Abstract
Purpose
This study aimed to evaluate the effect of advanced paternal age on pregnancy outcomes and sperm parameters following intrauterine insemination (IUI). We used IUI data rather than assisted reproductive technology data, which might mask the effects of sperm impairments.
Methods
We retrospectively analyzed 1576 IUI cycles in women under 40 years old between April 2012 and May 2016 at the National Center for Child Health and Development in Japan. The main outcomes were clinical pregnancy and live birth.
Results
The mean male age was significantly lower in cycles that resulted in pregnancy compared with those without pregnancy (38.0 vs 39.1 years; P < 0.001), with a similar trend for live‐birth cycles. However, there was no relationship between advanced paternal age and pregnancy outcomes after adjusting for confounding factors and correlations within patients using generalized estimating equations, and the age of the female partner was the only factor affecting pregnancy rate. Furthermore, advanced paternal age had no effect on sperm parameters.
Conclusions
Advanced paternal age alone does not adversely affect pregnancy or live‐birth rates or sperm parameters following IUI.
Keywords: advanced paternal age, generalized estimating equations, intrauterine insemination, pregnancy outcome, sperm parameter
1. INTRODUCTION
The age of couples at the birth of their first child has been gradually increasing in developed countries over recent decades.1 Although pregnancy rate is known to decrease with advancing maternal age,2, 3 the effect of advancing paternal age on pregnancy outcome is less clear, given that men can continue to produce sperm and retain a certain level of reproductive function into old age.4, 5 Furthermore, the association between advanced paternal age and sperm anomalies is also controversial.1, 6
Intrauterine insemination (IUI) has been widely used for the treatment of infertile couples with mild male factor or unexplained infertility, or with hyposecretion of cervical mucus after conization of the cervix.7 Compared with assisted reproductive technology (ART), IUI provides a more natural fertilization environment, is less invasive and expensive, and is easier to perform.6 Furthermore, Japanese culture tends to disapprove of the embryo manipulation involved in ART, and most patients, including older couples, prefer to conceive by non‐ART methods.
Numerous studies have investigated the relationships between ART or IUI and pregnancy rate; however, many of these did not take account of multiple results from the same patient when calculating the pregnancy rate. If repeatedly unsuccessful patients are included in the sample, the basic backgrounds of these patients are also included multiple times, thus distorting the pregnancy rate. We therefore analyzed the relationships between advanced paternal age and pregnancy outcomes in couples undergoing IUI using generalized estimating equations (GEE) to adjust for correlations within patients. GEE extends the generalized linear model algorithm to accommodate the modeling of stratification in correlated data. This method is particularly effective when the same patients are included multiple times in the total cohort, as for IUI or ART. We previously analyzed online Japanese ART data adjusting for correlations within clinics using this method.8 The reason why we selected IUI data to detect any effects of advanced paternal age because ART could mask sperm impairments such as fertility disorders. This study aimed to evaluate the relationships between paternal age and pregnancy outcomes and sperm parameters following IUI, after adjusting for multiple outcomes in the same patients.
2. MATERIALS AND METHODS
2.1. Study subjects, recruitment, and eligibility
This retrospective study of couples undergoing IUI was carried out at the National Center for Child Health and Development (NCCHD), Tokyo, Japan, between April 2012 and May 2016. The study protocol was approved by the NCCHD (approval number 922). Each IUI procedure was performed after acquiring informed consent. IUI was restricted to procedures using the husband's sperm and not from donor's sperm. NCCHD is a national facility where patients of any age may undergo self‐funded treatment for infertility. We collected baseline demographic and clinical data, including maternal and paternal ages, smoking status, and the ovulation induction agent used. The main outcomes were clinical pregnancy, defined as the detection of a gestational sac by transvaginal ultrasonography, and live birth.
A total of 2807 IUI cycles were performed between April 2012 and May 2016. Among these, 65 samples that used precipitated semen or thawed sperm were excluded, along with 85 cycles that involved double insemination and eight cycles where the results of IUI were unknown. A further 1073 cycles involving women > 40 years were excluded because of the low pregnancy rate expected in this age group.2 A total of 1576 cycles were included in the final analysis.
