Abstract
Chlortetracycline (CTC), one kind of common antibiotic for prevention and treatment of various diseases, also exhibits good performance in accelerating the growth of livestock. Macleaya cordata, a traditional Chinese medicine, is usually used as a natural additive in livestock because of its anti-microbial, anti-fungal, anti-inflammatory, and pesticidal activity. In this work, we studied whether M. cordata helps regulate the growth-promoting effect of CTC on broiler chickens. It is demonstrated that M. cordata improves the growth-promoting effect of CTC on growth performance indices of broiler chickens, such as survival rate, daily weight, and feed to weight rate. M. cordata also delays the maximum of CTC residues in plasma. It may depend on the higher values of operational taxonomic unit (OTU) and the indices of α diversity driven by simultaneous use of CTC and M. cordata.
Keywords: Chlortetracycline, Macleaya cordata, Broiler chicken, Growth promotion, Gut flora
1 Introduction
Chlortetracycline (CTC), one kind of tetracyline (Chopra and Roberts, 2001; Motamedi et al., 2010; Nelson et al., 2011), is widely used in the production feeding of livestock. As is known, CTC can not only prevent diseases through the inhibition of protein synthesis in the microorganism (Aarestrup et al., 1998; de Ruyck et al., 1999; Salama et al., 2011; Milbradt et al., 2014), but also accelerate the growth of simple-stomach animals, such as swine and broiler chickens (Kilroy et al., 1990). Several studies have demonstrated that changes of intestinal microbiota induced by CTC are central to growth promotion (Gaskins et al., 2002; Dibner and Richards, 2005). Kim et al. (2012) and Looft et al. (2014) have reported that usage of CTC influences the gut flora of swine, which is correlative with weight change. Unno et al. (2015) have reported that CTC significantly accretes the richness of gut microbes during the post-weaning period (four weeks old) after the stabilization of gut microbiota.
Macleaya cordata, a traditional Chinese medicine, is composed of a variety of active alkaloids such as sanguinarine and chelerythrine (Kosina et al., 2010; Shi et al., 2015), and is usually used as a natural additive in livestock because of its properties, including anti-microbial, anti-fungal, anti-inflammatory, and pesticidal activity (Walterová et al., 1995; Newman et al., 1999; Pang et al., 2005; Psotova et al., 2006; Kosina et al., 2010; Kantas et al., 2015). Kantas et al. (2015) have reported the beneficial effects of M. cordata on growth-promoting performance in weaning pigs. A similar result has also been gained by Khadem et al. (2014) in chickens.
The changes of growth level, CTC residue, and gut microbiota were studied in this work, when M. cordata was added. As far as we know, this study is the first to investigate the growth-promoting effect on broiler chickens by simultaneous application of CTC and M. cordata. This should be helpful for guidance in feeding.
2 Materials and methods
2.1 Chemicals and materials
CTC premix and M. cordata were provided from the Central China Charoen Pokphand Group (Zhumadian, China). Sulfuric acid (H2SO4), sodium tungstate (Na2WO4), and other chemicals were obtained from the Nanjing Chemical Agent Company, China and were used without further purification.
Mcllvaine buffer was prepared by mixing 0.1 mol/L citric acid and 0.2 mol/L NaH2PO4 (8:5 (v/v), pH 4.0). Sodium ethylenediamine tetraacetic acid (Na2EDTA)-Mcllvaine buffer (0.1 mol/L) was prepared by dissolving 37.23 g of Na2EDTA in Mcllvaine buffer and diluting to 1 L. All solutions, including 12.5 mol/L H2SO4 and 0.3 mol/L Na2WO4, were prepared by double-distilled water (ddH2O), which was purified with a Milli-Q purification system (Branstead, USA) to a resistance of 18.2 MΩ∙cm and stored at 4 °C.
2.2 Animals and group design
Seven hundred and twenty (360 males and 360 females) healthy 7-d-old broiler chickens were randomly divided into three groups, a control group (CT) and two experimental groups (T1 and T2). Broiler chickens in each group eat and drank freely, and all drugs were added by mixing in feed. CTC premix (50 mg/kg) was added to T1, which was replaced by 50 mg/kg CTC premix and 50 mg/kg M. cordata in T2. After a four-week feeding, there was a 5-d drug cessation.
2.3 Analysis of growth performance indices
Growth performance data, including death number, feed intake, and weights of ten stochastic chickens, were recorded weekly. Growth performance indices were calculated based on the above data, and the calculation formulae were as follows: survival rate=(sum−fatality)/total sum; feed to gain rate=feed intake/weight gain.
2.4 Detection of CTC in plasma by HPLC-MS/MS
Plasma samples at the 0, 7th, 14th, 21st, and 28th days during the four-week feeding were collected and purified based on the following procedure before high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS). Blood samples (750 µl) were first added into an equivalent volume of acetonitrile and centrifuged at 12 000 r/min for 25 min at 4 °C. Then, 1 ml of supernatant was collected and filtrated by 0.22 µm microporous membrane for Agilent 1290N-AB550 Qtrap HPLC-MS/MS (Agilent, USA) analysis using a 3-µm particle size Waters Atlantis dC18 column (3.0 mm×150 nm). The mobile phase is dynamic and is listed in Table S1. Briefly, the mobile phase is a combination of 0.2% (v/v) methyl alcohol (A) and acetonitrile (B) according to corresponding ratios (A:B) at different time points. Every new cycle consisted of 10 min. The ratio of A to B was 9:1 at the first 5 min. Then, it changed into 1:1 at the 6th minute. At last 4 min, the ratio turned back into 9:1. Primary parameters are listed in Tables S2,S3,S4.
