Abstract
Objectives
To compare population-based incidence rates of new-onset depression or self-harm in patients initiating incretin-based therapies with that of sulfonylureas (SU) and other glucose-lowering agents.
Design
Population-based cohort study.
Setting
Patients attending primary care practices registered with the UK-based Clinical Practice Research Datalink (CPRD).
Participants
Using the UK-based CPRD, we identified two incretin-based therapies cohorts: (1) dipeptidyl peptidase-4 inhibitor (DPP-4i)-cohort, consisting of new users of DPP-4i and SU and (2) glucagon-like peptide-1 receptor agonists (GLP-1RA)-cohort, consisting of new users of GLP-1RA and SU, between January 2007 and January 2016. Patients with a prior history of depression, self-harm and other serious psychiatric conditions were excluded.
Main outcome measures
The primary study outcome comprised a composite of new-onset depression or self-harm. Unadjusted and adjusted Cox proportional hazards regression was used to quantify the association between incretin-based therapies and depression or self-harm. Deciles of High-Dimensional Propensity Scores and concurrent number of glucose-lowering agents were used to adjust for potential confounding.
Results
We identified new users of 6206 DPP-4i and 22 128 SU in the DPP-4i-cohort, and 501 GLP-1RA and 16 409 SU new users in the GLP-1RA-cohort. The incidence of depression or self-harm was 8.2 vs 11.7 events/1000 person-years in the DPP-4i-cohort and 18.2 vs 13.6 events/1000 person-years in the GLP-1RA-cohort for incretin-based therapies versus SU, respectively. Incretin-based therapies were not associated with an increased or decreased incidence of depression or self-harm compared with SU (DPP-4i-cohort: unadjusted HR 0.70, 95% CI 0.51 to 0.96; adjusted HR 0.80, 95% CI 0.57 to 1.13; GLP-1RA-cohort: unadjusted HR 1.36, 95% CI 0.72 to 2.58; adjusted HR 1.25, 95% CI 0.63 to 2.50). Consistent results were observed for other glucose-lowering comparators including insulin and thiazolidinediones.
Conclusions
Our findings suggest that the two incretin-based therapies are not associated with an increased or decreased risk of depression or self-harm.
Keywords: cohort study, type 2 diabetes, dipeptidyl-peptidase 4 inhibitors, glucagon-like receptor 1 agonists, depression, suicide, self-harm
Strengths and limitations of this study.
This study used a new-user active comparator design with High-Dimensional Propensity Scores to control for confounding.
Depression is likely underestimated using diagnostic codes, although previous studies have shown positive predictive values around 90% or greater.
There were a limited number of self-harm events and the study was not powered to detect clinically relevant differences across exposure groups for this component of the composite outcome.
This study cannot rule out small or modest difference in risk of depression or self-harm between incretin-based therapy users and other glucose-lowering due to study power limitations.
Introduction
Patients with diabetes frequently have coexisting depression with a prevalence ranging from 12% to 27%.1 Depression is associated with diabetes and with an increased risk of diabetes-related complications,2 decreased quality of life3 and decreased life expectancy.4 Diabetes is also associated with new-onset depression; however, the temporal association between diabetes and depression remains unclear.5 6 Moreover, diabetes is associated with an increased risk of intentional self-harm,7 8 although there is significant heterogeneity between studies assessing the association between diabetes and suicide.9 It has been postulated that certain glucose-lowering pharmacotherapies may have a positive influence on the symptoms of depression, although the evidence is sparse.10–15 The incretin-based therapies in particular may have neuropsychiatric effects given the presence of glucagon-like peptide-1 (GLP-1) receptors in the central nervous system.16 17
Concerns surrounding central nervous system effects stem from a case report of exenatide-induced depression and from pooled adverse event data from premarketing clinical trials for sitagliptin.18–20 Pooled event rates for the latter suggested a fourfold increased risk of suicidal ideation and completed suicide in sitagliptin users compared with non-users.19 21 Animal models suggest that adverse neuropsychiatric effects are biologically plausible given the expression of GLP-1 receptors in the brain.20 Furthermore, studies have shown low dipeptidyl peptidase-4 (DPP-4) activity is correlated with depression.22–24 Although the case report mentioned above suggested a potential increased risk of depression, a recent study reported positive effects of GLP-1 receptor agonists on patients well-being.25 Therefore, alternations in DPP-4 enzymatic activity may modulate the pathophysiology of neuropsychiatric conditions such as major depression.
Using data from a population-based cohort of patients with type 2 diabetes, we aimed to quantify the association between incretin-based therapies and the composite of new-onset depression and self-harm.
Methods
Study design and data sources
We conducted a population-based cohort study using data from the Clinical Practice Research Datalink (CPRD), which captures electronic medical information for primary care encounters by general practitioners in the UK.26 The CPRD contains de-identified individual-level longitudinal data collected from a subset of primary care practices (~700) in the UK. The CPRD data are a representative sample that is similar to the overall UK population in age, sex and ethnicity.27 The database includes sociodemographic and lifestyle variables (eg, alcohol consumption), physiological measures (eg, blood pressure), laboratory testing (eg, glycated haemoglobin A1c (HbA1c)), physician-assigned diagnoses using the Read classification system and prescription records from general practitioner records. Data quality checks are performed in accordance with standardised guidelines that certify practices as up to standard. Furthermore, over 350 validation studies have been performed using the CPRD.28 29 Information on hospitalisations and causes of death is available for a subset of CPRD patients through linkages with the external databases. Details regarding the data quality, linkages and utility are available elsewhere.30 The CPRD has been used extensively to study associations between drugs and depression and self-harm.31–35
Study cohorts
Our source population consisted of all patients over 18 years of age with a minimum of 12 months of up-to-standard medical history in the CPRD database that received a new diagnosis for type 2 diabetes or a new prescription for any glucose-lowering therapy between 1 January 2001 and the February 2016 CPRD dataset build. We used a 365-day washout period to define a new diagnosis or new glucose-lowering therapy use. A subcohort of patients (~58%) selected from the source population was linked to Hospital Episode Statistics (HES—follow-up until 31 March 2014), Office for National Statistics (ONS—follow-up until 30 April 2014), and Index of Multiple Deprivation (IMD (2010)) data to capture hospital records, causes of death and socioeconomic status information, respectively. Women with polycystic ovarian syndrome, gestational diabetes or whom were pregnant during the study period were excluded. In addition, we excluded patients with a study entry date prior to 1 January 2007 as the first incretin-based therapies became available in the UK in early 2007.
