Same year | 13.86 | 1.00 | −1.10 | −2.93 | 0.74 |
| Recruitment | Precipitation | Year before | 25.85 | 1.00 | 0.61 | −0.03 | 1.25 |
| Survival | Precipitation | Same year | 26.77 | 1.00 | 2.78 | 1.17 | 4.39 |
| Recruitment | Average PMDI fall | Same year | 15.88 | 1.00 | 0.34 | −0.08 | 0.78 |
| Recruitment | Average PMDI fall | Year before | 16.61 | 1.00 | 0.21 | −0.08 | 0.50 |
| Recruitment | Maximum temperature | Same year | 28.77 | 1.00 | −0.36 | −0.58 | 0.13 |
| Recruitment | Rainfall summer | Same year | 17.47 | 1.00 | −0.16 | −0.47 | 0.15 |
| Recruitment | Rainfall summer | Year before | 17.47 | 1.00 | 0.16 | −0.16 | 0.49 |
| Recruitment | Rainfall fall | Year before | 17.73 | 1.00 | 0.36 | −0.45 | 1.62 |
| Recruitment | Rainfall winter | Same year | 29.91 | 1.00 | −0.16 | −0.47 | 0.15 |
| Recruitment | Maximum temperature | Year before | 30.08 | 1.00 | −0.40 | −0.51 | 0.28 |
| Recruitment | Average PMDI | Year before | 30.10 | 1.00 | 0.07 | −0.17 | 0.31 |
| Recruitment | Minimum PMDI | Same year | 30.11 | 1.00 | 0.06 | −0.15 | 0.27 |
| Recruitment | Minimum PMDI | Year before | 30.15 | 1.00 | 0.06 | −0.13 | 0.25 |
| Survival | Average PMDI winter | Same year | 18.40 | 1.00 | 6.40 | 0.86 | 11.94 |
| Recruitment | Average PMDI summer | Year before | 18.56 | 1.00 | −0.01 | −0.28 | 0.26 |
| Survival | Minimum PMDI | Same year | 32.39 | 1.00 | 9.42 | 4.24 | 14.61 |
| Survival | Average PMDI | Year before | 32.39 | 1.00 | 10.53 | 4.74 | 16.32 |
| Survival | Minimum PMDI | Year before | 32.39 | 1.00 | 8.26 | 3.72 | 12.80 |
| Survival | Average PMDI fall | Same year | 23.88 | 1.00 | 2.58 | 0.64 | 4.51 |
| Survival | Maximum temperature | Same year | 36.96 | 1.00 | −1.40 | −2.77 | 0.70 |
| Survival | Average PMDI spring | Same year | 28.36 | 1.00 | 6.34 | 1.23 | 11.46 |
| Survival | Hot days | Year before | 44.21 | 1.00 | −0.08 | −0.13 | −0.02 |
| Survival | Maximum temperature | Year before | 44.76 | 1.00 | −1.24 | −2.07 | −0.41 |
| Survival | Precipitation | Year before | 46.66 | 1.00 | 1.98 | 0.65 | 3.31 |
| Survival | Rainfall summer | Year before | 37.67 | 1.00 | 0.70 | 0.03 | 1.37 |
| Survival | Average PMDI summer | Year before | 39.15 | 1.00 | 0.69 | −0.47 | 1.86 |
| Survival | Rainfall fall | Year before | 41.40 | 1.00 | 0.59 | −0.82 | 2.00 |
| Survival | Rainfall fall | Same year | 41.50 | 1.00 | −0.59 | −2.12 | 0.94 |
| Survival | Rainfall winter | Same year | 41.57 | 1.00 | 0.20 | −0.37 | 0.78 |
| Survival | Rainfall summer | Same year | 41.57 | 1.00 | 0.20 | −0.37 | 0.78 |
Figure 2.
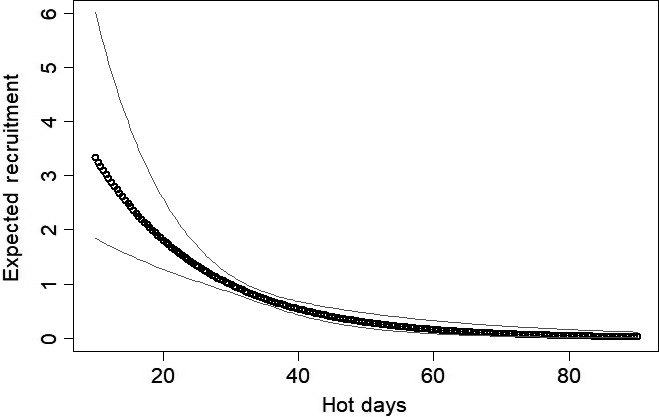
The number of annual days with a maximum temperature >90th percentile (hot days) negatively influenced lesser prairie‐chicken (Tympanuchus pallidicinctus; n = 49 leks) recruitment in Milnesand Prairie Preserve, New Mexico, from 2004 to 2015. Gray lines represent 95% confidence intervals
Figure 3.
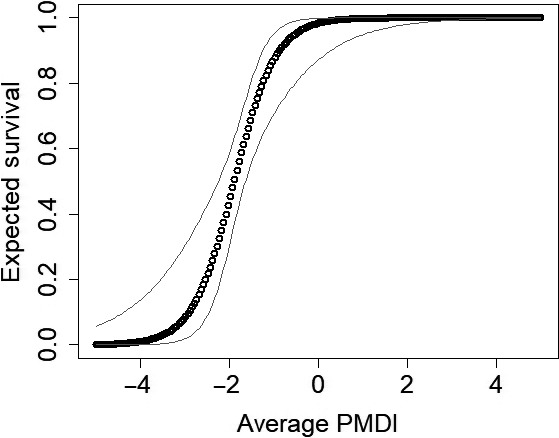
Average annual Palmer’Modified Drought Index (PMDI) positively influenced lesser prairie‐chicken (Tympanuchus pallidicinctus; n = 49 leks) survival in Milnesand Prairie Preserve, New Mexico, from 2004 to 2015. Gray lines represent 95% confidence intervals
Figure 4.
