Abstract
Lymphangiomas are most commonly described as a small painless mass in the neck or a vesicular rash in an infant patient. Ninety per cent of cases are diagnosed before the age of 2. Treatment usually involves surgical resection. Intra-abdominal lymphangiomas and mesenteric lymphangiomas, as described in our case report, represent a rare pathology. The exact prevalence of this condition is unclear but it has been suggested in the literature that there have been as few as 820 cases since the 16th century. The clinical presentation is usually subacute and diagnosis made incidentally during a workup of chronic gastrointestinal symptoms. Acute abdominal symptoms, as in our case presentation, are unusual but may be explained by the mass effect of a large intra-abdominal lesion. Cross-sectional imaging is key in preoperative workup and operative planning. Complete surgical resection is recommended and curative in the majority of cases with a low risk of local recurrence.
Keywords: surgical oncology, surgery, gastrointestinal surgery
Background
Lymphangiomas are benign, cystic, lymphatic malformations that are typically diagnosed through history and physical examination within the first few years of life as superficial vesicular lesions called capillary (or simple) lymphangioma. Larger, and often deeper, lesions are termed cavernous lymphangioma and often present as nodular swelling of the skin overlying the neck or head. Cystic hygromas are a third subtype, which are pathologically similar to cavernous lymphangioma but clinically less firm and tend to be located in the axillae, groin and neck.1
While lymphangiomas are frequently congenital conditions, they can be acquired later in life through various mechanisms, such as infection, trauma or prior surgical interventions, that lead to obstruction of the lymphatic system.2 Congenital lymphangiomas are typically due to a failure of lymphatic cisterns to appropriately form connections to the systemic lymphatic system. As these cisterns grow and extend outward, they exert a mass effect on nearby structures that may be clinically symptomatic. However, the typical patient is an infant with a painless mass of 1–3 cm in the neck or a vesicular rash. In fact, 90% of all cases are diagnosed before the age of 2.3 Abdominal lymphangiomas are more likely to be discovered incidentally with imaging for an unrelated reason, or during workup for gastrointestinal symptoms due to their mass effect. Imaging studies such as ultrasound, CT or MRI are the best options for diagnosing abdominal lymphangiomas. Laboratory studies usually do not add any diagnostic value in the case of lymphangioma, unless secondary complications from the mass are present.4
The presence of abdominal lymphangiomas in adults is uncommon, but the literature remains conflicted on exact numbers. Many sources reference an unclear estimate of 1/20 000 to 1/250 000 admissions per year5 but Liew and colleagues note that there have been only 820 cases of mesenteric cysts, of any aetiology, including lymphatic, documented since 1507.6 Of all abdominal lymphangiomas, <1% involve the mesentery and 90% of these present symptomatically before the age of 1.7 If symptomatic, adult patients with abdominal lymphangiomas rarely present acutely, then non-specific symptoms (such as pain, fullness and distension)8 are more commonly insidious in nature. Acute symptoms are likely secondary to complications like rupture, haemorrhage, obstruction or infection.5 The diagnosis of abdominal lymphangioma is almost never made on clinical examination alone; these non-specific symptoms typically lead to cross-sectional imaging, which is often diagnostic. Among all mesenteric lymphangiomas, the most common origin is the small bowel mesentery,9–13 which accounts for approximately 70% of cases, followed by masses arising from the colonic mesentery (approximately 26% of cases).14–16
The core treatment for lymphangiomas is complete surgical resection after appropriate planning with MRI, or another imaging modality such as ultrasound or CT, to assess the depth of the lesion, the tissue of origin and the involvement of surrounding structures.4 8 It is critical to be aware of infiltration of surrounding structures due to high recurrence rates in the setting of incomplete resection.5 Sclerotherapy, cryotherapy, laser therapy and cautery have also been used successfully to treat lymphangioma.17 Surgery is almost always curative as long as complete excision has been achieved.5
This report describes the case of a female adult patient with a large intra-abdominal lymphangioma arising from the colonic mesentery. This is both a rare diagnosis, as well as an atypical presentation given her age, the size of the mass and the acute onset of her symptoms.