2.5 Detection of CTC in tissues by HPLC
Muscle, kidney, fat, and liver were collected at the 0, 3rd, and 5th days of the drug cessation period and purified by the following procedure before HPLC. First, 2 g of tissues were homogenized with a mixed solution containing 2 ml of 12.5 mol/L H2SO4, 2 ml of 0.3 mol/L Na2WO4, and 8 ml of Na2EDTA-Mcllvaine buffer for 10 min, followed by centrifugation at 12 000 r/min for 10 min at 4 °C. Then, 5 ml of supernatant was collected and purified by Waters Oasis HLB solid phase Extraction Cartridge (Waters, USA), which was previously treated with 5 ml each of methanol and ddH2O. The cartridge was washed with ddH2O and methanol after completion of above supernatant filtration, and then aspirated by aurilave. After that, CTC attached on cartridges was eluted with 8 ml of methyl alcohol and concentrated in DC12H Termovap Sample Concentrator (Anpel Technology, China). After dissolution with 1 ml of 20% (v/v) methyl alcohol and filtration by 0.22 µm microporous membrane, CTC solution was prepared for Agilent 1260 HPLC (Agilent, USA) analysis, and the concentration was calculated by the standard linear equation.
2.6 Pathological section and staining of duodenums
Duodenums of chickens in each group were first collected at the 0, 3rd, and 5th days of the drug cessation period. After being fixed with formalin, tissues were trimmed and flushed by water for at least 4 h, and then dehydrated with alcohol solutions of different concentrations ranging from 75% to 100% with a 5% increase for about 1 h at each alcohol solution. After being soaked in xylene for 90 s and waxed for 3 h at 60 °C, tissues were cut into slices with a thickness of 5 µm. After being parched for 3 h at 60 °C, the slices were stained by hematoxylin and eosin (H&E) (Gu et al., 2017).
2.7 DNA extraction, PCR, and sequencing
DNA of duodenum contents was extracted using the TIANamp Stool DNA Kit (TianGen Biotech, China). The DNA quality was determined by 0.8% agarose gel electrophoresis and RS232G spectrophotometer (Eppendorf, Germany).
The V4 region of the bacterial 16S ribosomal RNAs (rRNA) gene was amplified by polymerase chain reaction (PCR). The sequences of primers were as follows: forward: 5'-GCACCTAAYTGGGYDTA AGNG-3'; reverse: 5'-TACNVGGGTATCTAATCC-3'. The barcode of the forward primer was a seven-base oligonucleotide which was used for distinguishing samples from one library. The PCR kit (Biolabs, New England) consisted of Q5 high-fidelity DNA polymerase, 5×reaction buffer, and 5×high glycine carbonate (GC) buffer. In a typical PCR system, 0.25 μl of Q5 high-fidelity DNA polymerase, 5 μl of 5×reaction buffer, 5 μl of 5×high GC buffer, 1 μl of 10 μmol/L each primer, 0.5 μl of 10 mmol/L deoxyribonucleoside triphosphate (dNTP), and 1 μl of template DNA were mixed together to a final volume of 25 μl with ddH2O. After undergoing the following reaction condition, thus 98 °C for 30 s, 25 cycles of 98 °C for 15 s, 50 °C for 30 s and 72 °C for 30 s, and a final extension at 72 °C for 5 min, PCR products were extracted from 2% agarose gels and purified by the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, USA). Finally, the extracted PCR products were quantified by Quant-iT PicoGreen dsDNA Assay Kit (Invitrogen, UK) and FLx Microplate reader (Biotek, USA).
The library construction was carried out using TruSeq Nano DNA LT Library Prep Kit (Illumina, USA). Previously, the ends of genes were repaired by the above kit according to the instructions. After construction, the quality of library was determined by Agilent Bioanalyzer 2100 and Agilent High Sensitivity DNA Kit (Agilent Technologies, USA). According to requirements, the library was diluted to 2 nmol/L and denatured to a single chain with 0.1 mol/L NaOH. Finally, the mixed library was sequenced (double-end, 2×300 bp) by MiSeq Reagent Kit V3 (600 cycles; Illumina, USA) and the concentration controlled between 15 and 18 pmol/L.
2.8 Statistical analyses
All statistical procedures were analyzed using statistical software SPSS 19.0. Data were expressed as mean±standard error of the mean (SEM). Differences were evaluated by one-way analysis of variance (ANOVA) followed by post-hoc tests. Differences were considered significant at P<0.05.
3 Results
3.1 Growth performance indices of broiler chickens
Growth performance indices of each group, including survival rate, daily gain, and feed to weight rate, are displayed in Table 1. The survival rate from 7 to 42 d old chickens in T2 (86.18%) was higher than those of other two groups, i.e. CT (58.21%) and T1 (75.34%). The daily gain and feed to weight rate of broiler chickens in T2 also displayed the best growth performance by comparison with other groups. It is indicated that simultaneous treatment of CTC and M. cordata effectively improves the growth performance of broiler chickens.