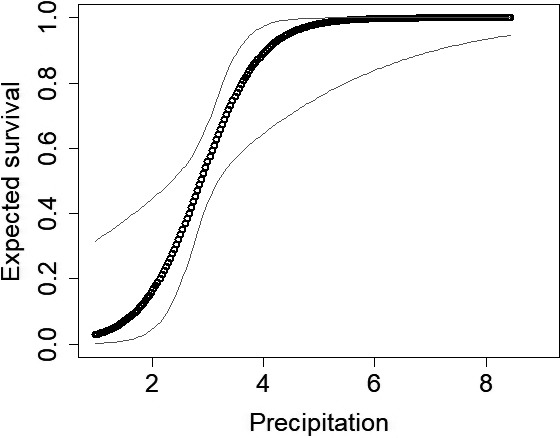
Spring precipitation (cm) positively influenced lesser prairie‐chicken (Tympanuchus pallidicinctus; n = 49 leks) survival in Milnesand Prairie Preserve, New Mexico, from 2004 to 2015. Gray lines represent 95% confidence intervals
Grazing had positive influences on lesser prairie‐chicken survival (cow days/ha logit‐scale β = 0.54, SE = 0.07, z = 8.16, 95% CI = 0.41–0.67; Figure 5) and recruitment predrought (cow days/ha log‐scale β = 0.19, SE = 0.02, z = 9.41, 95% CI = 0.15–0.23; Figure 6), but no influence postdrought. As the number of cow days/ha increased by 1 predrought, both survival and recruitment increased by approximately 0.8 (SE = 0.01 and 0.04, respectively).
Figure 5.
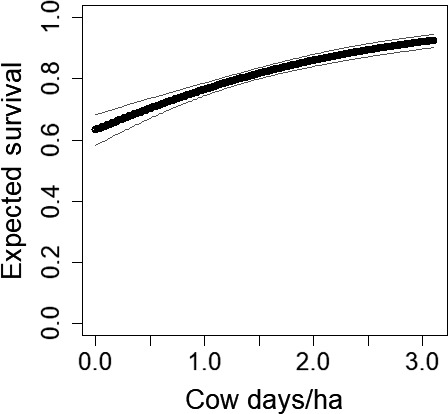
The number of cow days/ha positively influenced lesser prairie‐chicken (Tympanuchus pallidicinctus; n = 49 leks) survival predrought in Milnesand Prairie Preserve, New Mexico, from 2004 to 2015. Gray lines represent 95% confidence intervals
Figure 6.
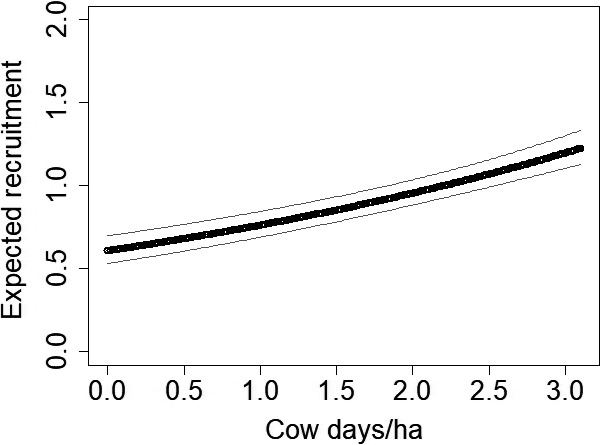
The number of cow days/ha positively influenced lesser prairie‐chicken (Tympanuchus pallidicinctus; n = 49 leks) recruitment predrought in Milnesand Prairie Preserve, New Mexico, from 2004 to 2015. Gray lines represent 95% confidence intervals
4. DISCUSSION
Using the lesser prairie‐chicken population on the Southern High Plains as a bioindicator of wildlife response to intense drought supports burgeoning evidence that wildlife is negatively affected by above average frequencies of drought and, over the long‐term, may not be adapted to the magnitude of drought expected with climate change. In fact, negative effects of increases in drought magnitude and frequency have been documented for all taxa, including amphibians (Mac Nally, Horrocks, & Lada, 2017), reptiles (Westphal, Stewart, Tennant, Butterfield, & Sinervo, 2016), fish (Jaeger, Olden, & Pelland, 2014), mammals (Ahlers et al., 2015), and birds (Selwood et al., 2015). Like other wildlife species in arid and semiarid grasslands, lesser prairie‐chickens on the Southern High Plains have adaptations that increase resilience to extreme environments and fluctuating weather patterns; however, environmental conditions expected from climate change may be outside of their adaptive potential, particularly in the time frame weather changes are expected to occur. It was apparent from 12‐year population models that lesser prairie‐chicken populations in this ecosystem exhibited a boom–bust life history strategy in which abundance was linked to drought conditions. Typically following a less intense drought followed by a bust period, such as that experienced in 2008, the population rebounds. However, if another drought occurs at a quicker return interval or that is greater in magnitude, populations may not boom before the subsequent bust. Our estimates indicated that the population failed to rebound for at least 4 years following the 2011 drought, suggesting the extreme environmental conditions during 2011 may have been beyond that to which the lesser prairie‐chicken is adapted and/or that the return interval following the 2008/2009 dry period and ensuing low population numbers in 2010 was too short for the population to recover enough to be resilient to the 2011 drought. This bust in population abundance with no subsequent boom has been documented in other bird species as well and indicates low resilience and stability to intensified drought conditions (Selwood et al., 2015).
Declines in lesser prairie‐chicken abundance during periods of drought have been documented throughout the species’ range (Giesen, 1998; Grisham et al., 2013; Merchant, 1982; Rodgers, 2016; Ross, Haukos, Hagen, & Pitman, 2016b). The decreases in abundance observed in our study resulted from both low adult survival and reduced reproductive output, which reduced lesser prairie‐chicken numbers on the Southern High Plains. The number of hot days had the greatest effect on recruitment, which corroborates previous studies that recorded reduced recruitment rates during drought years (Giesen, 1998; Grisham et al., 2014; Merchant, 1982; Ross et al., 2016a). Grisham et al. (2013) suggested aboveaverage winter temperatures, which often are correlated to La Niña events such as in 2011, negatively influence reproductive output, and may lead to nest survival below levels viable for population persistence. Although several mechanisms can result in reduced reproductive output, we surmise that decreased nesting effort and increased nest abandonment ultimately led to fewer broods produced during our study (Grisham et al., 2014; Merchant, 1982). Whether eggs die before or after nest abandonment is unknown, but environmental thresholds of temperature and vapor pressure exist for nest survival (Grisham, Godar, Boal, et al., 2016). Nest success and chick survival greatly influence population growth (Hagen, Sandercock, Pitman, Robel, & Applegate, 2009), both vital to near‐term population persistence. In Sand Shinnery Oak Prairies in Texas, 12 of 15 (80%) radiotagged hens failed to incubate eggs when conditions were similar (e.g., same year and habitat type, but different state) to those in our study (Grisham et al., 2014). Moreover, several lesser prairie‐chicken nest abandonments occurred in 2009 when ambient temperatures exceeded 38°C between four and seven consecutive days (Grisham, 2012). However, precipitation in the 2009 late summer may have increased survival so the effects to the population were not as long‐term as in 2011. Fields, White, Gilgert, and Rodgers (2006) found lower brood survival in Kansas when temperatures exceeded 35º C compared to cooler temperatures, a phenomenon also seen with other grouse species. For example, when operative temperatures were >35°C, nest survival diminished for greater prairie‐chickens (Tympanuchus cupido) in Oklahoma (Hovick, Elmore, Allred, Fuhlendorf, & Dahlgren, 2014). Therefore, we suspect thermal stress on incubating hens, eggs, and chicks was responsible for reduced recruitment during the drought in this study.
