Summary
Nucleotide‐binding, oligomerization domain (NOD)‐like receptor family, pyrin domain containing 3 (NLRP3) gene polymorphism was reported to be associated with susceptibility, disease activity or anti‐tumour necrosis factor (TNF) treatment response in rheumatoid arthritis (RA). However, the roles of NLRP3 inflammasome in the development of RA have not yet been elucidated fully. The present study aimed to study the role of NLRP3 inflammasome in RA. NLRP3 inflammasome activation in synovial tissues from RA and osteoarthritis (OA) patients were assessed by Western blot. Active caspase‐1 in synovia was stained by a FAM‐FLICA caspase‐1 probe. Mice with collagen‐induced arthritis (CIA) were treated with MCC950, a selective NLRP3 inhibitor, or vehicle for 2 weeks. The clinical score of arthritis, synovial inflammation and cartilage erosion were assessed. Proinflammatory cytokines were measured by enzyme‐linked immunosorbent assay (ELISA). The results showed that NLRP3 inflammasome was highly activated in both synovia from RA patients and CIA mice. Activation of NLRP3 inflammasome occurred mainly in the infiltrating monocyte/macrophages in synovia, but not in fibroblast‐like synoviocytes. Treatment with MCC950 resulted in significantly less severe joints inflammation and bone destruction. NLRP3 inflammasome activation in the synovia was inhibited significantly by MCC950 with reduced production of interleukin (IL)‐1β. The inhibition of NLRP3 inflammasome activation by MCC950 was confirmed further in a human monocytic cell line, THP‐1. In conclusion, NLRP3 inflammasome is involved in the pathogenesis of RA. Targeting NLRP3 inflammasome with a small molecule inhibitor might be a novel therapeutic strategy for RA.
Keywords: NLRP3 inflammasome, NLRP3 inhibitor, rheumatoid arthritis
Introduction
Rheumatoid arthritis (RA) is a chronic and systemic autoimmune disease characterized by persistent synovial inflammation in small diarthrodial joints, progressive cartilage and bone destruction and autoantibody production 1. The application of disease‐modifying anti‐rheumatic drugs (DMARDs) and biological agents have improved prognosis dramatically 2. Nevertheless, a proportion of patients fail to respond to current therapies, and the biological therapy raises the risks of serious infection 1, 3. Thus, the development of a novel therapeutic target for RA treatment remains urgent.
Cytokines are crucial in the pathogenesis of RA, including tumour necrosis factor (TNF), interleukin (IL)‐1β and IL‐6 4. IL‐1β is expressed abundantly in synovial membrane, permitting the activation of chondrocytes and osteoclasts which cause cartilage erosion and bone destruction 1, 5. IL‐1β also facilitates the differentiation of T helper type 17 (Th17) cells, which contribute to the development of RA 6. On the other hand, collagen‐induced arthritis (CIA) in IL‐18–/– mice was significantly less severe than in wild‐type (WT) controls 7, and the severity of CIA was reduced significantly after treatment with either IL‐18 neutralizing antibodies or recombinant IL‐18 binding protein 8. Nucleotide‐binding, oligomerization domain (NOD)‐like receptor family, pyrin domain containing 3 (NLRP3) inflammasome is found to be an important platform responsible for the maturation and secretion of IL‐1β and IL‐18 9. Upon activation, NLRP3 engages pro‐caspase‐1 through adapter molecule ASC to form NLRP3 inflammasome 10. Thereafter, the activated caspase‐1 catalyzes proteolytic cleavage of precursors of IL‐1β and IL‐18 into their mature forms and initiates inflammation 10.
NLRP3 inflammasome has been well elucidated in the pathogenesis of gouty arthritis 11. Recently, components of NLRP3 inflammasome were found expressed in synovia of RA patients 12. However, it is still not clear how importantly NLRP3 inflammasome contributes to the pathogenesis of RA. In the present study, we investigate the roles of NLRP3 inflammasome activation in the development of RA.
Materials and methods
Animals
Eight‐week‐old male DBA/1J mice were obtained from SLAC Laboratory Animal Centre (Shanghai, China) and maintained at the Laboratory Animal Centre, Sun Yat‐Sen University. Mice had free access to standard rodent diet and water ad libitum. The experimental protocol was approved by the Ethics Committee of the First Affiliated Hospital, Sun Yat‐Sen University. All procedures were performed in accordance with the National Institute of Health guide for Care and Use of Animals.
Collagen‐induced arthritis
Eight‐week‐old mice were used to establish the CIA model as described previously 13. Briefly, lyophilized bovine type II collagen (CII, Chondrex Inc., Redmond, WA, USA) was dissolved in 0·05 M acetic acid by stirring gently at 4°C overnight to form stock solution with a concentration of 2 mg/ml. On day 0, CII was emulsified with complete Freund’s adjuvant (CFA; Chondrex Inc.) containing high‐quality M. Tuberculosis at an equal volume and mice received 100 μl emulsion intradermally at the base of the tail, respectively. On day 21, a boosted vaccination was given with 100 μl emulsion in which CII was emulsified with incomplete Freund’s adjuvant (IFA; Chondrex Inc.).
Pharmacological inhibition of NLRP3
Established CIA mice were randomized to control (n = 8 mice) or treatment groups (n = 9 mice). MCC950 (MedChemExpress, Monmouth Junction, NJ, USA) was applied to treat mice. In brief, MCC950 was diluted at a concentration of 1·5 mg/ml in vehicle (saline). Mice were injected intraperitoneally with MCC950 (10 mg/kg) or vehicle once every 2 days beginning from day 6 after boosted immunization when evident arthritic symptoms were present, and the treatment lasted for 2 weeks 13, 14. Thereafter the mice were anaesthetized and killed. Sera and hind limbs were collected for enzyme‐linked immunosorbent assay (ELISA), Western blot and staining analysis.
Clinical score of CIA
Macroscopic evidence of arthritis was collected and assessed as described previously 15. The total clinical score consisted of two parts, including digit inflammation score (0–2 per animal) and paw swelling score (0–3 per paw), with a maximum clinical score of 14 per animal. Generally, the procedure was conducted specifically as follows every 2 days. Digit inflammation scores: score 0 = no inflamed digit; score 0·5 = 1–5 digits inflamed; score 1 = 6–10 digits inflamed; score 1·5 = 11–15 digits inflamed; and score 2 = 16 or more digits inflamed. Paw swelling (paw thickness increase percentage compared to day 0 before induction of CIA): score 0 ≤ 30% increase; score 1 > 30% increase; score 2 > 50% increase and score 3 > 80% increase.
Microcomputed tomography (micro‐CT) evaluation
A radiological test was used to access bone destruction. Mice experienced micro‐computerized tomography (CT) examination of the hind limb under anaesthesia. The procedure was performed employing a Siemens Inveon (Munich, Germany) CT/positron emission tomography (PET) multi‐modality system.