We identified two main study cohorts. Specifically, the first cohort consisted of new users of DPP-4 inhibitors and new users of sulfonylureas (DPP-4 inhibitor cohort) and the second cohort consisted of new users of GLP-1 receptor agonists and new users of sulfonylureas (GLP-1 receptor agonist cohort). Although new users of sulfonylureas served as the reference population for both cohorts, these individuals were selected separately for each cohort as prior use of other non-incretin glucose-lowering agents was permitted. To minimise potential selection bias within the above cohorts, we excluded patients with a history of depression, self-harm, anxiety and other serious psychiatric conditions in the year prior to a patient’s cohort entry date.
Exposure and outcome definitions
Within each incretin-based therapy cohort, we defined person-time exposure to all classes of glucose-lowering therapy including (1) DPP-4 inhibitors, (2) GLP-1 receptor agonists, (3) sulfonylureas, (4) metformin, (5) thiazolidinediones (TZDs), (6) sodium glucose cotransporter-2 inhibitors, (7) meglitinides, (8) acarbose, (9) insulin and (10) no glucose-lowering drug therapy (ie, diet/lifestyle). Patient’s contributed person-time to each of the aforementioned categories on the day of their first prescription or date of diagnosis (defined as the patient’s index date) until a patient discontinued the drug, left a CPRD practice, died or on the final date of follow-up, whichever occurred first. To account for potential non-adherence, we included a portion of follow-up time following the end of the expected medication supply that was equivalent to 50% of the prescription duration as a period of exposure.
Our primary outcome is the composite of either new-onset depression or self-harm, including suicide and suicidal ideation. If a patient experienced more than one event, the date of the first event was used. New-onset depression or episodes of self-harm were identified using diagnostic codes from either the CPRD, HES or ONS data sources (specific codes available in online supplementary appendices A and B).
bmjopen-2018-023830supp001.pdf (307.3KB, pdf)
Statistical analysis
Unadjusted and adjusted Cox proportional hazards regression was used to quantify the association between incretin-based therapies and depression or self-harm. Our primary exposure contrasts of interest were DPP-4 inhibitors versus sulfonylureas and GLP-1 receptor agonists versus sulfonylureas within the DPP-4 inhibitor and GLP-1 receptor agonists cohorts, respectively. Sulfonylureas were chosen a priori as the main reference group given their use in clinical practice as second or third agents resembles incretin-based therapies. Patients contributed follow-up time from the initiation of the incretin-based therapy of interest or comparator until they experienced the composite outcome of interest or were censored. Censoring occurred on the earliest date of the following events: discontinuation of the incretin-based therapy of interest or comparator, switching between an incretin-based therapy to the comparator (or vice versa), leaving a CPRD practice site, death, end of study period.
To adjust for potential confounders, we used a High-Dimensional Propensity Score (hdPS) algorithm to select up to 40 empirical covariates.36 Using a multivariable logistic regression model that included the both empirically derived and predefined (age, sex, alcohol abuse, body mass index (BMI), duration of treated diabetes, comorbidities, number of hospitalisations, HbA1c, prior medications use, smoking status, socioeconomic status (quintiles of the IMD), use of other glucose-lowering therapies, year of cohort entry. A detailed list of covariates forced into propensity score model is shown in online supplementary appendix C) covariates, we calculated the probability of initiating a DPP-4 inhibitor versus a sulfonylurea (or another comparator for secondary analysis). Patients with overlapping propensity scores were included in the analysis. A separate hdPS procedure was run for the GLP-1 receptor agonist cohort. Adjusted HRs and 95% CIs were calculated using a Cox proportional hazards regression model with deciles of the hdPS and variable indicating the number of glucose-lowering agents during follow-up (1, 2, 3 or more). We used standard graphical approaches to assess model assumptions for which no violations were noted.
Secondary analyses included alternative comparator groups and components of composite outcome (ie, depression and self-harm as separate outcomes). In addition, we conducted several additional sensitivity analyses. First, we used two alternative methods to adjust for potential confounding including a matched propensity score approach (1:1—matching using greedy nearest neighbour approach with a calliper set at 0.2 times the SD of the natural logarithm of the propensity score) and grouping patients with identical patterns of glucose-lowering therapies prior to and following cohort initiation. For the latter approach, an example of how we grouped patients is as follows. Patients who started with metformin monotherapy and added an incretin-based therapy would be grouped with patients who also started metformin monotherapy and then added the comparator drug of interest. Groups with less than 25 patients were excluded from this analysis. We used a categorical variable to adjust for all groups within our multivariable Cox proportional hazards model. Second, we ran several analyses using restricted cohorts including restricting our cohort to patients eligible for HES/ONS linkage (ie, patients with hospital and death certificate records), restricting to monotherapy users, restricting to a cohort of metformin monotherapy users who added the incretin-based therapy of interest or a sulfonylurea. Third, we added BMI (as a categorical variable) to Cox proportional hazards model given that weight may be a confounding factor.37 38 Fourth, we used time-dependent variables to classify our exposures of interest throughout follow-up time. All analyses were conducted with R V.3.3.3.
Patient and public involvement
No patients were involved in any aspect of the study.
