Abstract
A 46-year-old woman with quiescent lupus presented with worsening pleuritic chest pain and dyspnoea. Bedside echocardiogram confirmed large pericardial effusion with cardiac tamponade. Emergency bedside pericardiocentesis was performed. Pericardial fluid cytology confirmed diffuse large B cell lymphoma, stage four on positron emission tomography. Conventional rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone chemotherapy achieved good response in all sites except the pericardium. Progressive cardiac involvement was complicated by atrioventricular conduction block requiring permanent pacemaker. Second-line palliative chemotherapy was performed.
Keywords: cardiovascular medicine, pericardial disease, pacing and electrophysiology, haematology (incl blood transfusion), cancer - see oncology
Background
This is a rare initial presentation of non-Hodgkin’s lymphoma (NHL) in an immunosuppressed individual, which demonstrated resistance to conventional rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone (R-CHOP) chemotherapy.
Case presentation
A 46-year-old indigenous woman presented to a rural hospital with a short history of progressive dyspnoea and pleuritic chest pain. Her background included WHO stage V lupus nephritis (baseline creatinine 400 µmol/L) and antiphospholipid syndrome. This disease was quiescent on maintenance warfarin, prednisolone and azathioprine. The patient had previously received cyclophosphamide and rituximab therapy for prior relapses. She also had anal carcinoma in situ recently diagnosed, which was awaiting definitive therapy.
Examination revealed stable observations and no fever. Jugular venous pressure was elevated. A lymph node was palpable in the right supraclavicular fossa. There were no signs of active lupus.
Investigations
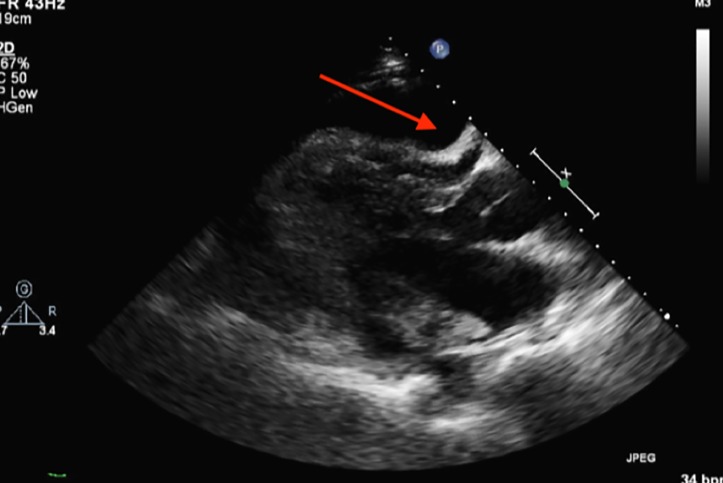
ECG demonstrated sinus tachycardia with low voltage and T wave inversion inferolaterally. Chest X-ray revealed an enlarged cardiac silhouette with mild pulmonary congestion. Bedside transthoracic echocardiography (TTE) confirmed a large, circumferential pericardial effusion with tamponade physiology (figure 1). The pericardium was diffusely thickened, with prominent echodensities at both sides of the atrioventricular (AV) groove. Inflammatory markers were elevated [white cell count 6.67×109/L (reference interval (RI) 4-11×109/L), C-reactive protein 13 mg/L (RI <5 mg/L), erythrocyte sedimentation rate 85 (RI 1-15 mm/h)] with low complement levels [C3 0.70 g/L (RI 0.88–1.98 g/L), C4 0.15 g/L (RI 0.16–0.52 g/L)]. Anti-DNA antibody was 10 IU/mL (RI <7 IU/mL), consistent with quiescent lupus. Blood cultures and viral serology (Epstein-Barr virus, cytomegalovirus, hepatitis B virus, hepatitis C virus, HIV) did not support active infection.
Figure 1.
Transthoracic echocardiogram (parasternal long-axis view) showing large circumferential pericardial effusion (arrow).