Histological evaluation of arthritis
At the end of MCC950 treatment, mice were killed under anaesthesia. Hind limbs were dissected and fixed in 10% neutral buffered formalin for 24 h and then transferred to 10% ethylenediamine tetraacetic acid (EDTA) solution for 4 weeks. When decalcification finished, limbs were embedded in paraffin. Knee joint sections (5 μm) were stained with haematoxylin and eosin (H&E). Safranin O‐fast green staining was also performed to reveal cartilage destruction. Histopathological evaluations were executed independently by two observers blind to the protocol 15, 16. The evaluation was composed of synovial inflammation and cartilage erosion. Synovial inflammation was scored from 0 = no inflammation, 1 = slight thickening of the lining layer or some infiltrating cells in sublining layer, 2 = slight thickening of the lining layer plus some infiltrating cells in the sublining layer, 3 = thickening of the lining layer, influx of cells in the sublining layer and the presence of cells in the synovial space to 4 = synovium highly infiltrated with many inflammatory cells. Cartilage erosion was determined by Safranin O‐fast green staining and graded from 0 = no destruction, 1 = minimal erosion limited to single spots, 2 = slight to moderate erosion in a limited area, 3 = more extensive areas of erosion to 4 = general destruction.
Cytokine detection
Sera were obtained at the end of the experiment. Knee joint synovial tissues were separated and snap‐frozen in liquid nitrogen, and then homogenized mechanically using cell lysis buffer (Cell Signaling Technology, Danvers, MA, USA). Tissue homogenate supernatants were collected by centrifugation at 15 000 g following lysis. The collected sera and supernatants were then subjected to ELISA for detection of cytokine IL‐1β (R&D Systems, Minneapolis, MN, USA), TNF (eBioscience, San Diego, CA, USA) and IL‐6 (eBioscience), according to the manufacturer’s instructions.
Cell culture
The THP‐1 cell line was maintained in complete medium supplemented with RPMI‐1640 (Gibco, Carlsbad, CA, USA), 2 mM glutamine, 100 U/ml penicillin 100 μg/ml streptomycin and 10% heat‐inactivated FBS (Hyclone, South Logan, Utah, USA). Prior to stimulation, cells were seeded to six‐well plates and treated with 10 ng/ml phorbol 12‐myristate 13‐acetate (PMA; Sigma, St Louis, MO, USA) for 24 h. When differentiated completely to macrophages, cells were then washed with phosphate‐buffered saline (PBS) (Hyclone) and grown in RPMI‐1640 medium for 24 h. The overnight medium was replaced with RPMI‐1640 medium. Cells were stimulated with 10 ng/ml lipopolysaccharide (LPS) and 10 μM MCC950 for 4 h; 5 mM adenosine triphosphate (ATP) was added for the last 30 min. Following different interventions, supernatants and cell lysates were collected and subjected to Western blot.
Western blot
Proteins from knee joint synovial tissues or cell line culture were extracted. Bicinchoninic acid (BCA) protein assay (Pierce ThermoScientific, Waltham, MA, USA) was applied to determine the concentrations of proteins and 1 × RIPA buffer (Cell Signaling Technology) was used to balance concentrations of samples before electrophoresis. Protein extracts were loaded onto a sodium dodecyl sulphate‐polyacrylamide gel electrophoresis (SDS‐PAGE) 10% gel for electrophoresis and then transferred to a polyvinylidene fluoride (PVDF) membrane (Merck Millipore, Darmstadt, Germany). After blockade with 5% non‐fat dry milk (NFDM), membranes were incubated with primary antibodies including mouse anti‐NLRP3 (Adipogen, San Diego, CA, USA), mouse anti‐caspase‐1 p20 (Adipogen), rabbit anti‐IL‐1β (Santa Cruz Inc., Santa Cruz, CA, USA), rabbit anti‐glyceraldehyde phosphate dehydrogenase (GAPDH) (Cell Signaling Technology) overnight at 4°C, respectively. Secondary antibodies included horseradish peroxidase (HRP)‐conjugated anti‐mouse immunoglobulin (Ig)G (Cell Signaling Technology) and HRP‐conjugated anti‐rabbit IgG (Cell Signaling Technology). Finally, visualization of target proteins was achieved by detection of fluorescence produced by enhanced chemiluminescence (ECL; Merck Millipore).
Human synovial tissues
Fresh synovial tissues were obtained from six patients with RA and six patients with osteoarthritis (OA) who received knee joint replacement surgery. Synovial tissues were cut into small pieces and homogenized with 1 × RIPA buffer, as described above. The prepared tissue samples were then subjected to SDS‐PAGE. Primary antibodies used in the detection of target molecules included mouse anti‐NLRP3, mouse anti‐caspase‐1 p20 (Adipogen) and rabbit anti‐GAPDH. Secondary antibodies were identical to those mentioned above.
In addition, acetone‐fixed cryostat sections (4 μm) of the synovia biopsies were washed with PBS and blocked for 1 h with 5% bovine serum albumin (BSA) in PBS. AlexaFluor A594‐conjugated anti‐CD14 antibody (Santa Cruz Inc.) was used to label monocytes/macrophages. AlexaFluor A594‐conjugated anti‐human CD3 (R&D Systems) was used to label T cells. AlexaFluor A594‐conjugated anti‐cadherin‐11 (Bioss, Shanghai, China) was used to label fibroblast‐like synoviocytes (FLS) 17. FAM‐FLICA caspase‐1 probe (ImmunoChemistry Technologies, Bloomington, MN, USA) and Vectashield anti‐fade mounting media with 4',6‐diamidino‐2‐Phenylindole (DAPI) (Vector Laboratories, Burlingame, CA, USA) were also used. A Zeiss LSM800 confocal microscope was applied.
All RA patients fulfilled the 1987 American College of Rheumatology Criteria for the classification of RA. Informed consent was obtained from all patients. The protocol was approved by the Ethics Committee of the First Affiliated Hospital, Sun Yat‐Sen University.
Statistical analysis
All data collected were expressed as mean ± standard deviation (s.d.). spss software version 16 was used to analyse data. Differences were assessed by Student’s t‐test, one‐way analysis of variance (anova) or non‐parametric Wilcoxon’s rank‐sum test. P‐values less than 0·05 were considered statistically significant.
Results
NLRP3 inflammasome was activated in synovia of RA patients
Synovia from OA patients and RA patients were collected and the expression of NLRP3 inflammasome pathway was analysed. As shown in Fig. 1, NLRP3 expression was significantly higher in RA patients than that in OA patients (Fig. 1 and Supporting information, Fig. 1). Caspase‐1 p20, the active form of caspase‐1, was expressed almost exclusively in RA patients but not in OA patients (Fig. 1 and Supporting information, Fig. 1).
Figure 1.
