Abstract
The present study aimed to evaluate the anti-diabetic property of peanut shell polyphenol extracts (PSPEs). Diabetic rats were oral-administrated with PSPE at doses of 50, 100, and 200 mg/kg body weight (BW) per day for 28 consecutive days, with metformin (Met) as a positive control. The results showed that, similar to the Met treatment, administration of PSPE caused significant decreases in food intake, water intake, fasting blood glucose, total cholesterol, triglyceride, low-density lipoprotein cholesterol, and methane dicarboxylic aldehyde in serum, and significant increases in BW, insulin level, high-density lipoprotein cholesterol, superoxide dismutase, glutathione, and liver glycogen. Further, glucose tolerance was markedly improved in the PSPE-treated diabetic groups. Histopathological results showed that PSPE improved cellular structural and pathological changes in liver, kidney, and pancreatic islets. Collectively, the results indicated that the hypoglycemic effects of PSPE on high-fat diet/streptozotocin (HFD/STZ)-induced diabetes are comparable to Met, though their exact mechanism actions are still under investigation. Therefore, the current study suggests that PSPE could be a potential health-care food supplement in the management of diabetes.
Keywords: Peanut shell polyphenol extract (PSPE), Anti-diabetic activity, Streptozotocin (STZ)
1. Introduction
Diabetes mellitus (DM), a chronic metabolic disease characterized by hyperglycemia and impaired glucose tolerance, leads to more than 2.9 million deaths annually and this number is projected to rise over the next 30 years (Yadav et al., 2009). DM is becoming the third-ranked killer of human beings just after cancer and cardiovascular disease (Li et al., 2004). Currently, there are about 380 million individuals worldwide who have diabetes.
Diabetes is mainly classified into type 1 DM (T1DM) and T2DM based on the impaired-insulin action or secretion. T1DM results from a failure in production of insulin from the pancreas. About 5%−10% of all DM cases are type 1. T2DM is a long-term metabolic disorder which begins with insulin resistance and then develops into hyperglycemia and hypoinsulinemia. An estimate of 90% of DM cases belong to T2DM (Stumvoll et al., 1900; Gavin et al., 2003). T2DM is a life-threatening disease with high mortality and morbidity in both developing and developed countries. Metformin (Met) and rosiglitazone, commonly prescribed drugs for T2DM, cause various types of undesirable side-effects, e.g. stomach ache, loss of appetite, diarrhea (Korejo et al., 2016). Therefore, new antidiabetic drugs with fewer side-effects are currently under development. It has been confirmed that several plant-derived extracts exhibit hypoglycemic effects. These can be used as new anti-diabetic remedies (Lü et al., 2009a, 2009b; Patel et al., 2012).
Hyperglycemia is the key contributor to pathological changes in DM, including retinopathy, nephropathy, and neuropathy (Krentz et al., 2007; Saini et al., 2007). Persistent hyperglycemia is also one of the risk factors associated with meta-inflammation, insulin resistance, cardiovascular disease, hypertension, and hyperlipidemia (King, 2008; Lü et al., 2009a). One therapeutic approach for the treatment of hyperglycemia is to lower the gastrointestinal glucose absorption through inhibition of carbohydrate-hydrolyzing enzymes, such as α-glucosidase and α-amylase (Gray and Flatt, 1997; Kim et al., 2004; Shobana et al., 2009). Therefore, inhibitors of these enzymes might be considered as potential drugs for hyperglycemia. DM-associated hyperglycemia triggers reactive oxygen species (ROS) production, which could lead to elevation of oxidative stress (Yeh et al., 2016). Exacerbated oxidative stress negatively impacts pancreas functions such as impairment of insulin production (Chandirasegaran et al., 2017). Thus, mitigation of oxidative stress could serve as an alternative therapeutic approach for the treatment of hyperglycemia. Medicinal plants are widely used as antioxidants for the treatment or prevention of diseases, such as diabetes, cardiovascular disease, and obesity (Russo et al., 2015).
Peanut shell, a by-product of the peanut industry, has been reported rich in polyphenols, isosaponaretin, and other bioactive compounds (Han et al., 2008). Gao et al. (2011) found that peanut shells contain about 71.3 mg total phenolics per gram of defatted dry shells. Intriguingly, peanut shell polyphenol extracts (PSPEs) function as strong antioxidants with less toxicity (Yen and Duh, 1994; Duh and Yen, 1995). Besides, Yu et al. (2013) reported that PSPE exerts an inhibitory effect on α-amylase activity. Therefore, there is a need to discover the anti-diabetic property of PSPE. In the current study, we wish to elucidate the anti-hyperglycemic and antioxidant activity of PSPE in diabetic rats.
2. Materials and methods
2.1. Materials
Peanut shells (place of origin: Changchun, China) purchased from a local market were stored at 4 °C until use. Pyrogallol, catechol, phloroglucinol, quercetin, luteolin, streptozotocin (STZ), and Met were purchased from Sigma (St. Louis, MO, USA). An insulin enzyme-linked immunosorbent assay (ELISA) kit was purchased from Seikagaku Industries (Tokyo, Japan). The test kits of total cholesterol (TC), triglyceride (TG), high density lipoprotein-cholesterol (HDL-C), low density lipoprotein-cholesterol (LDL-C), liver glycogen (LG), methane dicarboxylic aldehyde (MDA), superoxide dismutase (SOD), and glutathione (GSH) were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). All other chemicals were purchased from Peking Chemical Plant (Beijing, China) and were of the highest purity available.
2.2. Preparation and purification of peanut shell polyphenol extracts
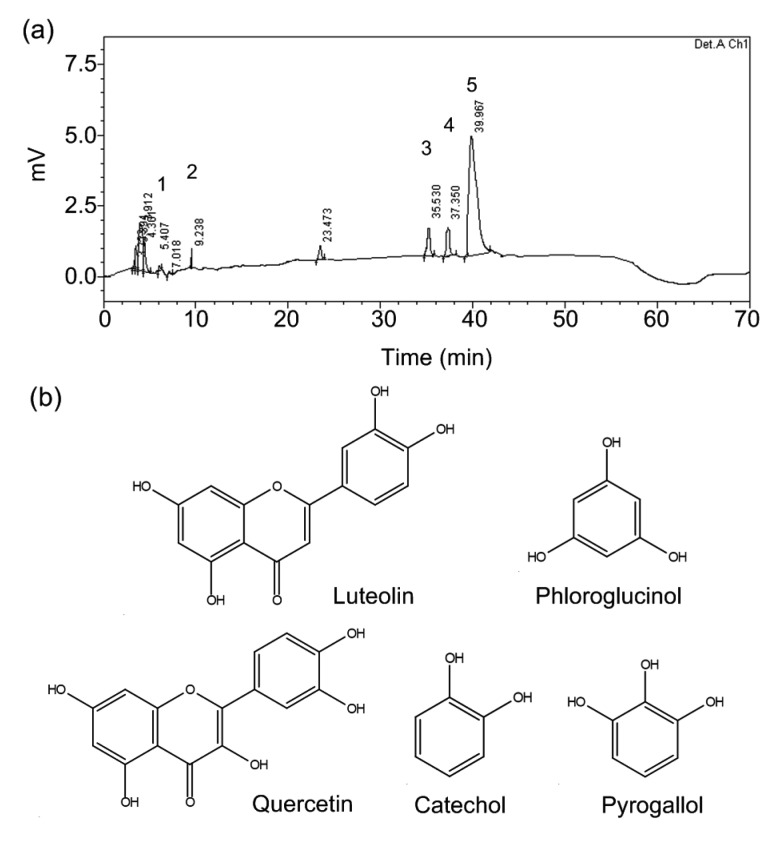
PSPEs were prepared following previously published methods (Gao et al., 2011). The peanut shells were defatted (1:68, w/v) for 10 h at room temperature using n-hexane and n-hexane was removed under reduced pressure. The defatted peanut shells (10 g) were macerated for 4 min with 250 ml aqueous ethanol (75%, v/v) in a high-pressure facility (DL700, Dalong High-Pressure Equipment Plant of Shanghai, China) at room temperature and 300 MPa. The extracts were purified using the static and dynamic adsorption-desorption performance of NKA-9 macroporous adsorption resin (#090216, Tianjin Haiguang Chemical Co., Ltd., China) to remove the water-soluble polysaccharide, glycosides, and protein from the extracts (Zhang et al., 2013). The purified extracts were evaporated to a final volume of 10 ml in a rotary evaporator (RE-52 Model, Anting Electronic Instruments Plant of Shanghai, China) at 35 °C. The concentrated solution was lyophilized with a freeze dryer system (FD-1C, Kangbo Experiment Instruments Ltd., Beijing, China) to obtain the PSPE powder. Based on characteristic analysis by high-performance liquid chromatography (HPLC), PSPE is a mixture of compounds including pyrogallol, luteolin, phloroglucinol, catechol, and quercetin (Fig. 1). The total phenolic content of PSPE was 71.3 mg/g, with luteolin at the highest concentration (Gao et al., 2011).
