Abstract
Background
Infections caused by Gram-negative pathogens resistant to carbapenems have limited treatment options and are associated with increased morbidity and mortality. We evaluated the rates, infection sources, and pathogen types associated with carbapenem-nonsusceptible (Carb-NS) Gram-negative isolates in intensive care unit (ICU) and non-ICU settings in a large US hospital database.
Methods
We conducted a retrospective cross-sectional analysis of carbapenem susceptibility of all nonduplicate isolates of Gram-negative pathogens collected from January 1, 2017, to December 31, 2017, at 358 US hospitals in the BD Insights Research Database. Carb-NS isolates included all pathogens reported at the institutional level as intermediate or resistant.
Results
Of 312 075 nonduplicate Gram-negative isolates, 10 698 (3.4%) were Carb-NS. Respiratory samples were the most frequent source of Carb-NS isolates (35.2%); skin/wound accounted for 23.6%. Pseudomonas aeruginosa was the most common Carb-NS pathogen (58.5% of isolates), and about 30% were Enterobacteriaceae. The highest rates of Carb-NS were found in Acinetobacter spp. (35.6%) and P. aeruginosa (14.6%). The rate of Carb-NS was significantly higher in ICU (5.4%) vs non-ICU settings (2.7%; P < .0001 in univariate analysis). This difference remained significant in multivariable analysis after adjusting for infection and hospital characteristics (odds ratio, 1.35; 95% confidence interval, 1.17–1.56; P < .0001).
Conclusions
Infections caused by Carb-NS isolates pose a significant clinical problem across different sources of infection, species of pathogen, and hospital settings. Widespread infection prevention and antimicrobial stewardship initiatives, in combination with new treatment options, may be required to reduce the burden of carbapenem resistance in health care settings.
Keywords: carbapenems, surveillance, Gram-negative pathogens, susceptibility, intensive care unit
First introduced in 1985, carbapenems continue to play a critical role as some of the agents of last resort for the treatment of antibiotic-resistant Gram-negative pathogens [1]. Increasing resistance to carbapanems thus jeopardizes patient outcomes and results in a significant economic burden [2, 3]. The increased morbidity and mortality associated with antibiotic resistance is of particular concern in vulnerable populations such as patients in intensive care units (ICUs) [4]. In recognition of this critical risk to public health, the Centers for Disease Control and Prevention has identified carbapenem-resistant Enterobacteriaceae (CRE) as an urgent threat [5], and the World Health Organization has prioritized the development of antibiotics against CRE and carbapenem-resistant Acinetobacter baumannii and Pseudomonas aeruginosa [6].
Hospital antibiograms and susceptibility data provide key information about the ecology of Gram-negative pathogens and the prevalence of resistance. This information can inform treatment decisions when selecting appropriate empiric treatments and therapies to address confirmed cases of carbapenem resistance. Effective therapy is critical for successful treatment of patients presenting with serious infections [7]. The objective of this study was to use a large multicenter, real-world database to evaluate carbapenem nonsusceptibility (Carb-NS) in Gram-negative bacteria in both ICU and non-ICU settings in US hospitals, with the goal of gaining insight into common culture sources and settings for infection and key pathogens.
METHODS
Study Design
This was a retrospective cross-sectional study of antimicrobial susceptibility of all nonduplicate (first isolate in 30 days) Enterobacteriaceae, P. aeruginosa, and Acinetobacter spp. isolates from ICU and non-ICU patients collected from January 1, 2017, to December 31, 2017. Reporting institutions comprised 358 US hospitals included in the BD Insights Research Database (Becton, Dickinson and Company, Franklin Lakes, NJ). The electronic surveillance system and clinical research database (formerly the CareFusion Clinical Research Database) have been previously described [8–10]. This database provides good geographical representation across the United States and includes both small and large hospitals in urban and rural areas.
The analyses reported here include all nonduplicate Enterobacteriaceae, P. aeruginosa, and Acinetobacter spp. (A. baumannii and A. haemolyticus) isolates from blood, respiratory, urine, skin/wound, intraabdominal, and other sources. Isolates from each source were considered separately; for example, if the patient had a blood and respiratory isolate for P. aeruginosa within 30 days, then an isolate was counted for each source. Isolates from the same patient within 30 days were included if they had different drug susceptibilities (>1 susceptibility difference). Isolates were classified as Carb-NS based on facility reports of intermediate susceptibility or resistance to at least 1 of the following agents: (a) ertapenem, imipenem, meropenem, or doripenem for Escherichia coli, Klebsiella pneumoniae, Enterobacter aerogenes, Enterobacter cloacae, Serratia marcescens, and Citrobacter freundii; (b) ertapenem, meropenem, or doripenem for Proteus mirabilis and Morganella morganii; and (c) imipenem, meropenem, or doripenem for P. aeruginosa and Acinetobacter spp.