Land management practices, including grazing, can offset adverse effects of climate change (Greenwood, Mossman, Suggitt, Curtis, & Maclean, 2016; Mawdsley et al., 2009; Pyke & Marty, 2005), particularly if management remains adaptive. In our study, although the directly measured benefits of grazing diminished following the 2011 drought, grazing at substantially reduced rates during and following the drought did not negatively affect lesser prairie‐chicken populations. It is likely that a quadratic relationship between grass canopy cover and demographics exists, as has been found between VOR and nest bowl, nest site selection, and brood locations (Lautenbach 2015), suggesting a tradeoff between cover, movement ability, and escape from predators (Bergerud and Gratson 1988, Hagen, Pitman, Sandercock, Robel, & Applegate, 2007). Tall, thick vegetation can hinder lesser prairie‐chicken ability to move broods through the landscape and reduce their ability to detect predators (Hagen et al., 2007), and grazing can be used to decrease vegetation, particularly in wet years. However, bare ground >35% may be detrimental to nesting hens (Fritts et al., 2016); thus, increases in grazing pressure during or following extreme drought (i.e., comparable to 2011 drought event) may negatively affect survival and recruitment. Measurements of grazing such as utilization and the number of cow days/ha have the potential to provide inference that is prescriptive for land owners and managers on the influence of grazing on wildlife populations.
The timing and magnitude of precipitation events affect plant and wildlife populations (Dale et al., 2001; Fravolini et al., 2005; Heffelfinger, Guthery, Olding, Cochran, & McMullen, 1999; Lusk, Guthery, & DeMaso, 2001; Robertson, Bell, Zak, & Tissue, 2010). Wetter conditions often are optimal for lesser prairie‐chickens on the Southern High Plains, but the importance of precipitation is complex. The timing of precipitation can negatively impact nest and brood survival in the spring, especially when paired with low temperatures (Fields et al., 2006). In addition, the monthly or annual amount of precipitation can be a misleading indicator for lesser prairie‐chicken population resilience and recovery if the magnitude and frequency of precipitation events are ignored. For example, drought conditions, although to a lesser extreme than in 2011, occurred in 2005 and population numbers decreased; however, populations rebounded in 2006 as precipitation increased, particularly during the nesting season (March, 2.59 cm across seven precipitation events, and April, 1.50 cm across four precipitation events, had aboveaverage rain for the month) and end of the summer. Although 2013 was a relatively wet year, 40% of the rain occurred in August and September and may not have led to adequate nesting and brood rearing habitat in March (0.15 cm across two precipitation events) or April (0.05 cm across one precipitation event). March (0.71 cm across four precipitation events) and April 2014 (1.32 cm across three precipitation events) experienced belowaverage precipitation as well.
Climate change and habitat fragmentation interact to affect wildlife populations and communities (Christie, Jensen, Schmidt, & Boyce, 2015; Opdam & Wascher, 2004). Because of large variation in annual habitat conditions, broad landscapes are necessary to provide selection based on habitat quality for wildlife during years of poor primary productivity. Lesser prairie‐chickens are a landscape‐scale species requiring several thousand hectares to fulfill the life history needs of a population (Haukos & Zavaleta, 2016). Lesser prairie‐chickens on the Southern High Plains occupy the Sand Shinnery Oak Prairie ecosystem, which has been considered threatened in New Mexico (Bailey & Painter, 1994) and Texas (Dhillion et al., 1994) for several decades.
In addition to the dry/drought effects experienced in our study following 2011 when the population decline became pronounced, habitat loss and fragmentation in Sand Shinnery Oak Prairies may have exacerbated population declines and contributed to declines prior to the 2011 drought. Although belowoptimal conditions, such as drought, led to the “bust” in the boom–bust population cycle (Hagen et al., 2009), past populations likely had greater potential to recover because of increased habitat connectivity and dispersal ability and greater initial metapopulation numbers to bolster the populations that had been negatively affected (Davis, Horton, Odell, Rodgers, & Whitlaw, 2008; Grisham, 2012). Thus, the low resilience and resistance of lesser prairie‐chicken populations to recent drought likely is exacerbated by habitat loss and fragmentation (Davis et al., 2008; Grisham, 2012; Oliver et al., 2013). Over the long term, intensive, adaptive habitat management will be vital to population persistence.
The resilience and resistance of species and ecosystems to changing environmental conditions depend on several factors. More intact ecosystems likely can better withstand increases in stochastic events (Oliver et al., 2013) as larger landscapes provide broader ranges of microclimates and resources (Hodgson, Moilanen, Wintle, & Thomas, 2011; Oliver, Roy, Hill, Brereton, & Thomas, 2010). Landscapes and terrain that provide microclimates can limit exposure of individuals to extreme environmental conditions and help regulate body temperature and water loss (Dobrowski, 2011; Williams, Shoo, Isaac, Hoffmann, & Langham, 2008). Increased habitat connectivity facilitates an individual's movement across a landscape, as mentioned above; thus, more mobile species may benefit inequitably. If a species is not as mobile, it may, through evolutionary time, alter fitness‐related traits by plastic change or genetic adaptation (Chevin, Lande, & Mace, 2010; Moritz & Agudo, 2013). Lesser prairie‐chickens can have home ranges of thousands of hectares, but they exhibit high site fidelity (Giesen 1994, Riley et al. 1994). The population in our study system not only experiences warmer and drier conditions compared to other populations due to it being the most extreme south and west population, but also the most extreme microclimates, particularly during incubation (Grisham, Godar, & Griffin, 2016; Grisham, Godar, Boal, et al., 2016; Grisham, Zavaleta, et al., 2016). Lesser prairie‐chicken resilience and stability may be increased by efforts to restore marginal croplands back to prairie habitats and to improve habitat quality and connectivity of populations (Ross et al., 2016a), thereby allowing increased immigration and dispersal abilities.
