Abstract
Cav3.2 T-type calcium channels are important for the signaling of nociceptive information in the primary afferent pain pathway. During neuropathy and peripheral inflammation, Cav3.2 channels are upregulated due to an increased association with the deubiquitinase USP5. Disrupting these interactions in male mice by the use of cell permeant peptides reverses mechanical and thermal hypersensitivity. Here we explore the effects of interfering with USP5 binding to the channel in female mice with synchronized estrous cycle. We show that intrathecal delivery of a cell-penetrating TAT peptide corresponding to the UBPc domain of USP5 fully reverses mechanical hypersensitivity in mice intraplantarly injected with Complete Freund’s Adjuvant. Hence, the USP5 mediated dysregulation of Cav3.2 channel activity does not exhibit sex differences, and potential therapeutics targeting this interaction should be effective in both male and female subjects.
Electronic supplementary material
The online version of this article (10.1186/s13041-018-0405-4) contains supplementary material, which is available to authorized users.
Keywords: T-type calcium channel, Ubiquitination, Inflammation, Pain, Sex differences, USP5
Cav3.2 T-type calcium channels are important for the propagation and transmission of nociceptive information in the afferent pain pathway [1]. Cav3.2 channel activity is aberrantly upregulated in a number of painful conditions, including visceral inflammation [2], diabetic neuropathy [3] and following nerve injury [4] whereas inhibition of T-type channel activity mediates analgesia [5, 6]. We reported that this enhancement of Cav3.2 channel activity in dorsal root ganglion neurons and spinal cord arises at least in part from an aberrant upregulation of USP5, a deubiquitinating enzyme that binds to the domain III-IV linker region of the Cav3.2 α1 subunit and deubiquitinates the channel [5]. As a result, this channel is protected from degradation, leading to an accumulation of channels in the plasma membrane and consequently larger whole cell currents. This upregulation of USP5 is observed after intraplantar injection of CFA, following peripheral nerve injury, during visceral inflammation, in diabetic mice, and in a model of post-surgical pain [5, 7, 8]. Intrathecal delivery of cell permeant peptides corresponding to either the USP5 interaction site on the channel, or to the Cav3.2 binding site on USP5 reverses mechanical and thermal hypersensitivity associated with these conditions [5, 8, 9]. The studies described above were performed exclusively in male mice. Because there is evidence that certain aspects of pain signaling in the spinal cord exhibit marked sex differences [10, 11], it is important to determine whether disrupting USP5 interactions with Cav3.2 is equally protective in female mice.
To address this issue, we tested the analgesic effects of a Tat-cUBP1-USP5 peptide in female mice. This peptide corresponds to the Cav3.2 interaction site on USP5 and was previously shown by us to be effective in alleviating both mechanical hypersensitivity in CFA injected or nerve-injured male mice [9]. All animal experiments were approved by the Animal care committee of the University of Calgary. Female C57BL/6 J mice were purchased from Jackson laboratories. Their estrous cycle was synchronized and only mice under the proestrus phase were tested. Mice received 20 μl of CFA by intraplantar injection (i.pl.) 2 days prior to behavioral assessment. On the testing day, they were placed individually in plexiglass chambers on top of a grid floor and allowed to habituate for at least 90 min before testing. The Tat-cUBP1-USP5 peptide (10 μg) or vehicle (10 μl) were delivered via intrathecal injections according to methods described previously [12], at a dose that we had previously found effective in male mice [5]. Mice were then tested for mechanical hyperalgesia by assessing mechanical withdrawal threshold via a Dynamic Plantar Aesthesiometer (Ugo Basile, Varese, Italy). Data were then analyzed statistically using two-way analysis of variance.