Care settings were classified using the Centers for Disease Control and Prevention (CDC) National Healthcare Safety Network classification and further classified as ICU (critical care) and non-ICU (inpatient adult wards, specialty care areas, and step-down wards). Hospital-onset isolates were defined as those occurring >3 days after inpatient admission or within 14 days of previous discharge, whereas admission isolates were defined as those occurring ≤3 days of inpatient admission with no previous admission within the past 14 days. Admission period isolates were classified as ICU-associated if the isolate was collected in the admission period and the patient was admitted to an ICU within 3 days of inpatient admission, and they were classified as non-ICU-associated if the isolate was collected in the admission period and the patient was admitted to a non-ICU location within 3 days of inpatient admission and did not have an ICU admission within 3 days of the inpatient admission. Hospital-onset isolates were classified as ICU-associated if the patient was admitted to an ICU on the specimen collection date and as non-ICU-associated if the patient was admitted to a non-ICU location on the specimen collection date with no ICU admission on that date. The study was approved by the New England Institutional Review Board (Wellesley, MA).
Outcomes
The primary outcome was the rate of Carb-NS isolates as determined by local laboratory breakpoints and practices per routine clinical standard of care.
Statistical Analysis
Statistical analysis included descriptive analysis, univariate analysis, and multivariable statistical modeling of the data. The univariate analysis was conducted to examine the associations between Carb-NS and ICU status, as well as other potential factors or confounders, including onset period, culture source of isolates collected, pathogen, and hospital characteristics (teaching status, bed size, urban/rural, and geographic location). Chi-square tests (or Fisher’s exact tests when expected frequency <5) were used to assess statistical significance in the univariate (unadjusted) analysis. In the multivariable (adjusted) analysis phase, we used a generalized linear mixed model (GLMM) method with hospital as a random effect to assess the effect of ICU status on Carb-NS rates. Specifically, the Carb-NS rates were modeled using random intercept logistic regression models with hospital as a random effect. Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated. All analyses were conducted using SAS, version 9.4 (SAS Institute, Cary, NC).
RESULTS
Of the 312 075 nonduplicate isolates tested, 80 310 (25.7%) were collected in the ICU setting and 74 991 (24.0%) were from hospital-onset infections (Table 1). The most common source was urine (59.4%), followed by skin/wound, respiratory, and blood. Over 90% of isolates were collected in urban hospitals. The most common pathogens isolated were E. coli (47.9%), K. pneumoniae (15.5%), and P. aeruginosa (13.7%) (Table 2).
Table 1.
Distribution of Isolates, Carb-NS Rates, and Univariate Analysis Results
| Total Isolates | Carb-NS Isolates | P Valuea | |||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Overall | 312 075 | 100.0 | 10 698 | 3.4 | |
| ICU status | <.0001 | ||||
| Non-ICU | 231 765 | 74.3 | 6344 | 2.7 | |
| ICU | 80 310 | 25.7 | 4354 | 5.4 | |
| Onset | <.0001 | ||||
| Admission | 237 084 | 76.0 | 5606 | 2.4 | |
| Hospital | 74 991 | 24.0 | 5092 | 6.8 | |
| Source | <.0001 | ||||
| Urine | 185 339 | 59.4 | 3311 | 1.8 | |
| Skin/wound | 48 589 | 15.6 | 2521 | 5.2 | |
| Respiratory | 32 778 | 10.5 | 3775 | 11.5 | |
| Blood | 30 499 | 9.8 | 588 | 1.9 | |
| Other sources | 7969 | 2.6 | 362 | 4.5 | |
| Intra-abdominal | 6901 | 2.2 | 141 | 2.0 | |
| Teaching hospital | <.0001 | ||||
| Nonteaching | 162 287 | 52.0 | 4802 | 3.0 | |
| Teaching | 149 788 | 48.0 | 5896 | 3.9 | |
| Bed size | <.0001 | ||||
| >300 | 180 097 | 57.7 | 7283 | 4.0 | |
| 100–300 | 114 052 | 36.5 | 3026 | 2.7 | |
| <100 | 17 926 | 5.7 | 389 | 2.2 | |
| Urban/rural | <.0001 | ||||
| Urban | 286 398 | 91.8 | 10 163 | 3.5 | |
| Rural | 25 677 | 8.2 | 535 | 2.1 | |
| Geographic region | <.0001 | ||||
| South | 136 421 | 43.7 | 4781 | 3.5 | |
| Midwest | 80 270 | 25.7 | 2828 | 3.5 | |
| Northeast | 51 769 | 16.6 | 1927 | 3.7 | |
| West | 43 615 | 14.0 | 1162 | 2.7 | |
Abbreviations: Carb, carbapenem; ICU, intensive care unit; NS, nonsusceptible.
aUnivariate analysis of clinical and hospital factors correlating with Carb-NS.
Table 2.