As climate change leads to more variability in rainfall patterns (Christian et al., 2015; Cook et al., 2015), biotic systems will continue to be affected. System responses to climate change are complex and likely nonlinear; therefore, using suitable species as bioindicators will be important for predicting the influences of global change and assessing the role of land management in conservation. For lesser prairie‐chickens, adaptive habitat conservation and management are vital for population persistence beyond 2050 given projections of increased drought frequency and intensity (Christian et al., 2015; Cook et al., 2015; Grisham, Zavaleta, et al., 2016), which, based on results, may further disrupt the boom–bust life history strategy by either reducing the population to levels that cannot rebound quickly or not allowing enough time following an extreme drought to rebound to levels that will be resilient to another drought in a quicker return interval. Lesser prairie‐chickens and other wildlife likely will benefit from habitat management that remains adaptive with continuous monitoring to ensure adequate habitat, including microhabitat, is available particularly during altered weather conditions. Based on results, specific adaptive measures that benefit wildlife on the Southern High Plains include destocking cattle during and immediately following severe drought, resting pastures between grazing events, and restocking at decreased levels following rainfall events in early spring. In addition, we suggest improving habitat quality and quantity to maintain stability and resiliency of species and entire systems through predicted changes in climate.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTION
All authors contributed to the manuscript including fund acquisition, data collection, data analysis, and writing/editing.
ACKNOWLEDGMENTS
We thank Milnesand Prairie Preserve, D. Davis, M. Massey, and G. Beauprez of New Mexico Department of Game and Fish for support. We also thank the Grasslans Charitable Trust, Center of Excellence, Texas Tech University Department of Natural Resources, U.S. Geological Survey, The Nature Conservancy, New Mexico Game and Fish, Texas Parks and Wildlife Department, U.S. Fish and Wildlife Service, and the Great Plains Landscape Conservation Cooperative for providing financial and logistical support. Support for C. A. Hagen was provided by Grant Agreement #LPCI‐2016‐04 between Oregon State University and Pheasants Forever. Thanks to A. Wood and C. Dixon. Thanks to A. Erickson (NMACD), J. Swafford (Pheasants Forever), and other staff for vegetation sampling. Thank you to the Texas Tech University Climate Science Center. The authors acknowledge the Texas Tech High Performance Computing Center for providing computing resources that have contributed to the research results reported within this article. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Fritts SR, Grisham BA, Cox RD, et al. Interactive effects of severe drought and grazing on the life history cycle of a bioindicator species. Ecol Evol. 2018;8:9550–9562. 10.1002/ece3.4432
REFERENCES
- Ahlers, A. A. , Cotner, L. A. , Wolff, P. J. , Mitchell, M. A. , Heske, E. J. , & Schooley, R. L. (2015). Summer precipitation predicts spatial distributions of semiaquatic mammals. PLoS ONE, 10(8), e0135036 10.1371/journal.pone.0135036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike, H. (1998). Information theory and an extension of the maximum likelihood principle In Selected papers of hirotugu akaike (pp. 199–213). New York, NY: Springer. [Google Scholar]
- Albertson, F. W. , Tomanek, G. W. , & Riegel, A. (1957). Ecology of drought cycles and grazing intensity on grasslands of central Great Plains. Ecological Monographs, 27(1), 27–44. 10.2307/1948569 [DOI] [Google Scholar]
- Arthington, A. H. , & Balcombe, S. R. (2011). Extreme flow variability and the ‘boom and bust’ ecology of fish in arid‐zone floodplain rivers: A case history with implications for environmental flows, conservation and management. Ecohydrology, 4(5), 708–720. 10.1002/eco.221 [DOI] [Google Scholar]
- Bailey, J. , & Painter, C. (1994). What good is this lizard. New Mexico Wildlife, 39(4), 22–23. [Google Scholar]
- Bausch, J. D. , & Carson, T. L. (1981). Oak poisoning in cattle. Iowa State University Veterinarian, 43(2), 108–111. [Google Scholar]
- Benson, A. , Zhu, P. , Farmer, M. , & Villalobos, C. (2011). Profitability of a dryland grazing system suitable for the Texas High Plains. Texas Journal of Agriculture and Natural Resources, 24, 62–73. [Google Scholar]
- Bergerud, A. T. , & Gratson, M. W. (1988). Adaptive strategies and population ecology of northern grouse. Vol. 2 Minneapolis, MN: University of Minnesota Press. [Google Scholar]
- Bidwell, T. G. , Fuhlendorf, S. , Gillen, B. , Harmon, S. , Horton, R. , Manes, R. , … Wolfe, D. (2003). Ecology and mangaement of the lesser prairie‐chicken in Oklahoma. Oklahoma State University Extension Circular E‐970. Stillwater, OK: Oklahoma Cooperative Extension Unit. [Google Scholar]
- Chambers, J. C. , Maestas, J. D. , Pyke, D. A. , Boyd, C. S. , Pellant, M. , & Wuenschel, A. (2017). Using resilience and resistance concepts to manage persistent threats to sagebrush ecosystems and Greater Sage‐Grouse. Rangeland Ecology & Management, 70(2), 149–164. 10.1016/j.rama.2016.08.005 [DOI] [Google Scholar]
- Charmantier, A. , & Gienapp, P. (2014). Climate change and timing of avian breeding and migration: Evolutionary versus plastic changes. Evolutionary Applications, 7(1), 15–28. 10.1111/eva.12126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, P. , & Newman, M. (1998). Rossby wave propagation and the rapid development of upper‐level anomalous anticyclones during the 1988 US drought. Journal of Climate, 11(10), 2491–2504. [DOI] [Google Scholar]