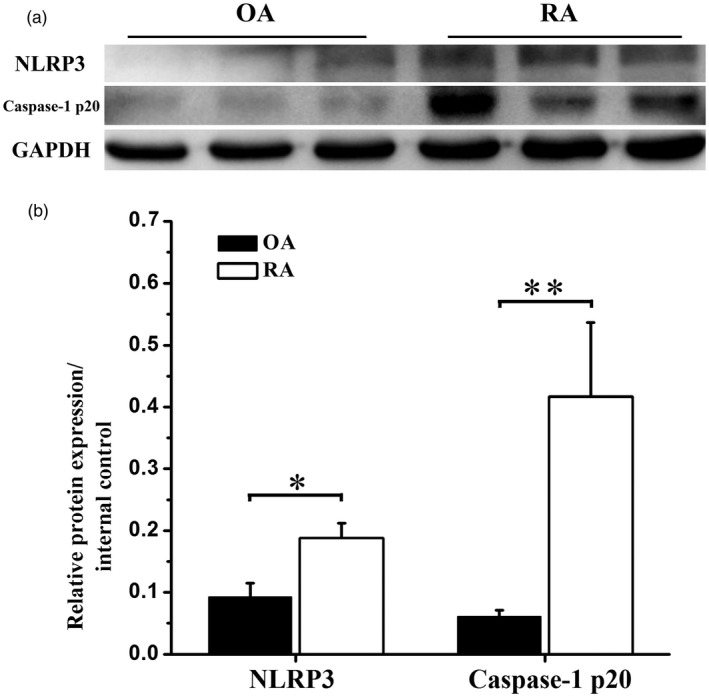
Nucleotide‐binding, oligomerization domain (NOD)‐like receptor family, pyrin domain containing 3 (NLRP3) inflammasome pathway was activated in synovia of rheumatoid arthritis (RA) patients. Human synovial tissues from RA or osteoarthritis (OA) patients were prepared and subjected to Western blot. (a) Representative Western blot bands of NLRP3 and caspase‐1 p20 expression in synovia of RA patients compared to OA patients. (b) Statistical analysis of relative target protein expression/internal control ratio. Data were recorded as mean ± standard deviation (s.d.), *P < 0·05, **P < 0·01, six patients per group.
CD14‐positive cells were the major sources of active caspase‐1 in synovia
To elucidate further the sources of NLRP3 inflammasome in synovia of RA, a fluorescence‐labelled caspase‐1 probe was used to detect active caspase‐1 enzyme. A large amount of CD14‐positive cells were observed in synovia of RA, while only a few CD14‐positive cells were found in synovia of OA (Fig. 2a). Similarly, many CD3‐positive T cells infiltrated and aggregated in synovia of RA patients, but only scattered T cells were found in synovia of OA (Fig. 2b). Cadherin‐11 positive FLS were also increased in synovia of RA (Fig. 2c). Active caspase‐1 fluorescence increased significantly in synovia of RA compared to OA. The fluorescence of active caspase‐1 was co‐localized almost exclusively with CD14 (Fig. 2a). However, no significant co‐localization between active caspase‐1 and CD3 or cadherin‐11 was observed in synovia of RA (Fig. 2b,c).
Figure 2.
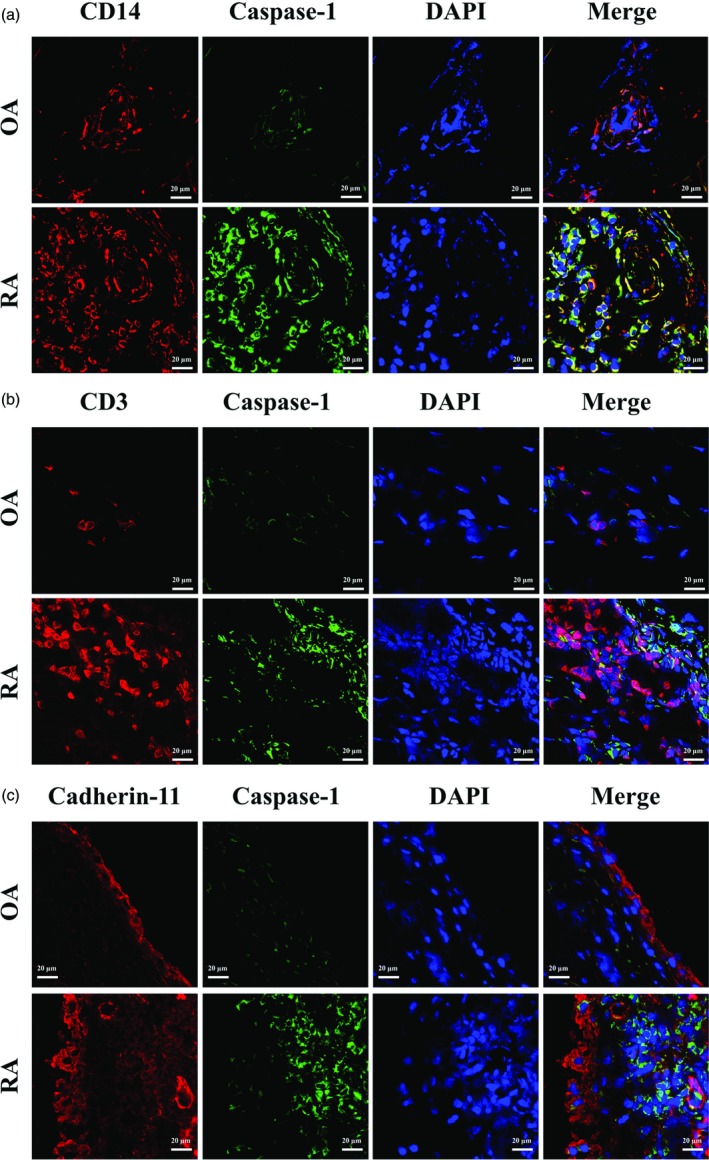
CD14 co‐localized with active caspase‐1 in synovia. Synovia from rheumatoid arthritis (RA) or osteoarthritis (OA) patients were collected for immunofluorescence staining. Slides were stained for active caspase‐1 (green) and CD14 (red) (a), CD3 (red) (b) or cadherin‐11 (red) (c). Original magnification ×20.
MCC950 treatment alleviated arthritis in CIA mice
To study the roles of NLRP3 inflammasome activation in the pathogenesis of RA, we treated mice with MCC950, a selective NLRP3 inhibitor, after the induction of CIA. The increase in the mean clinical score (Supporting information, Table 1) of the MCC950 group was inhibited significantly by MCC950 treatment at day 8 after boosted immunization, and this trend was maintained from day 10 to the end of the study (Fig. 3a). Following intervention, redness and swelling of the paws were attenuated markedly in the MCC950 group (Fig. 3b). Consistently, micro‐CT imaging indicated that joint destructions in MCC950 group mice were also reduced significantly (Fig. 3b).
Figure 3.