Case presentation
The patient is a 44-year-old woman with a medical history notable for gastro-oesophageal reflux disease and surgical history notable for open umbilical hernia repair with mesh in 2012. She initially presented in October 2015 to a local community health centre with a 1-month history of nausea and burning right upper quadrant (RUQ) pain that felt unlike her typical reflux. Her pain was worse after eating and improved with Alka-Seltzer. Her examination revealed no hepatomegaly and she had a negative Murphy’s sign. Pertinent laboratory values were unremarkable. Without further imaging, and given her positive family history for cholelithiasis, she was diagnosed with biliary colic and was advised to follow-up in outpatient clinic.
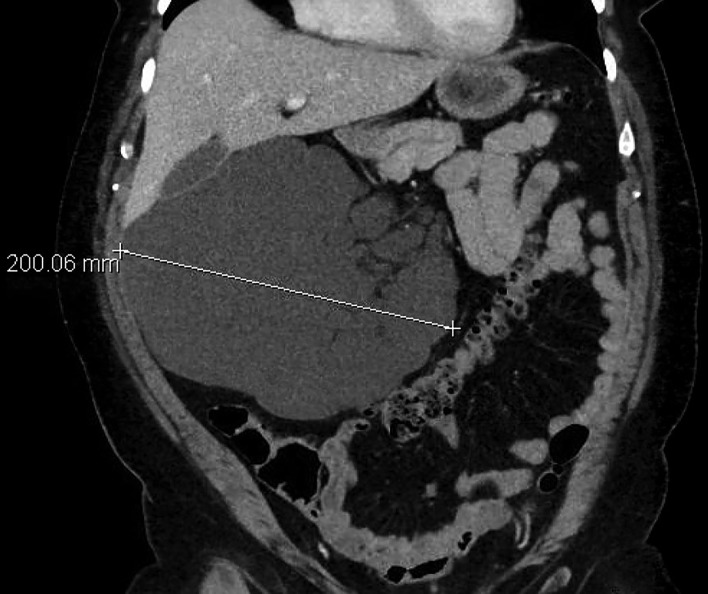
She eventually re-presented to the emergency department in August 2016 with a 1-day history of acute right abdominal, flank and back pain, 9-10/10 in intensity and burning in quality. She was afebrile and vital signs were within normal limits. On examination, she was tender to palpation over her RUQ without guarding, distension or overlying skin changes and she did not appear jaundiced. The remainder of her examination was unremarkable. She endorsed early satiety and denied any other symptoms, including any abnormal bowel movements. A contrast-enhanced CT scan of her abdomen and pelvis was obtained that revealed a 20.1×15.7×10.5 cm multiloculated, cystic, intra-abdominal mass that crossed her midline and extended from the subhepatic space down the right flank and displaced colon, duodenum and kidney (figure 1). Her symptoms resolved with bowel rest and symptomatic management and was discharged. She was referred to a surgical oncologist shortly after.
Figure 1.
CT abdomen/pelvis with intravenous contrast demonstrating a multiloculated cystic intra-abdominal mass measuring 20.1×10.5×15.7 cm with displaced liver as well as large and small bowel.
Due to this unusual finding and presentation, a preoperative MRI by her surgical oncologist was ordered to better evaluate the mass including its origin and relationship with surrounding structures. This imaging demonstrated a large, non-enhancing, T2 hyperintense mass with multiple non-enhancing internal septations and no obvious communication with adjacent structures. Given these results, our differential diagnoses at the time included a veno-lymphatic malformation, peritoneal inclusion cyst and mesenteric lymphangioma.
Treatment
In early November, the decision was made to perform an elective exploratory laparotomy given the patient’s symptomatic presentation. On opening of the abdominal cavity, the lymphatic tissue-appearing mass was immediately visualised in the RUQ and was bluntly dissected away from surrounding connective tissue. After further dissection down, we encountered a stalk that appeared to be originating from the transverse mesocolon mesentery. The mass was excised without complications (figure 2) and submitted for pathological evaluation. The resected specimen was a 25×18×8.2 cm, 2000 g, multiloculated cystic structure with areas of haemorrhage and septa of variable thickness, separating cystic spaces containing a variable amount of white, cloudy fluid. Standard sampling of the mass was performed for microscopic examination. Histologically, the mass was composed of variably sized cystic structures lined by an attenuated endothelial type lining and contained patchy foci of smooth muscle, inflammatory cells and lymphoid aggregates within the cyst walls (figure 3). There was no histological evidence of malignancy.
