Abstract
This case series documents three patients referred to the Intensive Dietary Management clinic in Toronto, Canada, for insulin-dependent type 2 diabetes. It demonstrates the effectiveness of therapeutic fasting to reverse their insulin resistance, resulting in cessation of insulin therapy while maintaining control of their blood sugars. In addition, these patients were also able to lose significant amounts of body weight, reduce their waist circumference and also reduce their glycated haemoglobin level.
Keywords: diet, obesity (nutrition), metabolic disorders, diabetes, endocrine system
Background
Type 2 diabetes (T2D) is a chronic disease closely linked to the epidemic of obesity that requires long-term medical attention to limit the development of its wide range of microvascular, macrovascular and neuropathic complications. Many of these complications arise from the combination of resistance to insulin action, inadequate insulin secretion, and excessive or inappropriate glucagon secretion. Approximately 10% of the population of the USA and Canada have a diagnosis of T2D, and the morbidity and mortality rates associated with it are fairly high. The economic burden of T2D in the USA is $245 billion.1 2
These three cases exemplify that therapeutic fasting may reduce insulin requirements in T2D. Given the rising cost of insulin, patients may potentially save significant money. Further, the reduced need for syringes and blood glucose monitoring may reduce patient discomfort.
Although lifestyle modifications are universally acknowledged to be the first-line treatment of T2D, adequate glycaemic control is difficult to achieve in majority of obese patients. Bariatric surgery is an effective treatment option for obese patients with T2D, but is invasive, costly and not without its risks. Long-term effects have not been definitively established, and failure of the surgical intervention may occur due to non-compliance with diet and lifestyle factors. In addition, many patients require surgical reversal.3 4 Medications help manage the symptoms of diabetes, but they cannot prevent the progression of the disease.5
Therapeutic fasting has the potential to fill this gap in diabetes care by providing similar intensive caloric restriction and hormonal benefits as bariatric surgery without the invasive surgery. Therapeutic fasting is defined as the controlled and voluntary abstinence from all calorie-containing food and drinks from a specified period of time.6 This differs from starvation, which is neither deliberate nor controlled. During fasting periods, patients are allowed to drink unlimited amounts of very low-calorie fluids such as water, coffee, tea and bone broth. A general multivitamin supplement is encouraged to provide adequate micronutrients. Precise fasting schedules vary depending primarily on the patient’s preference, ranging from 16 hours to several days. On eating days, patients are encouraged to eat a diet low in sugar and refined carbohydrates, which decreases blood glucose and insulin secretion. The full manual of the dietary regimen used in this study has been published and is quoted in the references.7
As such, patients with T2D can reverse their diseases without the worry of side effects and financial burden of many pharmaceuticals, as well as the unknown long-term risks and uncertainty of surgery, all by means of therapeutic fasting.
Case presentation
Our case series involved three patients. Chart reviews of each patient were completed in November 2016, which included printed notes from the referring physicians, blood work and Intensive Dietary Management (IDM) clinic notes from each visit. On the initial consultation, all patients had been receiving various pharmacological therapies for their T2D, including at least 70 units of insulin daily. Patients were then seen monthly thereafter. Patient characteristics are summarised in table 1.
Table 1.
Patient characteristics
| Age | Sex | Years with type 2 diabetes | Comorbidities | Fasting frequency/duration | |
| Patient 1 | 40 | Male | 20 | Hypertension. Hypercholesterolaemia. |
3×/week for 7 months |
| Patient 2 | 52 | Male | 25 | Chronic kidney disease. Renal cell carcinoma (nephrectomy 2004). Hypertension. Hypercholesterolaemia. |
3×/week for 11 months |
| Patient 3 | 67 | Male | 10 | Hypertension. Hypercholesterolaemia. |
Alternating days for 11 months |
Patient 1 is a 40-year-old man diagnosed with T2D for 20 years. Other significant medical history includes hypertension and hypercholesterolaemia. His diabetic pharmacotherapy at the time of admission was insulin glargine 58 units at bedtime, insulin aspart 22 units twice daily, canagliflozin 300 mg once daily and metformin 1 g twice daily.
Patient 2 is a 52-year-old man diagnosed with T2D for 25 years. Other significant medical history includes chronic kidney disease, renal cell carcinoma (treated with previous nephrectomy), hypertension and hypercholesterolaemia. His diabetic pharmacotherapy at the time of admission consisted of insulin lispro mix units −38/32 25 IU twice daily.
Patient 3 is a 67-year-old man diagnosed with T2D for 10 years. Other significant medical history includes hypertension and hypercholesterolaemia. His diabetic pharmacotherapy at the time of admission consisted of metformin 1 g twice daily and insulin lispro mix 25–30 units in the morning and 20 units at night.