2.2. Induction of ovulation
Ovulation induction with clomiphene citrate 50‐150 mg was started on day 3 or 5 of the cycle, or induction with gonadotropin 37.5‐75 IU was started on day 3 of the cycle. Serial transvaginal ultrasonography was performed to determine the day on which IUI should be performed. Human chorionic gonadotropin 10 000 IU was administered if necessary when the diameter of the leading follicle was >18 mm. We selected normal ovulation induction in women with normal menstrual cycles, and used clomiphene citrate in women with irregular menstrual cycles. We used gonadotropin in women with polycystic ovarian syndrome in whom the menstrual cycle could not be regulated with clomiphene induction. We only carried out IUI in women with an endometrial thickness >7 mm, especially in cases of repeated clomiphene induction.
2.3. Semen analysis
The collected semen samples were kept at room temperature for 20 min to liquefy, and their characteristics were then examined under a microscope. Sperm samples were processed by continuous‐step Percoll density gradient centrifugation (ISolate™; Irvine Scientific, Santa Ana, CA, USA). After 20 minutes of centrifugation at 600 × g, the pellet was collected, resuspended in sperm‐washing medium, and centrifuged for 7 minutes at 200 × g. The supernatant was then removed using a transfer pipette and the resulting pellet was resuspended in 0.5 mL of medium. Insemination was performed using an intrauterine catheter.
2.4. Statistical analysis
We investigated the associations among male and female ages, the distribution of sperm parameters, and pregnancy outcomes. Continuous variables with a normal distribution were analyzed by Mann‐Whitney U tests. We compared patient background characteristics stratified by pregnancy outcomes using χ2 or Student's t tests. We selected male age, female age, smoking status, and ovulation induction agent as confounding factors in the analysis.9 Subjects were classified into age groups according to 3‐year intervals. We also analyzed smoking status, with the inclusion of an additional group including subjects for whom the smoking history was unclear. Spearman's rank correlation coefficients and linear regression lines were calculated for male age, female age, and sperm parameters. We used GEE adjusting for correlations within the same patients, for a binomial family using the logit‐link function. Crude odds ratios (ORs) and 95% confidence intervals (CIs) were calculated for comparisons of pregnancy outcomes. Adjusted ORs were calculated for the confounders male age, female age, smoking status, and ovulation induction agent. A two‐tailed P < 0.05 was considered to be statistically significant. All analyses were performed using IBM SPSS Statistics version 22.0 software (IBM Corp., Armonk, NY, USA).
3. RESULTS
The mean ages for both sexes and sperm parameters stratified by pregnancy and live birth are shown in Table 1. The mean male and female ages were both significantly younger in pregnancy cycles than in nonpregnancy cycles (38.0 vs 39.1 years, P < 0.001, and 35.9 vs 36.6 years, P = 0.002, respectively) (Table 1, Figure 1A, B). A similar trend was seen for live‐birth cycles (37.8 vs 39.1 years, P = 0.001, and 35.6 vs 36.6 years, P = 0.001, respectively) (Table 1), suggesting that advanced paternal age could reduce the rates of clinical pregnancy and live birth.
Table 1.
Mean age and sperm parameters stratified by pregnancy outcomes
| Characteristic | Pregnancy cycles (n = 111) | Nonpregnancy cycles (n = 1465) | P valuea | Live‐birth cycles (n = 86) | Nonlive‐birth cycles (n = 1490) | P valuea |
|---|---|---|---|---|---|---|
| Male age (years) | 38.0 (5.0) | 39.1 (4.7) | <0.001 | 37.8 (5.1) | 39.1 (4.7) | 0.001 |
| Female age (years) | 35.9 (3.0) | 36.6 (3.1) | 0.002 | 35.6 (3.1) | 36.6 (3.1) | 0.001 |
| Sperm characteristics | ||||||
| Semen volume (mL) | 2.2 (1.4) | 2.2 (1.3) | 0.651 | 2.1 (1.3) | 2.2 (1.3) | 0.470 |
| Sperm concentration (×106) | 6949 (4482) | 6264 (4930) | 0.028 | 6700 (4496) | 6289 (4925) | 0.176 |
| Sperm motility (%) | 42.7 (17.7) | 39.0 (18.6) | 0.044 | 42.3 (17.4) | 39.1 (18.6) | 0.132 |
| Total motile sperm count (×106) | 6557 (5962) | 5830 (7314) | 0.015 | 6112 (5558) | 5869 (7313) | 0.124 |
Data are presented as mean (standard deviation) for continuous variables and n (%) for dichotomous variables.