Figure 2.
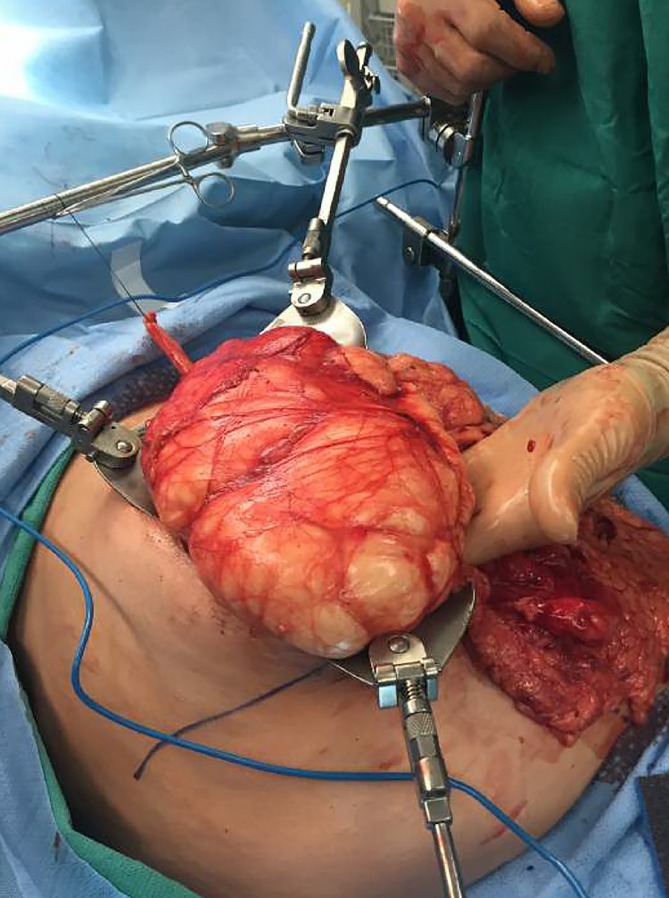
Intra-operative image taken of intra-abdominal mass delivered out of abdominal cavity. Capsule-like attachments are visible.
Figure 3.
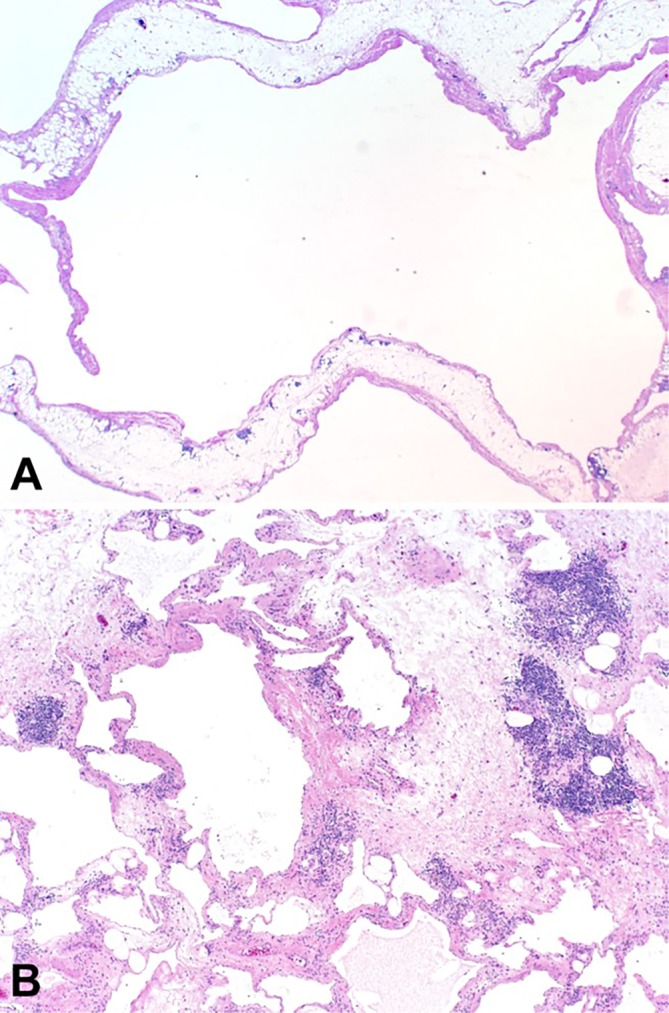
Routine H&E-stained sections showing (A) large, dilated lymphovascular spaces lined by a simple endothelium, and (B) areas of septal thickening with occasional lymphoid aggregates, interstitial inflammation and focal perivascular smooth muscle (A; 1X, B; 4X).