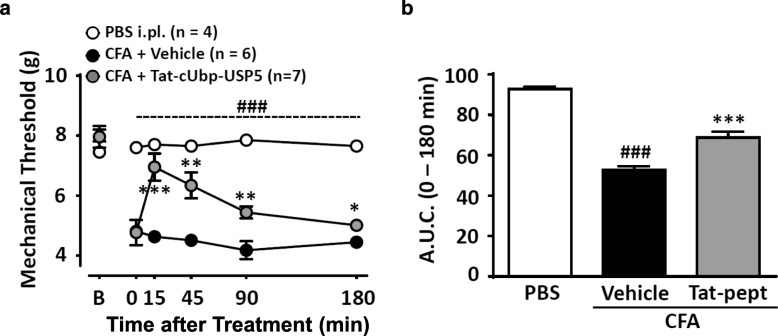
Figure 1 shows the result of this experiment. Following CFA injection, mechanical withdrawal threshold decreased significantly compared to animals that had received a PBS injection into the hindpaw. Within 15 min of delivery of the Tat-cUBP1-USP5 peptide, there was a near complete reversal of mechanical hypersensitivity that declined over time, but remained statistically different from that observed following intrathecal delivery of vehicle. As in our previous studies with male mice, no behavioral abnormalities were observed following Tat-cUBP1-USP5 treatment. These data indicate that disrupting USP5/Cav3.2 interactions mediate analgesia irrespective of the sex of the animals, and by inference, that the USP5/Cav3.2 pathway is dysregulated in a non-sexually dimorphic way in mice following peripheral inflammation.
Fig. 1.
Effects of the Tat-cUBP1-USP5 peptide on inflammatory pain induced by CFA in female mice. a Time-dependent effect and (b) Area under curve bar representation of Tat-cUBP1-USP5 delivered intrathecally to female mice in the proestrus phase of the estrous cycle. Each point (Panel a) represents the mean ± SEM of mechanical withdrawal thresholds of female mice. Bars (Panel b) represent mean ± SEM of area under curve for mechanical withdrawal threshold (n = 4–7). ### P < 0.001 indicates difference between Sham and vehicle control groups and * P < 0.05, ** P < 0.01, *** P < 0.001 denotes significance between vehicle control and Tat-cUBP1-USP5 peptide groups (Two-way ANOVA followed by a Newman-Keuls test)
Closer inspection of the data, however, reveals some differences between male and female mice. First, at the same dose as the one examined here, the maximal effect of the Tat-cUBP1-USP5 peptide in the CFA model was lower in male mice compared to female mice, with males showing a maximal effect of 70–75% reversal of mechanical hypersensitivity. In this context it is noteworthy, however, that full reversal was observed with this dose in a nerve injury model [9]. Second, the kinetics of the action of the tat peptide were different between males and females, such that analgesia peaked after 45 min and declined more slowly in males compared to what we report here. The cellular and molecular basis of these differences is unclear at this point.
Chronic pain is a debilitating condition that is often refractory to treatment, and new avenues for therapeutic intervention are sorely needed. T-type calcium channels have been pursued as a potential pharmacological target for some time. Direct inhibitors of these channels are efficacious in various preclinical pain models, however, there has not been a successful human clinical phase II trial for T-type channel inhibitors [6]. Targeting dysregulation of ion channels is an attractive alternative approach to inhibiting ion flux through the channels, as such an approach that selectively targets channels that are being dysregulated while sparing their normal physiological function is an attractive alternative [13]. This may involve the disruption of protein-protein interactions as such as the one described here, and can potentially be accomplished with small organic molecules rather than cell permeant peptides. Indeed, we have reported that small organic molecules identified in a compound library screen against the USP5/Cav3.2 interaction can show efficacy in various preclinical pain models [7]. The observation that disrupting the regulation of Cav3.2 by USP5 mediates analgesia in both female and male mice further underscores the utility of targeting Cav3.2 channel dysregulation in chronic pain as a possible therapeutic approach.
Additional file
Extended Methodology. (DOCX 24 kb)
Acknowledgements
We thank Dr. Agustin Garcia-Caballero for providing the Tat-cUBP1-USP5 peptide.
Funding
This work was supported by a Foundation Grant for the Canadian Institutes of Health Research and a Grant from Alberta Innovates. VMG is supported by the Vi Riddell program in Pediatric Pain. GWZ is a Canada Research Chair.
Availability of data and materials
All data generated or analysed during this study are included in this published article (and its Additional file 1).
Abbreviations
- CFA
Complete Freund’s adjuvant
- PBS
Phosphate buffer solution
- USP5
Ubiquitin specific peptidase 5
Authors’ contributions
VG and GWZ conceived the study and wrote the manuscript, VF performed experiments and analyzed the data, GWZ supervised the study. Both authors read and approved the final manuscript.