Results
DPP-4 inhibitor cohort
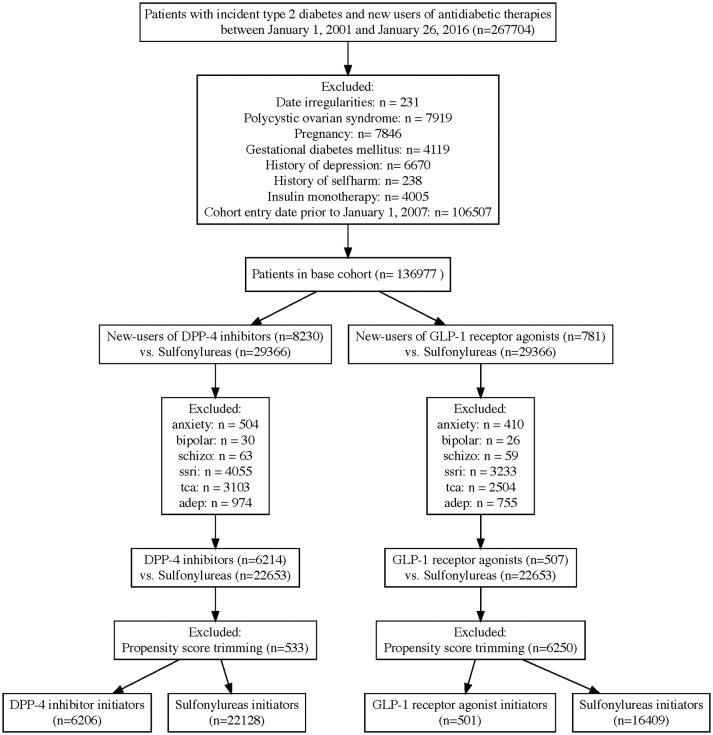
Within the DPP-4 inhibitor new user cohort, there were 6206 initiators of a DPP-4 inhibitor and 22 128 initiators of a sulfonylurea (figure 1). The mean (SD) follow-up time was 324 (362) days for DPP-4 inhibitor users and 299 (385) days for sulfonylurea users. Compared with sulfonylurea users, DPP-4 inhibitor users were on average younger, had fewer hospitalisations in the year prior to cohort entry and less likely to have impaired kidney function. Patient characteristics were well balanced following propensity score matching (table 1). There were a total 264 patients identified with new-onset depression or self-harm.
Figure 1.
Flow diagram to identify initiators of DPP-4 inhibitors and sulfonylureas (DPP-4 inhibitor cohort), and GLP-1 receptor agonists and sulfonylureas (GLP-1 receptor agonist cohort). adep, other antidpetressents; DPP-4, dipeptidyl peptidase-4; GLP-1, glucagon-like peptide-1; ssri, selective serotonin reuptake inhibitors; tca, tricyclic antidepressants.
Table 1.
Patient characteristics of new-user DPP-4i cohort before and after propensity score matching
| DPP-4i new user cohort before propensity score matching | DPP-4i new user cohort after propensity score matching | |||
| DPP-4i (n=6206) |
SU (n=22 128) |
DPP-4i (n=6008) |
SU (n=6008) |
|
| Age in years (SD) | 58 (12.2) | 60.5 (13.8) | 58.1 (12.2) | 58.2 (12.5) |
| Female | 2258 (36.4%) | 8107 (36.6%) | 2189 (36.4%) | 2187 (36.4%) |
| Measure of deprivation | ||||
| Least | 624 (10.1%) | 2492 (11.3%) | 603 (10%) | 594 (9.9%) |
| Most | 615 (9.9%) | 2342 (10.6%) | 603 (10%) | 614 (10.2%) |
| Unknown | 2862 (46.1%) | 8780 (39.7%) | 2739 (45.6%) | 2683 (44.7%) |
| Diabetes duration in years (SD) | 2.0 (1.8) | 1.0 (1.5) | 1.9 (1.7) | 1.9 (1.8) |
| Body mass index >30 | 4162 (67.1%) | 10 661 (48.2%) | 3994 (66.5%) | 3978 (66.2%) |
| No of hospitalisations in year prior to cohort entry | ||||
| 0 | 5647 (91%) | 18 516 (83.7%) | 5452 (90.7%) | 5470 (91%) |
| 1 | 378 (6.1%) | 2105 (9.5%) | 375 (6.2%) | 379 (6.3%) |
| 2 | 109 (1.8%) | 784 (3.5%) | 109 (1.8%) | 92 (1.5%) |
| 3+ | 72 (1.2%) | 723 (3.3%) | 72 (1.2%) | 67 (1.1%) |
| No of drugs in year prior to cohort entry | ||||
| 0–4 | 721 (11.6%) | 3098 (14%) | 703 (11.7%) | 671 (11.2%) |
| 5–10 | 3204 (51.6%) | 10 379 (46.9%) | 3081 (51.3%) | 3119 (51.9%) |
| 11+ | 2281 (36.8%) | 8651 (39.1%) | 2224 (37%) | 2218 (36.9%) |
| HbA1c | ||||
| <6.5% | 242 (3.9%) | 1393 (6.3%) | 238 (4%) | 233 (3.9%) |
| 6.5%–7.5% | 1104 (17.8%) | 3349 (15.1%) | 1049 (17.5%) | 1053 (17.5%) |
| 7.5%–9% | 2831 (45.6%) | 7121 (32.2%) | 2701 (45%) | 2694 (44.8%) |
| 9%+ | 2000 (32.2%) | 9833 (44.4%) | 1991 (33.1%) | 2007 (33.4%) |
| Unknown | 29(<1%) | 432 (2%) | 29(<1%) | 21(<1%) |
| eGFR <60 | 883 (14.2%) | 4429 (20%) | 857 (14.3%) | 890 (14.8%) |
| Diagnoses in year prior to cohort entry | ||||
| Heart failure | 68 (1.1%) | 369 (1.7%) | 68 (1.1%) | 51(<1%) |
| Hypertension | 1095 (17.6%) | 4475 (20.2%) | 1066 (17.7%) | 1087 (18.1%) |
| Dyslipidaemia | 213 (3.4%) | 1093 (4.9%) | 213 (3.5%) | 212 (3.5%) |
| Ischaemic heart disease | 174 (2.8%) | 1033 (4.7%) | 171 (2.8%) | 168 (2.8%) |
| Peripheral vascular disease | 25(<1%) | 145(<1%) | 25(<1%) | 24(<1%) |
| Prescription drug use in year prior to cohort entry | ||||
| Metformin | 5775 (93.1%) | 16 534 (74.7%) | 5578 (92.8%) | 5638 (93.8%) |
| Acarbose | S | 8(<1%) | S | S |
| SGLT2 inhibitors | 38(<1%) | 93(<1%) | 38(<1%) | 40(<1%) |
| Meglitinide | 47(<1%) | 39(<1%) | 38(<1%) | 29(<1%) |
| Thiazolidinedione | 252 (4.1%) | 403 (1.8%) | 222 (3.7%) | 209 (3.5%) |
| Insulin | 82 (1.3%) | 331 (1.5%) | 80 (1.3%) | 86 (1.4%) |
| Hypnotic | 332 (5.3%) | 1486 (6.7%) | 328 (5.5%) | 324 (5.4%) |
| Mood | 85 (1.4%) | 280 (1.3%) | 81 (1.3%) | 83 (1.4%) |
| Anticonvulsant | 271 (4.4%) | 832 (3.8%) | 260 (4.3%) | 266 (4.4%) |
| Antipsychotics | 176 (2.8%) | 829 (3.7%) | 172 (2.9%) | 171 (2.8%) |
S, suppressed due to low number of events.