Outcome and follow-up
Postoperative recovery was largely uncomplicated with mild abdominal and back pain well-controlled on oral medication and a postoperative ileus that resolved within 2 days. The patient was discharged home on postoperative day 2 and was first seen for follow-up in the outpatient setting 2 weeks later. At that time, she was noted to be clinically well, with normal eating and bowel habits and without any complaints or analgesic requirements.
Discussion
This patient presented with an atypical case of a rare disease in adults. Her age, the size of the mass, its location and its acutely symptomatic presentation were highly unusual for any lymphangioma. The vast majority of these cases are 1–3 cm subcutaneous masses in an infant, which is in stark contrast to our patient’s age of 44 and the 25 cm mass arising from her transverse mesocolon mesentery. The fact that her tumour arose from the mesocolon confers a notable level of rarity on this case. While often noting mesenteric lymphangiomas to be uncommon, the literature does not report an exact prevalence rate. However, one publication reports that there have been 820 cases of any type of mesenteric cyst reported between 1507 and 1994.6 Of these mesenteric cysts, if the statistic that 90% present symptomatically prior to the age of 17 is applied, then it could be inferred that only an estimated 10%, or 82 total documented cases, have occurred symptomatically in individuals older than 1 in the last 500 years. Although the data are lacking, that population of 82 would presumably be narrowed down even further once only adults are considered and all non-lymphangioma mesenteric cyst cases are excluded. It should be noted that this calculation must be considered with caution, given its reliance on the sole study6 that reported an exact rate of this disease. Additional studies that examine registries or databases to better characterise the true prevalence of this condition are needed.
In the limited number of other case reports of mesenteric lymphangiomas, patients’ age range from 2 to 48 years.18–21 Among adults, the non-specific symptoms of abdominal pain, distension and discomfort are among the most common, though none of these are described as severe or as sudden as in the patient we present here.19 21 Laboratory studies were largely normal among all patients, though leucocytosis was occasionally present.20 21 Imaging options, employed nearly universally in these studies, included some combination of abdominal plain films, ultrasonography and CT. All patients were found to have a large, cystic abdominal mass (range 7.8–25 cm in the longest diameter), and small bowel displacement or compression, occasionally leading to complete obstruction.18–21 All patients underwent complete resection, with intraoperative and pathological findings consistent with mesenteric lymphangiomas and no postoperative complications were described in any patient.18–21 The similarities between these recent cases and our patient’s presentation highlight the importance of imaging, the likelihood of bowel displacement or obstruction, and the success of operative management of mesenteric lymphangiomas.
Our patient’s presentation with sudden, severe abdominal pain is notably uncommon. The literature notes that this is often a result of rupture, haemorrhage, bowel obstruction of infection.5 7 22 23 The reason for her acute onset of pain remains unclear as the tumour was not found to be infiltrating surrounding organs on imaging or intraoperatively. Partial bowel obstruction secondary to mass effect remains a possibility as a potential cause for her acute presentation, though she reported normal bowel function during her initial emergency department presentation. The other causes of acute symptomatic presentation—rupture, haemorrhage and infection—are additionally unlikely given the lack of gross visual evidence intraoperatively and pathologically and her normal vital signs on presentation. Ultimately, her presenting symptoms prompted the elective laparotomy and excision of her lymphangioma. The report from her MRI correctly predicted the final diagnosis of lymphangioma by noting the mass’ enhancing and septation pattern, but a broad differential that includes other primary mesenteric tumours, metastatic disease, tuberculosis, pseudocysts, cystic teratomas, enteric duplication cysts and ovarian cysts should be considered when an abdominal mass is discovered.8 24 Given this broad list of differential diagnoses, surgery should be the treatment of choice, even in asymptomatic patients, as pathology gives a final diagnosis. For symptomatic lesions that cause pain or obstructive symptoms, complete surgical excision is recommended.