Fig. 1.
Profiles of peanut shell polyphenol extracts
(a) Peanut shell polyphenols by HPLC. 1, pyrogallol; 2, catechol; 3, phloroglucinol; 4, quercetin; 5, luteolin. (b) Molecular structures of luteolin, pyrogallol, catechol, phloroglucinol, and quercetin
2.3. Experimental animals
We used 60 three-month-old male Wistar rats ((190±10) g body weight (BW)) as experimental animals, which were purchased from the Center of Experimental Animals (School of Basic Medical Sciences, Jilin University, China). The rats were housed at constant temperature (22±2) °C and humidity (55±5)% with a 12-h light/dark cycle. The animals were fed a chow diet consisting of 53% carbohydrate, 5% fat, and 23% protein (Xietong Organism adjusted fat diet, Xietong Pharmaceutical Biological Engineering Co., Ltd., Nanjing, China), and water was given ad libitum for adaptation for one week. All procedures were approved by the Animal Welfare Committees of Jilin University, Changchun, China, and performed strictly following the “Principles of Laboratory Animal Care and Use in Research” (State Council of China, 1988).
2.4. Experimental induction of diabetes in rats
After one-week adaptation, diabetic rat models were developed using a high-fat diet (HFD) containing 41% carbohydrate, 24% fat, and 24% protein (Xietong Pharmaceutical Biological Engineering Co., Ltd., Nanjing, China) for 4 weeks. The control groups were fed a chow diet. After 4 weeks of HFD intervention, the diabetic group rats were intraperitoneally (i.p.) injected with STZ (at 60 mg/kg BW, dissolved in 0.1 mol/L citrate buffer (pH 4.5)), while the other groups received 0.1 mol/L citrate buffer (i.p., 1 ml/kg) as controls. Fasting blood glucose (FBG) was tested 1 week post-injection (Zhang et al., 2013). The rats with hyperglycemia (FBG level ≥11.1 mmol/L) were considered as diabetic and used for the experiments (Wang et al., 2013). Before necropsy, rats were fasted for 16 h.
2.5. Experimental design
All the rats were randomly divided into six groups, with seven rats per group: normal control group (NCG, the normal rats given water), diabetic control group (DCG, the diabetic rats given water), Met group (MG, diabetic rats given 200 mg/kg Met hydrochloride), high-dose PSPE group (PSPE 200, the diabetic rats given 200 mg/kg PSPE), medium-dose PSPE group (PSPE 100, diabetic rats given 100 mg/kg PSPE), and low-dose PSPE group (PSPE 50, the diabetic rats given 50 mg/kg PSPE). PSPE solutions were given through daily oral gavages (at 10 ml/kg BW) for 4 weeks.
2.6. Measurement of body weight and average daily food and water intake
The animal BW was monitored before gavage on the 7th, 14th, 21st, and 28th days of PSPE administration. The food and water intake were monitored weekly.
2.7. Measurement of fasting blood glucose, serum insulin level, and serum lipids
Blood samples obtained from the tail vein were used for FBG tests weekly by glucometer (Sannuo, Beijing, China). Serum samples were prepared by centrifuging (3000 r/min for 15 min, 4 °C) blood samples collected from rats’ eye sockets. The serum insulin level was determined using an insulin ELISA kit (Seikagaku Industries, Tokyo, Japan). Serum TC, TG, HDL-C, and LDL-C were determined using kits purchased from Jiancheng Bioengineering Institute (Nanjing, China). All blood samples were collected from overnight fasting rats.
2.8. Oral glucose tolerance test
Oral glucose tolerance test (OGTT) was performed on the 3rd week of PSPE administration. Diabetic and control rats were divided into six groups as previously described (Section 2.5). After 12-h fasting, 2 g/kg BW of glucose was oral-administered to all rats. The blood glucose level was measured from the tail vein at 0, 60, 120, and 180 min post the glucose injection.
2.9. Biochemical and histological analyses
Animals were euthanized at the end of the experiments after overnight fasting. Blood samples were collected by retro-orbital sinus puncture using capillary tubes under diethyl ether anesthesia and kept under room temperature for 40 min. Then, serums were prepared as previously described (Section 2.7).
The levels of SOD, GSH, MDA, and LG were measured using enzymatic kits according to the manufacturer’s instructions (Jiancheng Bioengineering Institute, Nanjing, China).
Tissues, like liver, kidney, and pancreas, were collected and fixed in 10% neutral buffered formalin solution. Histologic sections were prepared and stained with hematoxylin and eosin (H&E) as previously described (Fischer et al., 2008). Images of sections (liver, kidney, and pancreas) were acquired with a Nikon E2000 microscope (Tokyo, Japan) under objective 20×, 20×, and 40×, respectively.
2.10. Statistical analysis
Experiments were repeated at least three times. The experimental data were expressed as mean±standard deviation (SD). Statistical analyses were carried out by one-way analysis of variance (ANOVA) using SPSS Version 20.0 software (SPSS Inc., Chicago, USA). P-value lower than 0.05 was considered significant.
3. Results
Effects of PSPE on body weight and average daily food and water intake in diabetic rats
Table 1 shows the changes in BW and average daily intake of food and water in diabetic rats weekly.
Table 1.