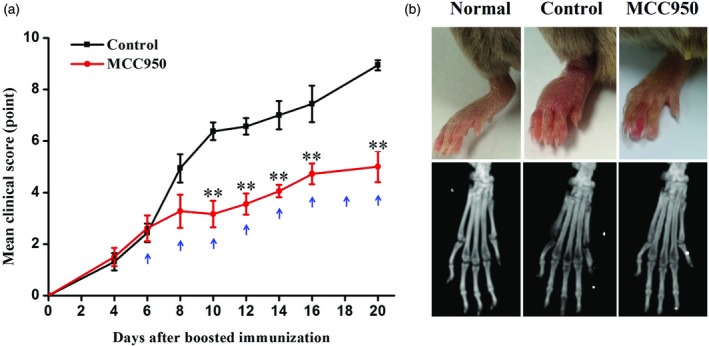
MCC950 treatment alleviated severity of collagen‐induced arthritis (CIA). Established CIA mice were randomized into control or treatment groups. Mice were injected intraperitoneally with MCC950 (10 mg/kg) or vehicle once every 2 days from day 6 after boosted immunization for 2 weeks. (a) Mean clinical scores of CIA mice treated with MCC950 (nine mice) and control group (eight mice). Blue arrows indicate the time when treatments were given. (b) Macroscopic observation and microcomputed tomography analysis of joints under anesthesia using a Siemens Inveon CT/PET multi‐modality system. Data were recorded as mean ± standard deviation (s.d.), **P < 0·01; normal group, eight mice.
MCC950 treatment attenuated synovial inflammation and cartilage erosion in CIA mice
To evaluate further the effect of MCC950 on synovial pathology and cartilage destruction, histological evaluation was performed at the end of the experiment. Significant hyperplasia of synovia, pannus formation and infiltration of inflammatory cells was observed in CIA mice (Fig. 4a). In contrast, only a few localized hyperplasia of synovia were found following MCC950 treatment (Fig. 4a). CIA mice also exhibited complete loss of Safranin O staining (Fig. 4a). Diseased synovia destroyed bone continuum from bilateral sides and spread into bone marrow cavities (Fig. 4a), while in the MCC950‐treated group, continuum of Safranin O staining lining the joints was found and there was less severe bone destruction (Fig. 4a). Semi‐quantitative histological score confirmed further that MCC950 treatment reduced the severity of both synovial inflammation and cartilage erosion in CIA mice (Fig. 4b).
Figure 4.
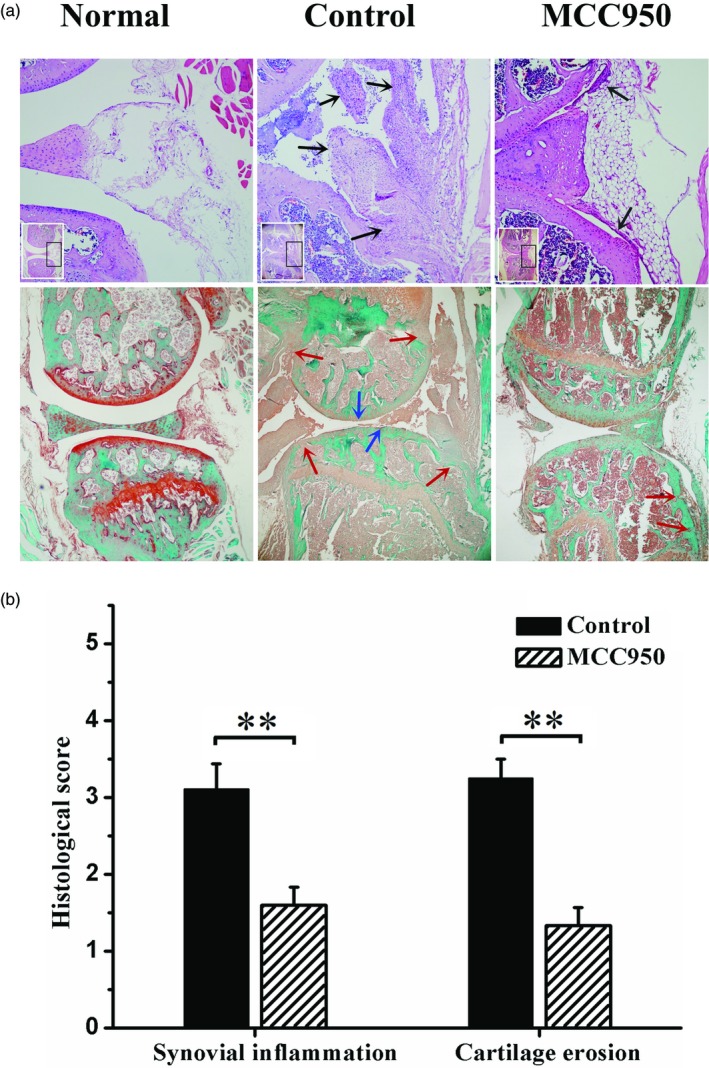
MCC950 treatment attenuated histological change in CIA mice. (a) (Up) Representative haematoxylin and eosin (H&E) staining images and amplification of respective typical lesion (×100). Black arrow, infiltration of inflammatory cells or hyperplasia of synovia. (Low) Representative safranin O‐fast green staining images (×40). Red arrow, bone erosion; blue arrow, cartilage loss. (b) Semiquantitative score of synovial inflammation and cartilage erosion. Data were recorded as mean ± standard deviation (s.d.), **P < 0·01.
MCC950 treatment inhibited NLRP3 inflammasome activation in CIA mice
Knee joint synovial tissues were abstracted and subjected to Western blot. In mice with CIA, NLRP3 expression in the knee joint synovia was increased significantly (Fig. 5 and Supporting information, Fig. 2). The expression of caspase‐1 p20, a functional subunit of caspase‐1, was also increased significantly compared to normal mice (Fig. 5 and Supporting information, Fig. 2). Following MCC950 treatment, both NLRP3 and caspase‐1 p20 expression were inhibited significantly (Fig. 5 and Supporting information, Fig. 2).
Figure 5.
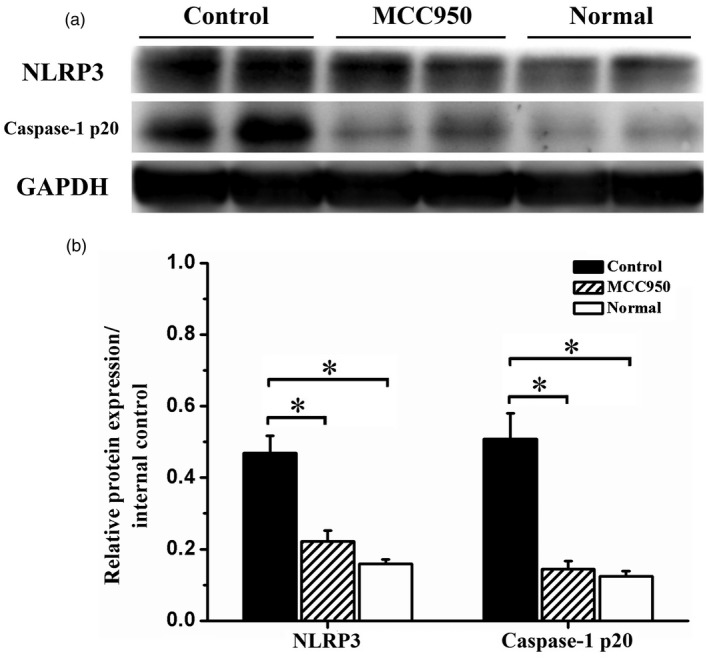
MCC950 treatment inhibited Nucleotide‐binding, oligomerization domain (NOD)‐like receptor family, pyrin domain containing 3 (NLRP3) inflammasome pathway activation. Following MCC950 treatment, knee joint synovial tissues were subjected to Western blot to evaluate NLRP3 inflammasome activation. (a) Representative Western blot bands of NLRP3 and caspase‐1 p20 expression in vehicle‐treated, MCC950‐treated and normal group. (b) Statistical analysis of relative target protein expression/internal control ratio. Data were recorded as mean ± standard deviation (s.d.), *P < 0·05.