DPP-4i, dipeptidyl peptidase-4 inhibitor; eGFR, estimated glomerular filtration rate; GLP-1, glucagon-like peptide-1 receptor agonist; HbA1c, glycated haemoglobin A1c; SGLT2, sodium-dependent glucose cotransporter-2; SU, sulfonylureas.
The incidence of depression or self-harm was 8.2 per 1000 person-years in DPP-4 inhibitor users compared with 11.7 per 1000 person-years in sulfonylurea users (unadjusted HR 0.70, 95% CI 0.51 to 0.96 (table 2)). Similarly, the crude incidence rates were smaller for DPP-4 inhibitor users versus other comparators (10.0 vs 10.8 per 1000 person-years for TZDs; 9.8 vs 20.7 for insulin users). However, following adjustment for potential confounding variables, there was no significant association between DPP-4 inhibitor use and the risk of depression or self-harm for all comparator groups (sulfonylurea comparator: adjusted HR 0.80, 95% CI 0.57 to 1.13; TZD comparator: adjusted HR 1.17, 95% CI 0.70 to 1.96; insulin comparator: adjusted HR 0.98, 95% CI 0.53 to 1.83). Online supplementary appendices D and E show the results for the risks of depression and self-harm separately.
Table 2.
Measures of frequency and association for depression or self-harm in new users of dipeptidyl peptidase-4 inhibitors (DPP-4i) or new users of glucagon-like peptide-1 receptor agonists (GLP-1RA) versus sulfonylureas (SU), thiazolidinediones (TZD) or insulin
| DPP-4i new user cohort | GLP-1RA new user cohort | |||
| Comparator: SU | ||||
| DPP-4i | SU | GLP-1RA | SU | |
| No of patients | 6206 | 22 128 | 501 | 16 409 |
| Person-years of follow-up | 5589 | 18 596 | 549 | 13 418 |
| No of events | 46 | 218 | 10 | 183 |
| Incidence per 1000 person-years (95% CI) | 8.2 (6.2 to 11) | 11.7 (10.3 to 13.4) | 18.2 (10 to 33.5) | 13.6 (11.8 to 15.8) |
| Crude HR | 0.70 (0.51–0.96) | Ref | 1.36 (0.72–2.58) | Ref |
| Adjusted HR | 0.80 (0.57–1.13) | Ref | 1.25 (0.63–2.50) | Ref |
| Comparator: TZD | ||||
| DPP-4i | TZD | GLP-1RA | TZD | |
| No of patients | 9565 | 2512 | 851 | 2011 |
| Person-years of follow-up | 9190 | 2786 | 1035 | 2165 |
| No of events | 92 | 30 | 17 | 27 |
| Incidence per 1000 person-years (95% CI) | 10.0 (8.2 to 12.3) | 10.8 (7.6 to 15.4) | 16.4 (10.3 to 26.3) | 12.5 (8.6 to 18.1) |
| Crude HR | 0.90 (0.59–1.36) | Ref | 1.32 (0.72–2.42) | Ref |
| Adjusted HR | 1.17 (0.70–1.96) | Ref | 1.18 (0.53–2.65) | Ref |
| Comparator: insulin | ||||
| DPP-4i | Insulin | GLP-1RA | Insulin | |
| No of patients | 10 049 | 3600 | 854 | 2745 |
| Person-years of follow-up | 9878 | 1161 | 1033 | 919 |
| No of events | 97 | 24 | 14 | 19 |
| Incidence per 1000 person-years (95% CI) | 9.8 (8.1 to 12) | 20.7 (13.9 to 30.8) | 13.6 (8.1 to 22.7) | 20.7 (13.3 to 32.3) |
| Crude HR | 0.54 (0.34–0.87) | Ref | 0.74 (0.35–1.56) | Ref |
| Adjusted HR | 0.98 (0.53–1.83) | Ref | 1.07 (0.39–2.94) | Ref |
GLP-1 receptor agonist cohort
Within the GLP-1 receptor agonist cohort, there were 501 initiators of a GLP-1 receptor agonist and 16 409 initiators of a sulfonylurea (figure 1). The mean (SD) follow-up time was 397 (409) days for GLP-1 receptor agonist users and 292 (373) days for sulfonylurea users. Compared with sulfonylurea users, GLP-1 receptor agonist users were on average younger, more likely female, used more drugs in the year prior to cohort entry, had a lower baseline HbA1c, more likely to have used several medications prior to cohort entry including insulin. Following propensity score matching, baseline patient characteristics were well balanced (table 3). There were a total 193 patients identified with new-onset depression or self-harm.
Table 3.