Effects of PSPE on body weight and average daily food and water intake in diabetic rats
| Group | Body weight (g) |
||||
| Week 0 | Week 1 | Week 2 | Week 3 | Week 4 | |
| NCG | 246.00±20.54 | 269.17±19.88 | 297.11±22.57 | 315.12±18.88 | 328.90±22.52 |
| DCG | 232.31±17.36# | 225.17±8.63## | 218.87±13.99## | 213.75±10.67## | 210.22±15.31## |
| MG | 233.53±16.75 | 236.82±16.15 | 248.06±15.73 | 255.42±17.92* | 264.58±18.23* |
| PSPE 50 | 229.60±17.15 | 226.68±18.36 | 222.77±20.33 | 219.29±14.85 | 223.14±17.61 |
| PSPE 100 | 230.13±17.05 | 227.07±16.73 | 223.92±12.06 | 231.17±15.58 | 238.28±15.02 |
| PSPE 200 | 229.08±11.47 | 231.61±18.58 | 235.43±12.19 | 241.47±22.57 | 247.76±23.03* |
|
| |||||
| Group | Food (g/d) | ||||
|
| |||||
| Week 0 | Week 1 | Week 2 | Week 3 | Week 4 | |
|
| |||||
| NCG | 17.09±0.74 | 19.28±1.47 | 20.01±2.09 | 24.18±0.90 | 27.10±1.13 |
| DCG | 20.32±0.83## | 25.69±1.91## | 33.69±1.54## | 50.67±1.16## | 62.29±3.53## |
| MG | 20.56±1.42 | 22.59±0.48** | 25.91±2.54** | 31.37±1.66** | 30.16±0.84** |
| PSPE 50 | 20.27±1.12 | 25.57±0.64 | 33.49±0.47 | 50.44±0.88 | 49.54±1.33** |
| PSPE 100 | 19.97±1.23 | 24.60±1.08 | 33.71±1.45 | 48.57±2.66 | 47.62±0.70** |
| PSPE 200 | 20.14±0.50 | 24.90±0.82 | 33.04±2.06 | 44.98±1.80** | 46.05±0.27** |
|
| |||||
| Group | Water (ml/d) | ||||
|
| |||||
| Week 0 | Week 1 | Week 2 | Week 3 | Week 4 | |
|
| |||||
| NCG | 53.81±1.80 | 63.10±4.19 | 67.86±0.71 | 71.43±7.14 | 85.00±6.10 |
| DCG | 86.33±2.95## | 119.76±10.93## | 177.86±15.67## | 199.52±14.31## | 244.29±14.34## |
| MG | 84.52±5.77 | 93.81±4.76* | 141.43±3.78** | 144.76±8.73** | 139.05±11.69** |
| PSPE 50 | 85.95±6.40 | 113.33±4.59 | 173.33±5.82 | 199.76±11.83 | 190.71±6.35** |
| PSPE 100 | 86.43±7.56 | 112.62±10.16 | 163.81±6.79 | 185.71±2.47 | 177.62±6.86** |
| PSPE 200 | 84.29±3.27 | 114.05±24.71 | 160.48±9.72* | 178.10±3.60* | 165.00±10.08** |
Results are expressed as the mean±SD (n=7). PSPE: peanut shell polyphenol extract; NCG: normal control group; DCG: diabetic control group; MG: diabetic rats treated with metformin (Met; 200 mg/kg); PSPE 50: diabetic rats treated with PSPE (50 mg/kg, oral); PSPE 100: diabetic rats treated with PSPE (100 mg/kg, oral); PSPE 200: diabetic rats treated with PSPE (200 mg/kg, oral).
P<0.05,
P<0.01, compared with NCG;
P<0.05,
P<0.01, compared with DCG
At baseline, the BW of NCG was significantly higher than that of DCG, MG, PSPE 50, PSPE 100, and PSPE 200, while there was no significant difference among the diabetic rat groups. The BW of the DCG decreased during the onset of hyperglycemia (e.g. diabetes for 4 weeks). The BW of the PSPE 50 reduced in the first 3 weeks. The BW of the PSPE 100 reduced in the first 2 weeks. The BWs of the MG and PSPE 200 continuously increased. At the end of the experimental period, there was a slight weight gain in all PSPE groups compared with baseline (before treatments) (Table 1). The final BW gains (the BW on the 28th day minus the initial BW) of NCG, MG, PSPE 100, and PSPE 200 were 33.70%, 13.30%, 3.54%, and 8.15%, respectively. During the 4-week experiment diabetic rats were found to have significant BW loss compared with NCG (P<0.01). The final BW of the PSPE 200 group significantly increased compared with DCG (P<0.05) after 4-week administration. Data indicated that PSPE affected BW. PSPE at 200 mg/kg could effectively reverse the effect of BW loss in diabetic rats.
The food and water intake of the diabetic rats increased throughout the study period compared with NCG (Table 1). However, MG rats showed significantly lower food and water intake than the DCG rats. PSPE treatments (50, 100, and 200 mg/kg) at the 4th week of the study showed a significant reduction in both the daily food and water intake compared with the DCG rats (P<0.01). Therefore, we concluded that PSPE could improve the symptoms of diabetic rats.
3.2. Effects of PSPE on FBG and serum levels of insulin and lipids
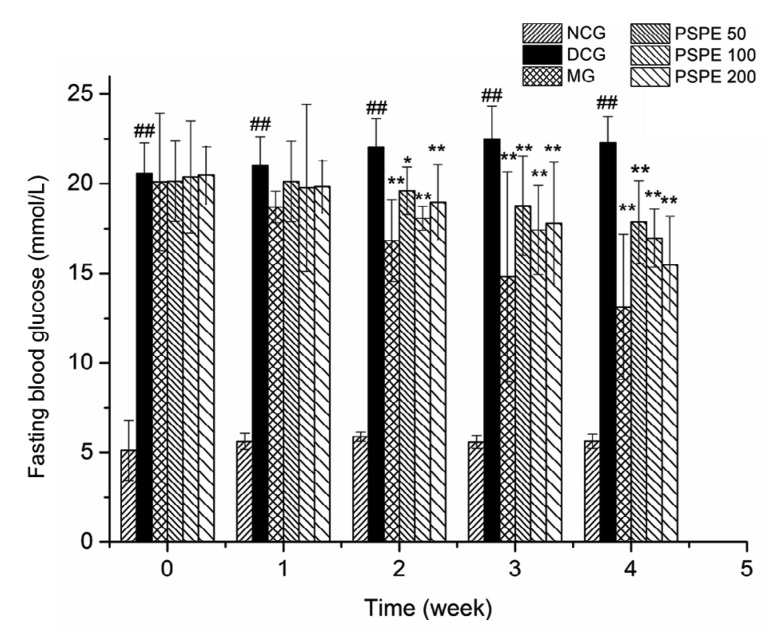
Fig. 2 shows the weekly FBG levels of experimental rats. The FBG levels of NCG rats were under the normal range, from (5.12±0.67) to (5.63±0.41) mmol/L. DCG rats presented higher FBG levels than NCG (P<0.01) throughout the experiment. FBG levels of DCG rats were elevated from (20.56±1.72) to (22.47±1.85) mmol/L at the end of the experiment with a 9.29% rise. After treatment with Met, the FBG levels of MG rats were significantly lower (P<0.01) than those of DCG rats from the 2nd week to the 4th week, and at the 4th week, the levels further decreased by 34.71%. After 1-week treatment with PSPE, the FBG levels of PSPE 50, PSPE 100, and PSPE 200 rats showed no significant decline. However, from the 2nd week of the PSPE treatment, the FBG levels in PSPE 50, PSPE 100, and PSPE 200 rats significantly decreased through to the end of the experiment compared with DCG (P<0.01). At the 4th week, the FBG levels of PSPE 50, PSPE 100, and PSPE 200 rats were reduced by 20.47%, 24.52%, and 31.06%, respectively, compared with DCG. Collectively, data indicate that PSPE treatments could effectively improve hyperglycemia in diabetic rats.
Fig. 2.
Effect of PSPE on fasting blood glucose level in diabetic rats
Results are expressed as the mean±SD (n=7). NCG: normal control group; DCG: diabetic control group; MG: diabetic rats treated with Met (200 mg/kg); PSPE 50: diabetic rats treated with PSPE (50 mg/kg, oral); PSPE 100: diabetic rats treated with PSPE (100 mg/kg, oral); PSPE 200: diabetic rats treated with PSPE (200 mg/kg, oral). ## P<0.01, compared with NCG; * P<0.05, ** P<0.01, compared with DCG
Table 2 shows the serum insulin and lipids of experimental rats at the end of the study. Compared with NCG, serum insulin levels of DCG rats were much higher. After administration with PSPE for 28 d, the serum insulin levels of MG, PSPE 50, PSPE 100, and PSPE 200 rats were significantly lower than those of DCG rats.
Table 2.