Table 1.
Growth performance indexes of broiler chickens
| Group | Survival rate (%) | Daily gain (g) | Feed to gain rate |
| CT | 58.21 | ||
| The 1st week | 30.39 | 1.128 | |
| The 2nd week | 44.98 | 1.395 | |
| The 3rd week | 49.67 | 1.570 | |
| The 4th week | 49.75 | 1.705 | |
| The 5th week | 72.19 | 1.801 | |
| 1–5 weeks | 49.40 | 1.520 | |
| T1 | 75.34 | ||
| The 1st week | 32.96 | 1.094 | |
| The 2nd week | 46.80 | 1.332 | |
| The 3rd week | 63.56 | 1.481 | |
| The 4th week | 60.34 | 1.638 | |
| The 5th week | 85.13 | 1.664 | |
| 1–5 weeks | 57.76 | 1.442 | |
| T2 | 86.18 | ||
| The 1st week | 34.39 | 1.079 | |
| The 2nd week | 50.51 | 1.297 | |
| The 3rd week | 65.96 | 1.458 | |
| The 4th week | 65.34 | 1.633 | |
| The 5th week | 90.41 | 1.652 | |
| 1–5 weeks | 61.32 | 1.424 |
Values are expressed as mean of triplicate experiments for survival rate of 7–42 d old chickens, and mean of ten times for daily gain and feed to gain rate. CT: control; T1: Experiment 1 (50 mg/kg CTC premix); T2: Experiment 2 (50 mg/kg CTC premix and 50 mg/kg M. cordata)
3.2 Detection of CTC residue in plasmas and tissues
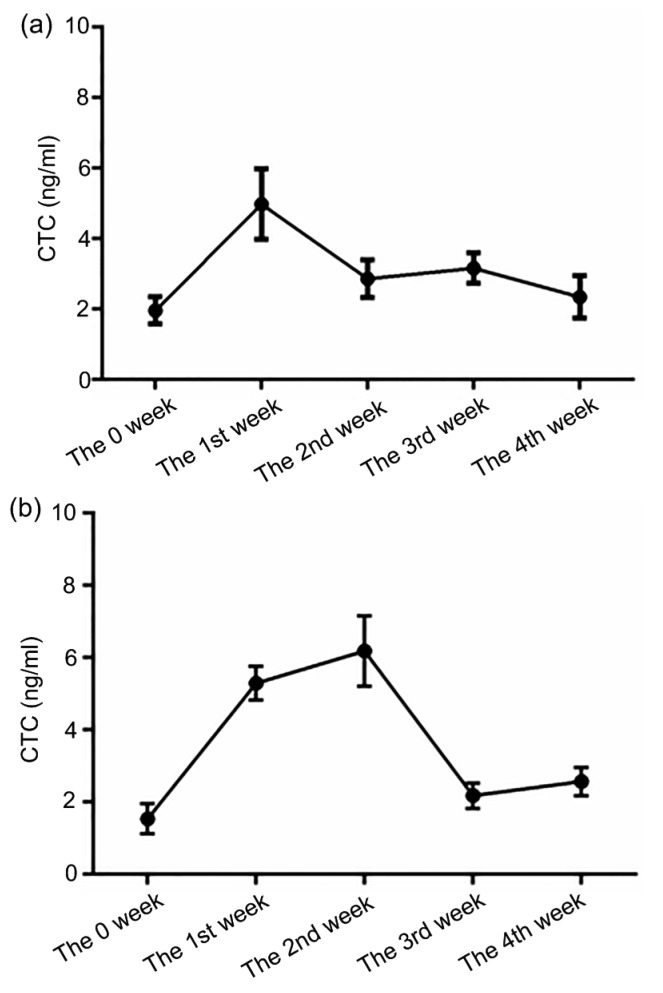
The CTC concentration in the blood was measured at the first day of each week i.e. the 0, 7th, 14th, 21st, and 28th days during the four-week feeding. Experimental results showed that the CTC concentration of the T1 group reached a maximum at the 1st week and the maximum of the T2 group was postponed to the 2nd week (Fig. 1). Then, CTC concentrations of both groups declined accompanied by continued feeding.
Fig. 1.