Ethics approval and consent to participate
This study was approved by the University of Calgary’s animal care committee.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Vinicius M. Gadotti, Email: vgadotti@ucalgary.ca
Gerald W. Zamponi, Email: zamponi@ucalgary.ca
References
- 1.Bourinet E, Alloui A, Monteil A, Barrère C, Couette B, Poirot O, Pages A, et al. Silencing of the Cav3.2 T-type calcium channel gene in sensory neurons demonstrates its major role in nociception. EMBO J. 2005;24(2):315–324. doi: 10.1038/sj.emboj.7600515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marger F, Gelot A, Alloui A, Matricon J, Ferrer JF, Barrère C, Pizzoccaro A, et al. T-type calcium channels contribute to colonic hypersensitivity in a rat model of irritable bowel syndrome. Proc Natl Acad Sci U S A. 2011;108(27):11268–11273. doi: 10.1073/pnas.1100869108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jagodic MM, Pathirathna S, Nelson MT, Mancuso S, Joksovic PM, Rosenberg ER, Bayliss DA, et al. Cell-specific alterations of T-type calcium current in painful diabetic neuropathy enhance excitability of sensory neurons. J Neurosci. 2007;27(12):3305–3316. doi: 10.1523/JNEUROSCI.4866-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jagodic MM, Pathirathna S, Joksovic PM, Lee W, Nelson MT, Naik AK, Su P, et al. Upregulation of the T-type calcium current in small rat sensory neurons after chronic constrictive injury of the sciatic nerve. J Neurophysiol. 2008;99(6):3151–3156. doi: 10.1152/jn.01031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.García-Caballero A, Gadotti VM, Stemkowski P, Weiss N, Souza IA, Hodgkinson V, Bladen C, et al. The deubiquitinating enzyme USP5 modulates neuropathic and inflammatory pain by enhancing Cav3.2 channel activity. Neuron. 2014;83(5):1144–1158. doi: 10.1016/j.neuron.2014.07.036. [DOI] [PubMed] [Google Scholar]
- 6.Snutch TP, Zamponi GW. Recent advances in the development of T-type calcium channel blockers for pain intervention. Br J Pharmacol. 2018;175(12):2375–2383. doi: 10.1111/bph.13906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gadotti VM, Caballero AG, Berger ND, Gladding CM, Chen L, Pfeifer TA, Zamponi GW. Small organic molecule disruptors of Cav3.2 - USP5 interactions reverse inflammatory and neuropathic pain. Mol Pain. 2015;11:12. doi: 10.1186/s12990-015-0011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joksimovic SL, Joksimovic SM, Tesic V, García-Caballero A, Feseha S, Zamponi GW, Jevtovic-Todorovic V, et al. Selective inhibition of Cav3.2 channels reverses hyperexcitability of peripheral nociceptors and alleviates postsurgical pain. Sci Signal. 2018;11:545. doi: 10.1126/scisignal.aao4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia-Caballero Agustin, Gadotti Vinicius M, Chen Lina, Zamponi Gerald W. A cell-permeant peptide corresponding to the cUBP domain of USP5 reverses inflammatory and neuropathic pain. Molecular Pain. 2016;12:174480691664244. doi: 10.1177/1744806916642444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sorge RE, Mapplebeck JC, Rosen S, Beggs S, Taves S, Alexander JK, Martin LJ, et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci. 2015;18(8):1081–1083. doi: 10.1038/nn.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mapplebeck JCS, Dalgarno R, Tu Y, Moriarty O, Beggs S, Kwok CHT, Halievski K, et al. Microglial P2X4R-evoked pain hypersensitivity is sexually dimorphic in rats. Pain. 2018;159(9):1752–1763. doi: 10.1097/j.pain.0000000000001265. [DOI] [PubMed] [Google Scholar]
- 12.Hylden JL, Wilcox GL. Intrathecal morphine in mice: a new technique. Eur J Pharmacol. 1980;67(2–3):313–316. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- 13.Feldman P, Khanna R. Challenging the catechism of therapeutics for chronic neuropathic pain: Targeting CaV2.2 interactions with CRMP2 peptides. Neurosci Lett. 2013;557:27–36. doi: 10.1016/j.neulet.2013.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Extended Methodology. (DOCX 24 kb)
Data Availability Statement
All data generated or analysed during this study are included in this published article (and its Additional file 1).