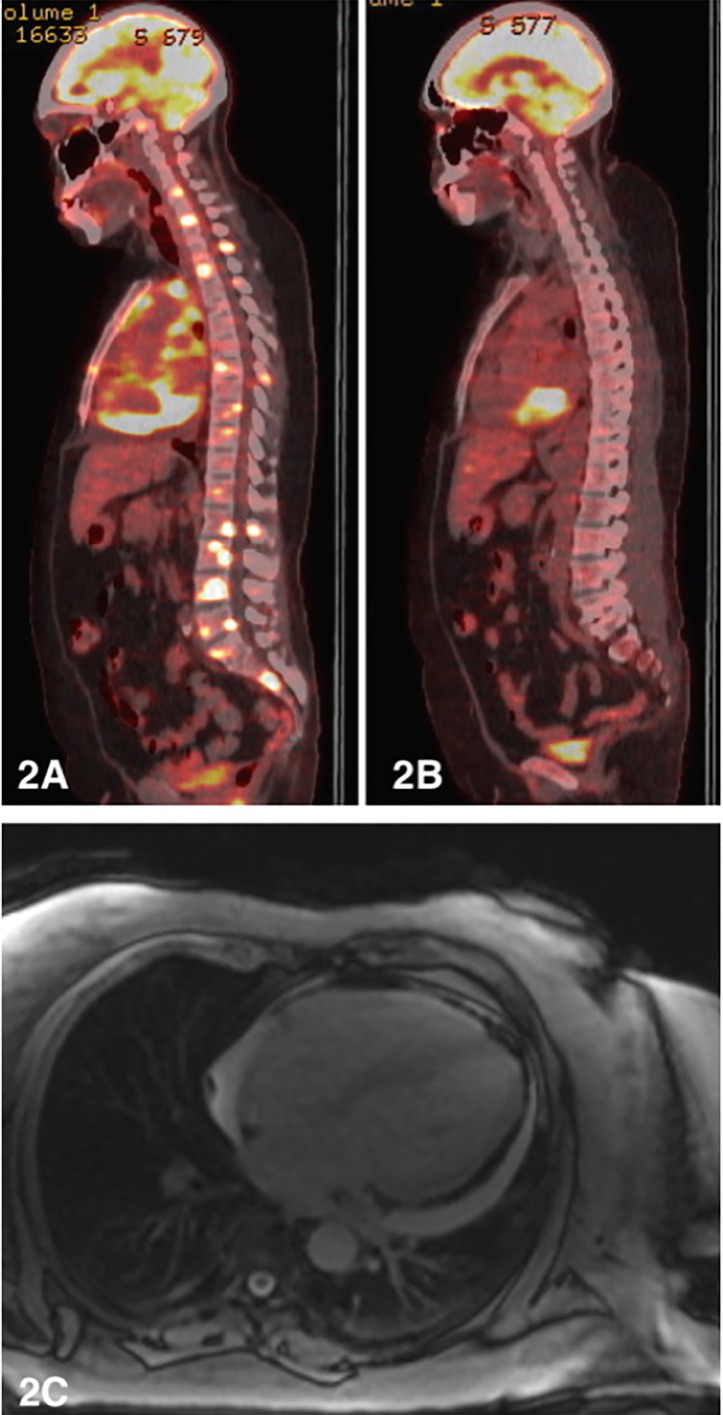
Bedside pericardiocentesis yielded 800 mL of turbid fluid. Biochemistry was consistent with an exudate. Microbiological cultures were negative. Cytology demonstrated a lymphoid infiltrate of large cells with immunophenotype CD10+/CD20+/CD79A+, Epstein-Barr virus-encoded small RNA positivity and ki67 over 90%. Hence, a diagnosis of diffuse large B cell lymphoma (DLBCL, of germinal centre subtype by Hans criteria) was made and stage four disease confirmed on positron emission tomography (PET) scan (figure 2A).
Figure 2.
(A) Initial staging positron emission tomography (PET) scan (sagittal view) showing stage four disease. (B) Postchemotherapy PET scan showing resolution of osseous lesions with persistent high grade metabolic activity in the posteroinferior pericardial recess. (C) Cardiac MRI showing diffuse pericardial thickening and small to moderate circumferential pericardial effusion (12 mm posteriorly).