Patient characteristics of new-user GLP-1RA cohort before and after propensity score matching
| GLP-1RA new user cohort before propensity score matching | GLP-1RA new user cohort after propensity score matching | |||
| GLP-1RA (n=501) |
SU (n=16 409) |
GLP-1RA (n=488) |
SU (n=488) |
|
| Age in years (SD) | 49.4 (11.3) | 57.8 (12.9) | 49.7 (11.2) | 49.2 (12.6) |
| Female | 204 (40.7%) | 6021 (36.7%) | 198 (40.6%) | 174 (35.7%) |
| Measure of deprivation | ||||
| Least | 40 (8%) | 1688 (10.3%) | 40 (8.2%) | 29 (5.9%) |
| Most | 56 (11.2%) | 1770 (10.8%) | 56 (11.5%) | 52 (10.7%) |
| Unknown | 240 (47.9%) | 6784 (41.3%) | 230 (47.1%) | 214 (43.9%) |
| Diabetes duration in years (SD) | 1.7 (1.6) | 1.2 (1.6) | 1.7 (1.6) | 1.7 (1.8) |
| Body mass index >30 | 470 (93.8%) | 10 481 (63.9%) | 458 (93.9%) | 452 (92.6%) |
| No of hospitalisations in year prior to cohort entry | ||||
| 0 | 456 (91%) | 14 170 (86.4%) | 445 (91.2%) | 437 (89.5%) |
| 1 | 29 (5.8%) | 1344 (8.2%) | 28 (5.7%) | 27 (5.5%) |
| 2 | 10 (2%) | 499 (3%) | 9 (1.8%) | 17 (3.5%) |
| 3+ | 6 (1.2%) | 396 (2.4%) | 6 (1.2%) | 7 (1.4%) |
| No of drugs in year prior to cohort entry | ||||
| 0–4 | 17 (3.4%) | 1660 (10.1%) | 17 (3.5%) | 18 (3.7%) |
| 5–10 | 195 (38.9%) | 7899 (48.1%) | 192 (39.3%) | 208 (42.6%) |
| 11+ | 289 (57.7%) | 6850 (41.7%) | 279 (57.2%) | 262 (53.7%) |
| HbA1c | ||||
| <6.5% | 66 (13.2%) | 1085 (6.6%) | 62 (12.7%) | 66 (13.5%) |
| 6.5%–7.5% | 99 (19.8%) | 2593 (15.8%) | 97 (19.9%) | 99 (20.3%) |
| 7.5%–9% | 150 (29.9%) | 5357 (32.6%) | 145 (29.7%) | 134 (27.5%) |
| 9%+ | 179 (35.7%) | 7068 (43.1%) | 177 (36.3%) | 178 (36.5%) |
| Unknown | 7 (1.4%) | 306 (1.9%) | 7 (1.4%) | 11 (2.3%) |
| eGFR <60 | 36 (7.2%) | 2821 (17.2%) | 35 (7.2%) | 40 (8.2%) |
| Diagnoses in year prior to cohort entry | ||||
| Heart failure | 5 (1%) | 244 (1.5%) | 5 (1%) | 6 (1.2%) |
| Hypertension | 107 (21.4%) | 3398 (20.7%) | 106 (21.7%) | 104 (21.3%) |
| Dyslipidaemia | 16 (3.2%) | 771 (4.7%) | 16 (3.3%) | 23 (4.7%) |
| Ischaemic heart disease | 11 (2.2%) | 644 (3.9%) | 11 (2.3%) | 9 (1.8%) |
| Peripheral vascular disease | S | 106 (<1%) | S | S |
| Prescription drug use in year prior to cohort entry | ||||
| Metformin | 457 (91.2%) | 13 542 (82.5%) | 445 (91.2%) | 449 (92%) |
| Acarbose | S | 7(<1%) | S | S |
| SGLT2 inhibitors | 5 (1%) | 87(<1%) | 5 (1%) | 5 (1%) |
| Meglitinide | 11 (2.2%) | 39(<1%) | 10 (2%) | 10 (2%) |
| Thiazolidinedione | 38 (7.6%) | 376 (2.3%) | 38 (7.8%) | 41 (8.4%) |
| Insulin | 65 (13%) | 307 (1.9%) | 55 (11.3%) | 59 (12.1%) |
| Hypnotic | 32 (6.4%) | 1093 (6.7%) | 32 (6.6%) | 35 (7.2%) |
| Mood | 10 (2%) | 228 (1.4%) | 10 (2%) | 8 (1.6%) |
| Anticonvulsant | 33 (6.6%) | 682 (4.2%) | 31 (6.4%) | 32 (6.6%) |
| Antipsychotics | 12 (2.4%) | 507 (3.1%) | 12 (2.5%) | 12 (2.5%) |
S, suppressed due to low number of events.
DPP-4i, dipeptidyl peptidase-4 inhibitor; eGFR, estimated glomerular filtration rate; GLP-1RA, glucagon-like peptide-1 receptor agonist; HbA1c, glycated haemoglobin A1c; SGLT2, sodium-dependent glucose cotransporter-2; SU, sulfonylureas.
The incidence rate of depression or self-harm was non-significantly higher for GLP-1 receptor users compared with sulfonylurea users (18.2 vs 13.6 per 1000 person-years; unadjusted HR 1.36, 95% CI 0.72 to 2.58; adjusted HR 1.25, 95% CI 0.63 to 2.50), TZDs (16.4 vs 12.5 per 1000 person-years; unadjusted HR 1.32, 95% CI 0.72 to 2.42; adjusted HR 1.18, 95% CI 0.53 to 2.65) and insulin users (13.6 vs 20.7 per 1000 person-years; unadjusted HR 0.74, 95% CI 0.35 to 1.56; adjusted HR 1.07, 95% CI 0.39 to 2.94). All measured associations remained non-significant following adjustment for potential confounders (table 2). Online supplementary appendix D shows the results for depression analysed as a separate outcome. We were unable to analyse results for self-harm separately, due to small numbers of events (online supplementary appendix E).
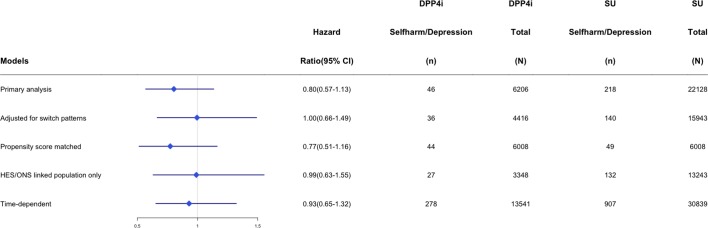
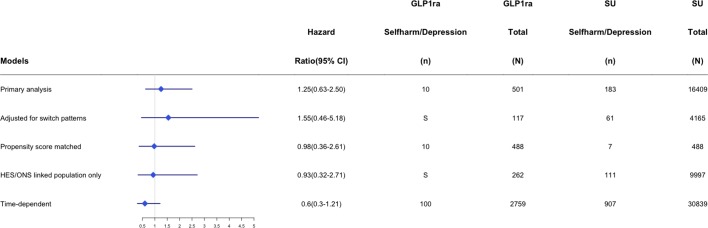
Sensitivity analyses
Figures 2 and 3 provide the number of events per treatment exposure group and measures of association for selected sensitivity analyses across the main DPP-4 inhibitor and GLP-1 receptor agonist cohorts. There were too few events to run a stable statistical model for all prespecified sensitivity analyses (eg, new monotherapy users); however, findings from models that were run were consistent with our main results suggesting that DPP-4 inhibitor use did not have an increased or decreased risk of new-onset depression (online supplementary appendices F–L).
Figure 2.