Chlortetracycline determination of plasma samples
During four-week feeding, CTC concentrations (ng/ml) at the 0, 7th, 14th, 21st, and 28th days in the T1 (a) and T2 (b) groups were determined by HPLC-MS/MS. Data are presented as the mean±SEM (n=5)
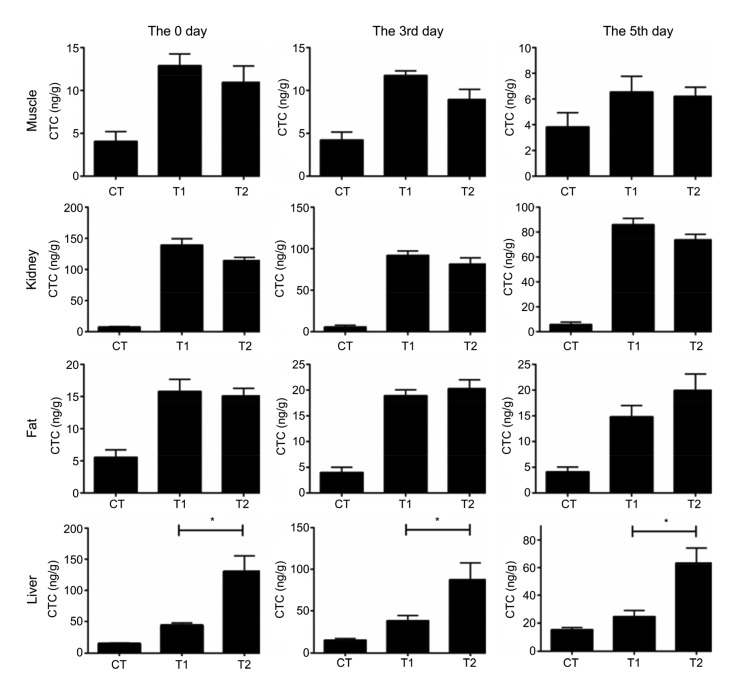
CTC concentrations of four tissues at the 0, 3rd, and 5th days during drug cessation were detected and calculated according to the standard linear equation (Fig.S1). CTC concentrations of T2 group were not significantly different from T1 in muscle, kidney, or fat, while the difference was significant in the liver. This indicated that M. cordata increased the CTC residues of liver (Fig. 2).
Fig. 2.
Chlortetracycline determination of tissue samples
After four-week feeding, muscle, kidney, fat, and liver samples were collected at the 0, 3rd, and 5th days during drug cession and CTC concentrations (ng/g) were determined by HPLC. CT: control; T1: Experiment 1 (50 mg/kg CTC premix); T2: Experiment 2 (50 mg/kg CTC premix and 50 mg/kg M. cordata). Data are presented as mean±SEM (n=5). * P<0.05, significantly different between the indicated groups
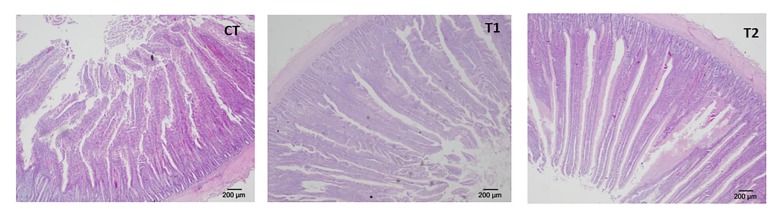
3.3 Pathological section of duodenums
Histomorphological sections of duodenums showed that the intestinal mucous membrane and villus construction structure in the experimental groups were integrated compared with CT (Fig. 3). Therefore, it could be concluded that CTC and M. cordata have little negative effect on the structure of the small intestine.
Fig. 3.
Small intestine pathological tissue slices
CT: control; T1: Experiment 1 (50 mg/kg CTC premix); T2: Experiment 2 (50 mg/kg CTC premix and 50 mg/kg M. cordata)
3.4 Analysis of gut flora
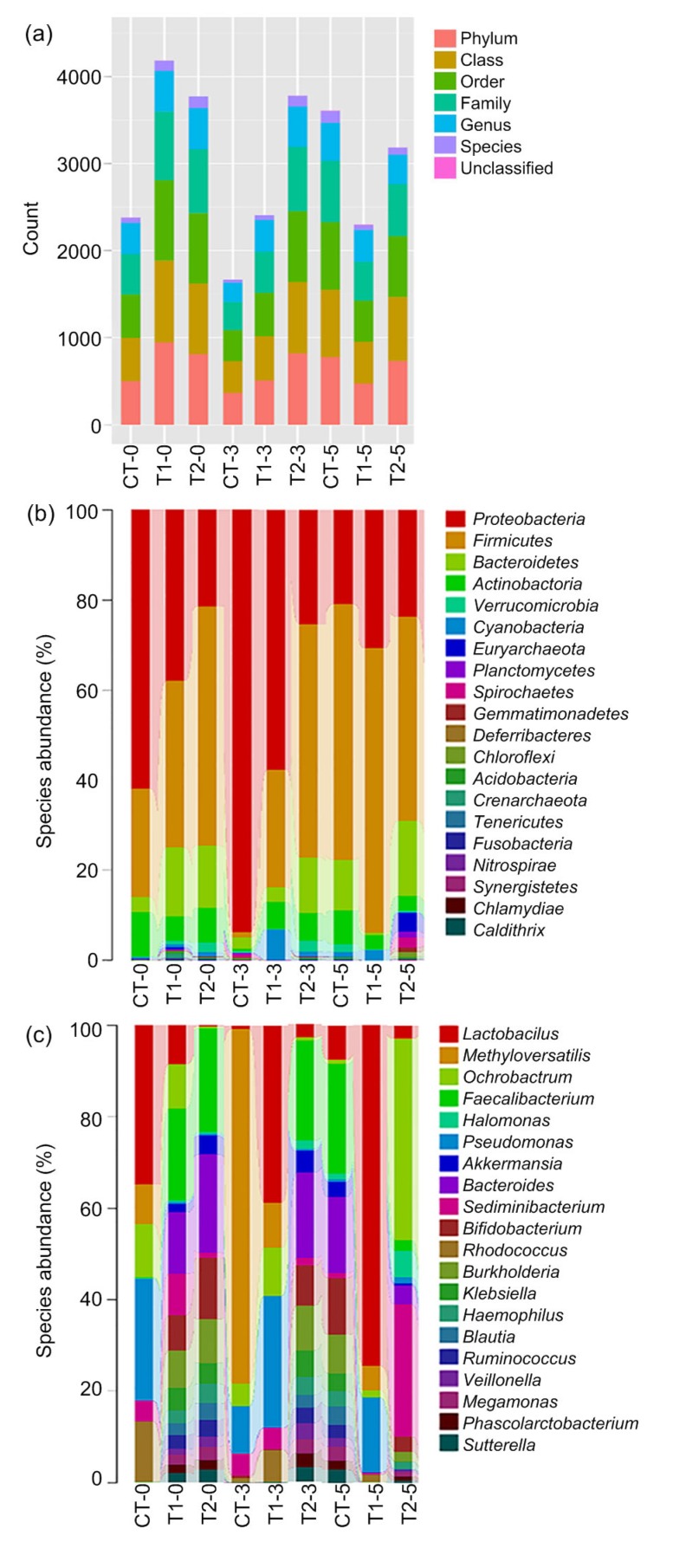
The numbers of operational taxonomic units (OTUs) in T1 and T2 were 4181 and 3987, respectively, at the 0 day of the drug cessation period, and both were much higher than that of CT (Fig. 4). OTUs at the 3rd day showed the same trends as the 0 day, but the value of OTU in T1 was lower than that in T2. However, from the value of OTUs at the 5th day, experimental groups had a decreasing tendency compared with CT. As for the 3rd day, the OTU of T1 was lower than that of T2.
Fig. 4.
Gut flora sequencing
Small intestine was collected at the 0 (CT-0, T1-0, and T2-0), 3rd (CT-3, T1-3, and T2-3), and 5th (CT-5, T1-5, and T2-5) days after drug to microbial sequencing. (a) OTU; (b) Genus level; (c) Phylum level. CT: control; T1: Experiment 1 (50 mg/kg CTC premix); T2: Experiment 2 (50 mg/kg CTC premix and 50 mg/kg M. cordata)