The aetiology of our patient’s cavernous lymphangioma, congenital versus acquired, remains unknown given the fact that she never had abdominal imaging studies at a younger age. Her episode of what was presumed to be biliary colic has the potential to be either an inciting, inflammatory event that led to the formation of her mass, or the first clinical presentation of her mass that was misdiagnosed at the time. In a 2018 case report, Rojas and Molina24 suggested that their patient’s small bowel mesentery cavernous lymphangioma may have been a result of her prior cholecystectomy, appendectomy or hysterectomy. Their report, as well as others,25 demonstrate surgery as a potential risk factor for the development of abdominal lymphangiomas, but it is unlikely that our patients open umbilical hernia repair caused any trauma at the transverse mesocolon mesentery. Thus, it remains unclear what the inciting event was for our patient’s mesenteric lymphangioma.
Our patient will continue to have follow-up with her surgical and primary care teams for any complications, but she has had no abdominal complaints since surgical excision and her overall prognosis for this benign tumour is excellent.
Learning points.
Mesenteric lymphangiomas represent a rare cause of a spectrum of abdominal symptoms from mild discomfort and fullness to severe pain in patients of all ages.
Imaging is a critical means of diagnosis as laboratory studies are infrequently remarkable.
Determining the pathogenesis of mesenteric lymphangioma in adults remains challenging and may require a thorough medical and procedural history.
Treatment with complete surgical resection is often curative and patients fare well postoperatively following these procedures.
Footnotes
Contributors: All authors contributed to the planning, writing and editing of this case report.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Hoda SA, Cheng E. Robbins basic pathology. Am J Clin Pathol 2017:557 10.1093/ajcp/aqx09528472207 [DOI] [Google Scholar]
- 2.Prabhakar C, Shah NK. Lymphatic malformations: a dilemma in diagnosis and management. Contemp Clin Dent 2014;5:119–22. 10.4103/0976-237X.128689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giguère CM, Bauman NM, Smith RJ. New treatment options for lymphangioma in infants and children. Ann Otol Rhinol Laryngol 2002;111:1066–75. 10.1177/000348940211101202 [DOI] [PubMed] [Google Scholar]
- 4.Romeo V, Maurea S, Mainenti PP, et al. . Correlative imaging of cystic lymphangiomas: ultrasound, CT and MRI comparison. Acta Radiol Open 2015;4 10.1177/2047981614564911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allen JG, Riall TS, Cameron JL, et al. . Abdominal lymphangiomas in adults. J Gastrointest Surg 2006;10:746–51. 10.1016/j.gassur.2005.10.015 [DOI] [PubMed] [Google Scholar]
- 6.Liew SC, Glenn DC, Storey DW. Mesenteric cyst. Aust N Z J Surg 1994;64:741–4. 10.1111/j.1445-2197.1994.tb04530.x [DOI] [PubMed] [Google Scholar]
- 7.Losanoff JE, Richman BW, El-Sherif A, et al. . Mesenteric cystic lymphangioma. J Am Coll Surg 2003;196:598–603. 10.1016/S1072-7515(02)01755-6 [DOI] [PubMed] [Google Scholar]
- 8.Naganuma H, Ishida H, Komatsuda T, et al. . Sonographic findings in two cases of lymphangioma of the mesocolon in adults. J Clin Ultrasound 2018;46:78–81. 10.1002/jcu.22488 [DOI] [PubMed] [Google Scholar]
- 9.Tian C, Zheng Y, Ren X, et al. . A giant abdominal cystic tumour: mesentery cystic lymphangioma. Dig Liver Dis 2015;47:816–7. 10.1016/j.dld.2015.05.008 [DOI] [PubMed] [Google Scholar]
