Abstract
Background
The present study aims to improve the M-stage classification of pancreatic neuroendocrine neoplasms (pNENs).
Methods
Two thousand six hundred sixty six pNENs were extracted from the Surveillance, Epidemiology, and End Results database to explore the metastatic patterns of pNENs. Metastatic patterns were categorized as single, two, or multiple (three or more) distant organ metastasis. The mean overall survival and hazard rate of different metastatic patterns were calculated by Kaplan-Meier and Cox proportional hazards models, respectively. The discriminatory capability of the modified M-stage classification was evaluated by Harrell’s concordance index.
Results
The overall survival time significantly decreased with an increasing number of metastatic organs. In addition, pNENs with only liver metastasis had better prognosis when compared to other metastatic patterns. Thus, we modified the M-stage classification (mM-stage) as follows: mM0-stage, tumor without metastasis; mM1-stage, tumor only metastasized to liver; mM2-stage, tumor metastasized to other single distant organ (lung, bone, or brain) or two distant organs; mM3-stage, tumor metastasized to three or more distant organs. Harrell’s concordance index showed that the modified M-stage classification had superior discriminatory capability than both the American Joint Committee on Cancer (AJCC) and the European Neuroendocrine Tumor Society (ENETS) M-stage classifications.
Conclusions
The modified M-stage classification is superior to both AJCC and ENETS M-stage classifications in the prognosis of pNENs. In the future, individualized treatment and follow-up programs should be explored for patients with distinct metastatic patterns.
Electronic supplementary material
The online version of this article (10.1186/s12902-018-0301-z) contains supplementary material, which is available to authorized users.
Keywords: Metastasis, Survival, Prognosis, Pancreas, Cancer
Background
Pancreatic neuroendocrine neoplasms (pNENs) are relatively rare tumors. However, a recent population study showed that the incidence of pNENs increased more than 4-fold from 1973 to 2012 [1]. Moreover, pNENs are considered the most serious neuroendocrine neoplasms (NENs), due to the patients have a shorter median overall survival times (3.6 years) when compared to those with tumors located in lung (5.5 years), rectum (24.6 years), and appendix (more than 30.0 years) [1].
Cancer staging classification systems are used to codify the extent of cancer. They allow clinicians to quantify prognosis and plan treatment for individual patients. Two widely used tumor staging classification systems, which are proposed by the American Joint Committee on Cancer (AJCC) and the European Neuroendocrine Tumor Society (ENETS), describe M0-stage as having no distant metastasis and M1-stage as having at least one distant metastasis [2, 3]. However, several studies demonstrated that pNENs with liver metastasis have better prognosis than other metastatic patterns [4–6].
Therefore, we utilized the Surveillance, Epidemiology, and End Result (SEER) database to explore the prognosis of different metastatic patterns of pNENs and propose a modified M-stage classification. This modified M-stage classification proves to be superior to both AJCC and ENETS M-stage classifications in prognosis.
Methods
Study cohort
As published previously [3], we utilized the topography codes (C25.0 to C25.9) and histology codes (8150, 8151, 8152, 8153, 8154, 8155, 8156, 8157, 8240, 8241, 8242, 8243, 8244, 8245, 8246, and 8249) of the International Classification of Diseases for Oncology (third edition) to identify pNENs.
Outcomes and variables
The primary outcome was overall survival. Demographic data included age, sex, and race; tumor characteristics included tumor size, primary site, differentiation, 7th AJCC T-stage, and N-stage; treatment information included surgery and radiotherapy. Single organ metastasis was defined as the tumor spreading from pancreas to another single distant organ [7]. Similarly, two organ metastases were defined as the tumor spreading from pancreas to two distant organs. Tumors spreading from pancreas to three or more distant organs were defined as multiple metastases.
Inclusion and exclusion criteria
Patients microscopically diagnosed as pNENs were included in the present study. We excluded cases with unclear or incomplete information about metastasis. In addition, we also excluded cases without information about survival time.
Statistical analyses
To compare the constituent ratio of variables among patients, we broke the continuous variables (age, tumor size) into binary variables. Survival time was plotted using the Kaplan-Meier estimator and Cox proportional hazards model. The results were presented as mean and hazard ratio, respectively, each with a 95% confidence interval (CI). Harrell’s concordance index was used to evaluate the discriminatory capability of the modified M stage classification. An index value of greater than 0.70 suggests the classification has an acceptable discriminatory capability [8]. Differences with P ≤ 0.05 divided by the number of meaningful comparisons, Bonferroni correction, were considered to be significant. Differences with P ≤ 0.1 divided by the number of meaningful comparisons, were considered to indicate a tendency. All statistical analyses were performed using SPSS 19.0 (IBM, New York, USA) or R (version 3.5.0).