MCC950 treatment inhibited IL‐1β production in the CIA model
In accordance with severe histological change, levels of IL‐1β, TNF and IL‐6 in knee joint synovial tissues and sera were increased significantly in control group mice following CIA induction (Fig. 6). The concentrations of IL‐1β in synovia were much higher than those in sera (Fig. 6a). Following 2 weeks of MCC950 treatment, IL‐1β production in synovia was reduced significantly (Fig. 6a). The levels of IL‐1β in sera were also reduced significantly following MCC950 treatment (Fig. 6a). However, the levels of both TNF and IL‐6 in synovia and sera were not affected by MCC950 treatment (Fig. 6b,c).
Figure 6.
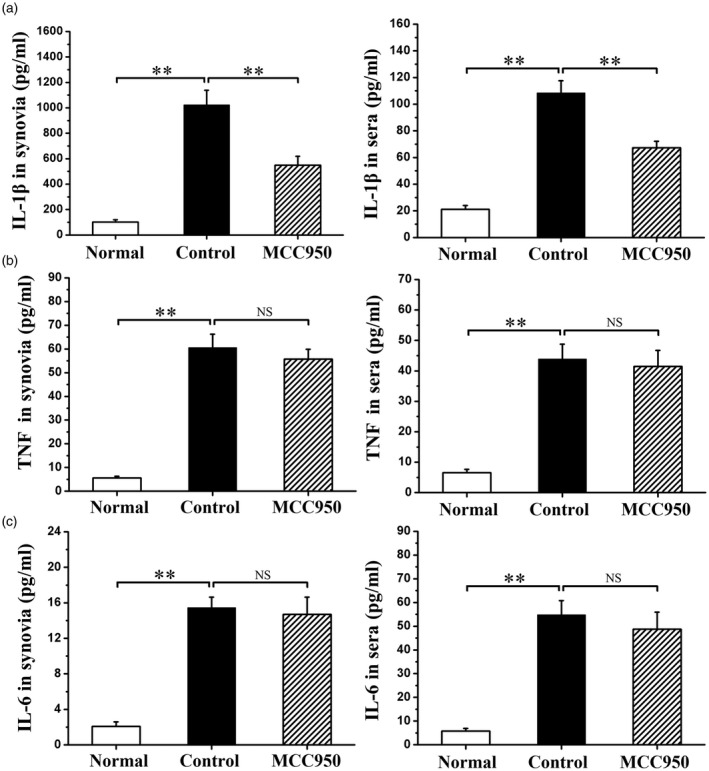
Effects of MCC950 treatment on proinflammatory cytokine production. (a) Interleukin (IL)‐1β levels in knee joint synovia and sera. (b) Tumour necrosis factor (TNF) levels in knee joint synovia and sera. (c) IL‐6 levels in knee joint synovia and sera. Data were recorded as mean ± standard deviation (s.d.), **P < 0·01, NS = not significant.
MCC950 inhibited NLRP3 inflammasome pathway activation in vitro
The THP‐1 cell line was used to investigate the inhibitory effects of MCC950 in regulation of NLRP3 inflammasome activation. As shown by Western blot, the classical combination of LPS + ATP activated significantly the NLRP3 inflammasome pathway presented as the markedly increased levels of NLRP3 and caspase‐1 p20 (Fig. 7 and Supporting information, Fig. 3). Also, levels of precursor and active forms of IL‐1β in the supernatant were apparently increased simultaneously (Fig. 7a and Supporting information, Fig. 3). Pretreated with MCC950 before ATP, levels of caspase‐1 p20 in the lysate and pro‐IL‐1β and IL‐1β in the supernatant were decreased significantly (Fig. 7a and Supporting information, Fig. 3). However, MCC950 did not affect the expression of NLRP3 (Fig. 7 and Supporting information, Fig. 3).
Figure 7.
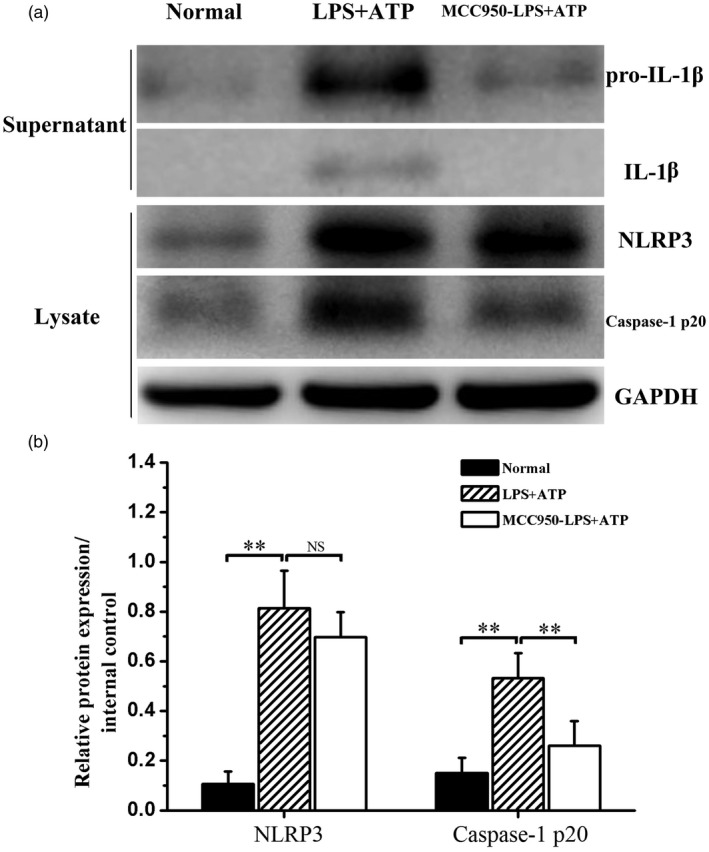
MCC950 treatment affected Nucleotide‐binding, oligomerization domain (NOD)‐like receptor family, pyrin domain containing 3 (NLRP3) inflammasome pathway activation. Cell lysates and supernatants were prepared and subjected to Western blot. (a) Representative Western blot bands of NLRP3, caspase‐1 p20, interleukin (IL)‐1β and pro‐IL‐1β in normal, lipopolysaccharide + adenosine triphosphate (LPS + ATP) and MCC950‐LPS + ATP groups. (b) Statistical analysis of relative target protein expression/internal control ratio. Data were recorded as mean ± standard deviation (s.d.), **P < 0·01, NS = not significant.