HRs and number of events within dipeptidyl peptidase-4 inhibitor (DPP-4i) and sulfonylurea (SU) users across sensitivity analysis. HES, Hospital Episode Statistics; ONS, Office of National Statistics.
Figure 3.
HRs and number of events within glucagon-like peptide-1 receptor agonist (GLP1RA) and sulfonylurea (SU) users across sensitivity analysis. HES, Hospital Episode Statistics; ONS, Office of National Statistics; S, supressed due to low number of events.
Discussion
New users of DPP-4 inhibitors and new users of GLP-1 receptor agonists did not have an increased or decreased risk of a new diagnosis of depression or episode of self-harm. These findings extend our current knowledge regarding the relative safety of the incretin-based therapies used to manage hyperglycaemia in patients with type 2 diabetes.
The impetus for our study was the safety signal generated by randomised controlled trials and a case report suggesting that incretin-based therapies may affect the risk of depression or self-harm. Specifically, early trial data found a four-time greater risk of suicidal ideation or completed suicide in sitagliptin users versus glipizide users.19 21 A higher incidence of depression was also observed in the long-term safety population among phase-3 clinical trial in sitagliptin 100 mg users (13/429) compared with placebo (0/154); however, the incidence of psychiatric events was no different among pooled phase 3 studies (3.0% in sitagliptin 100 mg users; 2.4% in sitagliptin 200 mg users and 3.2% in placebo users).20 Moreover, a case report has also been published regarding exenatide-induced depression.18
Despite our findings suggesting a lack of association between incretin-based agents and depression or self-harm, there is a substantial evidence base from animal models that suggest incretin-based therapies may affect mood disorders. Anderberg et al found differential effects of acute versus chronic exposure to a GLP-1 receptor agonist.39 Acute activation of GLP-1 receptors was associated with anxiogenic effects, whereas chronic GLP-1 receptor activation did not elicit anxiogenic effects in Sprague Dawley rats. In fact, chronic exposure to a GLP-1 receptor agonist was associated with a decrease in depressive-like behaviour. Furthermore, acute stimulation of GLP-1 receptors affected serotonin turnover and serotonin receptor expression in the amygdala; however, chronic stimulation did not affect serotonin turnover or receptor expression. In addition to effects on serotonin, activation of GLP-1 may have mood effects through impacting central dopamine levels.40 A mice model suggests that liraglutide, a GLP-1 receptor agonist, has antipsychotic properties possibly through its affecting dopamine activity in the brain.41 Interestingly, the DPP-4 inhibitor sitagliptin did not exhibit the same antipsychotic properties.
Another possible mechanism by which glucose-lowering therapies may affect mood disorders is through the reduction in inflammatory cytokines/mediators. Moulton et al reported improvement in depressive symptoms over 1 year in a cohort of 1735 newly diagnosed patients with type 2 diabetes.10 The improvement in depressive symptoms measure by the Patient Health Questionnaire-9 (PHQ-9) was independent of change in glycaemic control and was correlated with a change in the inflammatory marker high sensitivity C reactive protein (CRP). Furthermore, a meta-analysis found that pioglitazone was associated with a reduction in symptoms of depression compared with placebo (pooled OR 3.3, 95% CI 1.4 to 7.8).11 A 12-week open-label study also found that pioglitazone was associated with a reduction in depression symptoms as well as a decrease in CRP and decreased insulin resistance.12 Indeed, a population-based cross-sectional study found that numerous inflammatory markers (eg, CRP, inerleukin-1 receptor agonist, monocyte chemotactic protein-1, white cell count, triglyceride) were associated with depression in patients with type 2 diabetes.42 To further test this hypothesis among DPP-4 inhibitor users, there is an ongoing small clinical trial evaluating the effect of sitagliptin on symptoms of depression in the elderly (EudraCT Number: 2015-004527-32).43
Our study is subject to the standard limitations of observational cohort studies including the potential for residual and unmeasured confounding. Although we adjusted for over 70 potential confounders using an HdPS approach, we were not able to capture all relevant potential confounders such as severity of depressive symptoms and patient-level socioeconomic status. Our follow-up time was also limited (DPP-4 cohort mean follow-up time=305 days; GLP-1 receptor agonist cohort mean follow-up=296 days), therefore, it is possible that a longer time frame was required to detect an association. However, it would be expected that an effect on depression symptoms mediated by serotonin or dopaminergic central pathways would be apparent after 4–6 weeks or sooner. There were a limited number of self-harm events and our study was not powered to detect clinically relevant differences across exposure groups for this component of our composite outcome. Similarly, given the lower and upper limits of the 95% CIs, our study cannot rule out small or moderate differences in the risk of depression across exposure groups. Misclassification of the exposure or outcome variables of interest may have also impacted our findings. Our exposure variables of interest (incretin-based therapies) were measured based on primary care prescription records and therefore may overestimate true exposure due to primary and secondary non-adherence. In addition, prescriptions written by specialists are not captured in the CPRD. It is possible that when the incretin-based therapies were introduced, they were more frequently prescribed by specialists and our study would miss the initial prescription, however, subsequent prescriptions written by general practitioners would be captured. Previous studies have shown that depression is likely underestimated using diagnostic codes, although positive predictive values have generally been greater than 90% using the 10th version of the International Statistical Classification of Diseases and Related Health Problems (ICD-10) codes.44 Underascertainment of depression would likely be non-differential between our exposure groups of interest and therefore bias our findings towards the null. Suicide and self-harm have also been shown to be underestimated using CPRD data and the use of linked mortality data via the ONS improves the sensitivity for capturing suicide and self-harm; however, under-reporting of events is still expected.45 In addition, the role of incretin-based agents may have shifted over time whereby when they were first introduced to the market were not used commonly as second-line agents and sulfonylureas may have been used as first or second-line agents. We attempted to control for both temporal trends and timing of therapy by using calendar time, duration of and prior exposure of glucose-lowering therapies as covariates in the propensity score.
Our findings provide some reassurance regarding the safety of the incretin-based therapies in the treatment of type 2 diabetes. Specifically, our study results suggest that there is not a clinically relevant association between either DPP-4 inhibitors or GLP-1 receptor agonists and depression or self-harm.