Alpha diversity reflecting the diversity of samples has been widely used in the analysis of gut flora. The indices of α diversity involved Chao1 and ACE and focused on community richness, as well as Shannon and Simpson which concentrated on evenness in one sample or site. The result showed that four indices of two experimental groups were much larger than those of CT at the 0 day of drug cessation (Table 2). Four α diversity indices at the 3rd day in T1 and T2 had an increasing tendency compared with CT, and the latter was larger. At the 5th day, those indices of T1 had a decreasing tendency compared with CT, while no obvious change was observed between T2 and CT. The above results of α diversity were coincident with the analysis of OTU.
Table 2.
Alpha diversity analysis
| Group | Chao1 | ACE | Simpson | Shannon |
| The 0 day | ||||
| CT | 405 | 448.211 479 2 | 0.798 423 671 | 3.957 269 039 |
| T1 | 686 | 790.230 189 9 | 0.946 040 868 | 6.468 898 868 |
| T2 | 565 | 669.565 936 5 | 0.973 247 532 | 6.423 953 528 |
| The 3rd day | ||||
| CT | 310 | 333.116 964 2 | 0.773 986 413 | 3.425 416 740 |
| T1 | 415 | 455.977 386 0 | 0.899 740 275 | 4.809 963 993 |
| T2 | 548 | 698.595 888 5 | 0.973 121 886 | 6.343 162 141 |
| The 5th day | ||||
| CT | 525 | 633.091 183 2 | 0.941 098 871 | 5.973 159 100 |
| T1 | 340 | 369.322 265 6 | 0.824 612 753 | 4.008 542 852 |
| T2 | 645 | 671.597 426 7 | 0.926 718 147 | 5.940 116 568 |
CT: control; T1: Experiment 1 (50 mg/kg CTC premix); T2: Experiment 2 (50 mg/kg CTC premix and 50 mg/kg M. cordata)
The taxonomic composition analysis of the flora gut (Figs. 4b and 4c) showed that CTC causes the changes at phylum and gene levels of the intestinal bacteria community. There was a significant increase of Firmicutes and Bacteroidetes in T1 and T2 at the phylum level. The kinds of genus composition of intestinal flora in T1 and T2 were more diversified, such as the higher proportion of Bacteroides, Bifidobacterium, Burkholderia, and Faecalibacterium.
4 Discussion
It is well-known that CTC, one kind of antibiotic, promotes the growth of broilers (Anadón et al., 2012). M. cordata, a traditional Chinese medicine possessing analgesic and antiedemic properties (Zdarilova et al., 2008), also promotes growth for broilers (Khadem et al., 2014). The effect of growth by simultaneous use of CTC and M. cordata in broilers is studied in this work. Experimental results show that CTC clearly improves the growth level of broilers, and it is worth noting that the promoting-growth effect of simultaneous use of CTC and M. cordata is superior to that of CTC. Despite the survival rate, daily gain and feed to gain rate at the 4th week are inferior to normal levels because of two diseases (Escherichia coli-influenced enteritis and respiratory infection). The same conclusion still can be found by comparison of the results of all the groups (Table 2). It proves that simultaneous use of CTC and M. cordata possesses an overlaying effect of growth-promotion, which is instructive for the application of CTC.