Discussion
The discovery of NLRP3 inflammasome as a platform sensing endogenous or exogenous stimuli and controlling the maturation and secretion of IL‐1β and IL‐18 was a milestone in innate immunity 18. Growing evidence has implicated NLRP3 inflammasome in the pathogenesis of autoinflammatory diseases 19 and autoimmune diseases 20. Of which the role of NLRP3 inflammasome in gouty arthritis has been well characterized 11. Blockade of IL‐1 with rilonacept was proved beneficial in management of gouty arthritis 21. Here, we documented that NLRP3 inflammasome was activated highly in both synovia from rheumatoid arthritis patients and CIA mice. Inhibition of NLRP3 with a highly selective NLRP3 inhibitor MCC950 was effective in ameliorating arthritic symptoms and cartilage erosion in CIA mice.
Current studies have taken into consideration NLRP3 inflammasome in the pathogenesis of rheumatoid arthritis. CIA mice, which are used widely as an RA mouse model, share typical pathological features with RA, including mononuclear cell infiltration, synovial hyperplasia and cartilage erosion 22. Zhang et al. showed that NLRP3 was expressed highly in the synovial proliferation areas and subchondral vasculitis areas in the paws of CIA mice compared to control mice 23. In A20myel‐knock‐out (KO) mice, synovial inflammation and cartilage destruction were protected markedly by NLRP3, caspase‐1 or IL‐1 deletion 24. In humans, the NLRP3 gene polymorphism was reported to be associated with susceptibility, disease activity or anti‐TNF treatment response in RA 25, 26, 27, 28. NLRP3 mRNA levels were also found to be up‐regulated in the synovia of RA patients compared to OA patients 29. Consistently, our study documented that NLRP3 inflammasome was activated highly in synovia from both RA patients and CIA mice. Treatment with NLRP3 selective inhibitor MCC950 showed obvious effectiveness in inhibiting NLRP3 inflammasome activation both in vivo and in vitro and amelioration of arthritic symptoms and cartilage destruction in CIA.
Regarding the location of the repertories of NLRP3 inflammasome responsible for the pathogenesis of RA, the reported results were conflicting. Rosengren et al. documented that enhanced NLRP3 expression in RA synovia was due to the accumulation of a large number of macrophages 29. In addition to macrophages, endothelial cells and neutrophils expressed high levels of NLRP3 inflammasome components, but synovial fibroblasts did not 12. Choulaki et al. found that higher basal amounts of NLRP3 were observed in whole blood cells from active RA patients compared to health control, and activation of NLRP3 inflammasome was more likely to be induced by LPS + ATP in peripheral blood cells from active RA 30. These data indicated that endothelial cells and myeloid‐derived cells might be the major source of NLRP3 inflammasome in RA. In contrast, fibroblast‐like synoviocytes isolated from the adjuvant‐induced arthritis model were reported to express higher levels of NLRP3 inflammasome compared to normal mice 31. In this study, we found that active caspase‐1, a downstream molecule of NLRP3 activation, was increased significantly in synovia of RA and was expressed almost exclusively in CD14‐positive cells but not in CD3‐positive cells and cadherin‐11‐positive FLS, indicating that monocytes and macrophages were the major cells with activated NLRP3 inflammasome in the synovia of RA. This increased the credence that myeloid‐derived cells were active participants in the pathogenesis of RA. Interestingly, Dennis et al. documented evidence for four major phenotypes of RA synovium – lymphoid, myeloid, low inflammatory and fibroid 32. Patients with the myeloid phenotype exhibited the most robust response to anti‐TNF 32. Together with our findings, this raised the possibility that NLRP3 inflammasome inhibition might be effective in treating the myeloid subtype of RA. However, more investigation is needed to confirm the respective contribution of different cell types in the pathogenesis of RA.
Proinflammatory cytokines are key functional molecules in the pathogenesis and progression of rheumatoid arthritis, including TNF, IL‐6 and IL‐1β 3, 33. Of which IL‐1β plays an important role in the pathogenesis of RA 5. It is accepted widely that IL‐1β is produced mainly by macrophages 5. IL‐1β could activate monocytes–macrophages, inducing cell adhesion molecules, chemokines and other inflammatory mediators, and thus contribute to exacerbated inflammation in joints 5. Also, IL‐1β also induces synovial cell proliferation and matrix metalloproteinases (MMP) production by chondrocytes and synovial cell and degrade cartilage 5. In this study, we found that levels of TNF, IL‐6 and IL‐1β were increased dramatically in synovia and sera following CIA induction, especially IL‐1β in the synovia. After MCC950 treatment, levels of IL‐1β in the synovia and sera were reduced significantly and joint pathology was improved significantly. On the other hand, MCC950 treatment did not down‐regulate the levels of TNF and IL‐6. These results indicated that MCC950 might inhibit relatively specifically the production of IL‐1β instead of TNF and IL‐6.
Although inhibition of NLRP3 with MCC950 presented good efficacy in CIA mice in this study, anti‐IL‐1Ra therapy was not remarkably effective and not recommended in the current treatment guidelines for RA. The first clinical trial reported that, in combination with MTX, anakinra achieved a 43% ACR20 response rate compared to 27% in the placebo group at 24 weeks 34. The following II/III phage clinical studies confirmed further that at 24 weeks anakinra achieved an approximately 38% response rate compared to 22–23% in the placebo group 35, 36. In contrast, the ACR20 response rate achieved at 24 weeks in approximately 60% by TNF inhibitor compared to 11–20% in the placebo group 37, 38, 39, 40 and 50–60% by IL‐6R inhibitor compared to 25–26% in the placebo group 41, 42. The differences between anti‐IL‐1R and NLRP3 inhibition for the treatment of RA should be elucidated further. IL‐18 and other non‐IL‐1‐dependent functions of the NLRP3 may also contribute.
The relation between NLRP3 inflammasome and autoimmunity should be taken into consideration. Growing evidence has revealed that NLRP3 was no longer confined to innate immune cell, but also was involved in adaptive immunity. Arbore et al. reported that there was NLRP3 inflammasome assembly within CD4+ T cells and contributed to Th1 differentiation through IFN‐γ induced by caspase‐1‐dependent IL‐1β secretion in an autocrine manner 43. Bruchard documented that NLRP3 but not ASC or caspase‐1 regulated a Th2 programme specifically as a transcriptional regulator 44. It indicated that blockade NLRP3 might affect Th2 differentiation and antibodies production in an IL‐1‐independent fashion. This might account partially for the different efficacy between anti‐IL‐1R therapy and NLRP3 selective inhibitor treatment therapy. However, activated caspase‐1 was not detected in CD3+ cells by immunofluorescence staining, suggesting that the abundance of activated NLRP3 in T cells is much lower than that of monocytes or macrophages.
In summary, NLRP3 inflammasome was activated in the synovia from both RA and CIA mice. Selective NLRP3 inhibitor MCC950 treatment attenuated arthritic symptoms and cartilage destruction, suggesting that targeting NLRP3 inflammasome with small molecule chemical inhibitor might be a potential therapeutic strategy for the treatment of RA.