P values for all factors except year were assessed using χ2 or Student's t tests.
Figure 1.
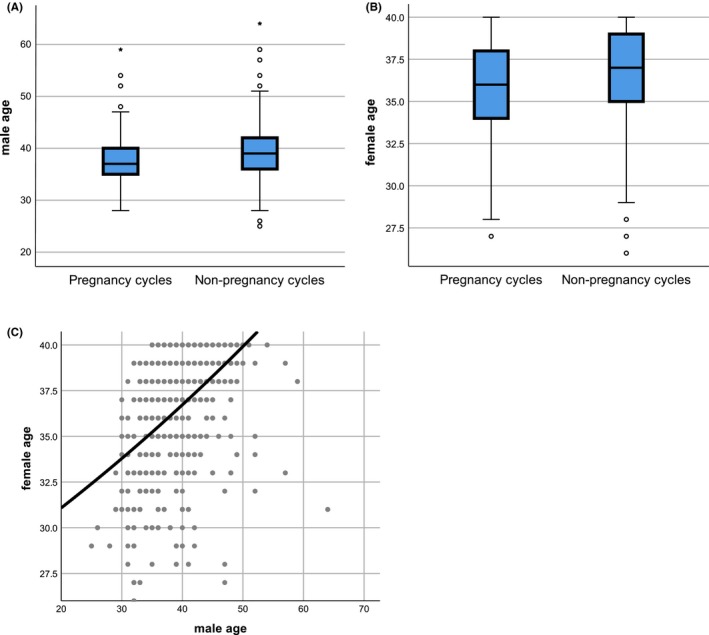
Male age (A) and female age (B) stratified by pregnancy outcome. Mean age was significantly younger for both sexes in pregnancy compared with nonpregnancy cycles. (C) Scatter plot showing a significant correlation between male age and female age
We also compared the background characteristics stratified by pregnancy outcomes (Table 2). The overall clinical pregnancy and live‐birth rates were 7.0% (111/1576) and 3.6% (86/1576), respectively. The pregnancy outcomes also included 18 spontaneous abortions, three artificial abortions, and four unknown pregnancy outcomes. Smoking status and ovulation induction agent were comparable among pregnancy outcomes.
Table 2.
Background characteristics stratified by pregnancy outcomes
| Characteristic | Total cycles (n = 1576) | Pregnancy cycles (n = 111) | Pregnancy rate (%) | P valuea | Live‐birth cycles (n = 86) | Live‐birth rate (%) | P valuea |
|---|---|---|---|---|---|---|---|
| Male age (years) | |||||||
| 34≥ | 358 (22.7) | 33 (29.7) | 9.2 | 0.002 | 28 (32.6) | 7.8 | 0.001 |
| 35‐37 | 217 (13.8) | 28 (25.2) | 12.9 | 22 (25.6) | 10.1 | ||
| 38‐40 | 473 (30.0) | 25 (22.5) | 5.3 | 18 (20.9) | 3.8 | ||
| 41‐43 | 294 (18.7) | 13 (11.7) | 4.4 | 10 (11.6) | 3.4 | ||
| 44‐46 | 131 (8.3) | 5 (4.5) | 3.8 | 2 (2.3) | 1.5 | ||
| ≥47 | 103 (6.5) | 7 (6.3) | 6.8 | 6 (7.0) | 5.8 | ||
| Female age (years) | |||||||
| 34≥ | 511 (32.4) | 46 (41.4) | 9.0 | 0.002 | 38 (44.2) | 7.4 | 0.002 |
| 35‐37 | 298 (18.9) | 29 (26.1) | 9.7 | 22 (25.6) | 7.4 | ||
| 38‐40 | 767 (48.7) | 36 (32.4) | 4.7 | 26 (30.2) | 3.4 | ||
| Male smoking (%) | |||||||
| Yes | 474 (30.1) | 36 (32.4) | 7.6 | 0.126 | 27 (31.4) | 5.7 | 0.252 |
| No | 986 (62.6) | 62 (55.9) | 6.3 | 49 (57.0) | 5.0 | ||
| Unknown | 116 (7.4) | 13 (11.7) | 11.2 | 10 (8.6) | 8.6 | ||
| Female smoking (%) | |||||||
| Yes | 97 (6.2) | 8 (7.2) | 8.2 | 0.330 | 6 (7.0) | 6.2 | 0.242 |
| No | 1407 (89.3) | 95 (85.6) | 6.8 | 73 (84.9) | 5.2 | ||
| Unknown | 72 (4.6) | 8 (7.2) | 11.1 | 7 (8.1) | 9.7 | ||
| Ovulation induction agent (%) | |||||||
| Natural cycle | 931 (59.1) | 63 (56.8) | 6.8 | 0.938 | 47 (54.7) | 5.0 | 0.689 |
| Clomiphene citrate | 380 (24.1) | 28 (25.2) | 7.4 | 22 (25.6) | 5.8 | ||
| Gonadotropin | 228 (14.5) | 18 (16.2) | 7.9 | 16 (18.6) | 7.0 | ||