Carb-NS Rates by Pathogen
| Pathogen | Total Isolates | Carb-NS Isolatesa | |||
|---|---|---|---|---|---|
| No. | % of Total | No. | % of Total Isolates | % of Carb-NS Isolates | |
| Any | 312 075 | 100.0 | 10 698 | 3.4 | 100 |
| Enterobacteriaceae | 265 781 | 85.2 | 3227 | 1.2 | 30.2 |
| Escherichia coli | 149 420 | 47.9 | 458 | 0.3 | 4.3 |
| Klebsiella pneumoniae | 48 453 | 15.5 | 1375 | 2.8 | 12.9 |
| Proteus mirabilis | 26 585 | 8.5 | 131 | 0.5 | 1.2 |
| Enterobacter cloacae | 12 971 | 4.2 | 696 | 5.4 | 6.5 |
| Klebsiella oxytoca | 7422 | 2.4 | 65 | 0.9 | 0.6 |
| Serratia marcescens | 6553 | 2.1 | 176 | 2.7 | 1.6 |
| Enterobacter aerogenes | 5087 | 1.6 | 218 | 4.3 | 2.0 |
| Morganella morganii | 4866 | 1.6 | 21 | 0.4 | 0.2 |
| Citrobacter freundii | 4424 | 1.4 | 87 | 2.0 | 0.8 |
| Pseudomonas aeruginosa | 42 880 | 13.7 | 6256 | 14.6 | 58.5 |
| Acinetobacter spp. | 3414 | 1.1 | 1215 | 35.6 | 11.4 |
Abbreviations: Carb, carbapenem; NS, nonsusceptible.
a P < .0001 for correlation of pathogen with Carb-NS.
Carbapenem Nonsusceptibility in Hospital Isolates
Overall, 10 698 nonduplicate Carb-NS pathogens were isolated, resulting in a Carb-NS rate of 3.4% (Table 1). The majority (80.3%) of Carb-NS pathogens were reported as resistant, and the remainder (19.7%) had intermediate susceptibility (Supplementary Table 1). Respiratory samples were the most frequent source for Carb-NS isolates (n = 3775; 35.3%), followed by urine (30.9%), skin/wound (23.6%), and blood (5.5%). The highest Carb-NS rates were found in respiratory samples (11.5%), followed by skin/wound (5.2%) and other sources (2.0%).
P. aeruginosa accounted for over half (58.5%) of all Carb-NS pathogens, followed by Enterobacteriaceae (30.2%, with K. pneumoniae constituting 12.9% of all Carb-NS pathogens and 42.6% of Carb-NS Enterobacteriaceae) and Acinetobacter spp. (11.4%) (Table 2). The pathogens with the highest rates of Carb-NS were Acinetobacter spp. (35.6%) and P. aeruginosa (14.6%) (Table 2). The lowest Carb-NS rates were observed in E. coli (0.3%) and M. morganii (0.4%). Univariate analysis indicated that Carb-NS rates were significantly influenced by pathogen type (P < .0001).
The contributions of different pathogens to Carb-NS rates varied depending on the specimen source (Supplementary Table 2). P. aeruginosa was the most common Carb-NS pathogen for all specimen sources except blood and intra-abdominal (second to Enterobacteriaceae). The contribution of Acinetobacter spp. to Carb-NS isolates was fairly minor (≤5%) for urine and intra-abdominal sources, but this pathogen accounted for approximately 20% and 15% of Carb-NS isolates for skin/wound and blood, respectively.
Carb-NS rates also varied across geographic regions (Supplementary Table 3). The highest rates for Carb-NS Enterobacteriaceae and Acinetobacter spp. were in US Department of Health and Human Services (HHS) Region 2 (2.2% and 53.5%, respectively), which includes New York and New Jersey. The highest Carb-NS P. aeruginosa rates were observed in HHS Region 6 (17.1%), which includes states in the south-central region (Arkansas, Louisiana, New Mexico, Oklahoma, and Texas).
The majority of Carb-NS isolates were also multidrug-resistant (64.0% to 97.8%) and nonsusceptible to extended-spectrum cephalosporins (54.8% to 86.2%) and fluoroquinolones (57.4% to 97.7%) (Supplementary Table 4). Piperacillin-tazobactam had better activity against Carb-NS P. aeruginosa (34.8% nonsusceptible) than against Carb-NS Enterobacteriaceae (71.9% nonsusceptible) or Acinetobacter spp. (51.2% nonsusceptible).
Carbapenem Nonsusceptibility in ICU vs Non-ICU Settings
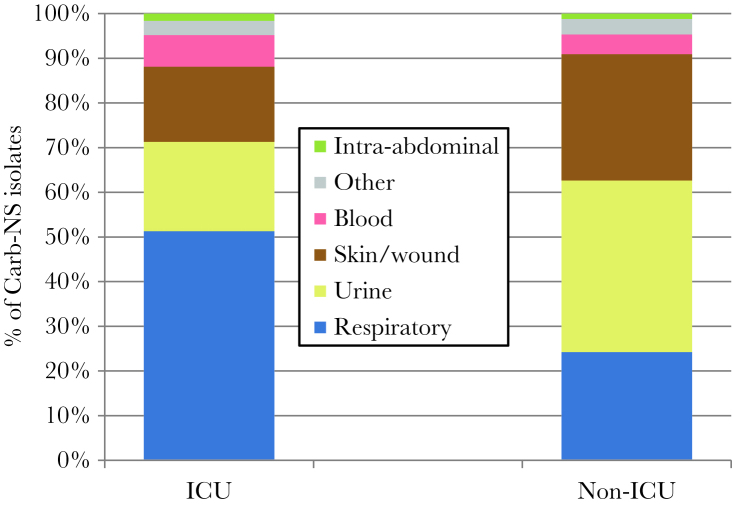
A total of 4354 Carb-NS isolates (40.7%) were obtained in the ICU, and the remaining 6344 (59.3%) were obtained in non-ICU settings. ICU and non-ICU Carb-NS isolates varied markedly by culture source. The most common source for ICU Carb-NS isolates was respiratory (51.3% vs 24.3% for non-ICU isolates), whereas the most common source for non-ICU Carb-NS isolates was urine (38.5% vs 20.0% for ICU isolates) (Figure 1, Table 3). Skin/wound was a more common source of Carb-NS isolates in non-ICU settings compared with ICUs (28.2% vs 16.8%). In contrast to the differences observed in the source of Carb-NS isolates, pathogen distribution between ICU and non-ICU settings was quite similar. Enterobacteriaceae accounted for 29.0% of Carb-NS isolates in the ICU compared with 31.0% in non-ICU settings. P. aeruginosa (58.4% in the ICU vs 58.5% for non-ICU) and Acinetobacter spp. (12.6% vs 10.5%) were also fairly equally distributed between the 2 settings (Table 3).