Treatment
All patients were seen in the IDM clinic after the initial educational seminar and dietary and insulin adjustments were made. Patients were followed in the clinic biweekly in the first few weeks until the insulin was discontinued.
The primary intervention used in this case series was dietary education and medically supervised therapeutic fasting. All patients were given detailed instructions on monitoring blood glucose, and insulin dosage was reduced prior to starting their fasting regimen in anticipation of the reduced dietary intake. Patients were closely monitored medically and instructed to stop fasting immediately if unwell for any reason.
All three patients participated in a 6-hour long nutritional training seminar which outlined many topics including the pathophysiology of diabetes, insulin resistance, education on macronutrients, and the principles of dietary management of diabetes including therapeutic fasting as well as safety.
After completing the educational training, the patients were instructed to follow a scheduled 24-hour fasts three times per week over a period of several months. Over the time period they were evaluated for glycaemic control and other diabetes-related health measures.
All patients followed similar dietary regimen. Patients 1 and 3 followed alternating-day 24-hour fasts, and patient 2 followed the triweekly 24-hour fasts schedule. On fasting days, the patients only consumed dinner, whereas on non-fasting days the patients consumed lunch and dinner. Low-carbohydrate meals were recommended when eating meals. Patients were examined on average twice a month and labs were recorded.
At each visit, patients’ daily blood sugar diaries were reviewed and further dietary and medication adjustments made if needed. Blood sugars were measured by patients at least four times daily during the insulin-weaning period. Target daily blood sugars were <10 during the initial insulin-weaning phase and <7 thereafter.
In addition, patients’ weight, waist circumference and blood pressures were measured and recorded at each visit.
Outcome and follow-up
There were five outcome measures in this case series:
Time to discontinuation of insulin.
Fasting blood glucose.
Serum A1C level (%, mmol/mol).
Patient weight (kg).
Patient waist circumference (cm).
The most noteworthy outcome from this case series is the complete discontinuation of insulin in all three patients. The changes in diabetic medications in all three patients are summarised in table 2. Both patients 2 and 3 discontinued all diabetic medications entirely. Patient 3 discontinued three out of four medications post fasting regimen. All three were able to discontinue their insulin. The minimum number of days to discontinuation of insulin was 5 and the maximum was 18. There was a general reduction of haemoglobin A1C (HbA1C) levels for all patients during the course of the fast. No symptomatic episodes of hypoglycaemia were reported in any of the patients.
Table 2.
Changes in glycaemic and other health parameters from baseline to end of follow-up
| Initiation HbA1c (%), (mmol/mol) | Final HbA1c (%), (mmol/mol) |
Initial diabetic medications | Final diabetic medications | Initial weight (kg) | Final weight (kg) | Initial waist circumference (cm) | Final waist circumference (cm) | Number of days to come off insulin | |
| Patient 1 | 11, 96.7 | 7, 53 | insulin glargine 58. insulin aspart 22. canagliflozin 300 mg. metformin 1 g. |
canagliflozin 300 mg | 83.8 | 73.8 | 100 | 87 | 5 |
| Patient 2 | 7.2, 55.2 | 6, 42.1 | insulin lispro mix 25–38/32 IU 25. | None | 61 | 50.4 | 89 | 70 | 18 |
| Patient 3 | 6.8, 50.8 | 6.2, 44.3 | metformin 1000 mg. insulin lispro mix 25–30/20 IU. |
None | 97.1 | 88.1 | 123 | 110 | 13 |
HbA1C, haemoglobin A1C.
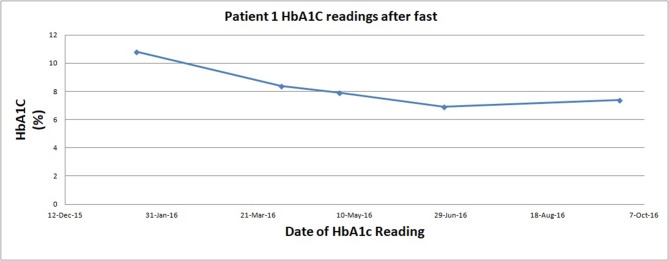
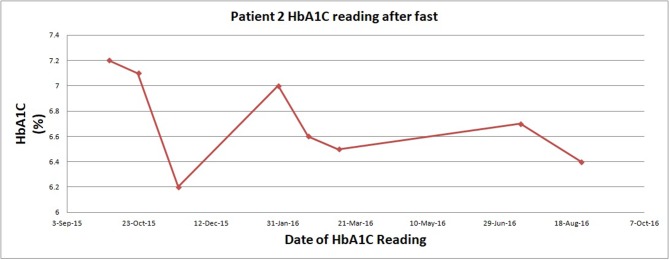
Patient 1, depicted in figure 1, fasted 3×/week and reported that he tolerated fasting without difficulty and felt ‘excellent’ on his fasting days. Blood glucose ranged from 5 to 10. Further, he had a 12% weight loss and 13% waist circumference reduction (table 2). Patient 2, who followed the same fasting regimen, significantly reduced his HbA1C overall, with some fluctuations as summarised in figure 2. Patient 2 also subjectively reported feeling ‘terrific’, and his daily blood sugars ranged from 5 to 6. Further, he had 18% weight loss and 22% waist circumference reduction (table 2).