| Clomiphene citrate + gonadotropin | 25 (1.6) | 1 (0.90) | 4.0 | 1 (1.2) | 4.0 | ||
| Other | 12 (0.76) | 1 (0.90) | 8.3 | 0 | 0 | ||
Data are presented as mean (standard deviation) for continuous variables and n (%) for dichotomous variables.
P values of all factors except year were assessed using χ2 or Student's t tests.
We also analyzed pregnancy outcomes using GEE adjusting for correlations within patients. Some patients underwent up to 18 cycles of IUI. There was no relationship between male age and pregnancy outcomes when the effects of confounding factors and correlations within patients were eliminated by adjusted ORs (Table 3), though the pregnancy rate was significantly lower in females aged 38‐40 years. Furthermore, a scatter plot (Figure 1C) revealed a significant correlation between male age and female age (r = 0.486). These results suggest that the reduced pregnancy rate in couples with advanced paternal age was actually due to the fact that the female partner was also likely to be older. A similar trend was seen for live‐birth cycles, though there was no relationship with either male of female age in terms of live births. Pregnancy outcomes were also unaffected by smoking status and ovulation induction agent.
Table 3.
Crude and adjusted odds ratios with confounding factors and correlations within patients
| Characteristic | Pregnancy cycles | Live‐birth cycles | ||||||
|---|---|---|---|---|---|---|---|---|
| Crude OR (95% Cl) | P value | Adjusted OR (95% Cl)b | P valuea | Crude OR (95% Cl) | P value | Adjusted OR (95% Cl)b | P valuea | |
| Male age (years) | ||||||||
| 34≥ | Reference | Reference | Reference | Reference | ||||
| 35‐37 | 1.50 (0.86‐2.47) | 0.159 | 1.54 (0.88‐2.68) | 0.129 | 1.33 (0.75‐2.35) | 0.327 | 1.43 (0.80‐2.55) | 0.228 |
| 38‐40 | 0.55 (0.31‐0.96) | 0.036 | 0.65 (0.36‐1.17) | 0.151 | 0.47 (0.25‐0.87) | 0.016 | 0.55 (0.28‐1.06) | 0.072 |
| 41‐43 | 0.46 (0.24‐0.88) | 0.020 | 0.56 (0.28‐1.12) | 0.100 | 0.42 (0.20‐0.88) | 0.022 | 0.50 (0.23‐1.08) | 0.079 |
| 44‐46 | 0.39 (0.15‐1.02) | 0.056 | 0.49 (0.17‐1.36) | 0.169 | 0.18 (0.04‐0.81) | 0.026 | 0.22 (0.05‐1.04) | 0.056 |
| ≥47 | 0.71 (0.32‐1.64) | 0.432 | 0.92 (0.40‐2.10) | 0.837 | 0.73 (0.30‐1.77) | 0.486 | 0.93 (0.38‐2.29) | 0.881 |
| Female age (years) | ||||||||
| 34≥ | Reference | Reference | Reference | Reference | ||||