- 10.Tsuboi M, Noda H, Watanabe F, et al. . Complete resection of a complicated huge mesenteric lymphangioma guided by mesenteric computed tomography angiography with three-dimensional reconstruction: report of a case. Int Surg 2015;100:574–8. 10.9738/INTSURG-D-14-00112.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maqueda Merino A, Sardón Ramos JD, Vitores Lopez JM, et al. . Mesenteric polycystic lymphangioma. A rare cause of acute surgical abdomen. An Pediatr 2014;81:59–61. 10.1016/j.anpedi.2013.10.041 [DOI] [PubMed] [Google Scholar]
- 12.Chung JC, Song OP. Cystic lymphangioma of the jejunal mesentery presenting with acute abdomen in an adult. Can J Surg 2009;52:E286–8. [PMC free article] [PubMed] [Google Scholar]
- 13.Watanabe A, Suzuki H, Kubo N, et al. . A case of mesenteric cystic lymphangioma in an adult which caused duodenal stenosis after resection. Int J Surg Case Rep 2013;4:212–5. 10.1016/j.ijscr.2012.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoon J, Ros PR. Tumors of the mesentery and omentum. Radiologic-pathologic correlations 2005:447–74. [Google Scholar]
- 15.Walker AR, Putnam TC. Omental, mesenteric, and retroperitoneal cysts: a clinical study of 33 new cases. Ann Surg 1973;178:13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nizami S, Mohiuddin K, Daudi I, et al. . Cavernous transverse mesocolonic lymphangioma in an adult. Am J Surg 2007;193:740–1. 10.1016/j.amjsurg.2006.06.037 [DOI] [PubMed] [Google Scholar]
- 17.Mirza B, Ijaz L, Saleem M, et al. . Different modalities used to treat concurrent lymphangioma of chest wall and scrotum. J Cutan Aesthet Surg 2010;3:189–90. 10.4103/0974-2077.74501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suthiwartnarueput W, Kiatipunsodsai S, Kwankua A, et al. . Lymphangioma of the small bowel mesentery: a case report and review of the literature. World J Gastroenterol 2012;18:6328–32. 10.3748/wjg.v18.i43.6328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen CW, Hsu SD, Lin CH, et al. . Cystic lymphangioma of the jejunal mesentery in an adult: a case report. World J Gastroenterol 2005;11:5084–6. 10.3748/wjg.v11.i32.5084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rami M, Mahmoudi A, El Madi A, et al. . Giant cystic lymphangioma of the mesentery: varied clinical presentation of 3 cases. Pan Afr Med J 2012;12:7. [PMC free article] [PubMed] [Google Scholar]
- 21.Tsukada H, Takaori K, Ishiguro S, et al. . Giant cystic lymphangioma of the small bowel mesentery: report of a case. Surg Today 2002;32:734–7. 10.1007/s005950200138 [DOI] [PubMed] [Google Scholar]
- 22.Takiff H, Calabria R, Yin L, et al. . Mesenteric cysts and intra-abdominal cystic lymphangiomas. Arch Surg 1985;120:1266–9. 10.1001/archsurg.1985.01390350048010 [DOI] [PubMed] [Google Scholar]
- 23.de Perrot M, Bründler M, Tötsch M, et al. . Mesenteric cysts. Toward less confusion? Dig Surg 2000;17:323–8. 10.1159/000018872 [DOI] [PubMed] [Google Scholar]
- 24.Rojas CL, Molina GA. Lymphangioma cavernous of the small bowel mesentery, an infrequent cause of acute abdomen in adult. J Surg Case Rep 2018;2018:rjy018 10.1093/jscr/rjy018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar B, Bhatnagar A, Upadhyaya VD, et al. . Small Intestinal Lymphangioma presenting as an acute abdomen with relevant review of literature. J Clin Diagn Res 2017;11:PD01–2. 10.7860/JCDR/2017/22703.9962 [DOI] [PMC free article] [PubMed] [Google Scholar]