Effects of PSPE on serum insulin and serum lipids in diabetic rats
| Group | Serum insulin (μIU/ml) | TC (mg/dl) | TG (mg/dl) | LDL-C (mg/dl) | HDL-C (mg/dl) |
| NCG | 8.51±0.34 | 87.13±2.97 | 95.45±2.97 | 14.83±0.86 | 53.83±0.65 |
| DCG | 16.91±0.42## | 137.12±2.51## | 158.65±3.86## | 54.17±1.33## | 26.44±0.87## |
| MG | 11.75±0.55* | 97.28±3.34** | 103.74±2.55** | 21.87±0.84** | 44.75±0.62** |
| PSPE 50 | 12.08±0.43* | 107.33±2.76** | 123.82±1.97** | 23.64±0.92* | 40.16±0.74** |
| PSPE 100 | 13.16±0.41* | 103.43±3.21** | 118.08±2.87** | 18.67±0.76** | 41.53±0.62** |
| PSPE 200 | 14.45±0.34* | 100.62±2.64** | 109.59±2.13** | 18.36±0.63** | 42.36±0.87** |
Results are expressed as the mean±SD (n=7). TC: total cholesterol; TG: triglycerides; LDL-C: low density lipoprotein-cholesterol; HDL-C: high density lipoprotein-cholesterol; NCG: normal control group; DCG: diabetic control group; MG: diabetic rats treated with Met (200 mg/kg); PSPE 50: diabetic rats treated with PSPE (50 mg/kg, oral); PSPE 100: diabetic rats treated with PSPE (100 mg/kg, oral); PSPE 200: diabetic rats treated with PSPE (200 mg/kg, oral).
P<0.01, compared with NCG;
P<0.05,
P<0.01, compared with DCG
As shown in Table 2, after 4-week oral administration, serum TC, TG, and LDL-C levels in DCG rats were significantly higher than those in NCG rats (P<0.01), while MG, PSPE 50, PSPE 100, and PSPE 200 rats showed significant decreases in TC, TG, and LDL-C, but a significant increase in HDL-C level, compared with DCG (P<0.01). Data indicate that PSPE treatments could effectively improve lipid metabolism in diabetic rats.
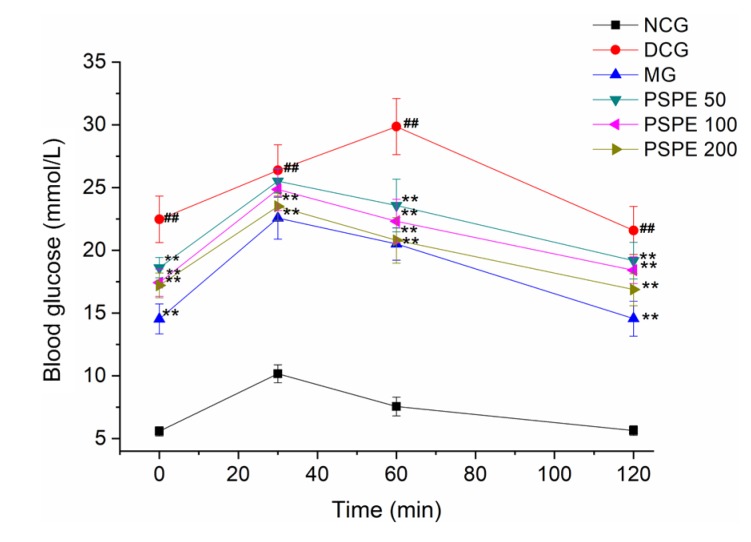
3.3. Effect of PSPE on OGTT
OGTT has been widely used to test the relative secretion and function of insulin in a population with glucose tolerance (Phillips et al., 1994; Stumvoll et al., 2000). Fig. 3 shows the effect of PSPE on OGTT. After glucose administration, the blood glucose level in NCG rats significantly increased at 30 min and decreased to normal levels at 120 min. The DCG rats presented significant glucose intolerance compared with NCG rats (P<0.01). MG, PSPE 100, and PSPE 200 showed significantly improved glucose intolerance (P<0.01). The baseline glucose level remained elevated in the DCG rats. However, MG, PSPE 50, PSPE 100, and PSPE 200 groups presented decreased blood glucose (35.49%, 24.77%, 25.86%, and 28.17%, respectively) at 120 min when compared with those at 30 min. PSPE 50, PSPE 100, and PSPE 200 groups exhibited much lower decreases in blood glucose compared with MG. Results indicate that Met and PSPE treatments could improve the glucose intolerance in diabetic rats.
Fig. 3.
Effect of PSPE on oral glucose tolerance test
Results are expressed as the mean±SD (n=7). NCG: normal control group; DCG: diabetic control group; MG: diabetic rats treated with Met (200 mg/kg); PSPE 50: diabetic rats treated with PSPE (50 mg/kg, oral); PSPE 100: diabetic rats treated with PSPE (100 mg/kg, oral); PSPE 200: diabetic rats treated with PSPE (200 mg/kg, oral). ## P<0.01, compared with NCG; ** P<0.01, compared with DCG
3.4. Effects of PSPE on the serum levels of SOD, GSH, and MDA
The effects of PSPE on oxidative stress were shown in Table 3. Compared with NCG, the levels of SOD and GSH reduced significantly (P<0.01), while the level of MDA was significantly increased in DCG (P<0.01). Compared with DCG rats, the levels of SOD and GSH in rats were significantly increased (P<0.01) by 50.49% and 37.63% with continuous 4-week PSPE treatments at 100 mg/kg, and by 86.49% and 61.66% at 200 mg/kg, respectively, while the MDA levels markedly decreased by 23.45% and 47.11%, respectively (P<0.01). In sum, the effects of PSPE on the levels of hepatic SOD, GSH, and MDA were dose-dependent.
Table 3.
Effects of PSPE on levels of serum SOD, GSH, MDA, and LG in diabetic rats
| Group | SOD (U/ml) | GSH (mg/L) | MDA (nmol/ml) | LG (mg/g) |
| NCG | 113.18±0.86 | 18.26±2.44 | 4.73±0.91 | 9.90±1.04 |
| DCG | 37.81±7.74## | 8.45±1.22## | 19.36±0.72## | 3.36±0.71## |
| MG | 76.14±2.44** | 15.89±1.26** | 8.79±1.45** | 7.28±0.90** |
| PSPE 50 | 47.56±4.15** | 9.80±0.60 | 17.54±0.96 | 3.83±0.16 |
| PSPE 100 | 56.90±2.68** | 11.63±1.23** | 14.82±2.12** | 5.17±0.22** |
| PSPE 200 | 70.51±2.49** | 13.66±1.00** | 10.24±1.11** | 6.33±0.40** |
Results are expressed as the mean±SD (n=7). SOD: superoxide dismutase; GSH: glutathione; MDA: methane dicarboxylic aldehyde; LG: liver glycogen; NCG: normal control group; DCG: diabetic control group; MG: diabetic rats treated with Met (200 mg/kg); PSPE 50: diabetic rats treated with PSPE (50 mg/kg, oral); PSPE 100: diabetic rats treated with PSPE (100 mg/kg, oral); PSPE 200: diabetic rats treated with PSPE (200 mg/kg, oral).
P<0.01, compared with NCG;
P<0.01, compared with DCG
3.5. Effect of PSPE on the level of glycogen in liver
Glycogen is the major storage form of glucose, and can be mainly found in liver and muscles and its function is to provide a readily available source of glucose for body (Chen et al., 2014). Compared with NCG, the glycogen content in liver decreased significantly in DCG (Table 3). Treatments with PSPE at the doses of 100 and 200 mg/kg for 4 weeks resulted in a significant increase in glycogen levels (P<0.01).