| 35‐37 | 1.09 (0.66‐1.80) | 0.736 | 1.07 (0.64‐1.80) | 0.806 | 0.99 (0.57‐1.73) | 0.978 | 1.01 (0.57‐1.79) | 0.965 |
| 38‐40 | 0.50 (0.31‐0.80) | 0.004 | 0.60 (0.36‐0.98) | 0.041 | 0.44 (0.26‐0.74) | 0.002 | 0.59 (0.34‐1.01) | 0.053 |
| Smoking status | ||||||||
| Male | 1.23 (0.79‐1.91) | 0.368 | 1.04 (0.66‐1.64) | 0.858 | 1.16 (0.70‐1.90) | 0.568 | 0.99 (0.60‐1.64) | 0.335 |
| Female | 1.24 (0.52‐2.94) | 0.623 | 1.50 (0.62‐3.65) | 0.368 | 1.21 (0.50‐2.91) | 0.679 | 1.58 (0.63‐3.98) | 0.970 |
| Ovulation induction agent | ||||||||
| Natural cycle | Reference | Reference | Reference | Reference | ||||
| Clomiphene citrate | 1.10 (0.69‐1.73) | 0.695 | 1.01 (0.63‐1.61) | 0.973 | 1.16 (0.68‐1.95) | 0.589 | 1.05 (0.61‐1.80) | 0.858 |
| Gonadotropin | 1.18 (0.67‐2.07) | 0.561 | 1.16 (0.65‐2.05) | 0.617 | 1.42 (0.78‐2.57) | 0.248 | 1.43 (0.77‐2.63) | 0.255 |
| Clomiphene citrate +gonadotropin | 0.57 (0.07‐4.57) | 0.600 | 0.58 (0.07‐4.75) | 0.607 | 0.78 (0.10‐6.27) | 0.818 | 0.76 (0.09‐6.46) | 0.802 |
| Others | 1.25 (0.19‐8.44) | 0.817 | 1.44 (0.17‐12.45) | 0.742 | NA | NA | ||
OR, odds ratio; CI, confidence interval.
P values for all factors except year were assessed using χ2 or Student's t tests.
Adjusted for male and female age, smoking status, ovulation induction agent and correlations within patients.
Sperm concentration, sperm motility, and total motile sperm count were significantly higher in pregnancy cycles compared with nonpregnancy cycles (Table 1). However, there was no relationship between advanced paternal age and sperm parameters, including semen volume, sperm concentration, sperm motility, or total sperm motility count (r = −0.117, 0.048, −0.117, and −0.074, respectively) (Figure 2).
Figure 2.
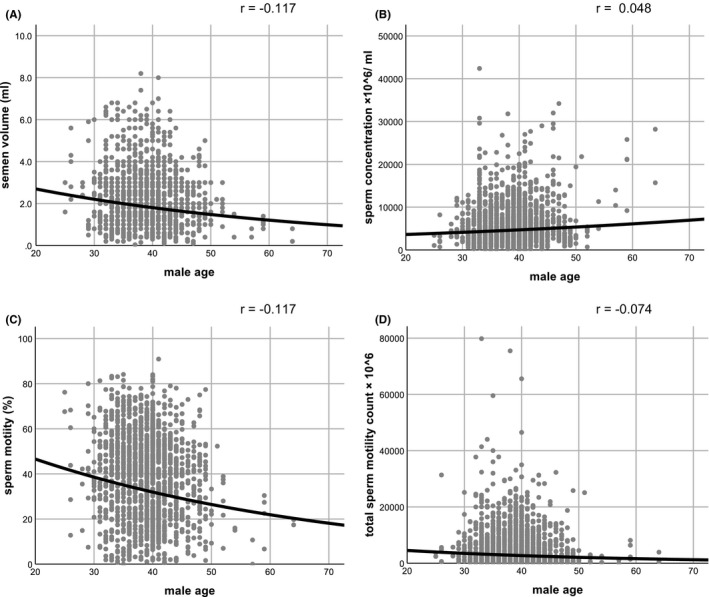
Scatter plot showing no significant correlation between male age and semen volume (A), sperm concentration (B), sperm motility (C), and total sperm motility count (D)
4. DISCUSSION
The results of this study showed that advanced paternal age had no adverse effect on pregnancy rates in couples undergoing IUI, and pregnancy outcomes depended on female age alone. Pregnancy outcomes were also unaffected by smoking status and ovulation induction agent. Our data also indicated that there was no relationship between advanced paternal age and semen volume or sperm concentration, sperm motility, or total motility count.