Supplementary Material
Acknowledgments
J-MG is supported as a New Investigator Award from the Canadian Institute of Health Research and a Clinician Scientist Award from Diabetes Canada. This study is based in part on data from the Clinical Practice Research Datalink obtained under licence from the UK Medicines and Healthcare products Regulatory Agency. However, the interpretation and conclusions contained in this study are those of the author/s alone.
Footnotes
Contributors: J-MG, EC, WKM, LKT and SRM were involved in the concept and design of the study. J-MG was responsible for drafting the first version of the manuscript. All authors contributed to the interpretation of data. J-MG, EC, WKM and LKT provided revisions to the manuscript. J-MG will act as guarantor for the study.
Funding: This work was supported by an operating grant from the Canadian Institute for Health Research (FRN173599-287647).
Competing interests: None declared.
Patient consent: Not required.
Ethics approval: Our study protocol was approved by the Independent Scientific Advisory Committee (ISAC 15_016RARA, August 2017) and received approval from the Health Research Ethics Board at Memorial University.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1. Holt RI, de Groot M, Lucki I, et al. . NIDDK international conference report on diabetes and depression: current understanding and future directions. Diabetes Care 2014;37:2067–77. 10.2337/dc13-2134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. de Groot M, Anderson R, Freedland KE, et al. . Association of depression and diabetes complications: a meta-analysis. Psychosom Med 2001;63:619–30. 10.1097/00006842-200107000-00015 [DOI] [PubMed] [Google Scholar]
- 3. Ali S, Stone M, Skinner TC, et al. . The association between depression and health-related quality of life in people with type 2 diabetes: a systematic literature review. Diabetes Metab Res Rev 2010;26:75–89. 10.1002/dmrr.1065 [DOI] [PubMed] [Google Scholar]
- 4. Holt RI, de Groot M, Golden SH. Diabetes and depression. Curr Diab Rep 2014;14:491 10.1007/s11892-014-0491-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Knol MJ, Twisk JW, Beekman AT, et al. . Depression as a risk factor for the onset of type 2 diabetes mellitus. A meta-analysis. Diabetologia 2006;49:837–45. 10.1007/s00125-006-0159-x [DOI] [PubMed] [Google Scholar]
- 6. Golden SH, Lazo M, Carnethon M, et al. . Examining a bidirectional association between depressive symptoms and diabetes. JAMA 2008;299:2751–9. 10.1001/jama.299.23.2751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rao Kondapally Seshasai S, Kaptoge S, Thompson A, et al. . Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 2011;364:829–41. 10.1056/NEJMoa1008862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Singhal A, Ross J, Seminog O, et al. . Risk of self-harm and suicide in people with specific psychiatric and physical disorders: comparisons between disorders using English national record linkage. J R Soc Med 2014;107:194–204. 10.1177/0141076814522033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang Y, Tang S, Xu S, et al. . Association between diabetes and risk of suicide death: a meta-analysis of 3 million participants. Compr Psychiatry 2016;71:11–16. 10.1016/j.comppsych.2016.08.006 [DOI] [PubMed] [Google Scholar]
- 10. Moulton CD, Pickup JC, Amiel SA, et al. . Investigating incretin-based therapies as a novel treatment for depression in type 2 diabetes: findings from the South London Diabetes (SOUL-D) Study. Prim Care Diabetes 2016;10:156–9. 10.1016/j.pcd.2015.06.003 [DOI] [PubMed] [Google Scholar]
- 11. Colle R, de Larminat D, Rotenberg S, et al. . Pioglitazone could induce remission in major depression: a meta-analysis. Neuropsychiatr Dis Treat 2017;13:9–16. 10.2147/NDT.S121149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kemp DE, Ismail-Beigi F, Ganocy SJ, et al. . Use of insulin sensitizers for the treatment of major depressive disorder: a pilot study of pioglitazone for major depression accompanied by abdominal obesity. J Affect Disord 2012;136:1164–73. 10.1016/j.jad.2011.06.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wahlqvist ML, Lee MS, Chuang SY, et al. . Increased risk of affective disorders in type 2 diabetes is minimized by sulfonylurea and metformin combination: a population-based cohort study. BMC Med 2012;10:150 10.1186/1741-7015-10-150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Berge LI, Riise T, Tell GS, et al. . Depression in persons with diabetes by age and antidiabetic treatment: a cross-sectional analysis with data from the Hordaland Health Study. PLoS One 2015;10:e0127161 10.1371/journal.pone.0127161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bode BW, Testa MA, Magwire M, et al. . Patient-reported outcomes following treatment with the human GLP-1 analogue liraglutide or glimepiride in monotherapy: results from a randomized controlled trial in patients with type 2 diabetes. Diabetes Obes Metab 2010;12:604–12. 10.1111/j.1463-1326.2010.01196.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alvarez E, Roncero I, Chowen JA, et al. . Expression of the glucagon-like peptide-1 receptor gene in rat brain. J Neurochem 1996;66:920–7. 10.1046/j.1471-4159.1996.66030920.x [DOI] [PubMed] [Google Scholar]
- 17. Lockie SH. Glucagon-like peptide-1 receptor in the brain: role in neuroendocrine control of energy metabolism and treatment target for obesity. J Neuroendocrinol 2013;25:597–604. 10.1111/jne.12039 [DOI] [PubMed] [Google Scholar]
- 18. Kohen I, Lester P. Exenatide-induced depression in a geriatric patient. Int J Geriatr Psychiatry 2008;23:443–4. 10.1002/gps.1937 [DOI] [PubMed] [Google Scholar]
- 19. Agency EM. Januvia: European Public Assessment Report (EPAR) - scientific discussion. London: European Medicines Agency, 2007. [Google Scholar]
- 20. U.S. Food and Drug Administration, 2006. Center For Drug Evaluation and Research. Application number: 21-995 Medical review. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2006/021995s000_MedR.pdf (accessed 24 Nov 2014).