3.6. Effects of PSPE on histopathology of liver, kidney, and pancreas tissues in diabetic rats
The effects of PSPE on histopathology of liver, kidney, and pancreas tissues (Figs. 4,5,6) in control and diabetic rats were evaluated as they were majorly affected by diabetes.
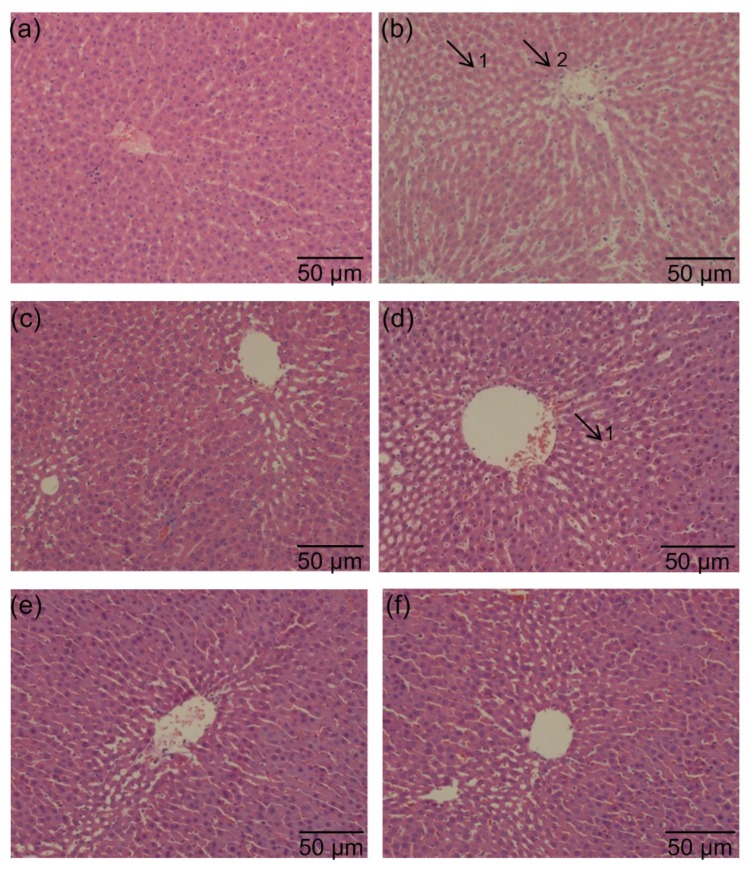
Fig. 4.
Effects of PSPE on morphological changes of liver in diabetic rats
(a) Normal control group; (b) Diabetic control group; (c) Diabetic rats treated with Met (200 mg/kg); (d) Diabetic rats treated with PSPE (50 mg/kg); (e) Diabetic rats treated with PSPE (100 mg/kg); (f) Diabetic rats treated with PSPE (200 mg/kg). Arrows: 1, swelling; 2, inflammatory cell infiltration
Fig. 5.
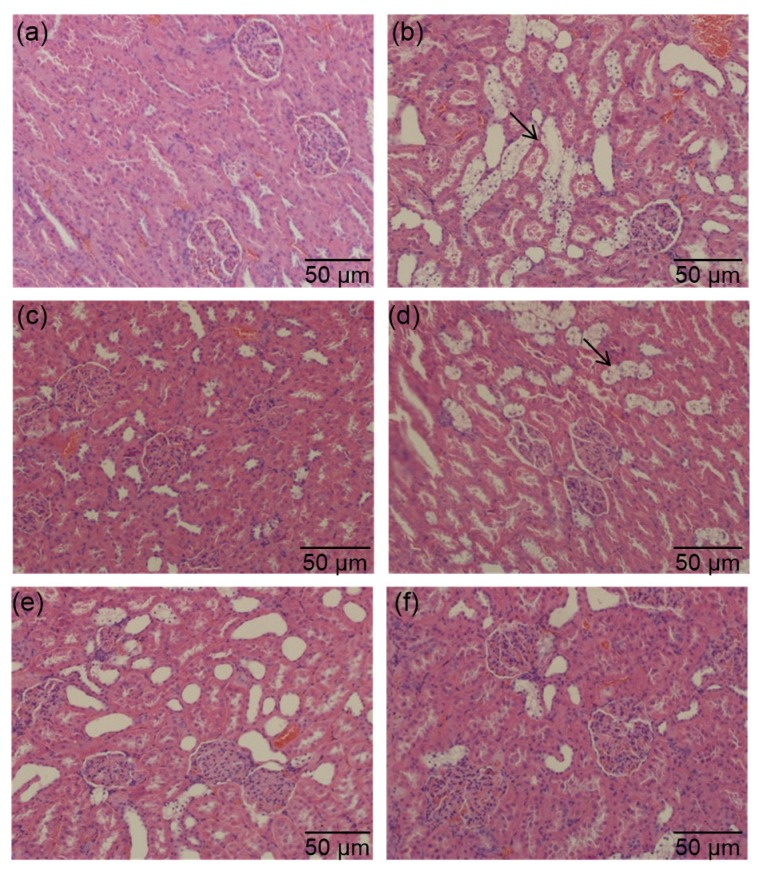
Effects of PSPE on morphological changes of kidneys in diabetic rats
(a) Normal control group; (b) Diabetic control group; (c) Diabetic rats treated with Met (200 mg/kg); (d) Diabetic rats treated with PSPE (50 mg/kg); (e) Diabetic rats treated with PSPE (100 mg/kg); (f) Diabetic rats treated with PSPE (200 mg/kg). Arrows: glomeruli with mesangiocapillary proliferation
Fig. 6.
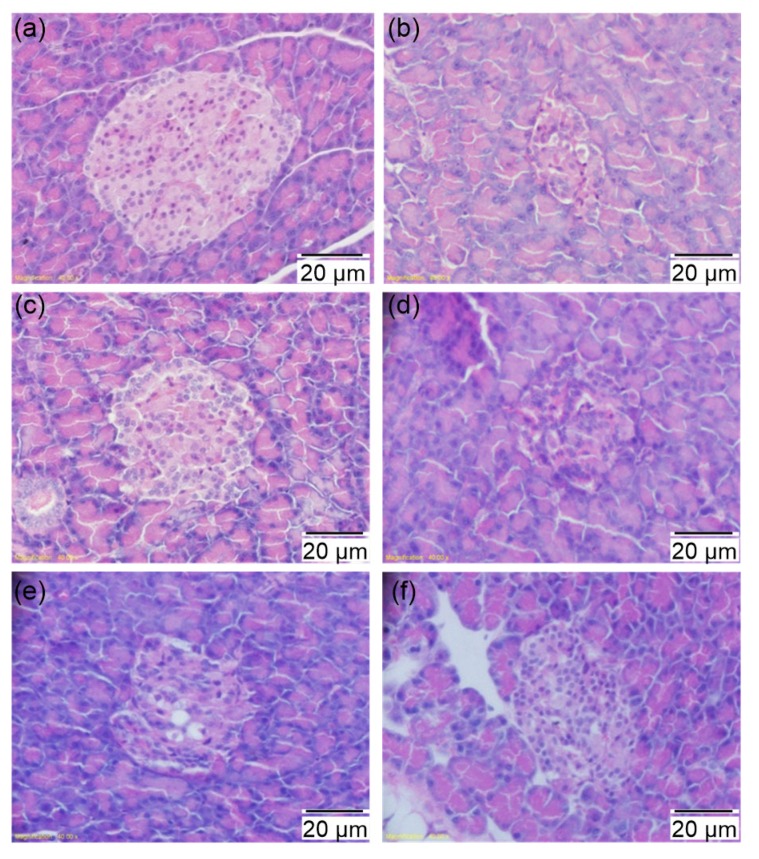
Effects of PSPE on morphological changes of pancreas in diabetic rats
(a) Normal control group; (b) Diabetic control group; (c) Diabetic rats treated with Met (200 mg/kg); (d) Diabetic rats treated with PSPE (50 mg/kg); (e) Diabetic rats treated with PSPE (100 mg/kg); (f) Diabetic rats treated with PSPE (200 mg/kg)
Fig. 4 shows the effects of PSPE on the histopathological changes in rat liver. Hepatic cells of DCG rats developed hepatic cellular edema and inflammatory cell infiltration (minor lesion) (Fig. 4b) compared with NCG (Fig. 4a). MG rats presented a similar liver histopathology to NCG rats (Fig. 4c). While in PSPE 100 (Fig. 4e) and PSPE 200 (Fig. 4f) groups, rat hepatic cells showed an improved liver histopathology compared with DCG rats. With a high dose of PSPE treatment, PSPE 200 led to the better improvement of liver tissue morphology and inflammatory cell infiltration (no lesion was observed).