Treatment
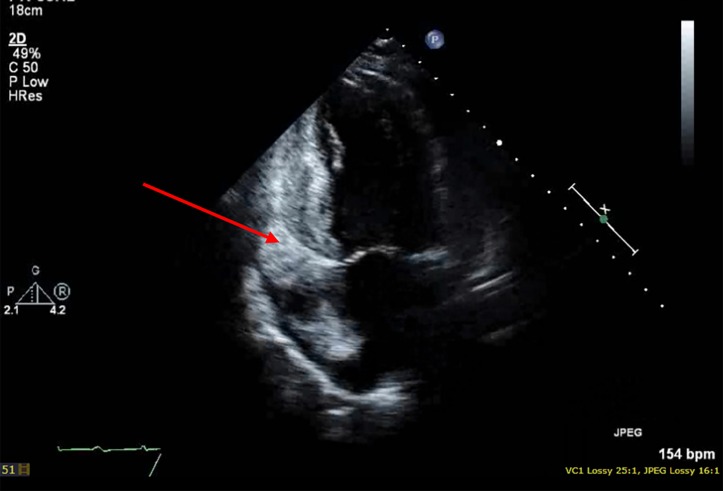
Azathioprine was stopped due to stable autoimmune disease and high-dose prednisolone was initiated for 7 days pretreatment. The patient received six cycles of R-CHOP chemotherapy at conventional dosing. Tumour lysis prophylaxis with allopurinol was given. Treatment was complicated by several admissions with febrile neutropenia (despite granulocyte-colony stimulating factor support) and acute on chronic renal impairment. End of therapy PET scan showed isolated high-grade activity at the posteroinferior pericardial recess, with remission at all other sites (figure 2B). Cardiac MRI showed diffuse pericardial enhancement and stable appearance of the pericardial masses without infiltrate (figure 2C); however, serial echocardiogram 10 days later indeed demonstrated myocardial lymphomata infiltration (figure 3).
Figure 3.
Transthoracic echocardiography (apical two-chamber left ventricle view) showing lymphomata infiltration of the left atrium and left ventricle.
Outcome and follow-up
Biopsy of the pericardial mass, formation of a pericardial window and re-drainage of the effusion was performed via video-assisted thorascopic surgery. Fluid cytology confirmed refractory lymphoma. While febrile on steroid therapy, the patient also developed intermittent high-grade heart block (due to presumed conduction system infiltration) requiring leadless pacemaker insertion. The patient was assessed not to be suitable for an aggressive salvage regimen due to the presence of comorbidities, particularly renal dysfunction. The palliative second-line chemotherapy regimen gemcitabine/vinorelbine was chosen with the intent of allowing the patient to travel back to her rural home between chemotherapy cycles. Unfortunately, the patient passed away from progressive disease.
Discussion
This case represents a rare initial presentation of DLBCL in the form of a large pericardial effusion in an immunocompromised individual. A review of the literature revealed only few similar cases. While primary cardiac lymphoma accounts for only 1%–2% of all cardiac tumours, secondary cardiac involvement in the setting of disseminated lymphoma is more common, occurring in up to 20% of patients with NHL.1–3 Cardiac involvement typically occurs 20 months post-lymphoma diagnosis and is often diagnosed at autopsy.4
DLBCL is the most common histological subtype of NHL and is implicated in over half of cardiac NHL cases, with a tendency to rapidly progress.2 Cardiac involvement in malignancy can occur haematogenously (as with lymphoma), via the lymphatic or transvenous route or via direct extension.5 6 Overall, the pericardium is the most frequently involved cardiac site in lymphoma and over half of the cases of pericardial involvement are DLBCL.2 5
The manifestations of cardiac involvement in lymphoma are highly variable and non-specific. They may mimic common presentations of heart failure, arrhythmia and pericarditis.2 4 5 In the majority, however, they remain asymptomatic.2 4 5 This can lead to diagnostic delay and advanced disease at presentation.2 In severe effusions without inflammatory signs, cardiac tamponade increases the likelihood of a malignant aetiology almost threefold.7
HIV is the most well-recognised associated factor for NHL with cardiac involvement and has been frequently described in the literature.2 8 However, a review by Gordon et al found that only 19% of patients with cardiac lymphomatous involvement were HIV positive.2 Our patient was HIV negative, but had been exposed to long-term immunosuppression for lupus nephritis, with prior rituximab therapy. Lymphoma is a recognised long-term complication of immunosuppressive therapy and clinical suspicion for malignancy should be raised at presentation of an otherwise unexplained pericardial effusion.