- 21. Prescrire Editorial Staff. Sitagliptin: new drug. Type 2 diabetes: limited efficacy, too many unknown risks. Prescrire Int 2008;17:12–15. [PubMed] [Google Scholar]
- 22. Maes M, De Meester I, Scharpe S, et al. . Alterations in plasma dipeptidyl peptidase IV enzyme activity in depression and schizophrenia: effects of antidepressants and antipsychotic drugs. Acta Psychiatr Scand 1996;93:1–8. 10.1111/j.1600-0447.1996.tb10612.x [DOI] [PubMed] [Google Scholar]
- 23. Maes M, De Meester I, Vanhoof G, et al. . Decreased serum dipeptidyl peptidase IV activity in major depression. Biol Psychiatry 1991;30:577–86. 10.1016/0006-3223(91)90027-J [DOI] [PubMed] [Google Scholar]
- 24. Maes M, De Meester I, Verkerk R, et al. . Lower serum dipeptidyl peptidase IV activity in treatment resistant major depression: relationships with immune-inflammatory markers. Psychoneuroendocrinology 1997;22:65–78. 10.1016/S0306-4530(96)00040-6 [DOI] [PubMed] [Google Scholar]
- 25. Grant P, Lipscomb D, Quin J. Psychological and quality of life changes in patients using GLP-1 analogues. J Diabetes Complications 2011;25:244–6. 10.1016/j.jdiacomp.2011.03.002 [DOI] [PubMed] [Google Scholar]
- 26. Gamble JM, Thomas JM, Twells LK, et al. . Comparative effectiveness of incretin-based therapies and the risk of death and cardiovascular events in 38,233 metformin monotherapy users. Medicine 2016;95:e3995 10.1097/MD.0000000000003995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Herrett E, Gallagher AM, Bhaskaran K, et al. . Data resource profile: Clinical Practice Research Datalink (CPRD). Int J Epidemiol 2015;44:827–36. 10.1093/ije/dyv098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Herrett E, Thomas SL, Schoonen WM, et al. . Validation and validity of diagnoses in the General Practice Research Database: a systematic review. Br J Clin Pharmacol 2010;69:4–14. 10.1111/j.1365-2125.2009.03537.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jick SS, Kaye JA, Vasilakis-Scaramozza C, et al. . Validity of the general practice research database. Pharmacotherapy 2003;23:686–9. 10.1592/phco.23.5.686.32205 [DOI] [PubMed] [Google Scholar]
- 30. Williams T, van Staa T, Puri S, et al. . Recent advances in the utility and use of the General Practice Research Database as an example of a UK Primary Care Data resource. Ther Adv Drug Saf 2012;3:89–99. 10.1177/2042098611435911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bornand D, Toovey S, Jick SS, et al. . The risk of new onset depression in association with influenza--A population-based observational study. Brain Behav Immun 2016;53:131–7. 10.1016/j.bbi.2015.12.005 [DOI] [PubMed] [Google Scholar]
- 32. Hagberg KW, Li L, Peng M, et al. . Incidence rates of suicidal behaviors and treated depression in patients with and without psoriatic arthritis using the Clinical Practice Research Datalink. Mod Rheumatol 2016;26:774–9. 10.3109/14397595.2015.1136726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jick H, Kaye JA, Jick SS. Antidepressants and the risk of suicidal behaviors. JAMA 2004;292:338–43. 10.1001/jama.292.3.338 [DOI] [PubMed] [Google Scholar]
- 34. Thomas KH, Martin RM, Davies NM, et al. . Smoking cessation treatment and risk of depression, suicide, and self harm in the Clinical Practice Research Datalink: prospective cohort study. BMJ 2013;347:f5704 10.1136/bmj.f5704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Welk B, McArthur E, Ordon M, et al. . Association of Suicidality and Depression With 5α-Reductase Inhibitors. JAMA Intern Med 2017;177:683–91. 10.1001/jamainternmed.2017.0089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schneeweiss S, Rassen JA, Glynn RJ, et al. . High-dimensional propensity score adjustment in studies of treatment effects using health care claims data. Epidemiology 2009;20:512–22. 10.1097/EDE.0b013e3181a663cc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sevilla-González MDR, Quintana-Mendoza BM, Aguilar-Salinas CA. Interaction Between Depression, Obesity, and Type 2 Diabetes: A Complex Picture. Arch Med Res 2017;48:582–91. 10.1016/j.arcmed.2018.02.004 [DOI] [PubMed] [Google Scholar]
- 38. Svenningsson I, Björkelund C, Marklund B, et al. . Anxiety and depression in obese and normal-weight individuals with diabetes type 2: a gender perspective. Scand J Caring Sci 2012;26:349–54. 10.1111/j.1471-6712.2011.00940.x [DOI] [PubMed] [Google Scholar]
- 39. Anderberg RH, Richard JE, Hansson C, et al. . GLP-1 is both anxiogenic and antidepressant; divergent effects of acute and chronic GLP-1 on emotionality. Psychoneuroendocrinology 2016;65:54–66. 10.1016/j.psyneuen.2015.11.021 [DOI] [PubMed] [Google Scholar]
- 40. Anderberg RH, Anefors C, Bergquist F, et al. . Dopamine signaling in the amygdala, increased by food ingestion and GLP-1, regulates feeding behavior. Physiol Behav 2014;136:135–44. 10.1016/j.physbeh.2014.02.026 [DOI] [PubMed] [Google Scholar]
- 41. Dixit TS, Sharma AN, Lucot JB, et al. . Antipsychotic-like effect of GLP-1 agonist liraglutide but not DPP-IV inhibitor sitagliptin in mouse model for psychosis. Physiol Behav 2013;114-115:38–41. 10.1016/j.physbeh.2013.03.008 [DOI] [PubMed] [Google Scholar]
- 42. Laake JP, Stahl D, Amiel SA, et al. . The association between depressive symptoms and systemic inflammation in people with type 2 diabetes: findings from the South London Diabetes Study. Diabetes Care 2014;37:2186–92. 10.2337/dc13-2522 [DOI] [PubMed] [Google Scholar]
- 43. Pilot randomised controlled trial of SITAgliptin for Depressive Symptoms in type 2 diabetes (EudraCT Number: 2015-004527-32. https://www.clinicaltrialsregister.eu/ctr-search/search?query=2015-004527-32 (accessed 7 Dec 2017).
- 44. Fiest KM, Jette N, Quan H, et al. . Systematic review and assessment of validated case definitions for depression in administrative data. BMC Psychiatry 2014;14:289 10.1186/s12888-014-0289-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Thomas KH, Davies N, Metcalfe C, et al. . Validation of suicide and self-harm records in the clinical practice research datalink: validation of suicide and self-harm records. Br J Clin Pharmacol 2013;76:145–57. 10.1111/bcp.12059 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2018-023830supp001.pdf (307.3KB, pdf)