Fig. 5 shows the effects of PSPE on the histopathological changes in rat kidneys. The renal tissue of kidneys in DCG (Fig. 5b) showed glomeruli with mesangiocapillary proliferation compared with NCG (Fig. 5a). Administration of PSPE at medium dose (Fig. 5e) and high dose (Fig. 5f) improved the glomeruli with mesangiocapillary proliferation in the kidneys compared with DCG rats.
Fig. 6 shows the effects of PSPE on the histopathological changes in rat pancreas. The NCG rats (Fig. 6a) showed the typical histological structure of pancreas with normal sized islets, while the islet of DCG (Fig. 6b) displayed shrinkage, and a decrease in number and destruction of β-cells. The PSPE 100 treatment showed an amelioration, but not significantly so, in histological signs (Fig. 6e). Met (Fig. 6c) and PSPE 200 (Fig. 6f) treatments restored such dysregulated structural changes. Both Met-(Fig. 6c) and PSPE-treated (Figs. 6e and 6f) diabetic rats presented prominent preservation and improvement of cellular structural and pathological changes in the pancreatic islets.
The investigation indicated that PSPE at doses of 100 and 200 mg/kg could improve the cellular structural and pathological changes in liver, kidney, and pancreatic islets, and may present potential beneficial effects against diabetes, indicating that PSPE may be a potential candidate in prevention and treatment of diabetes.
4. Discussion
Recently, plant-based treatment has been thought to be effective for the prevention and control of diabetes (Srivastava et al., 1993; Al-Attar and Zari, 2010; Wang et al., 2011; Chen et al., 2013). Peanut shells, a by-product of the peanut industry, are rich in polyphenols with less toxicological effects. PSPE presents a strong antioxidant capacity in vitro (Gao et al., 2011; Zhang et al., 2013) and inhibition of carbohydrate-hydrolyzing enzymes, such as α-amylase. Therefore, PSPE might also present potential anti-diabetic capacities. In this study, the anti-diabetic effect of PSPE has been investigated. Diabetes cause increases in food and water intake and BW loss. The present data showed that daily administration of PSPE for four weeks improved BW loss, and increased food and water intake (Table 1) in diabetic rats. This suggested that administration of the PSPE might prevent BW loss from excessive decrease in diabetic rats.
Hyperglycemia appears to be the key factor in the pathogenesis of diabetes. Therefore, most anti-diabetic drugs target lowering blood glucose effectively (Bell, 2001; Li et al., 2004). We found that the FBG was significantly increased in DCG compared with NCG, while PSPE treatment at a high dose of 200 mg/kg was able to effectively reduce the FBG level in diabetic rats, which is similar to the effect of Met (Fig. 2). The underlying mechanism of lowering blood glucose by PSPE could be due to: (1) diminished absorption of glucose decomposed from starch by inhibiting the activity of α-amylase in intestine (Yu et al., 2013); (2) promotion of absorption of glucose into the hepatic tissues in the form of glycogen (Sharma et al., 2006); (3) increased release of insulin from remaining β-cells and/or regeneration of β-cells (Esmaeili and Yazdanparast, 2004; Sharma et al., 2006). We found that PSPE showed elevated inhibition of α-amylase activity in vitro. PSPE is a potent reversible inhibitor associated with K m (Michaelis constant) value of 5.49 mg/ml (Yu et al., 2013). The liver is the most important organ in the regulation of glucose metabolism. It can absorb and convert circulated glucose into glycogen to lower blood glucose (Roden and Bernroider, 2003; Sharma et al., 2006). In the present study, the level of LG was decreased significantly in DCG compared with NCG (P<0.05). This indicates excessive hepatic gluconeogenesis and glucose production as the possible causes of hyperglycemia in diabetes (Zhang et al., 2014). The LG levels in PSPE 100 and PSPE 200 groups (Table 3) were higher than that of the DCG group, indicating that the defective glycogen storage in the diabetic state was partially reversed by PSPE administration. Our study also demonstrated the significant improvement of insulin resistance in diabetic rats (Table 3). Histopathological examinations of the pancreatic sections revealed that pancreatic β-cells of rats from the MG (Fig. 6c) and PSPE groups (Figs. 6e and 6f) had less damage than those in DCG, which further indicates that PSPE can repair the destroyed pancreatic β-cells to improve serum insulin levels in diabetic rats. Taken together, the results indicated that blood glucose level could be controlled by administering PSPE in diabetic rats.
OGTT is an important criterion for diagnosing diabetes (Shulman, 2000). OGTT results indicated that application of PSPE, to some extent, decreased blood glucose levels at 30, 60, and 120 min after glucose challenge when compared with the diabetic control group in Fig. 3. These results indicated that PSPE could efficiently improve glucose intolerance in diabetic rats.
Dyslipidemia, a common complication of diabetes, is the primary cause of cardiovascular disease in people with diabetes (Bertoni et al., 2004). Dyslipidemia is characterized by lipid abnormalities, including increased serum levels of TC, TG, and LDL-C, and a decrease in the level of HDL-C (Ginsberg, 2000). In this study, PSPE treatment for four weeks reduced serum levels of TG, TC, and LDL-C. Thus, PSPE may have a potential to ameliorate the symptoms of diabetes, which could further contribute to lowering the risk of cardiovascular complications in diabetic conditions.
Oxidative stress is defined as an imbalance between ROS production and antioxidant defense system of body (Kaur et al., 2016). Oxidative stress has recently been shown to play a key role in the pathogenesis of diabetes (Sepici-Dincel et al., 2007). MDA is an end product and an indicator of the lipid peroxidation process (Ong et al., 2011). GSH and SOD play important roles in the antioxidant defense system (Chen et al., 2013). Our results showed that HFD and STZ treatment caused a significant increase in the level of MDA, and significant decreases in the levels of GSH and SOD in DCG compared with NCG. The treatment with different doses of PSPE ameliorated the oxidative stress.
Oxidative stress can also impair the living cells (Zhang et al., 2011). This might contribute to a cellular structural abnormality of the liver, kidney, and pancreas observed in our histopathologic study. In the PSPE group, the liver, kidney, and islets were substantially protected from destruction compared with DCG (Figs. 4–6). Therefore, administration of PSPE reversed this structural damage possibly through anti-hyperglycemic and antioxidant activity.
Previous reports demonstrated that PSPE is rich in phenols and flavonoids such as pyrogallol, catechol, phloroglucinol, quercetin, and luteolin (which accounts for the highest amount). PSPE and its bioactive constituents showed a strong free radical scavenging activity in vitro (Upadhyay et al., 2010; Qiu et al., 2012; Zhang et al., 2013; Kasala et al., 2016; Zizkova et al., 2017), while luteolin and quercetin had been proved to have anti-diabetic effects (Torres-Piedra et al., 2010; Chen et al., 2016). Therefore, the anti-diabetic effect of PSPE might be due to these phytochemical constituents. Further studies are warranted to confirm these possibilities.