Results
Patient characteristics
In total, 2666 patients (mean age 60.9 years ±13.6 years; 55.7% male, 78.8% white) were included in the present study (Fig. 1). Many patients (55.4%) underwent surgery, and some (4.7%) were treated with radiation. The constituent ratios of tumor size, location, differentiation, T-stage, and N-stage were significantly (P < 0.05) different between patients with and without metastasis (Table 1).
Fig. 1.
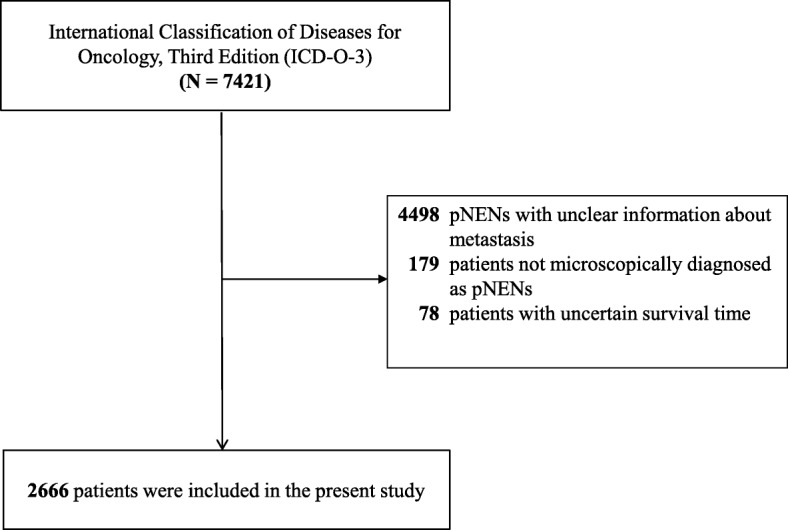
Flow chart of patient selection
Table 1.
Clinicopathological Characters
| Without Metastasis | Metastasis | P | |
|---|---|---|---|
| N = 1679 | N = 987 | ||
| Age (years) | 0.221b | ||
| ≤ 60 | 793 (47.2%) | 442 (44.8%) | |
| > 60 | 886 (52.8%) | 545 (55.2%) | |
| Sex | 0.338a | ||
| Male | 924 (55.0%) | 562 (56.9%) | |
| Female | 755 (45.0%) | 425 (43.1%) | |
| Race | 0.011a | ||
| White | 1314 (78.3%) | 787 (79.7%) | |
| Black | 191 (11.4%) | 130 (13.2%) | |
| Other | 174 (10.3%) | 70 (7.1%) | |
| Size (cm) | < 0.001b | ||
| ≤ 2 | 670 (39.9%) | 68 (6.9%) | |
| > 2 | 934 (55.6%) | 699 (70.8%) | |
| Unclear | 75 (4.5%) | 220 (22.3%) | |
| Primary Site | < 0.001b | ||
| Head | 502 (29.9%) | 258 (26.2%) | |
| Body | 295 (17.6%) | 106 (10.7%) | |
| Tail | 542 (32.3%) | 314 (31.8%) | |
| Other | 340 (20.2%) | 309 (31.3%) | |
| Differentiation | < 0.001b | ||
| Well | 1043 (62.1%) | 189 (19.1%) | |
| Moderately | 221 (13.2%) | 86 (8.7%) | |
| Poorly | 64 (3.8%) | 95 (9.6%) | |
| Undifferentiated | 17 (1.0%) | 28 (2.8%) | |
| Unclear | 334 (19.9%) | 589 (59.7%) | |
| T-sage | < 0.001b | ||
| T1 | 602 (35.9%) | 37 (3.7%) | |
| T2 | 538 (32.0%) | 276 (28.0%) | |
| T3 | 389 (23.2%) | 271 (27.5%) | |
| T4 | 67 (4.0%) | 102 (10.3%) | |
| Tx | 83 (4.9%) | 301 (30.5%) | |
| N-stage | < 0.001b | ||
| N0 | 1247 (74.3%) | 463 (46.9%) | |
| N1 | 401 (23.9%) | 338 (34.3%) | |
| Nx | 31 (1.8%) | 186 (18.8%) | |
| Surgery | < 0.001b | ||
| Yes | 1313 (78.2%) | 164 (16.6%) | |
| No | 339 (20.2%) | 813 (82.4%) | |
| Unclear | 27 (1.6%) | 10 (1.0%) | |
| Radiation | < 0.001b | ||
| Yes | 52 (3.1%) | 74 (7.5%) | |
| No | 1609 (95.8%) | 905 (91.7%) | |
| Unclear | 18 (1.1%) | 8 (0.8%) | |