Figure 1.
Distribution of Carb-NS ICU and non-ICU isolates by source. Abbreviations: Carb, carbapenem; ICU, intensive-care unit; NS, nonsusceptible.
Table 3.
Adjusted Effect of ICU Status on Carb-NS Rates: Overall and by Onset, Source, and Pathogen
| Characteristic or Pathogen | ICU | Non-ICU | ICU vs Non-ICU: Model-Estimated ORa |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NS | Tested | NS% | NS | Tested | NS% | OR | 95% CI | P Value | ||
| Overall | 4354 | 80 310 | 5.4 | 6344 | 231 765 | 2.7 | 1.35 | 1.17 | 1.56 | <.0001 |
| Onset | ||||||||||
| Admission | 2012 | 54 795 | 3.7 | 3594 | 182 289 | 2.0 | 1.53 | 1.32 | 1.78 | <.0001 |
| Hospital | 2342 | 25 515 | 9.2 | 2750 | 49 476 | 5.6 | 1.19 | 1.03 | 1.39 | .0223 |
| Source | ||||||||||
| Blood | 311 | 11 351 | 2.7 | 277 | 19 148 | 1.4 | 1.34 | 1.09 | 1.65 | .0062 |
| Intra-abdominal | 68 | 2076 | 3.3 | 73 | 4825 | 1.5 | 1.54 | 1.06 | 2.23 | .0236 |
| Other sources | 138 | 2106 | 6.6 | 224 | 5863 | 3.8 | 1.66 | 1.27 | 2.17 | .0002 |
| Respiratory | 2234 | 20 316 | 11.0 | 1541 | 12 462 | 12.4 | 0.93 | 0.80 | 1.08 | .3423 |
| Skin/wound | 732 | 9041 | 8.1 | 1789 | 39 548 | 4.5 | 1.63 | 1.40 | 1.91 | <.0001 |
| Urine | 871 | 35 420 | 2.5 | 2440 | 149 919 | 1.6 | 1.18 | 1.02 | 1.36 | .0292 |
| Pathogen | ||||||||||
| Enterobacteriaceae | 1263 | 65 684 | 1.9 | 1964 | 200 097 | 1.0 | 1.59 | 1.44 | 1.75 | <.0001 |
| E. coli | 153 | 32 685 | 0.5 | 305 | 116 735 | 0.3 | 1.70 | 1.38 | 2.09 | <.0001 |
| K. pneumoniae | 612 | 14 084 | 4.3 | 763 | 34 369 | 2.2 | 1.82 | 1.60 | 2.08 | <.0001 |
| P. mirabilis | 36 | 6749 | 0.5 | 95 | 19 836 | 0.5 | 1.03 | 0.70 | 1.53 | .8747 |
| E. cloacae | 241 | 3716 | 6.5 | 455 | 9255 | 4.9 | 1.23 | 1.03 | 1.47 | .0237 |
| K. oxytoca | 24 | 2046 | 1.2 | 41 | 5376 | 0.8 | 1.35 | 0.81 | 2.27 | .2526 |
| S. marcescens | 79 | 2477 | 3.2 | 97 | 4076 | 2.4 | 1.36 | 0.99 | 1.86 | .0570 |
| E. aerogenes | 84 | 1756 | 4.8 | 134 | 3331 | 4.0 | 1.18 | 0.88 | 1.59 | .2671 |
| M. morganii | 5 | 1161 | 0.4 | 16 | 3705 | 0.4 | 0.94 | 0.34 | 2.57 | .8995 |
| C. freundii | 29 | 1010 | 2.9 | 58 | 3414 | 1.7 | 1.61 | 1.02 | 2.56 | .0427 |
| P. aeruginosa | 2544 | 13 250 | 19.2 | 3712 | 29 630 | 12.5 | 1.55 | 1.41 | 1.70 | <.0001 |
| Acinetobacter spp. | 547 | 1376 | 39.8 | 668 | 2038 | 32.8 | 1.38 | 1.15 | 1.66 | .0006 |
Abbreviations: Carb, carbapenem; CI, confidence interval; ICU, intensive care unit; NS, nonsusceptible; OR, odds ratio.
aEffect of ICU status adjusted using generalized linear mixed models. Adjusting variables include onset, source, pathogen, and hospital characteristics (teaching status, bed size, urban/rural, geographic region).