- Chevin, L. M. , Lande, R. , & Mace, G. M. (2010). Adaptation, plasticity, and extinction in a changing environment: Towards a predictive theory. PLoS Biology, 8(4), e1000357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian, J. , Christian, K. , & Basara, J. B. (2015). Drought and pluvial dipole events within the Great Plains of the United States. Journal of Applied Meteorology and Climatology, 54(9), 1886–1898. 10.1175/JAMC-D-15-0002.1 [DOI] [Google Scholar]
- Christie, K. S. , Jensen, W. F. , Schmidt, J. H. , & Boyce, M. S. (2015). Long‐term changes in pronghorn abundance index linked to climate and oil development in North Dakota. Biological Conservation, 192(2015), 445–453. 10.1016/j.biocon.2015.11.007 [DOI] [Google Scholar]
- Cook, B. I. , Ault, T. R. , & Smerdon, J. E. (2015). Unprecedented 21st century drought risk in the American Southwest and Central Plains. Science Advances, 1(1), e1400082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dail, D. , & Madsen, L. (2011). Models for estimating abundance from repeated counts of an open metapopulation. Biometrics, 67(2), 577–587. 10.1111/j.1541-0420.2010.01465.x [DOI] [PubMed] [Google Scholar]
- Dale, V. H. , Joyce, L. A. , McNulty, S. , Neilson, R. P. , Ayres, M. P. , Flannigan, M. D. , … Wotton, B. M. (2001). Climate change and forest disturbances: Climate change can affect forests by altering the frequency, intensity, duration, and timing of fire, drought, introduced species, insect and pathogen outbreaks, hurricanes, windstorms, ice storms, or landslides. BioScience, 51(9), 723–734. 10.1641/0006-3568(2001)051[0723:CCAFD]2.0.CO;2 [DOI] [Google Scholar]
- Davis, D. M. , Horton, R. E. , Odell, E. A. , Rodgers, R. D. , & Whitlaw, H. A. (2008). Lesser prairie‐chicken conservation initiative. Lesser Prairie Chicken Interstate Working Group. Unpublished Report. Fort Collins, CO: Colorado Division of Wildlife. [Google Scholar]
- Dhillion, S. S. , McGinley, M. A. , Friese, C. F. , & Zak, J. C. (1994). Construction of sand shinnery oak communities of the Llano Estacado: Animal disturbances, plant community structure, and restoration. Restoration Ecology, 2(1), 51–60. 10.1111/j.1526-100X.1994.tb00041.x [DOI] [Google Scholar]
- Dickman, C. R. , Greenville, A. C. , Beh, C. L. , Tamayo, B. , & Wardle, G. M. (2010). Social organization and movements of desert rodents during population “booms” and “busts” in central Australia. Journal of Mammalogy, 91(4), 798–810. 10.1644/09-MAMM-S-205.1 [DOI] [Google Scholar]
- Diffenbaugh, N. S. , Giorgi, F. , & Pal, J. S. (2008). Climate change hotspots in the United States. Geophysical Research Letters, 35(16), L16709 10.1029/2008GL035075 [DOI] [Google Scholar]
- Dobrowski, S. Z. (2011). A climatic basis for microrefugia: The influence of terrain on climate. Global Change Biology, 17(2), 1022–1035. 10.1111/j.1365-2486.2010.02263.x [DOI] [Google Scholar]
- Ehleringer, J. R. , Schwinning, S. , & Gebauer, R. (1999). Water‐use in arid land ecosystems In Press M. C., Scholes J. D., & Barker M. G. (Eds.), Physiological plant ecology (pp. 347–365). Oxford, UK: Blackwell Science. [Google Scholar]
- Fields, T. L. , White, G. C. , Gilgert, W. C. , & Rodgers, R. D. (2006). Nest and brood survival of lesser prairie‐chickens in west central Kansas. Journal of Wildlife Management, 70(4), 931–938. 10.2193/0022-541X(2006)70[931:NABSOL]2.0.CO;2 [DOI] [Google Scholar]
- Fiske, I. , & Chandler, R. (2011). unmarked: An R package for fitting hierarchical models of wildlife occurrence and abundance. Journal of Statistical Software, 43(10), 1–23. [Google Scholar]
- Fiske, I. , Chandler, R. , Miller, D. , Royle, A. , Kery, M. , Hostetler, J. , & Chandler, M. R. (2017, April). Package “unmarked.”: Models for data for unmarked animals. Retrieved from https://cran.r-project.org/web/packages/unmarked/unmarked.pdf.
- Fravolini, A. , Hultine, K. R. , Brugnoli, E. , Gazal, R. , English, N. B. , & Williams, D. G. (2005). Precipitation pulse use by an invasive woody legume: The role of soil texture and pulse size. Oecologia, 144(4), 618–627. 10.1007/s00442-005-0078-4 [DOI] [PubMed] [Google Scholar]
- Fritts, S. R. , Grisham, B. A. , Haukos, D. A. , Boal, C. W. , Patten, M. A. , Wolfe, D. H. , … Heck, W. R. (2016). Long‐term lesser prairie‐chicken nest ecology in response to grassland management. The Journal of Wildlife Management, 80(3), 527–539. 10.1002/jwmg.1042 [DOI] [Google Scholar]
- Gibson, S. Y. , Van der Marel, R. C. , & Starzomski, B. M. (2009). Climate change and conservation of leading‐edge peripheral populations. Conservation Biology, 23(6), 1369–1373. 10.1111/j.1523-1739.2009.01375.x [DOI] [PubMed] [Google Scholar]
- Giesen, K. M. (1994). Movements and nesting habitat of lesser prairie‐chicken hens in Colorado. The Southwestern Naturalist, 39(1), 96–98. [Google Scholar]
- Giesen, K. M. (1998). Lesser prairie‐chicken (Tympanuchus pallidicinctus) In Poole A. & Gill F. (Eds.), The birds of North America (No. 364). Philadelphia, PA: The Birds of North America, Inc. [Google Scholar]
- Greenwood, O. , Mossman, H. L. , Suggitt, A. J. , Curtis, R. J. , & Maclean, I. (2016). Using in situ management to conserve biodiversity under climate change. Journal of Applied Ecology, 53(3), 885–894. 10.1111/1365-2664.12602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisham, B. A. (2012). The ecology of lesser prairie‐chickens in shinnery oak‐grassland communities in New Mexico and Texas with implications toward habitat management and future climate change. (Doctoral Dissertation). Lubbock, TX: Texas Tech University. [Google Scholar]