5. Conclusions
Our current study demonstrated that PSPE has a strong anti-diabetic effect on diabetic rats. PSPE significantly rescued the diabetic rats as demonstrated by restoring the parameters of BW gain, food and water intake, blood glucose levels, insulin level, lipid profile, and oxidative defense. PSPE played a preventive role in liver, renal, and pancreas destruction caused by STZ after 4-week treatment. The results suggested that the mechanism of anti-diabetic effect of PSPE was likely through inhibition of α-amylase activity, improving immune regulatory properties and oxidative stress. In conclusion, PSPE, which presents a protective property against diabetes with a relatively non-toxic nature, may serve as a promising candidate in improving the management of diabetes. Further studies need to be carried out to elucidate the actual mechanisms of this action.
Footnotes
Project supported by the China Scholarship Council (No. 201306175110) and the Major Scientific and Technological Research Project of Jilin Province (No. 20140204048NY), China
Compliance with ethics guidelines: Xiao-meng SUN, Hai-qing YE, Jing-bo LIU, Lei WU, Ding-bo LIN, Ya-li YU, and Feng GAO declare that they have no conflict of interest.
All institutional and national guidelines for the care and use of laboratory animals were followed.
References
- 1.Al-Attar AM, Zari TA. Influences of crude extract of tea leaves, Camellia sinensis, on streptozotocin diabetic male albino mice. Saudi J Biol Sci. 2010;17(4):295–301. doi: 10.1016/j.sjbs.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bell DS. Importance of postprandial glucose control. South Med J. 2001;94(8):804–809. doi: 10.1097/00007611-200194080-00011. [DOI] [PubMed] [Google Scholar]
- 3.Bertoni AG, Hundley WG, Massing MW, et al. Heart failure prevalence, incidence, and mortality in the elderly with diabetes. Diabetes Care. 2004;27(3):699–703. doi: 10.2337/diacare.27.3.699. [DOI] [PubMed] [Google Scholar]
- 4.Chandirasegaran G, Elanchezhiyan C, Ghosh K, et al. Berberine chloride ameliorates oxidative stress, inflammation and apoptosis in the pancreas of streptozotocin induced diabetic rats. Biomed Pharmacother. 2017;95:175–185. doi: 10.1016/j.biopha.2017.08.040. [DOI] [PubMed] [Google Scholar]
- 5.Chen FF, Xiong H, Wang JX, et al. Antidiabetic effect of total flavonoids from Sanguis draxonis in type 2 diabetic rats. J Ethnopharmacol. 2013;149(3):729–736. doi: 10.1016/j.jep.2013.07.035. [DOI] [PubMed] [Google Scholar]
- 6.Chen L, Tian GW, Tang WD, et al. Protective effect of luteolin on streptozotocin-induced diabetic renal damage in mice via the regulation of RIP140/NF-κB pathway and insulin signalling pathway. J Funct Foods. 2016;22:93–100. doi: 10.1016/j.jff.2016.01.023. [DOI] [Google Scholar]
- 7.Chen P, Zhang QX, Dang H, et al. Antidiabetic effect of Lactobacillus casei CCFM0412 on mice with type 2 diabetes induced by a high-fat diet and streptozotocin. Nutrition. 2014;30(9):1061–1068. doi: 10.1016/j.nut.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 8.Duh PD, Yen GC. Changes in antioxidant activity and components of methanolic extracts of peanut hulls irradiated with ultraviolet light. Food Chem. 1995;54(2):127–131. doi: 10.1016/0308-8146(94)00148-X. [DOI] [Google Scholar]
- 9.Esmaeili MA, Yazdanparast R. Hypoglycaemic effect of Teucrium polium: studies with rat pancreatic islets. J Ethnopharmacol. 2004;95(1):27–30. doi: 10.1016/j.jep.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 10.Fischer AH, Jacobson KA, Rose J, et al. Hematoxylin and eosin staining of tissue and cell sections. CSH Protoc, 2008:pdbprot4986. 2008 doi: 10.1101/pdb.prot4986. [DOI] [PubMed] [Google Scholar]
- 11.Gao F, Ye HQ, Yu YL, et al. Lack of toxicological effect through mutagenicity test of polyphenol extracts from peanut shells. Food Chem. 2011;129(3):920–924. doi: 10.1016/j.foodchem.2011.05.046. [DOI] [PubMed] [Google Scholar]
- 12.Gavin JR, Alberti KGMM, Davidson MB, et al. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26(S1):S5–S20. doi: 10.2337/diacare.26.2007.S5. [DOI] [PubMed] [Google Scholar]
- 13.Ginsberg HN. Insulin resistance and cardiovascular disease. J Clin Invest. 2000;106(4):453–458. doi: 10.1172/JCI10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gray AM, Flatt PR. Nature’s own pharmacy: the diabetes perspective. Proc Nutr Soc. 1997;56(1B):507–517. doi: 10.1079/PNS19970051. [DOI] [PubMed] [Google Scholar]
- 15.Han RP, Han P, Cai ZH, et al. Kinetics and isotherms of Neutral Red adsorption on peanut husk. J Environ Sci. 2008;20(9):1035–1041. doi: 10.1016/S1001-0742(08)62146-4. [DOI] [PubMed] [Google Scholar]
- 16.Kasala ER, Bodduluru LN, Barua CC, et al. Antioxidant and antitumor efficacy of Luteolin, a dietary flavone on benzo(a)pyrene-induced experimental lung carcinogenesis. Biomed Pharmacother. 2016;82:568–577. doi: 10.1016/j.biopha.2016.05.042. [DOI] [PubMed] [Google Scholar]
- 17.Kaur N, Kishore L, Singh R. Antidiabetic effect of new chromane isolated from Dillenia indica L. leaves in streptozotocin induced diabetic rats. J Funct Foods. 2016;22:547–555. doi: 10.1016/j.jff.2016.02.016. [DOI] [Google Scholar]
- 18.Kim YM, Wang MH, Rhee HI. A novel α-glucosidase inhibitor from pine bark. Carbohyd Res. 2004;339(3):715–717. doi: 10.1016/j.carres.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 19.King GL. The role of inflammatory cytokines in diabetes and its complications. J Periodontol. 2008;79(8S):1527–1534. doi: 10.1902/jop.2008.080246. [DOI] [PubMed] [Google Scholar]
- 20.Korejo NA, Wei QW, Shan AH, et al. Effects of concomitant diabetes mellitus and hyperthyroidism on testicular and epididymal histoarchitecture and steroidogenesis in male animals. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2016;17(11):850–863. doi: 10.1631/jzus.B1600136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krentz AJ, Clough G, Byrne CD. Interactions between microvascular and macrovascular disease in diabetes: pathophysiology and therapeutic implications. Diabetes Obes Metab. 2007;9(6):781–791. doi: 10.1111/j.1463-1326.2007.00670.x. [DOI] [PubMed] [Google Scholar]
- 22.Li WL, Zheng HC, Bukuru J, et al. Natural medicines used in the traditional Chinese medical system for therapy of diabetes mellitus. J Ethnopharmacol. 2004;92(1):1–21. doi: 10.1016/j.jep.2003.12.031. [DOI] [PubMed] [Google Scholar]
- 23.Lü H, Chen J, Li WL, et al. Hypoglycemic and hypolipidemic effects of the total triterpene acid fraction from Folium Eriobotryae. J Ethnopharmacol. 2009;122(3):486–491. doi: 10.1016/j.jep.2009.01.030. [DOI] [PubMed] [Google Scholar]