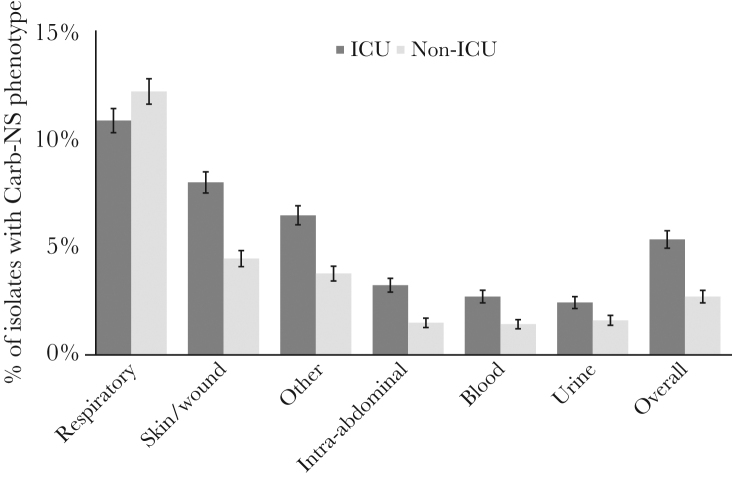
The rate of Carb-NS was significantly higher in ICU settings (5.4%) than in non-ICU settings (2.7%; P < .0001 in unadjusted [univariate] analysis) (Table 1). For both ICU and non-ICU settings, the sources with the highest Carb-NS rates were respiratory and skin/wound (Figure 2), and the pathogens with the highest Carb-NS rates were Acinetobacter spp. and P. aeruginosa (Table 3). In addition to ICU setting, other clinical and hospital factors associated with significantly higher Carb-NS rates included hospital-onset infections, isolate source, teaching vs nonteaching hospital, greater bed size, urban status, and non-Western US geographic region (all P < .0001) (Table 1).
Figure 2.
Carb-NS rates by ICU status and isolate source. Capped error bars indicate 95% confidence intervals. Abbreviations: Carb, carbapenem; ICU, intensive care unit; NS, nonsusceptible.
Multivariable Regression Analysis of the Association Between ICU Status and Carb-NS
The 2-fold difference in the Carb-NS rate in ICU vs non-ICU settings could potentially be explained by other factors associated with Carb-NS in univariate analyses. We therefore conducted a multivariable analysis using GLMM to evaluate the impact of the ICU setting on Carb-NS rates in greater detail.
The GLMM analysis, which was adjusted for infection onset, isolate source, pathogen, and hospital characteristics, confirmed that the Carb-NS rate was significantly higher in ICU vs non-ICU settings (OR, 1.35; 95% CI, 1.17–1.56; P < .0001). With the exception of respiratory isolates, isolates from all sources had significantly higher Carb-NS rates in ICU vs non-ICU settings (Table 3). The Carb-NS rate was also significantly higher for ICU vs non-ICU settings for both admission-onset and hospital-onset isolates (Table 3).
ICU status had varying effects on Carb-NS for different pathogens. The 3 most common pathogens, E. coli, K. pneumoniae, and P. aeruginosa, all had significantly higher Carb-NS rates in ICU vs non-ICU settings (ORs, 1.70, 1.82, and 1.55, respectively; all P < .0001), as did the Enterobacteriaceae family (OR, 1.59) (Table 3; Supplementary Figure 1). Carb-NS rates were also significantly higher in the ICU for E. cloacae, C. freundii, and Acinetobacter spp. ICU status did not have a significant effect on Carb-NS rates for the remaining pathogens.
DISCUSSION
Carbapenems are often used as one of the agents of last resort in the treatment of serious Gram-negative infections; therefore, carbapenem resistance is a critical clinical problem [11]. In the study reported here, we found an overall rate of 3.4% Carb-NS in 312 075 hospital Gram-negative bacterial isolates, with P. aeruginosa being the most frequent Carb-NS pathogen. Respiratory samples were the most common source of Carb-NS pathogens; skin/wound isolates accounted for almost one-quarter of isolates. Carb-NS rates were significantly higher in ICU vs non-ICU settings, although greater numbers of Carb-NS were identified in non-ICU settings.
Although almost unheard of in the United States until the 1990s, carbapenem resistance has become an important public health problem [12]. CRE is of particular concern. Not only are Enterobacteriaceae a frequent cause of common infections, but colonization with these bacteria allows efficient transfer of CRE between patients, particularly within health care settings [12]. CRE frequently carry plasmid-mediated carbapenem resistance determinants, such as K. pneumoniae carbapenemase (KPC), which can be easily disseminated to different pathogens [13]. The problem of carbapenem resistance in this pathogen is magnified by the paucity of options for the treatment of CRE [11], which may lead to inappropriate initial therapy [14]. At current rates, CRE infections are estimated to result in a 26% mortality rate and cost hospitals $275 million annually [2].