- Grisham, B. A. , Boal, C. W. , Haukos, D. A. , Davis, D. M. , Boydston, K. K. , Dixon, C. , & Heck, W. R. (2013). The predicted influence of climate change on lesser prairie‐chicken reproductive parameters. PLoS ONE, 8(7), e68225 10.1371/journal.pone.0068225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisham, B. A. , Borsdorf, P. K. , Boal, C. W. , & Boydston, K. K. (2014). Nesting ecology and nest survival of lesser prairie‐chickens on the Southern High Plains of Texas. The Journal of Wildlife Management, 78(5), 857–866. 10.1002/jwmg.716 [DOI] [Google Scholar]
- Grisham, B. A. , Godar, A. J. , Boal, C. W. , & Haukos, D. A. (2016). Interactive effects between nest microclimate and nest vegetation structure confirm microclimate thresholds for Lesser Prairie‐Chicken nest survival. The Condor, 118(4), 728–746. 10.1650/CONDOR-16-38.1 [DOI] [Google Scholar]
- Grisham, B. A. , Godar, A. J. , & Griffin, C. P. (2016). Climate Change In Haukos D. A. & Boal C. W. (Eds.). Ecology and conservation of lesser prairie‐chickens (pp. 221–242) Studies in avian biology vol. 48. Boca Raton, FL: CRC Press. [Google Scholar]
- Grisham, B. A. , Zavaleta, J. C. , Behney, A. C. , Borsdorf, P. K. , Lucia, D. R. , Boal, C. W. , & Haukos, D. A. (2016). Ecology and conservation of lesser prairie‐chickens in sand shinnery oak prairie In Haukos D. A. & Boal C. W. (Eds.), Ecology and conservation of lesser prairie‐chickens (pp. 315–344) Studies in avian biology vol. 48. Boca Raton, FL: CRC Press. [Google Scholar]
- Grover, H. D. , & Musick, H. B. (1990). Shrubland encroachment in southern New Mexico, USA: An analysis of desertification processes in the American Southwest. Climatic Change, 17(2), 305–330. 10.1007/BF00138373 [DOI] [Google Scholar]
- Hagen, C. A. , & Giesen, K. M. (2005). Lesser prairie‐chicken (Tympanuchus pallidinctus) In Poole A. (Ed.). The birds of North America (No. 364). Ithaca, NY: Cornell Lab of Ornithology. [Google Scholar]
- Hagen, C. A. , Grisham, B. A. , Boal, C. W. , & Haukos, D. A. (2013). A meta‐analysis of lesser prairie‐chicken nesting and brood‐rearing habitats: Implications for habitat management. Wildlife Society Bulletin, 37(4), 750–758. 10.1002/wsb.313 [DOI] [Google Scholar]
- Hagen, C. A. , Jamison, B. E. , Giesen, K. M. , & Riley, T. Z. (2004). Guidelines for managing lesser prairie‐chicken populations and their habitats. Wildlife Society Bulletin, 32(1), 69–82. 10.2193/0091-7648(2004)32[69:GFMLPP]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen, C. A. , Pitman, J. C. , Sandercock, B. K. , Robel, R. J. , & Applegate, R. D. (2007). Age‐specific survival and probable causes of mortality in female lesser prairie‐chickens. Journal of Wildlife Management, 71(2), 518–525. 10.2193/2005-778 [DOI] [Google Scholar]
- Hagen, C. A. , Sandercock, B. K. , Pitman, J. C. , Robel, R. J. , & Applegate, R. D. (2009). Spatial variation in lesser prairie‐chicken demography: A sensitivity analysis of population dynamics and management alternatives. Journal of Wildlife Management, 73(8), 1325–1332. 10.2193/2008-225 [DOI] [Google Scholar]
- Hannah, L. , Midgley, G. F. , Lovejoy, T. , Bond, W. J. , Bush, M. L. J. C. , Lovett, J. C. , … Woodward, F. I. (2002). Conservation of biodiversity in a changing climate. Conservation Biology, 16(1), 264–268. 10.1046/j.1523-1739.2002.00465.x [DOI] [PubMed] [Google Scholar]
- Haukos, D. A. (2011). Use of tebuthiuron to restore sand shinnery oak grasslands of the southern high plains In Hasaneen M. N. A. (Ed.), Herbicides – mechanisms and mode of action (pp. 103–124). Retrieved from http://www.intechopen.com/books/herbicides-mechanisms-and-mode-of-action [Google Scholar]
- Haukos, D. A. , & Smith, L. M. (1989). Lesser prairie‐chicken nest site selection and vegetation characteristics in tebuthiuron‐treated and untreated sand shinnery oak in Texas. The Great Basin Naturalist, 49(4), 624–626. [Google Scholar]
- Haukos, D. A. , & Zavaleta, J. C. (2016). Habitat In Haukos D. A. & Boal C. W. (Eds.). Ecology and conservation of lesser prairie‐chickens (pp. 99–132). Studies in avian biology vol. 48. Boca Raton, FL: CRC Press. [Google Scholar]
- Heffelfinger, J. R. , Guthery, F. S. , Olding, R. J. , Cochran, C. L. Jr , & McMullen, C. M. (1999). Influence of precipitation timing and summer temperatures on reproduction of Gambel's quail. The Journal of Wildlife Management, 63(1), 154–161. 10.2307/3802496 [DOI] [Google Scholar]
- Hodgson, J. A. , Moilanen, A. , Wintle, B. A. , & Thomas, C. D. (2011). Habitat area, quality and connectivity: Striking the balance for efficient conservation. Journal of Applied Ecology, 48(1), 148–152. 10.1111/j.1365-2664.2010.01919.x [DOI] [Google Scholar]
- Holechek, J. L. , Thomas, M. , Molinar, F. , & Galt, D. (1999). Stocking desert rangelands: What we've learned. Rangelands, 21(6), 8–12. [Google Scholar]
- Holling, C. S. (1973). Resilience and stability of ecological systems. Annual Review of Ecology and Systematics, 4(1), 1–23. 10.1146/annurev.es.04.110173.000245 [DOI] [Google Scholar]
- Hovick, T. J. , Elmore, R. D. , Allred, B. W. , Fuhlendorf, S. D. , & Dahlgren, D. K. (2014). Landscapes as a moderator of thermal extremes: A case study from an imperiled grouse. Ecosphere, 5(3), 1–12. [Google Scholar]
- Jackson, A. S. , & DeArment, R. (1963). The lesser prairie chicken in the Texas Panhandle. The Journal of Wildlife Management, 27(4), 733–737. 10.2307/3798489 [DOI] [Google Scholar]
- Jaeger, K. L. , Olden, J. D. , & Pelland, N. A. (2014). Climate change poised to threaten hydrologic connectivity and endemic fishes in dryland streams. Proceedings of the National Academy of Sciences, 111(38), 13894–13899. 10.1073/pnas.1320890111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl, T. R. , Melillo, J. , Peterson, T. C. , Anderson, D. M. , Boesch, D. F. , Burkett, V. , … Wuebbles, D. J. (2010). Global climate change impacts in the United States (p. 188). New York, NY: Cambridge University Press. [Google Scholar]