Pathological diagnosis of cardiac involvement prior to chemotherapy is essential to guide treatment. At a minimum, pericardial fluid should be sent for cytological analysis to confirm malignant involvement (Class I, B LOE).7 Pericardial/epicardial biopsy should also be considered to confirm malignant involvement (IIa, B), and tumour marker testing is controversial.7
Echocardiography remains the first-line imaging modality for assessing cardiac localisation of lymphomatous deposits and baseline left ventricular function pre-chemotherapy.9 However, reports suggest that cardiac MRI may provide better anatomical visualisation of epicardial borders and pericardial masses.9 Although it is difficult to distinguish between lymphomatous cardiac masses and other malignant tumours with cardiac MRI, it may allow earlier diagnosis and improve biopsy yield.9 In the case described by Monsuez et al, however, MRI-guided biopsy of a presumed residual tumour seen on MRI revealed only fibrotic tissue.9 Our patient similarly underwent biopsy of a persistent cardiac mass (after R-CHOP), which was negative for lymphoma. Serial echocardiography 10 days later detected first evidence of myocardial infiltration, coinciding with development of AV block. This highlights the potential limitations of cardiac MRI and the importance of serial echocardiography to assess response to therapy and diagnose treatment failure.
A case series of cardiac NHL cases from 1990 to 2015 reported heart failure as the most common presenting symptom in 34%, with only 4% of patients presenting with complete heart block.2 DLBCL subtype was slightly more likely to present with complete heart block.2 Although rare, cardiac conduction disturbance may be the first manifestation of cardiac lymphoma or of treatment failure.9 Improved prognosis has been observed in patients presenting with conduction system disease as first manifestation of disseminated lymphoma, compared with those presenting with heart failure.2 AV nodal block due to malignant infiltration of the myocardium is potentially reversible and may resolve completely following chemotherapy.10 It may therefore be reasonable to delay pacemaker insertion until after chemotherapy in stable patients.10 Unfortunately, our patient experienced symptomatic high-grade heart block following treatment failure, necessitating semiurgent permanent pacemaker insertion. Owing to difficulty in ruling out sepsis as a cause of fever and inflammatory response, as well as concerns regarding tissue healing on high dose prednisolone, she underwent insertion of a leadless, Medtronic ‘Micra’ pacemaker via transcatheter approach. This pacemaker is a safe and effective alternative to traditional transvensous devices with lower complication rate due to lack of pacemaker pocket and leads, making this a favourable option in individuals with high infection risk.11
Although R-CHOP chemotherapy improves the prognosis of extranodal disease significantly, data on the prognosis of patients with cardiac involvement of disseminated lymphoma is based on limited case reports.2 4 5 In the case series by Gordon et al, median survival for patients with B cell lymphoma with cardiac involvement was 4 months, with a trend towards improved survival in primary versus secondary cardiac NHL (6 months vs 2 months, respectively (HR 0.56; 95% CI 0.14 to 2.28; p=0.42)).2 Of the 34% of patients who received chemotherapy, median survival was 18 months versus 1 month for those who did not (HR 0.16; 95% CI 0.47 to 0.54; p=0.0003).2 The vast majority of patients diagnosed after the year 2000 received chemotherapy with overall better prognosis, likely due to widespread availability of rituximab.2
Our patient developed refractory disease involving the pericardium despite adequate response to R-CHOP chemotherapy at other sites. The authors postulate that poor chemotherapy penetration into the hypovascular pericardial region may have been a factor in limited treatment efficacy. In addition, large neoplastic pericardial effusions are known to have high recurrence rates of 40%–70%.7 The European Society of Cardiology 2015 Guidelines recommend pericardial drainage as the first line approach for large malignant effusions (Class I, B). Some cases report surgical management with a pericardial window (via mini-thoracotomy) being performed upfront for malignant cardiac tamponade. This may be considered a safe and effective alternative to pericardiocentesis in malignant cardiac tamponade; however, data is limited (Class IIb, B). Pericardiectomy is only rarely indicated, mainly in the case of complicated pericardial constriction. Apart from systemic chemotherapy, radiotherapy may serve as an effective adjunct in symptomatic control of secondary lymphomatous pericardial effusions. This, however, increases risk of myocarditis and chronic pericarditis.