Approximately 30% of the Carb-NS isolates identified in our study were Enterobacteriaceae; the most common individual Enterobacteriaceae pathogens were K. pneumoniae and E. cloacae. Both pathogens have seen rapid increases in Carb-NS rates in the past few years [15, 16]. In the United States, rates of carbapenem-resistant K. pneumoniae increased from 0.1% in 2002 to 4.5% in 2010 [15]. Although less common, E. cloacae had the highest Carb-NS rate among Enterobacteriaceae in our analysis. Recent studies have suggested that subsequent to the rapid increase that occurred in carbapenem-resistant K. pneumoniae, a “second epidemic” of carbapenem-resistant E. cloacae may be occurring [16].
Although early clinical attention was primarily focused on CRE, carbapenem-resistant nonfermenters, particularly P. aeruginosa and Acinetobacter spp., are now recognized as an increasing problem. These pathogens have always been difficult to treat, but carbapenem resistance further compounds the morbidity and mortality of associated infections [17]. Of note, carbapenem resistance in A. baumannii more than doubled between 2003/2005 and 2009/2012 (21.0% to 47.9%) [18]. The high rates of resistance in nonfermenters are of significant concern, as carbapenem resistance is associated with a more than 2-fold increase in mortality for both P. aeruginosa (adjusted OR, 2.38) [19] and Acinetobacter (adjusted OR, 2.49) [20].
The majority of Carb-NS pathogens in this study were multidrug-resistant and resistant to extended-spectrum cephalosporins, fluoroquinolones, and piperacillin-tazobactam. This finding provides strong support for further investigations into new antimicrobial options with activity against Carb-NS isolates.
Respiratory infections were the most common source of Carb-NS isolates, and P. aeruginosa accounted for >70% of the Carb-NS respiratory isolates. Pneumonia is a leading cause of death among hospital patients in the United States [21] and is a particular problem in the ICU. The mean hospital stay for P. aeruginosa pneumonia in US ICUs is 55.4 days per patient, and the mortality rate is >20% [22], a frequency that likely increases with carbapenem resistance [19]. These grim statistics highlight the clinical relevance of high Carb-NS rates in respiratory P. aeruginosa isolates and the vital need for more therapeutic options to treat carbapenem-resistant Gram-negative respiratory infections.
Our study also highlights skin and wound infections as an important potential source of Carb-NS pathogens. Recognition of Carb-NS pathogens in skin/wound samples is imperative from both the patient management and infection prevention perspectives, as wounds can serve as mobile reservoirs for CRE and other pathogens, thereby increasing their spread within and outside of hospitals [23]. Skin/wound infections are not typically included in routine surveillance of sterile site cultures, and there is thus limited data on carbapenem resistance from this source. In our study, skin/wound isolates showed a high rate of Carb-NS (5.2%; second only to respiratory isolates) and accounted for 23.6% of the total Carb-NS isolates. We hope this finding encourages clinical centers to include skin and wound infections in carbapenem resistance surveillance initiatives.
In our study, ICUs had significantly higher rates of Carb-NS Gram-negative pathogens than non-ICU settings. A significant difference in Carb-NS rates for ICU vs non-ICU settings was observed for all sources except respiratory and for the most common pathogens. It is unclear why respiratory specimens, the most common source of Carb-NS isolates overall and in the ICU and the source with the highest Carb-NS rate, did not show a difference for ICU vs non-ICU settings.
Higher Carb-NS rates for ICU vs non-ICU have also been noted in other studies [24–26]. This is perhaps not surprising given that many of the risk factors for carbapenem-resistant infections, such as antibiotic exposure, underlying diseases, invasive procedures, medical devices, and mechanical ventilation [27], are more common in the ICU than in general wards. The high Carb-NS rates in the ICU may be related to intestinal carriage of carbapenem-resistant Gram-negative bacteria in ICU patients, which have been found to increase rapidly during ICU stays (from 5.6% after 1 week to 58.6% after 6 weeks in the ICU) [28].
The impact of antimicrobial resistance in the ICU is magnified by the critical illnesses faced by this patient population. ICU patients are highly vulnerable to infections, which can be difficult to treat in ICU patients due to comorbidities and altered pharmacokinetic/pharmacodynamic parameters as a result of sepsis or augmented renal clearance [9, 29]. ICU patients are thus at high risk for poor outcomes subsequent to infections with Carb-NS pathogens. Antimicrobial resistance in Gram-negative pathogens has been shown to increase mortality, length of stay, and economic costs in ICU patients [30].
Although Carb-NS rates were higher in the ICU, it is important to note that non-ICU settings had greater overall numbers. These data serve as a reminder that although the ICU may serve as a locus for Carb-NS pathogens, infections are also common in the non-ICU setting. Accordingly, hospital-wide infection control practices are critical for preventing the spread of resistant pathogens. Hospital-onset rates of Carb-NS pathogens were about 2.5-fold higher than the rates of Carb-NS at admission (6.8% vs 2.4%), supporting intensive national efforts to reduce hospital-acquired infections.
Study limitations include the lack of standardization with respect to the use of methods for determining Carb-NS and the lack of a central laboratory to confirm results, as susceptibility results were based on local laboratory practices. As observed recently [13], methods for carbapenem testing vary widely across different clinical centers, which may have influenced the Carb-NS results reported. It is also important to note that data were collected and analyzed from the perspective of unique nonduplicated collected cultures and not from the perspective of unique patients. We were therefore unable to perform adjusted analyses for ICU vs non-ICU based on patient characteristics. Finally, a key limitation of all retrospective studies of antimicrobial resistance, including the one reported here, is that culturing of hospitalized patients relies on the clinician’s assessment of the need for a clinical culture. Accordingly, our findings are based on a potentially biased sample representing more severely ill patients who required clinical culture. We therefore cannot infer the rates of Carb-NS isolates to the hospitalized patient population as a whole, as not all patients provided samples for cultures.