- Kingsford, R. T. , Curtin, A. L. , & Porter, J. (1999). Water flows on Cooper Creek in arid Australia determine ‘boom’ and ‘bust’ periods for waterbirds. Biological Conservation, 88(2), 231–248. 10.1016/S0006-3207(98)00098-6 [DOI] [Google Scholar]
- Knopf, F. L. , & Samson, F. B. (1997). Conservation of grassland vertebrates Ecology and conservation of Great Plains vertebrates (pp. 273–289). New York, NY: Springer; 10.1007/978-1-4757-2703-6 [DOI] [Google Scholar]
- Lautenbach, J. M. (2015). Lesser prairie‐chicken reproductive success, habitat selection, and response to trees [Master's thesis]. Manhattan, KS: Kansas State University. [Google Scholar]
- Lawler, J. J. (2009). Climate change adaptation strategies for resource management and conservation planning. Annals of the New York Academy of Sciences, 1162(1), 79–98. 10.1111/j.1749-6632.2009.04147.x [DOI] [PubMed] [Google Scholar]
- Loeser, M. R. , Sisk, T. D. , & Crews, T. E. (2007). Impact of grazing intensity during drought in an Arizona grassland. Conservation Biology, 21(1), 87–97. 10.1111/j.1523-1739.2006.00606.x [DOI] [PubMed] [Google Scholar]
- Lusk, J. J. , Guthery, F. S. , & DeMaso, S. J. (2001). Northern bobwhite (Colinus virginianus) abundance in relation to yearly weather and long‐term climate patterns. Ecological Modelling, 146(1), 3–15. 10.1016/S0304-3800(01)00292-7 [DOI] [Google Scholar]
- Mac Nally, R. , Horrocks, G. F. , & Lada, H. (2017). Anuran responses to pressures from high‐amplitude drought–flood–drought sequences under climate change. Climatic Change, 141(2), 243–257. 10.1007/s10584-016-1890-z [DOI] [Google Scholar]
- Mawdsley, J. R. , O'Malley, R. , & Ojima, D. S. (2009). A review of climate‐change adaptation strategies for wildlife management and biodiversity conservation. Conservation Biology, 23(5), 1080–1089. 10.1111/j.1523-1739.2009.01264.x [DOI] [PubMed] [Google Scholar]
- McCaffery, R. , Nowak, J. J. , & Lukacs, P. M. (2016). Improved analysis of lek count data using N‐mixture models. The Journal of Wildlife Management, 80(6), 1011–1021. 10.1002/jwmg.21094 [DOI] [Google Scholar]
- McNaughton, S. J. , Ruess, R. W. , & Seagle, S. W. (1988). Large mammals and process dynamics in African ecosystems. BioScience, 38(11), 794–800. 10.2307/1310789 [DOI] [Google Scholar]
- Merchant, S. S. (1982). Habitat‐use, reproductive success, and survival of female lesser prairie chickens in two years of contrasting weather. (Master's thesis). Las Cruces, NM: New Mexico State University.
- Milchunas, D. G. , Lauenroth, W. K. , Chapman, P. L. , & Kazempour, M. K. (1989). Effects of grazing, topography, and precipitation on the structure of a semiarid grassland. Plant Ecology, 80(1), 11–23. 10.1007/BF00049137 [DOI] [Google Scholar]
- Milchunas, D. G. , Sala, O. E. , & Lauenroth, W. (1988). A generalized model of the effects of grazing by large herbivores on grassland community structure. The American Naturalist, 132(1), 87–106. 10.1086/284839 [DOI] [Google Scholar]
- Moritz, C. , & Agudo, R. (2013). The future of species under climate change: Resilience or decline? Science, 341(6145), 504–508. 10.1126/science.1237190 [DOI] [PubMed] [Google Scholar]
- National Oceanic and Atmospheric Administration [NOAA] , (2017a). Daily Summaries. Climate Data Online. Portales Station GHCND:USC00297008.
- National Oceanic and Atmospheric Administration [NOAA] (2017b). Monitoring content. Climate. US‐DIV02903. Retrieved from https://www.ncdc.noaa.gov/.
- Nielsen‐Gammon, J. W. (2012). The 2011 Texas drought. Texas Water Journal, 3(1), 59–95. [Google Scholar]
- Noy‐Meir, I. (1973). Desert ecosystems: Environment and producers. Annual Review of Ecology and Systematics, 4(1), 25–51. 10.1146/annurev.es.04.110173.000325 [DOI] [Google Scholar]
- Oliver, T. H. , Brereton, T. , & Roy, D. B. (2013). Population resilience to an extreme drought is influenced by habitat area and fragmentation in the local landscape. Ecography, 36(5), 579–586. 10.1111/j.1600-0587.2012.07665.x [DOI] [Google Scholar]
- Oliver, T. , Roy, D. B. , Hill, J. K. , Brereton, T. , & Thomas, C. D. (2010). Heterogeneous landscapes promote population stability. Ecology Letters, 13(4), 473–484. 10.1111/j.1461-0248.2010.01441.x [DOI] [PubMed] [Google Scholar]
- Opdam, P. , & Wascher, D. (2004). Climate change meets habitat fragmentation: Linking landscape and biogeographical scale levels in research and conservation. Biological Conservation, 117(3), 285–297. 10.1016/j.biocon.2003.12.008 [DOI] [Google Scholar]
- Patten, M. A. , Wolfe, D. H. , Shochat, E. , & Sherrod, S. K. (2005a). Habitat fragmentation, rapid evolution and population persistence. Evolutionary Ecology Research, 7(2), 235–249. [Google Scholar]
- Patten, M. A. , Wolfe, D. H. , Shochat, E. , & Sherrod, S. K. (2005b). Effects of microhabitat and microclimate selection on adult survivorship of the lesser prairie‐chicken. Journal of Wildlife Management, 69(3), 1270–1278. 10.2193/0022-541X(2005)069[1270:EOMAMS]2.0.CO;2 [DOI] [Google Scholar]
- Peterson, R. S. , & Boyd, C. S. (1998). Ecology and management of sand shinnery communities: A literature review (General technical report number RMRS‐GTR‐16). Fort Collins, CO: Department of Agriculture Forest Service, Rocky Mountain Research Station. [Google Scholar]
- Peterson, M. J. , & Silvy, N. J. (1994). Spring precipitation and fluctuations in Attwater's prairie‐chicken numbers: Hypotheses revisited. The Journal of Wildlife Management, 8(2), 5222–5229. [Google Scholar]
- Peterson, T. C. , Stott, P. A. , & Herring, S. (2012). Explaining extreme events of 2011 from a climate perspective. Bulletin of the American Meteorological Society, 93(7), 1041–1067. 10.1175/BAMS-D-12-00021.1 [DOI] [Google Scholar]
- Plumb, G. E. , & Dodd, J. L. (1993). Foraging ecology of bison and cattle on a mixed prairie: Implications for natural area management. Ecological Applications, 3(4), 631–643. 10.2307/1942096 [DOI] [PubMed] [Google Scholar]