Results of CTC concentrations in blood indicate that M. cordata slowly down-regulates the metabolism of CTC. In addition, CTC concentration in tissues during the drug cessation period is less than the maximal residue limits (100 µg/kg for muscle, 600 µg/kg for kidney, and 300 µg/kg for liver) (Anadón et al., 2012). CTC is continuously transferred from kidney and muscle to liver during the drug cessation period, because liver is the main metabolic organ leading to the increase of liver residue and the light decrease of CTC residues in other tissues. With the addition of M. cordata, CTC concentration in kidney and muscle decreases after four-week feeding. It indicates that M. cordata helps reduce CTC residues of muscle and kidney by accelerating the metabolism of CTC in the liver.
The intestinal microorganism is a significant factor in several biological functions, for example, the providing of nutriment and prevention of diseases (Mestecky and McGhee, 1987; Xing et al., 2005; Cerf-Bensussan and Gaboriau-Routhiau, 2010; Gerritsen et al., 2011; Zackular et al., 2013; Nie et al., 2015). Many studies have reported that CTC promotes growth of livestock via the regulation of gut microbiota (Kim et al., 2012; Looft et al., 2014; Unno et al., 2015). The gut flora of the duodenum that connects to the stomach is closely related to energy and nutrient absorption. OTU and α diversity analysis results indicate that CTC and M. cordata promote the growth of chicken via regulating the diversiform of gut flora in the duodenum, such as the improvement of a high proportion of Bacteroides, Faecalibacterium, and Bifidobacteria (Fig. 4 and Table 2). This conclusion is in good agreement with a previous report that high bacterial diversity is favorable to the health and productivity of animals (Hildebrand et al., 2013). It is known that Bacteroides can not only generate short-chain fatty acid to provide energy for hosts (Hooper et al., 2002), but also enhance gut mucosal immunity and defense against pathogens in gut colonization (Hooper et al., 2003; Rhee et al., 2004; Mazmanian and Kasper, 2006). Faecalibacterium regulates anti-inflammation pathway of the Clostridium leptum group (Pryde et al., 2002; Shen et al., 2006; Sokol et al., 2008), and Bifidobacteria enhances immunity to resist a pathogen (Ouwehand et al., 2002; Ventura et al., 2007; Russell et al., 2011). The better understanding of the growth-promoting mechanism, to some extent, has a significant role in seeking alternatives to antibiotics. The simultaneous use of CTC and M. cordata produces a better promoting-growth effect via the improvement of high proportion of Bacteroides, Faecalibacterium, and Bifidobacteria, which provides a more advisable use of CTC in chickens.
List of electronic supplementary materials
Mobile phase of HPLC-MS/MS
Chromatographic parameters of HPLC-MS/MS
Mass spectrometry parameters of HPLC-MS/MS
Mass spectrometry parameters of qualitative and quantitative ion pair in HPLC-MS/MS
Standard curve and peak figures
Footnotes
Project supported by the Natural Science Foundation of Jiangsu Province for Excellent Young Scholars (No. BK20170087) and the National Natural Science Foundation of China (Nos. 31502033, 31472164, and 31672515)
Electronic supplementary materials: The online version of this article (https://doi.org/10.1631/jzus.B1700435) contains supplementary materials, which are available to authorized users
Contributors: Bin LI, Jin-qiu ZHANG, Xian-gan HAN, and Zheng-lei WANG performed the experiments. Bin LI wrote the manuscript and prepared the figures. Yuan-yuan XU and Jin-feng MIAO conceived the project.
Compliance with ethics guidelines: Bin LI, Jin-qiu ZHANG, Xian-gan HAN, Zheng-lei WANG, Yuan-yuan XU, and Jin-feng MIAO declare that they have no conflict of interests.
All institutional and national guidelines for the care and use of laboratory animals were followed.