This patient presented several management challenges including comorbid renal dysfunction, concurrent anal carcinoma in situ, recurrent infections, long-term immunosuppression and requirement for long-term anticoagulation. In addition, she had to relocate from a rural location more than 1500 km to receive her care. Despite receiving adequate first line chemotherapy, her cardiac lymphoma progressed. Our case therefore highlights the importance of maintaining a high index of suspicion for lymphoma in an immunocompromised individual with a newly diagnosed pericardial effusion.
Learning points.
Pericardial effusion is as a rare initial manifestation of disseminated lymphoma in immunosuppressed individuals, but can occur in up to 20% of individuals with non-Hodgkin’s lymphoma.
Lymphomatous myocardial involvement should be considered in otherwise unexplained atrioventricular block.
Cardiac involvement in disseminated lymphoma may limit treatment efficacy. Pathological diagnosis is therefore important in guiding management.
Acknowledgments
The authors thank Dr Benedict Carnley, Consultant Haematologist, Royal Perth Hospital, the primary consultant for patient’s care.
Footnotes
Contributors: LG, MC and BK were involved in patient’s care. MC contributed significantly to authorship of the paper. BK contributed to authorship and final review of paper.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Vijayalakshmi IB, Govindappa S, Narasimhan C, et al. . Multiple intracardiac tumors secondary to non-Hodgkin lymphoma. J Echocardiogr 2015;13:113–5. 10.1007/s12574-015-0253-5 [DOI] [PubMed] [Google Scholar]
- 2.Gordon MJ, Danilova O, Spurgeon S, et al. . Cardiac non-Hodgkin’s lymphoma: clinical characteristics and trends in survival. Eur J Haematol 2016;97:445–52. 10.1111/ejh.12751 [DOI] [PubMed] [Google Scholar]
- 3.McDonnell PJ, Mann RB, Bulkley BH. Involvement of the heart by malignant lymphoma: a clinicopathologic study. Cancer 1982;49:944–51. [DOI] [PubMed] [Google Scholar]
- 4.O’Mahony D, Peikarz RL, Bandettini WP, et al. . Cardiac involvement with lymphoma: a review of the literature. Clin Lymphoma Myeloma 2008;8:249–52. 10.3816/CLM.2008.n.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bussani R, De-Giorgio F, Abbate A, et al. . Cardiac metastases. J Clin Pathol 2007;60:27–34. 10.1136/jcp.2005.035105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldberg AD, Blankstein R, Padera RF. Tumors metastatic to the heart. Circulation 2013;128:1790–4. 10.1161/CIRCULATIONAHA.112.000790 [DOI] [PubMed] [Google Scholar]
- 7.Adler Y, Charron P, Imazio M, et al. . 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: the task force for the diagnosis and management of pericardial diseases of the European Society of Cardiology (ESC)Endorsed by: the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2015;36:2921–61. 10.1093/eurheartj/ehv318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duong M, Dubois C, Buisson M, et al. . Non-Hodgkin’s lymphoma of the heart in patients infected with human immunodeficiency virus. Clin Cardiol 1997;20:497–502. 10.1002/clc.4960200519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monsuez JJ, Frija J, Hertz-Pannier L, et al. . Non-Hodgkin’s lymphoma with cardiac presentation: evaluation and follow-up with echocardiography and MR imaging. Eur Heart J 1991;12:464–7. 10.1093/oxfordjournals.eurheartj.a059918 [DOI] [PubMed] [Google Scholar]
- 10.Crisel RK, Knight BP, Kim SS. Reversible, complete atrioventricular block caused by primary cardiac lymphoma in a nonimmunocompromised patient. J Cardiovasc Electrophysiol 2012;23:1386–9. 10.1111/j.1540-8167.2012.02343.x [DOI] [PubMed] [Google Scholar]
- 11.Reynolds D, Duray GZ, Omar R, et al. . A leadless intracardiac transcatheter pacing system. N Engl J Med Overseas Ed 2016;374:533–41. 10.1056/NEJMoa1511643 [DOI] [PubMed] [Google Scholar]