Figure 1.
Change in glycosylated haemoglobin while on fast for patient 1. HbA1C, haemoglobin A1C.
Figure 2.
Change in glycosylated haemoglobin while on fast for patient 2. HbA1C, haemoglobin A1C.
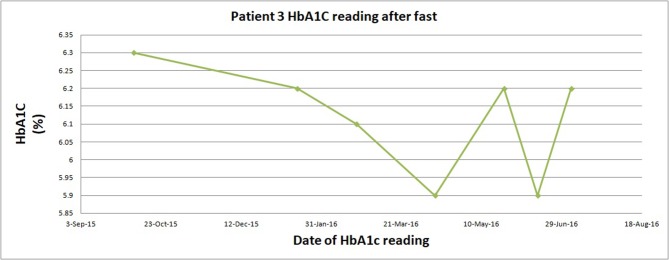
Patient 3 maintained a low HbA1C along the course of fasting scheduling as he eliminated his insulin and 75% of his oral hypoglycaemic medications (figure 3). He subjectively reported the fasting was ‘easy’ and does not have the carbohydrate cravings he once had before he started the diet, and he also experienced higher energy levels. Further, he had 10% weight loss and waist circumference reductions (table 2).
Figure 3.
Change in glycosylated haemoglobin while on fast for patient 3. HbA1C, haemoglobin A1C.
Despite its complete novelty of fasting for all three patients, it was well tolerated. No patient stopped fasting at any point out of choice. In general, our feedback from the patients in this programme was very positive, and a number of patients commented on enjoying being actively involved in the process of managing their diabetes.
Discussion
The goals in caring for patients with T2D are to reduce symptoms and to prevent or slow the development of complications. The main interventions for treating T2D have been lifestyle modification, pharmacological and in some cases surgical. The use of a therapeutic fasting regimen for treatment of T2D is virtually unheard of. This present case series showed that 24-hour fasting regimens can significantly reverse or eliminate the need for diabetic medication.
A case review by Unwin and Tobin8 documented that they were able to ‘deprescribe’ a 52-year-old man who was living with T2D for 14 years. He was suffering from gastrointestinal side effects from his metformin medication. Following a low-carbohydrate diet, the patient steadily lost a total of 16 kg over 7 months and successfully stopped all prescribed drugs, thereby achieving his goal of being medication-free.8
Caloric restriction and weight loss are important factors for remission of T2D, as recently demonstrated in an open-label Diabetes Remission Clinical Trial (DiRECT). The DiRECT study showed diabetes remission and maintenance through caloric restriction (~840 calories/day) and weight loss in a non-insulin-dependent diabetic population.9 To date, however, very few studies or cases have been documented or published with respect to therapeutic fasting as a cure for T2D, reversing it completely and eliminating the use of insulin. We were unable to locate a single case study published in the last 30 years.
In our study all three patients eliminated the need for insulin by initiating a therapeutic fasting regimen. All three patients succeeded within a month and one in as little as 5 days. Further, all patients improved in multiple other clinically significant health outcome measures, such as HbA1C, body mass index and waist circumference.
This reduction in risk factors will likely reduce the risk for further complications. A study by Wing et al 10 found that modest weight losses of 5%–10% have been associated with significant improvements in cardiovascular disease risk factors (ie, decreased HbA1C levels, reduced blood pressure, increase in HDL cholesterol, decreased plasma triglycerides) in patients with T2D. Risk factor reduction was even greater with losses of 10%–15% of body weight. In our present study, all three patients experienced weight loss of 10% or more.