- 24.Lü H, Chen J, Li WL, et al. Hypoglycemic effect of the total flavonoid fraction from Folium Eriobotryae . Phytomedicine. 2009;16(10):967–971. doi: 10.1016/j.phymed.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 25.Ong KW, Hsu A, Song LX, et al. Polyphenols-rich Vernonia amygdalina shows anti-diabetic effects in streptozotocin-induced diabetic rats. J Ethnopharmacol. 2011;133(2):598–607. doi: 10.1016/j.jep.2010.10.046. [DOI] [PubMed] [Google Scholar]
- 26.Patel DK, Prasad SK, Kumar R, et al. An overview on antidiabetic medicinal plants having insulin mimetic property. Asian Pacific J Trop Biomed. 2012;2(4):320–330. doi: 10.1016/S2221-1691(12)60032-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phillips DIW, Clark PM, Hales CN, et al. Understanding oral glucose tolerance: comparison of glucose or insulin measurements during the oral glucose tolerance test with specific measurements of insulin resistance and insulin secretion. Diabetic Med. 1994;11(3):286–292. doi: 10.1111/j.1464-5491.1994.tb00273.x. [DOI] [PubMed] [Google Scholar]
- 28.Qiu JY, Chen LL, Zhu QJ, et al. Screening natural antioxidants in peanut shell using DPPH-HPLC-DAD-TOF/MS methods. Food Chem. 2012;135(4):2366–2371. doi: 10.1016/j.foodchem.2012.07.042. [DOI] [PubMed] [Google Scholar]
- 29.Roden M, Bernroider E. Hepatic glucose metabolism in humans–its role in health and disease. Best Pract Res Clin Endocrinol Metab. 2003;17(3):365–383. doi: 10.1016/S1521-690X(03)00031-9. [DOI] [PubMed] [Google Scholar]
- 30.Russo D, Malafronte N, Frescura D, et al. Antioxidant activities and quali-quantitative analysis of different Smallanthus sonchifolius [(Poepp. and Endl.) H. Robinson] landrace extracts. Nat Prod Res. 2015;29(17):1673–1677. doi: 10.1080/14786419.2014.990906. [DOI] [PubMed] [Google Scholar]
- 31.Saini AK, Kumar HSA, Sharma SS. Preventive and curative effect of edaravone on nerve functions and oxidative stress in experimental diabetic neuropathy. Eur J Pharmacol. 2007;568(1-3):164–172. doi: 10.1016/j.ejphar.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 32.Sepici-Dincel A, Açıkgöz Ş, Çevik C, et al. Effects of in vivo antioxidant enzyme activities of myrtle oil in normoglycaemic and alloxan diabetic rabbits. J Ethnopharmacol. 2007;110(3):498–503. doi: 10.1016/j.jep.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 33.Sharma SB, Nasir A, Prabhu KM, et al. Antihyperglycemic effect of the fruit-pulp of Eugenia jambolana in experimental diabetes mellitus. J Ethnopharmacol. 2006;104(3):367–373. doi: 10.1016/j.jep.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 34.Shobana S, Sreerama YN, Malleshi NG. Composition and enzyme inhibitory properties of finger millet (Eleusine coracana L.) seed coat phenolics: mode of inhibition of α-glucosidase and pancreatic amylase. Food Chem. 2009;115(4):1268–1273. doi: 10.1016/j.foodchem.2009.01.042. [DOI] [Google Scholar]
- 35.Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106(2):171–176. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Srivastava Y, Venkatakrishna-Bhatt H, Verma Y, et al. Antidiabetic and adaptogenic properties of Momordica charantia extract: an experimental and clinical evaluation. Phytother Res. 1993;7(4):285–289. doi: 10.1002/ptr.2650070405. [DOI] [Google Scholar]
- 37.Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: principles of pathogenesis and therapy. Lancet. 1900;365(9467):1333–1346. doi: 10.1016/S0140-6736(05)61032-X. [DOI] [PubMed] [Google Scholar]
- 38.Stumvoll M, Mitrakou A, Pimenta W, et al. Use of the oral glucose tolerance test to assess insulin release and insulin sensitivity. Diabetes Care. 2000;23(3):295–301. doi: 10.2337/diacare.23.3.295. [DOI] [PubMed] [Google Scholar]
- 39.Torres-Piedra M, Ortiz-Andrade R, Villalobos-Molina R, et al. A comparative study of flavonoid analogues on streptozotocin-nicotinamide induced diabetic rats: quercetin as a potential antidiabetic agent acting via 11β-hydroxysteroid dehydrogenase type 1 inhibition. Eur J Med Chem. 2010;45(6):2606–2612. doi: 10.1016/j.ejmech.2010.02.049. [DOI] [PubMed] [Google Scholar]
- 40.Upadhyay G, Gupta SO, Singh MP. Pyrogallol-mediated toxicity and natural antioxidants: triumphs and pitfalls of preclinical findings and their translational limitations. Chem-Biol Interact. 2010;183(3):333–340. doi: 10.1016/j.cbi.2009.11.028. [DOI] [PubMed] [Google Scholar]
- 41.Wang LL, Duan GL, Lu Y, et al. The effect of simvastatin on glucose homeostasis in streptozotocin induced type 2 diabetic rats. J Diabetes Res, 2013:274986. 2013 doi: 10.1155/2013/274986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Y, Xin X, Jin ZD, et al. Anti-diabetic effects of pentamethylquercetin in neonatally streptozotocin-induced diabetic rats. Eur J Pharmacol. 2011;668(1-2):347–353. doi: 10.1016/j.ejphar.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 43.Yadav N, Morris G, Harding SE, et al. Various non-injectable delivery systems for the treatment of diabetes mellitus. Endocr Metab Immune Disord Drug Targets. 2009;9(1):1–13. doi: 10.2174/187153009787582405. [DOI] [PubMed] [Google Scholar]
- 44.Yeh PT, Huang HW, Yang CM, et al. Astaxanthin inhibits expression of retinal oxidative stress and inflammatory mediators in streptozotocin-induced diabetic rats. PLoS ONE. 2016;11(1):e0146438. doi: 10.1371/journal.pone.0146438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yen GC, Duh PD. Scavenging effect of methanolic extracts of peanut hulls on free-radical and active-oxygen species. J Agric Food Chem. 1994;42(3):629–632. doi: 10.1021/jf00039a005. [DOI] [Google Scholar]
- 46.Yu YL, Gao F, Deng XM, et al. Inhibitory effect of polyphenol extracts from peanut shells on the activity of pancreatic α-amylase activity in vitro. J Food Agric Environ. 2013;11(2):38–42. [Google Scholar]
- 47.Zhang GW, Hu MM, He L, et al. Optimization of microwave-assisted enzymatic extraction of polyphenols from waste peanut shells and evaluation of its antioxidant and antibacterial activities in vitro . Food Bioprod Process. 2013;91(2):158–168. doi: 10.1016/j.fbp.2012.09.003. [DOI] [Google Scholar]
- 48.Zhang SW, Liu L, Su YL, et al. Antioxidative activity of lactic acid bacteria in yogurt. Afr J Microbiol Res. 2011;5(29):5194–5201. doi: 10.5897/AJMR11.997. [DOI] [Google Scholar]
- 49.Zhang Y, Ren CJ, Lu GB, et al. Purification, characterization and anti-diabetic activity of a polysaccharide from mulberry leaf. Regul Toxicol Pharm. 2014;70(3):687–695. doi: 10.1016/j.yrtph.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 50.Zizkova P, Stefek M, Rackova L, et al. Novel quercetin derivatives: from redox properties to promising treatment of oxidative stress related diseases. Chem-Biol Interact. 2017;265:36–46. doi: 10.1016/j.cbi.2017.01.019. [DOI] [PubMed] [Google Scholar]