References
- 1.Aarestrup FM, Bager F, Jensen NE, et al. Resistance to antimicrobial agents used for animal therapy in pathogenic-, zoonotic- and indicator bacteria isolated from different food animals in Denmark: a baseline study for the Danish Integrated Antimicrobial Resistance Monitoring Programme (DANMAP) APMIS. 1998;106(7-12):745–770. doi: 10.1111/j.1699-0463.1998.tb00222.x. [DOI] [PubMed] [Google Scholar]
- 2.Anadón A, Gamboa F, Martínez MA, et al. Plasma disposition and tissue depletion of chlortetracycline in the food producing animals, chickens for fattening. Food Chem Toxicol. 2012;50(8):2714–2721. doi: 10.1016/j.fct.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 3.Cerf-Bensussan N, Gaboriau-Routhiau V. The immune system and the gut microbiota: friends or foes? Nat Rev Immunol. 2010;10(10):735–744. doi: 10.1038/nri2850. [DOI] [PubMed] [Google Scholar]
- 4.Chopra I, Roberts M. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol Mol Biol Rev. 2001;65(2):232–260. doi: 10.1128/MMBR.65.2.232-260.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Ruyck H, de Ridder H, van Renterghem R, et al. Validation of HPLC method of analysis of tetracycline residues in eggs and broiler meat and its application to a feeding trial. Food Addit Contam. 1999;16(2):47–56. doi: 10.1080/026520399284190. [DOI] [PubMed] [Google Scholar]
- 6.Dibner JJ, Richards JD. Antibiotic growth promoters in agriculture: history and mode of action. Poultry Sci. 2005;84(4):634–643. doi: 10.1093/ps/84.4.634. [DOI] [PubMed] [Google Scholar]
- 7.Gaskins HR, Collier CT, Anderson DB. Antibiotics as growth promotants: mode of action. Anim Biotechnol. 2002;13(1):29–42. doi: 10.1081/ABIO-120005768. [DOI] [PubMed] [Google Scholar]
- 8.Gerritsen J, Smidt H, Rijkers GT, et al. Intestinal microbiota in human health and disease: the impact of probiotics. Genes Nutr. 2011;6(3):209–240. doi: 10.1007/s12263-011-0229-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gu N, Ge KL, Hao C, et al. Neuregulin1β effects on brain tissue via ERK5-dependent MAPK pathway in a rat model of cerebral ischemia-reperfusion injury. J Mol Neurosci. 2017;61(4):607–616. doi: 10.1007/s12031-017-0902-4. [DOI] [PubMed] [Google Scholar]
- 10.Hildebrand F, Nguyen TLA, Brinkman B, et al. Inflammation-associated enterotypes, host genotype, cage and inter-individual effects drive gut microbiota variation in common laboratory mice. Genome Biol. 2013;14(1):R4. doi: 10.1186/gb-2013-14-1-r4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hooper LV, Midtvedt T, Gordon JI. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annu Rev Nutr. 2002;22:283–307. doi: 10.1146/annurev.nutr.22.011602.092259. [DOI] [PubMed] [Google Scholar]
- 12.Hooper LV, Stappenbeck TS, Hong CV, et al. Angiogenins: a new class of microbicidal proteins involved in innate immunity. Nat Immunol. 2003;4(3):269–273. doi: 10.1038/ni888. [DOI] [PubMed] [Google Scholar]
- 13.Kantas D, Papatsiros VG, Tassis PD, et al. The effect of a natural feed additive (Macleaya cordata), containing sanguinarine, on the performance and health status of weaning pigs. Anim Sci J. 2015;86(1):92–98. doi: 10.1111/asj.12240. [DOI] [PubMed] [Google Scholar]
- 14.Khadem A, Soler L, Everaert N, et al. Growth promotion in broilers by both oxytetracycline and Macleaya cordata extract is based on their anti-inflammatory properties. Brit J Nutr. 2014;112(7):1110–1118. doi: 10.1017/S0007114514001871. [DOI] [PubMed] [Google Scholar]
- 15.Kilroy CR, Hall WF, Bane DP, et al. Chlortetracycline in swine-bioavailability and pharmacokinetics in fasted and fed pigs. J Vet Pharmacol Ther. 1990;13(1):49–58. doi: 10.1111/j.1365-2885.1990.tb00747.x. [DOI] [PubMed] [Google Scholar]
- 16.Kim HB, Borewicz K, White BA, et al. Microbial shifts in the swine distal gut in response to the treatment with antimicrobial growth promoter, tylosin. Proc Natl Acad Sci USA. 2012;109(38):15485–15490. doi: 10.1073/pnas.1205147109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kosina P, Gregorova J, Gruz J, et al. Phytochemical and antimicrobial characterization of Macleaya cordata herb. Fitoterapia. 2010;81(8):1006–1012. doi: 10.1016/j.fitote.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 18.Looft T, Allen HK, Casey TA, et al. Carbadox has both temporary and lasting effects on the swine gut microbiota. Front Microbiol, 5:276. 2014 doi: 10.3389/fmicb.2014.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mazmanian SK, Kasper DL. The love-hate relationship between bacterial polysaccharides and the host immune system. Nat Rev Immunol. 2006;6(11):849–858. doi: 10.1038/nri1956. [DOI] [PubMed] [Google Scholar]
- 20.Mestecky J, McGhee JR. Immunoglobulin a (IgA): molecular and cellular interactions involved in IgA biosynthesis and immune response. Adv Immunol. 1987;40:153–245. doi: 10.1016/S0065-2776(08)60240-0. [DOI] [PubMed] [Google Scholar]
- 21.Milbradt EL, Okamoto AS, Rodrigues JCZ, et al. Use of organic acids and competitive exclusion product as an alternative to antibiotic as a growth promoter in the raising of commercial turkeys. Poultry Sci. 2014;93(7):1855–1861. doi: 10.3382/ps.2013-03593. [DOI] [PubMed] [Google Scholar]