- Pyke, C. R. , & Marty, J. T. (2005). Cattle Grazing mediates climate change impacts on ephemeral wetlands. Conservation Biology, 19(5), 1619–1625. [Google Scholar]
- R Core Team (2014). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Riley, T. Z. , Davis, C. A. , Candelaria, M. A. , & Suminski, H. R. (1994). Lesser prairie‐chicken movements and home ranges in New Mexico. Prairie Naturalist, 26, 183. [Google Scholar]
- Riley, T. Z. , Davis, C. A. , Ortiz, M. , & Wisdom, M. J. (1992). Vegetative characteristics of successful and unsuccessful nests of lesser prairie chickens. The Journal of Wildlife Management, 56(2), 383–387. 10.2307/3808839 [DOI] [Google Scholar]
- Robertson, T. , Zak, J. , & Tissue, D. (2010). Precipitation magnitude and timing differentially affect species richness and plant density in the Sotol Grassland of the Chihuahuan Desert. Oecologia, 162(1), 185–197. Retrieved from http://www.jstor.org/stable/40540155. [DOI] [PubMed] [Google Scholar]
- Rodgers, R. D. (2016). A history of lesser prairie‐chickens In Haukos D. A., & Boal C. W. (Eds.), Ecology and management of lesser prairie‐chickens (pp. 15–38). Boca Raton, FL: CRC Press. [Google Scholar]
- Ross, B. E. , Haukos, D. A. , Hagen, C. A. , & Pitman, J. C. (2016a). Landscape composition creates a threshold influencing lesser prairie‐chicken population resilience to extreme drought. Global Ecology and Conservation, 6, 179–188. 10.1016/j.gecco.2016.03.003 [DOI] [Google Scholar]
- Ross, B. E. , Haukos, D. A. , Hagen, C. , & Pitman, J. (2016b). The relative contribution of climate to changes in lesser prairie‐chicken abundance. Ecosphere, 7(6), e01323.F 10.1002/ecs2.1323 [DOI] [Google Scholar]
- Royle, J. A. (2004). N‐mixture models for estimating population size from spatially replicated counts. Biometrics, 60(1), 108–115. 10.1111/j.0006-341X.2004.00142.x [DOI] [PubMed] [Google Scholar]
- Samson, F. , & Knopf, F. (1994). Prairie conservation in North America. BioScience, 44(6), 418–421. 10.2307/1312365 [DOI] [Google Scholar]
- Samson, F. B. , & Knopf, F. L. (1996). Prairie conservation: Preserving North America's most endangered ecosystem (p. 351). Washington, DC: Island Press. [Google Scholar]
- Samson, F. B. , Knopf, F. L. , & Ostlie, W. R. (2004). Great plains ecosystems: Past, present, and future. Wildlife Society Bulletin, 32(1), 6–15. 10.2193/0091-7648(2004)32[6:GPEPPA]2.0.CO;2 [DOI] [Google Scholar]
- Savage, D. A. (1937). Drought survival of native grass species in the central and southern great plains, 1935. Beltsville, MD: US Department of Agriculture, National Agricultural Library, Alternative Farming Systems Information Center. [Google Scholar]
- Scheffer, M. , Carpenter, S. , Foley, J. A. , Folke, C. , & Walker, B. (2001). Catastrophic shifts in ecosystems. Nature, 413(6856), 591–596. 10.1038/35098000 [DOI] [PubMed] [Google Scholar]
- Schwinning, S. , & Sala, O. E. (2004). Hierarchy of responses to resource pulses in arid and semi‐arid ecosystems. Oecologia, 141(2), 211–220. 10.1007/s00442-004-1520-8 [DOI] [PubMed] [Google Scholar]
- Selwood, K. E. , Clarke, R. H. , Cunningham, S. C. , Lada, H. , McGeoch, M. A. , & Mac Nally, R. (2015). A bust but no boom: Responses of floodplain bird assemblages during and after prolonged drought. Journal of Animal Ecology, 84(6), 1700–1710. 10.1111/1365-2656.12424 [DOI] [PubMed] [Google Scholar]
- Shafer, M. , Ojima, D. , Antle, J. M. , Kluck, D. , McPherson, R. A. , Petersen, S. , … Sherman, K. (2014). Ch. 19: Great Plains. Climate change impacts in the United States: The third national climate assessment (pp. 441–461). Washington, DC: U.S. Global Change Research Program. [Google Scholar]
- Sherfy, M. H. , & Pekins, P. J. (1995). Influence of wind speed on sage grouse metabolism. Canadian Journal of Zoology, 73(4), 749–754. 10.1139/z95-088 [DOI] [Google Scholar]
- Silvy, N. J. , Peterson, M. J. , & Lopez, R. R. (2004). The cause of the decline of pinnated grouse: The Texas example. Wildlife Society Bulletin, 32(1), 16–21. 10.2193/0091-7648(2004)32[16:TCOTDO]2.0.CO;2 [DOI] [Google Scholar]
- Strahan, R. T. (2008). A floristic survey of the vascular plants of the Milnesand prairie preserve, Roosevelt County, New Mexico. Las Cruces, NM: New Mexico State University. [Google Scholar]
- Sullivan, R. M. , Hughes, J. P. , & Lionberger, J. E. (2001). Review of the historical and present status of the lesser prairie‐chicken (Tympanuchus pallidicinctus) in Texas. The Prairie Naturalist, 32(3). [Google Scholar]
- Thomas, C. D. , Cameron, A. , Green, R. E. , Bakkenes, M. , Beaumont, L. J. , Collingham, Y. C. , … Hughes, L. (2004). Extinction risk from climate change. Nature, 427(6970), 145–148. 10.1038/nature02121 [DOI] [PubMed] [Google Scholar]
- Weltzin, J. F. , Loik, M. E. , Schwinning, S. , Williams, D. G. , Fay, P. A. , Haddad, B. M. , … Zak, J. C. (2003). Assessing the response of terrestrial ecosystems to potential changes in precipitation. BioScience, 53(10), 941–952. [Google Scholar]
- Wester, D. B. , Sosebee, R. E. , Wester, D. B. , Britton, C. M. , McArthur, E. D. , & Kitchen, S. G. (2007, Aug.). The southern High Plains: A history of vegetation, 1540 to present In Proceedings: Shrubland Dynamics‐fire and Water (pp. 24–47). Lubbock, TX. [Google Scholar]
- Westphal, M. F. , Stewart, J. A. , Tennant, E. N. , Butterfield, H. S. , & Sinervo, B. (2016). Contemporary drought and future effects of climate change on the endangered blunt‐nosed leopard lizard, Gambelia sila . PLoS ONE, 11(5), e0154838 10.1371/journal.pone.0154838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, S. E. , Shoo, L. P. , Isaac, J. L. , Hoffmann, A. A. , & Langham, G. (2008). Towards an integrated framework for assessing the vulnerability of species to climate change. PLoS Biology, 6(12), e325 10.1371/journal.pbio.0060325 [DOI] [PMC free article] [PubMed] [Google Scholar]