We conclude from our findings that Carb-NS Gram-negative bacteria continue to be an important problem in hospitals. Carb-NS bacteria, especially CRE, are an important focus of national infection control efforts. The CDC has identified CRE as an “urgent” threat to human health [5] and has developed resources to help facilities control the spread of these organisms (https://www.cdc.gov/hai/organisms/cre/cre-toolkit/index.html). The challenge of carbapenem resistance is compounded by the continued emergence and spread of novel carbapenemases [13]. An early and aggressive response to imported novel carbapenemases could potentially prevent them from becoming endemic in the United States. To that end, the CDC has launched a containment strategy that comprises improved laboratory detection of novel carbapenemases and resources to perform colonization testing through the Antimicrobial Resistance Lab Network (AR Lab Network), along with improved response capacity at state health departments (https://www.cdc.gov/hai/containment/guidelines.html). Infectious disease clinicians are critical partners in this control effort as they are the primary point of contact for many patients with Carb-NS infections. They should be aware of AR Lab Network resources and should know which Carb-NS isolates to refer to their state laboratory for further testing.
Although overall Carb-NS rates are fairly low in US hospitals, high rates are observed for some sources such as respiratory, some pathogens such as P. aeruginosa and Acinetobacter spp., and some locations such as the ICU. Unexpected sources also contribute to Carb-NS isolates. Given the important contributions of skin/wound infections to Carb-NS hospital isolates, it is clear that infection prevention and antimicrobial stewardship initiatives need to address carbapenem resistance associated with all types of infection in both ICU and non-ICU settings. We hope our data will encourage hospitals to conduct evaluations at the facility level that can be used to guide local antimicrobial therapy. In addition to these efforts, continued research into therapeutic options to treat carbapenem-resistant infections is urgently needed, particularly for respiratory infections. A multifaceted approach to this clinically significant problem, as exemplified by the tools developed by the CDC, may help stem the tide of carbapenem resistance.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank Patrick R. Murray, PhD, Senior Director of Worldwise Scientific Affairs at BD Life Sciences, for his careful review and insightful comments, and Fusion MD Medical Science Network, Inc., for providing manuscript support with funding from Becton, Dickinson & Company.
Author contributions. All authors assisted in developing the study concept and design. E.M., A.S., D.D.D., G.Y., J.M., and V.G. were involved in data analysis and interpretation. G.Y. and V.G. drafted the manuscript. All authors read and provided feedback to the manuscript and approved the submitted version. G.Y., J.M., and V.G. had full access to all data and had final responsibility for the decision to submit for publication.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Financial support. This work was funded by a grant from Merck & Co., Inc., Kenilworth, New Jersey, to Becton, Dickinson, and Company, Franklin Lakes, New Jersey.
Potential conflicts of interest. E. McCann, C. A. DeRyke, and D. D. DePestel are employees of Merck & Co., Inc., Kenilworth, New Jersey. V. Gupta, J. Murray, and G. Ye are employees of Becton, Dickinson & Company, Franklin Lakes, New Jersey. A. Srinivasan has no conflicts of interest to report. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Papp-Wallace KM, Endimiani A, Taracila MA, Bonomo RA. Carbapenems: past, present, and future. Antimicrob Agents Chemother 2011; 55:4943–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bartsch SM, McKinnell JA, Mueller LE, et al. Potential economic burden of carbapenem-resistant Enterobacteriaceae (CRE) in the United States. Clin Microbiol Infect 2017; 23:48.39–48.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thaden JT, Pogue JM, Kaye KS. Role of newer and re-emerging older agents in the treatment of infections caused by carbapenem-resistant Enterobacteriaceae. Virulence 2017; 8:403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kollef MH, Bassetti M, Francois B, et al. The intensive care medicine research agenda on multidrug-resistant bacteria, antibiotics, and stewardship. Intensive Care Med 2017; 43:1187–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. https://www.cdc.gov/drugresistance/biggest_threats.html. Accessed 1 December 2017.
- 6. World Health Organization. Global priority list of antibiotic-resistant bacteria to guide research, discover, and development of new antibiotics 2017. http://apps.who.int/medicinedocs/documents/s23171en/s23171en.pdf. Accessed 5 May 2018.