- 22.Motamedi H, Darabpour E, Gholipour M, et al. In vitro assay for the anti-brucella activity of medicinal plants against tetracycline-resistant Brucella melitensis . J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2010;11(7):506–511. doi: 10.1631/jzus.B0900365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nelson KL, Brözel VS, Gibson SA, et al. Influence of manure from pigs fed chlortetracycline as growth promotant on soil microbial community structure. World J Microb Biot. 2011;27(3):659–668. doi: 10.1007/s11274-010-0504-6. [DOI] [Google Scholar]
- 24.Newman SE, Roll MJ, Harkrader RJ. A naturally occurring compound for controlling powdery mildew of greenhouse roses. Hortscience. 1999;34(4):686–689. [Google Scholar]
- 25.Nie YF, Hu J, Yan XH. Cross-talk between bile acids and intestinal microbiota in host metabolism and health. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2015;16(6):436–446. doi: 10.1631/jzus.B1400327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ouwehand AC, Salminen S, Isolauri E. Probiotics: an overview of beneficial effects. Antonie van Leeuwenhoek. 2002;82(1-4):279–289. doi: 10.1023/A:1020620607611. [DOI] [PubMed] [Google Scholar]
- 27.Pang JX, Ma RQ, Liu LM, et al. Total alkaloid of Macleaya cordata: in vitro cytotoxic effect on Hep3B cells and in vivo antitumor effect in mice. J First Mil Med Univ. 2005;25(3):325–328. doi: 10.3321/j.issn:1673-4254.2005.03.018. (in Chinese) [DOI] [PubMed] [Google Scholar]
- 28.Pryde SE, Duncan SH, Hold GL, et al. The microbiology of butyrate formation in the human colon. FEMS Microbiol Lett. 2002;217(2):133–139. doi: 10.1111/j.1574-6968.2002.tb11467.x. [DOI] [PubMed] [Google Scholar]
- 29.Psotova J, Vecera R, Zdarilova A, et al. Safety assessment of sanguiritrin, alkaloid fraction of Macleaya cordata, in rats. Vet Med. 2006;51(4):145–155. doi: 10.17221/5534-VETMED. [DOI] [Google Scholar]
- 30.Rhee KJ, Sethupathi P, Driks A, et al. Role of commensal bacteria in development of gut-associated lymphoid tissues and preimmune antibody repertoire. J Immunol. 2004;172(2):1118–1124. doi: 10.4049/jimmunol.172.2.1118. [DOI] [PubMed] [Google Scholar]
- 31.Russell DA, Ross RP, Fitzgerald GF, et al. Metabolic activities and probiotic potential of bifidobacteria. Int J Food Microbol. 2011;149(1):88–105. doi: 10.1016/j.ijfoodmicro.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 32.Salama NA, Abou-Raya SH, Shalaby AR, et al. Incidence of tetracycline residues in chicken meat and liver retailed to consumers. Food Addit Contam B. 2011;4(2):88–93. doi: 10.1080/19393210.2011.585245. [DOI] [PubMed] [Google Scholar]
- 33.Shen J, Zhang BR, Wei GF, et al. Molecular profiling of the Clostridium leptum subgroup in human fecal microflora by PCR-denaturing gradient gel electrophoresis and clone library analysis. Appl Environ Microbol. 2006;72(8):5232–5238. doi: 10.1128/AEM.00151-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi YB, Liu L, Shao W, et al. Microcalorimetry studies of the antimicrobial actions of Aconitum alkaloids. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2015;16(8):690–695. doi: 10.1631/jzus.B1500121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sokol H, Pigneur B, Watterlot L, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci USA. 2008;105(43):16731–16736. doi: 10.1073/pnas.0804812105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Unno T, Kim J, Guevarra RB, et al. Effects of antibiotic growth promoter and characterization of ecological succession in swine gut microbiota. J Microbol Biotechnol. 2015;25(4):431–438. doi: 10.4014/jmb.1408.08063. [DOI] [PubMed] [Google Scholar]
- 37.Ventura M, Canchaya C, Tauch A, et al. Genomics of Actinobacteria: tracing the evolutionary history of an ancient phylura. Microbol Mol Biol Rev. 2007;71(3):495–548. doi: 10.1128/MMBR.00005-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walterová D, Ulrichová J, Válka I, et al. Benzo[c] phenanthridine alkaloids sanguinarine and chelerythrine: biological activities and dental care applications. Acta Univ Palacki Olomuc Fac Med. 1995;139:7–16. [PubMed] [Google Scholar]
- 39.Xing HC, Li LJ, Xu KJ, et al. Intestinal microflora in rats with ischemia/reperfusion liver injury. J Zhejiang Univ SCI. 2005;6B(1):14–21. doi: 10.1631/jzus.2005.B0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zackular JP, Baxter NT, Iverson KD, et al. The gut microbiome modulates colon tumorigenesis. mBio. 2013;4(6):e00692–13. doi: 10.1128/mBio.00692-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zdarilova A, Vrublova E, Vostalova J, et al. Natural feed additive of Macleaya cordata: safety assessment in rats a 90-day feeding experiment. Food Chem Toxicol. 2008;46(12):3721–3726. doi: 10.1016/j.fct.2008.09.054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mobile phase of HPLC-MS/MS
Chromatographic parameters of HPLC-MS/MS
Mass spectrometry parameters of HPLC-MS/MS
Mass spectrometry parameters of qualitative and quantitative ion pair in HPLC-MS/MS
Standard curve and peak figures