Educating patients on the benefits of fasting in the management of T2D may aid in the remission of the disease and curtail the use of pharmacological interventions. A systematic review suggested that patients with T2D who have a baseline HbA1C of greater than 8% may achieve better glycaemic control when given individual education rather than usual care.11 Additionally, patients should be educated about and encouraged to follow an appropriate treatment plan tailored to them. Adherence to a fasting diet should continue to be stressed throughout treatment, because these lifestyle measures and modifications can have a large impact on the degree of diabetic control that patients can achieve, as seen with this case series. This is further emphasised by a study by Morrison et al,12 who found that more frequent visits with a primary care provider led to markedly rapid reductions in serum glucose, HbA1C and low-density lipoprotein cholesterol levels. In our study patients followed up with the treating physician on average every 2 weeks.
Learning points.
Medically supervised, therapeutic fasting regimens can help reverse type 2 diabetes (T2D) and minimise the use of pharmacological and possibly surgical interventions in patients with T2D.
Therapeutic fasting is an underutilised dietary intervention that can provide superior blood glucose reduction compared with standard pharmacological agents.
Fasting is a practical dietary strategy.
With proper education and support, we found compliance to be good.
Footnotes
Contributors: SF compiled all patient results and medical records for the purpose of analysing the data; completed 50% of the background literature review to help support the manuscript; assisted in writing approximately 50% of the manuscript. RE assisted in 50% of the background literature search and writing up 50% of the manuscript; created the graphs and figures for the manuscript. MR took all of the patients' measurements and medical histories for each appointment, and ensured that the data were correct and kept up-to-date, safe and confidential; arranged follow-up appointments for the patients and assisted with coaching and motivational interviewing at each visit; organised and retrieved all medical information to complete this study; ensured proper verbal and written patient consent for all three study subjects. JF provided clinical oversight for the management of each patient at each visit, including pharmacological management and deprescribing when needed; provided methodological support including design, analysis and interpretation of the results; proof-read the manuscript entirely at least three times.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Centers for Disease Control and Prevention. Diabetes Latest – Fact Sheet. 2016. https://www.cdc.gov/features/diabetesfactsheet/
- 2. Canadian Diabetes Association: Diabetes Charter for Canada. Diabetes in Canada Fact Sheet. 2016. https://www.diabetes.ca/getmedia/513a0f6c-b1c9-4e56-a77c-6a492bf7350f/diabetes-charter-backgrounder-national-english.pdf.aspx
- 3. Shoar S, Nguyen T, Ona MA, et al. . Roux-en-Y gastric bypass reversal: a systematic review. Surg Obes Relat Dis 2016;12:1366–72. 10.1016/j.soard.2016.02.023 [DOI] [PubMed] [Google Scholar]
- 4. Pucher PH, Lord AC, Sodergren MH, et al. . Reversal to normal anatomy after failed gastric bypass: systematic review of indications, techniques, and outcomes. Surg Obes Relat Dis 2016;12:1351–6. 10.1016/j.soard.2016.01.030 [DOI] [PubMed] [Google Scholar]
- 5. Brethauer SA, Aminian A, Romero-Talamás H, et al. . Can diabetes be surgically cured? Long-term metabolic effects of bariatric surgery in obese patients with type 2 diabetes mellitus. Ann Surg 2013;258:1 10.1097/SLA.0b013e3182a5034b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ku M, Ramos MJ, Fung J. Therapeutic fasting as a potential effective treatment for type 2 diabetes: A 4-month case study. Journal of Insulin Resistance 2017;1:5 10.4102/jir.v2i1.31 [DOI] [Google Scholar]
- 7. Fung J, Moore J. The complete guide to fasting. 1st edn Las Vegas: Victory Belt Publishing:39–45. [Google Scholar]
- 8. Unwin D, Tobin S. A patient request for some "deprescribing". BMJ 2015;351:h4023. [DOI] [PubMed] [Google Scholar]
- 9. Lean ME, Leslie WS, Barnes AC, et al. . Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet 2018;391 10.1016/S0140-6736(17)33102-1 [DOI] [PubMed] [Google Scholar]
- 10. Wing RR, Lang W, Wadden TA, et al. . Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care 2011;34:1481–6. 10.2337/dc10-2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Duke S-AS, Colagiuri S, Colagiuri R. Cochrane Metabolic and Endocrine Disorders Group. Individual patient education for people with type 2 diabetes mellitus. Cochrane Database Syst Rev 2009;22 10.1002/14651858.CD005268.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Morrison F, Shubina M, Turchin A. Encounter frequency and serum glucose level, blood pressure, and cholesterol level control in patients with diabetes mellitus. Arch Intern Med 2011;171:1542–50. 10.1001/archinternmed.2011.400 [DOI] [PMC free article] [PubMed] [Google Scholar]