Disclosure
None.
Author contributions
Study conception and design: C. G., R. F., S. W. and N. Y. Acquisition of data: C. G., R. F., S. W., Y. H., X. L., M. Z., J. Z. and N. Y. Analysis and interpretation of data: C. G., R. F., S. W., Y. H., X. L., M. Z., J. Z. and N. Y.
Supporting information
Fig. 1. Nucleotide‐binding, oligomerization domain (NOD)‐like receptor family, pyrin domain containing 3 (NLRP3) inflammasome pathway in synovia of osteoarthritis (OA) and rheumatoid arthritis (RA) patients. Human synovial tissues from six RA patients and six OA patients were prepared and subjected to Western blot. Blots of NLRP3 and caspase‐1 p20 expression in synovia were presented in groups of every three RA and three OA patients (a,b).
Fig. 2. Nucleotide‐binding, oligomerization domain (NOD)‐like receptor family, pyrin domain containing 3 (NLRP3) inflammasome pathway in joint synovial from collagen‐induced arthritis (CIA) mice. After MCC950 treatment, knee joint synovial tissues from CIA mice were collected and subjected to Western blot for detection of NLRP3 inflammasome pathway. Blots of NLRP3 and caspase‐1 p20 expression in synovia were presented in groups of every two vehicle‐treated, two MCC950‐treated and two normal mice (a–d).
Fig. 3. MCC950 inhibited nucleotide‐binding, oligomerization domain (NOD)‐like receptor family, pyrin domain containing 3 (NLRP3) inflammasome activation in the THP‐1 cell line. THP‐1 cells were seeded in six‐well plates and treated with lipopolysaccharide + adenosine triphosphate (LPS + ATP) in the presence or absence of MCC950. Cell lysate and supernatants were collected and subjected to Western blot. Blots of NLRP3, caspase‐1 p20, interleukin (IL)‐1β and pro‐IL‐1β in normal, LPS + ATP and MCC950‐LPS + ATP groups are presented. The experiments were repeated for three times as shown in (a), (b) and (c).
Table 1. Clinical scores of collagen‐induced arthritis (CIA) mice. Established CIA mice were divided into vehicle‐treated or MCC950‐treated groups. Clinical scores were recorded from day 0 after boosted immunization to day 20. Clinical scores of eight vehicle‐treated and nine MCC950‐treated mice are presented.
Acknowledgements
All authors were involved in drafting and revising the article, and all authors approved the final version to be published. N. Y. had full access to all of the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. This work was supported by grants from National Natural Science Foundation of China (81471598, 81671593 and 81701595), Guangzhou Science and Technology Planning Program (201707010093) and Natural Science Foundation of Guangdong Province (2016A030310172).
References
- 1. McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. New Engl J Med. 2011;365:2205–19. [DOI] [PubMed] [Google Scholar]
- 2. Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388:2023–38. [DOI] [PubMed] [Google Scholar]
- 3. Siebert S, Tsoukas A, Robertson J, McInnes I. Cytokines as therapeutic targets in rheumatoid arthritis and other inflammatory diseases. Pharmacol Rev. 2015;67:280–309. [DOI] [PubMed] [Google Scholar]
- 4. McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007;7:429–42. [DOI] [PubMed] [Google Scholar]
- 5. Dayer JM. The pivotal role of interleukin‐1 in the clinical manifestations of rheumatoid arthritis. Rheumatology (Oxf). 2003;42:3ii‐10. [DOI] [PubMed] [Google Scholar]
- 6. Zhang H, Wang S, Huang Y, et al. Myeloid‐derived suppressor cells are proinflammatory and regulate collagen‐induced arthritis through manipulating Th17 cell differentiation. Clin Immunol. 2015;157:175–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wei XQ, Leung BP, Arthur HM, Mclnnes IB, Liew FY. Reduced incidence and severity of collagen‐induced arthritis in mice lacking IL‐18. J Immunol. 2001;166:517–21. [DOI] [PubMed] [Google Scholar]
- 8. Plater‐Zyberk C, Joosten LA, Helsen MM, et al. Therapeutic effect of neutralizing endogenous IL‐18 activity in the collagen‐induced model of arthritis. J Clin Invest. 2001;108:1825–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wen H, Miao EA, Ting JP. Mechanisms of NOD‐like receptor‐associated inflammasome activation. Immunity. 2013;39:432–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Man SM, Kanneganti TD. Regulation of inflammasome activation. Immunol Rev. 2015;265:6–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pope RM, Tschopp J. The role of interleukin‐1 and the inflammasome in gout: implications for therapy. Arthritis Rheum. 2007;56:3183–8. [DOI] [PubMed] [Google Scholar]
- 12. Kolly L, Busso N, Palmer G, Talabot‐Ayer D, Chobaz V, So A. Expression and function of the NALP3 inflammasome in rheumatoid synovium. Immunology. 2010;129:178–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang H, Zhao J, Zhang H, et al. CARD11 blockade suppresses murine collagen‐induced arthritis via inhibiting CARD11/Bcl10 assembly and T helper type 17 response. Clin Exp Immunol. 2014;176:238–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Khachigian LM. Collagen antibody‐induced arthritis. Nat Protoc. 2006;1:2512–6. [DOI] [PubMed] [Google Scholar]
- 15. Camps M, Ruckle T, Ji H, et al. Blockade of PI3Kgamma suppresses joint inflammation and damage in mouse models of rheumatoid arthritis. Nat Med. 2005;11:936–43. [DOI] [PubMed] [Google Scholar]
- 16. Marty I, Peclat V, Kirdaite G, Salvi R, So A, Busso N. Amelioration of collagen‐induced arthritis by thrombin inhibition. J Clin Invest. 2001;107:631–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bartok B, Firestein G. Fibroblast‐like synoviocytes: key effector cells in rheumatoid arthritis. Immunol Rev. 2010;233:233–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Strowig T, Henao‐Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278–86. [DOI] [PubMed] [Google Scholar]
- 19. de Torre‐Minguela C, Mesa Del Castillo P, Pelegrin P. The NLRP3 and pyrin inflammasomes: implications in the pathophysiology of autoinflammatory diseases. Front Immunol. 2017;8:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. So A, Ives A, Joosten LA, Busso N. Targeting inflammasomes in rheumatic diseases. Nat Rev Rheumatol. 2013;9:391–9. [DOI] [PubMed] [Google Scholar]
- 21. Terkeltaub R, Sundy JS, Schumacher HR, et al. The interleukin 1 inhibitor rilonacept in treatment of chronic gouty arthritis: results of a placebo‐controlled, monosequence crossover, non‐randomised, single‐blind pilot study. Ann Rheum Dis. 2009;68:1613–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Williams RO. Collagen‐induced arthritis in mice: a major role for tumor necrosis factor‐alpha. Methods Mol Biol. 2007;361:265–84. [DOI] [PubMed] [Google Scholar]