The results of previous studies regarding the association between advanced paternal age and pregnancy rate have been controversial.3, 10, 11 Belloc et al reported a significantly decreased pregnancy rate in women older than 38 years in a series of >17 000 IUI cycles (OR 0.67, 95% CI 0.56‐0.80) and a marginally significant decrease in men older than 45 years old (OR 0.81, 95% CI 0.65‐1.02). These translated into pregnancy rates of 12.3% in men younger than 30 and 9.3% in men older than 45 years.3 Bellver et al, however, found no relationship between advanced paternal age and pregnancy rates in 2204 IUI cycles.11 However, these studies did not take account of correlations within patients, and the current study thus provides the first evidence demonstrating no significant effect of advanced paternal age on pregnancy outcome after adjusting for correlations within patients.
The pregnancy rate in our study (7.0%, 111/1576) was lower than in some previous studies because we included some patients with multiple unsuccessful IUI attempts, including some patients who underwent up to 18 cycles. However, the pregnancy rate per couple was 21.9% (104/475), and seven couples had several successful pregnancies. Our institute had no restriction on the number of IUI attempts that a couple would like to undergo. These results therefore also indicated that some patients chose to continue to receive IUI if the clinic allowed, and could achieve successful pregnancy as a result. Our results also suggested that ART should not necessarily be indicated on the basis of advanced male age in couples with a female partner aged 40 years or younger.
Although sperm concentration, sperm motility, and total motile sperm count were significantly higher in pregnancy cycles compared with nonpregnancy cycles, in line with previous studies,6 there was no relationship between advanced paternal age and semen volume or sperm concentration, sperm motility, or total motility count. These results also supported the lack of an effect of advanced male age on pregnancy rate in couples following IUI. During ART, the environment of an oocyte fertilized in vitro is affected by factors such as laboratory parameters, culture media, and duration of culture, and thus differs from the in vivo environment in the Fallopian tubes or uterus.12 Furthermore, the culture medium and long culture periods used in ART can lead to epigenetic changes that may affect the phenotype.12, 13 In contrast, fertilization during IUI takes place in the natural environment, and our study thus reflects the fertilizing ability of sperm from older men more accurately than ART data, which might mask sperm impairments such as fertilization disorders.14 IUI avoids these concerns and is thus attracting increasing attention as a less artificial alternative to ART.
An important limitation of this study was that it was a single‐institution study, and the results may therefore have been biased. Conversely, however, the single‐center nature of the study was also a strength, given that all patients were subject to the same criteria for IUI and there was no restriction on performing IUI, and the results may thus reflect the pregnancy rate more accurately, including multiple IUI attempts, compared with other institutions. Another limitation was that the cases were limited to women aged 40 years or younger, and therefore excluded many couples undergoing IUI. However, the pregnancy rates for women in their 40s during the study period were 3.0% (14/464) at 41‐42 years, 2.5% (11/435) at 43‐45 years, and 1.1% (2/174) at ≥46 years, which were obviously lower than the pregnancy rates in women aged 40 years or younger. These results suggested that it may be advisable for couples to switch to ART, according to female age. Finally, the study was also limited by a relatively small number of men not of general reproductive age. Although it is more important to consider men within the common‐sense range, further information on men of other ages is required.
Our results indicate that advanced paternal age alone does not affect pregnancy and live‐birth rates or sperm parameters in couples undergoing IUI. This suggests that ART should not be recommended solely on the basis of an older male partner, and some couples may have a good chance of pregnancy without the need for ART. Although ART currently tends to be the preferred treatment, our results suggest that non‐ART treatments may have good pregnancy outcomes. We therefore recommend IUI as an effective method for achieving pregnancy while minimizing undesirable effects on the fertilized egg, even in couples including a male partner of advanced age.
DISCLOSURES
Conflict of interests: The authors report no conflict of interest. Human rights statements and informed consent: The protocol for the research project including human subjects has been approved by NCCHD (approval number 922). Animal rights: This article does not contain any studies with animal subjects performed by any of the authors.