- 7. Micek ST, Welch EC, Khan J, et al. Empiric combination antibiotic therapy is associated with improved outcome against sepsis due to Gram-negative bacteria: a retrospective analysis. Antimicrob Agents Chemother 2010; 54:1742–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brossette SE, Hacek DM, Gavin PJ, et al. A laboratory-based, hospital-wide, electronic marker for nosocomial infection: the future of infection control surveillance?Am J Clin Pathol 2006; 125:34–9. [PubMed] [Google Scholar]
- 9. Tabak YP, Zilberberg MD, Johannes RS, et al. Attributable burden of hospital-onset Clostridium difficile infection: a propensity score matching study. Infect Control Hosp Epidemiol 2013; 34:588–96. [DOI] [PubMed] [Google Scholar]
- 10. Ridgway JP, Sun X, Tabak YP, et al. Performance characteristics and associated outcomes for an automated surveillance tool for bloodstream infection. Am J Infect Control 2016; 44:567–71. [DOI] [PubMed] [Google Scholar]
- 11. Morrill HJ, Pogue JM, Kaye KS, LaPlante KL. Treatment options for carbapenem-resistant Enterobacteriaceae infections. Open Forum Infect Dis 2015; 2:ofv050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gupta N, Limbago BM, Patel JB, Kallen AJ. Carbapenem-resistant Enterobacteriaceae: epidemiology and prevention. Clin Infect Dis 2011; 53:60–7. [DOI] [PubMed] [Google Scholar]
- 13. Bonomo RA, Burd EM, Conly J, et al. Carbapenemase-producing organisms: a global scourge. Clin Infect Dis 2018; 66:1290–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zilberberg MD, Nathanson BH, Sulham K, et al. Carbapenem resistance, inappropriate empiric treatment and outcomes among patients hospitalized with Enterobacteriaceae urinary tract infection, pneumonia and sepsis. BMC Infect Dis 2017; 17:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Braykov NP, Eber MR, Klein EY, et al. Trends in resistance to carbapenems and third-generation cephalosporins among clinical isolates of Klebsiella pneumoniae in the United States, 1999–2010. Infect Control Hosp Epidemiol 2013; 34:259–68. [DOI] [PubMed] [Google Scholar]
- 16. Wilson BM, El Chakhtoura NG, Patel S, et al. Carbapenem-resistant Enterobacter cloacae in patients from the US Veterans Health Administration, 2006–2015. Emerg Infect Dis 2017; 23:878–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cai B, Echols R, Magee G, et al. Prevalence of carbapenem-resistant Gram-negative infections in the United States predominated by Acinetobacter baumannii and Pseudomonas aeruginosa. Open Forum Infect Dis 2017; 4:ofx176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zilberberg MD, Nathanson BH, Sulham K, et al. Multidrug resistance, inappropriate empiric therapy, and hospital mortality in Acinetobacter baumannii pneumonia and sepsis. Crit Care 2016; 20:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu Q, Li X, Li W, et al. Influence of carbapenem resistance on mortality of patients with Pseudomonas aeruginosa infection: a meta-analysis. Sci Rep 2015; 5:11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lemos EV, de la Hoz FP, Einarson TR, et al. Carbapenem resistance and mortality in patients with Acinetobacter baumannii infection: systematic review and meta-analysis. Clin Microbiol Infect 2014; 20:416–23. [DOI] [PubMed] [Google Scholar]
- 21. Hall MJ, Levant S, DeFrances CJ. Trends in inpatient hospital deaths: National Hospital Discharge Survey, 2000–2010. NHS Data Brief 2013; (118):1–8. [PubMed] [Google Scholar]
- 22. Kyaw MH, Kern DM, Zhou S, et al. Healthcare utilization and costs associated with S. aureus and P. aeruginosa pneumonia in the intensive care unit: a retrospective observational cohort study in a US claims database. BMC Health Serv Res 2015; 15:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Henig O, Cober E, Richter SS, et al. A prospective observational study of the epidemiology, management, and outcomes of skin and soft tissue infections due to carbapenem-resistant Enterobacteriaceae. Open Forum Infect Dis 2017; 4:ofx157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sader HS, Farrell DJ, Flamm RK, Jones RN. Antimicrobial susceptibility of Gram-negative organisms isolated from patients hospitalized in intensive care units in United States and European hospitals (2009–2011). Diagn Microbiol Infect Dis 2014; 78:443–8. [DOI] [PubMed] [Google Scholar]
- 25. Sader HS, Castanheira M, Flamm RK, et al. Ceftazidime/avibactam tested against Gram-negative bacteria from intensive care unit (ICU) and non-ICU patients, including those with ventilator-associated pneumonia. Int J Antimicrob Agents 2015; 46:53–9. [DOI] [PubMed] [Google Scholar]
- 26. Lodise T, Ye MJ, Zhao Q. Prevalence of invasive infections due to carbapenem-resistant Enterobacteriaceae among adult patients in U.S. hospitals. Antimicrob Agents Chemother 2017; 61:e00228–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van Loon K, Voor In’t Holt AF, Vos MC. Systematic review and meta-analyses of the clinical epidemiology of carbapenem-resistant Enterobacteriaceae. Antimicrob Agents Chemother 2017; 62:e01730–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Armand-Lefèvre L, Angebault C, Barbier F, et al. Emergence of imipenem-resistant Gram-negative bacilli in intestinal flora of intensive care patients. Antimicrob Agents Chemother 2013; 57:1488–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. MacVane SH. Antimicrobial resistance in the intensive care unit: a focus on gram-negative bacterial infections. J Intensive Care Med 2017; 32:25–37. [DOI] [PubMed] [Google Scholar]
- 30. Shorr AF. Review of studies of the impact on Gram-negative bacterial resistance on outcomes in the intensive care unit. Crit Care Med 2009; 37:1463–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.