- 23. Zhang Y, Zheng Y, Li H. NLRP3 inflammasome plays an important role in the pathogenesis of collagen‐induced arthritis. Mediators Inflamm. 2016;2016:9656270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vande Walle L, Van Opdenbosch N, Jacques P, et al. Negative regulation of the NLRP3 inflammasome by A20 protects against arthritis. Nature. 2014;512:69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jenko B, Praprotnik S, Tomsic M, Dolzan V. NLRP3 and CARD8 polymorphisms influence higher disease activity in rheumatoid arthritis. J Med Biochem. 2016;35:319–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yang Z, Cao J, Yang Q, Zhang Y, Han L. NLRP3 p. Q705K and CARD8 p.C10X single nucleotide polymorphisms are not associated with susceptibility to rheumatoid arthritis: a meta‐analysis. Int J Rheum Dis 2017; 20:1481–91. [DOI] [PubMed] [Google Scholar]
- 27. Sode J, Vogel U, Bank S, et al. Anti‐TNF treatment response in rheumatoid arthritis patients is associated with genetic variation in the NLRP3‐inflammasome. PLOS ONE. 2014;9:e100361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mathews RJ, Robinson JI, Battellino M, et al. Evidence of NLRP3‐inflammasome activation in rheumatoid arthritis (RA); genetic variants within the NLRP3‐inflammasome complex in relation to susceptibility to RA and response to anti‐TNF treatment. Ann Rheum Dis. 2014;73:1202–10. [DOI] [PubMed] [Google Scholar]
- 29. Rosengren S, Hoffman HM, Bugbee W, Boyle DL. Expression and regulation of cryopyrin and related proteins in rheumatoid arthritis synovium. Ann Rheum Dis. 2005;64:708–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Choulaki C, Papadaki G, Repa A, et al. Enhanced activity of NLRP3 inflammasome in peripheral blood cells of patients with active rheumatoid arthritis. Arthritis Res Ther. 2015;17:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li XF, Shen WW, Sun YY, et al. MicroRNA‐20a negatively regulates expression of NLRP3‐inflammasome by targeting TXNIP in adjuvant‐induced arthritis fibroblast‐like synoviocytes. Joint Bone Spine. 2016;83:695–700. [DOI] [PubMed] [Google Scholar]
- 32. Dennis G Jr, Holweg CT, Kummerfeld SK, et al. Synovial phenotypes in rheumatoid arthritis correlate with response to biologic therapeutics. Arthritis Res Ther. 2014;16:R90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brennan FM, McInnes IB. Evidence that cytokines play a role in rheumatoid arthritis. J Clin Invest. 2008;118:3537–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bresnihan B, Alvaro‐Gracia JM, Cobby M, et al. Treatment of rheumatoid arthritis with recombinant human interleukin‐1 receptor antagonist. Arthritis Rheum. 1998;41:2196–204. [DOI] [PubMed] [Google Scholar]
- 35. Cohen SB, Moreland LW, Cush JJ, et al. A multicentre, double blind, randomised, placebo controlled trial of anakinra (Kineret), a recombinant interleukin 1 receptor antagonist, in patients with rheumatoid arthritis treated with background methotrexate. Ann Rheum Dis. 2004;63:1062–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mertens M, Singh JA. Anakinra for rheumatoid arthritis. Cochrane Database Syst Rev. 2009;CD005121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Maini R, St Clair EW, Breedveld F, et al. Infliximab (chimeric anti‐tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. Lancet. 1999;354:1932–9. [DOI] [PubMed] [Google Scholar]
- 38. Weinblatt ME, Keystone EC, Furst DE, et al. Adalimumab, a fully human anti‐tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum. 2003;48:35–45. [DOI] [PubMed] [Google Scholar]
- 39. Moreland LW, Schiff MH, Baumgartner SW, et al. Etanercept therapy in rheumatoid arthritis. A randomized, controlled trial. Ann Intern Med. 1999;130:478–86. [DOI] [PubMed] [Google Scholar]
- 40. Strand V, Mease P, Burmester GR, et al. Rapid and sustained improvements in health‐related quality of life, fatigue, and other patient‐reported outcomes in rheumatoid arthritis patients treated with certolizumab pegol plus methotrexate over 1 year: results from the RAPID 1 randomized controlled trial. Arthritis Res Ther. 2009;11:R170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Genovese MC, McKay JD, Nasonov EL, et al. Interleukin‐6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease‐modifying antirheumatic drugs: the tocilizumab in combination with traditional disease‐modifying antirheumatic drug therapy study. Arthritis Rheum. 2008;58:2968–80. [DOI] [PubMed] [Google Scholar]
- 42. Smolen JS, Beaulieu A, Rubbert‐Roth A, et al. Effect of interleukin‐6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double‐blind, placebo‐controlled, randomised trial. Lancet. 2008;371:987–97. [DOI] [PubMed] [Google Scholar]
- 43. Arbore G, West EE, Spolski RT, et al. T helper 1 immunity requires complement‐driven NLRP3 inflammasome activity in CD4(+) T cells. Science. 2016;352:aad1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bruchard M, Rebe C, Derangere V, et al. The receptor NLRP3 is a transcriptional regulator of TH2 differentiation. Nat Immunol. 2015;16:859–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. 1. Nucleotide‐binding, oligomerization domain (NOD)‐like receptor family, pyrin domain containing 3 (NLRP3) inflammasome pathway in synovia of osteoarthritis (OA) and rheumatoid arthritis (RA) patients. Human synovial tissues from six RA patients and six OA patients were prepared and subjected to Western blot. Blots of NLRP3 and caspase‐1 p20 expression in synovia were presented in groups of every three RA and three OA patients (a,b).
Fig. 2. Nucleotide‐binding, oligomerization domain (NOD)‐like receptor family, pyrin domain containing 3 (NLRP3) inflammasome pathway in joint synovial from collagen‐induced arthritis (CIA) mice. After MCC950 treatment, knee joint synovial tissues from CIA mice were collected and subjected to Western blot for detection of NLRP3 inflammasome pathway. Blots of NLRP3 and caspase‐1 p20 expression in synovia were presented in groups of every two vehicle‐treated, two MCC950‐treated and two normal mice (a–d).
Fig. 3. MCC950 inhibited nucleotide‐binding, oligomerization domain (NOD)‐like receptor family, pyrin domain containing 3 (NLRP3) inflammasome activation in the THP‐1 cell line. THP‐1 cells were seeded in six‐well plates and treated with lipopolysaccharide + adenosine triphosphate (LPS + ATP) in the presence or absence of MCC950. Cell lysate and supernatants were collected and subjected to Western blot. Blots of NLRP3, caspase‐1 p20, interleukin (IL)‐1β and pro‐IL‐1β in normal, LPS + ATP and MCC950‐LPS + ATP groups are presented. The experiments were repeated for three times as shown in (a), (b) and (c).
Table 1. Clinical scores of collagen‐induced arthritis (CIA) mice. Established CIA mice were divided into vehicle‐treated or MCC950‐treated groups. Clinical scores were recorded from day 0 after boosted immunization to day 20. Clinical scores of eight vehicle‐treated and nine MCC950‐treated mice are presented.