Tatsumi T, Ishida E, Tatsumi K, et al. Advanced paternal age alone does not adversely affect pregnancy or live‐birth rates or sperm parameters following intrauterine insemination. Reprod Med Biol. 2018;17:459–465. 10.1002/rmb2.12222
REFERENCES
- 1. Belloc S, Hazout A, Zini A, et al. How to overcome male infertility after 40: Influence of paternal age on fertility. Maturitas. 2014;78(1):22‐29. 10.1016/j.maturitas.2014.02.011 [DOI] [PubMed] [Google Scholar]
- 2. Hourvitz A, Machtinger R, Maman E, Baum M, Dor J, Levron J. Assisted reproduction in women over 40 years of age: how old is too old? Reprod Biomed Online. 2009;19(4):599‐603. [DOI] [PubMed] [Google Scholar]
- 3. Belloc S, Cohen‐Bacrie P, Benkhalifa M, et al. Effect of maternal and paternal age on pregnancy and miscarriage rates after intrauterine insemination. Reprod Biomed Online. 2008;17(3):392‐397. [DOI] [PubMed] [Google Scholar]
- 4. de La Rochebrochard E, de Mouzon J, Thepot F, Thonneau P. Fathers over 40 and increased failure to conceive: the lessons of in vitro fertilization in France. Fertil Steril. 2006;85(5):1420‐1424. 10.1016/j.fertnstert.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 5. Wu Y, Kang X, Zheng H, Liu H, Liu J. Effect of Paternal Age on Reproductive Outcomes of In Vitro Fertilization. PLoS ONE. 2015;10(9):e0135734 10.1371/journal.pone.0135734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zadehmodarres S, Oladi B, Saeedi S, Jahed F, Ashraf H. Intrauterine insemination with husband semen: an evaluation of pregnancy rate and factors affecting outcome. J Assist Reprod Genet. 2009;26(1):7‐11. 10.1007/s10815-008-9273-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Honda T, Tsutsumi M, Komoda F, Tatsumi K. Acceptable pregnancy rate of unstimulated intrauterine insemination: a retrospective analysis of 17,830 cycles. Reprod Med Biol. 2015;14:27‐32. 10.1007/s12522-014-0192-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tatsumi T, Jwa SC, Kuwahara A, Irahara M, Kubota T, Saito H. Pregnancy and neonatal outcomes following letrozole use in frozen‐thawed single embryo transfer cycles. Hum Reprod. 2017;32(6):1244‐1248. 10.1093/humrep/dex066 [DOI] [PubMed] [Google Scholar]
- 9. Gordon JD. Handbook for clinical gynecologic endocrinology and infertility. Philadelphia, PA, USA: Lippincott Williams & Wilkins; 2002. [Google Scholar]
- 10. Humm KC, Sakkas D. Role of increased male age in IVF and egg donation: is sperm DNA fragmentation responsible? Fertil Steril. 2013;99(1):30‐36. 10.1016/j.fertnstert.2012.11.024 [DOI] [PubMed] [Google Scholar]
- 11. Bellver J, Garrido N, Remohi J, Pellicer A, Meseguer M. Influence of paternal age on assisted reproduction outcome. Reprod Biomed Online. 2008;17(5):595‐604. [DOI] [PubMed] [Google Scholar]
- 12. Pinborg A, Loft A, Romundstad LB, et al. Epigenetics and assisted reproductive technologies (ART). Acta Obstet Gynecol Scand. 2016;95(1):10‐5. [DOI] [PubMed] [Google Scholar]
- 13. El Hajj N, Haaf T. Epigenetic disturbances in in vitro cultured gametes and embryos: implications for human assisted reproduction. Fertil Steril. 2013;99(3):632‐641. 10.1016/j.fertnstert.2012.12.044 [DOI] [PubMed] [Google Scholar]
- 14. Begueria R, Garcia D, Obradors A, Poisot F, Vassena R, Vernaeve V. Paternal age and assisted reproductive outcomes in ICSI donor oocytes: is there an effect of older fathers? Hum Reprod. 2014;29(10):2114‐2122. 10.1093/humrep/deu189 [DOI] [PMC free article] [PubMed] [Google Scholar]