aChi-square test; bKruskal-Wallis test
Metastatic patterns and survival
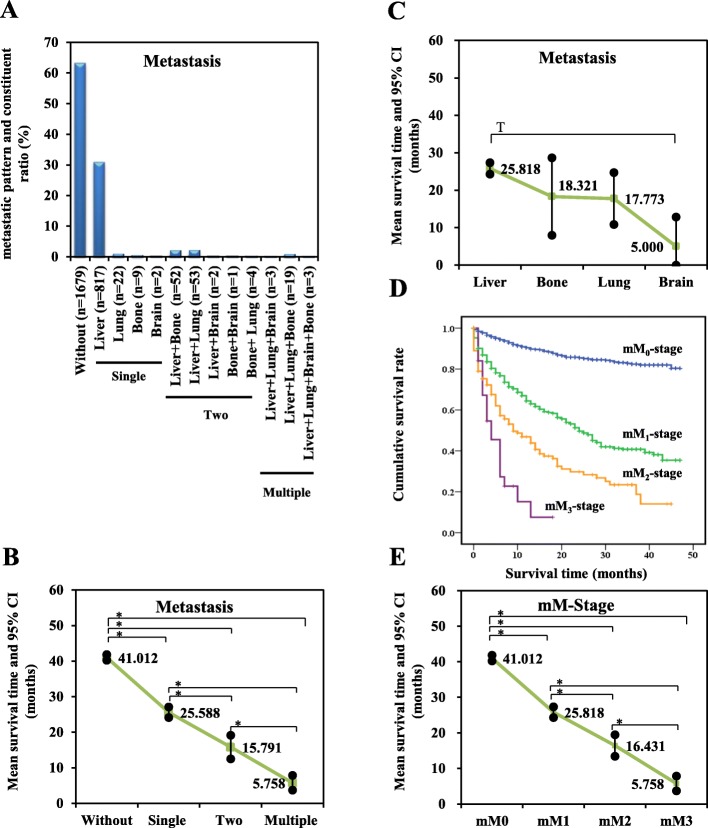
At the time of diagnosis, 1679 (62.98%) patients showed no metastasis. As shown in Fig. 2a, single organ metastases comprised 850 (31.88%) patients, including 817 liver (30.64%), 22 lung (0.83%), nine bone (0.34%), and two brain (0.07%) cases. One hundred and twelve patients (4.20%) showed two-organ metastases, including 52 liver plus bone (1.95%), 53 liver plus lung (1.99%), four bone plus lung (0.15%), two liver plus brain (0.08%), and one bone plus brain (0.04%) cases. Twenty-five patients (0.94%) presented multiple organ metastases, including 19 cases of liver plus lung plus bone (0.71%), three cases of liver plus lung plus brain (0.11%), and three cases of liver plus lung plus brain plus bone (0.11%).
Fig. 2.
a Metastatic patterns of pNENs. b Survival time of patients different metastatic patterns. c Survival time of patients with single distant organ metastasis. d Kaplan-Meier curve of overall survival of patients with modified M-stage classification. e Survival time of patients with modified M-stage classification. * Significant difference: P < 0.008; T Tendentious difference: P < 0.017
To assess survival time of different metastatic patterns, we compared the survival time of patients without metastasis to those with single distant organ metastasis, two-organ metastases, and multiple organ metastases. As the number of metastatic organs increased, survival time was significantly (P < 0.001) reduced (Fig. 2b). In addition, patients with only liver metastasis had a longer survival time than did other single-organ metastases (Fig. 2c), whereas patients with bone, lung or two-organ metastasis had similar mean survival time (bone, 18.32 months ± 5.27 months; lung, 17.77 months ± 3.54 months, two organs metastases, 15.79 months ± 1.70 months).
Modified M-stage classification and discriminatory capability
Thus, based on the observed metastatic patterns and survival times, we modified the M-stage classification (mM-stage) as shown in Table 2. Tumor without metastasis was defined as mM0-stage. Tumor spread from pancreas only to liver was defined as mM1-stage. Tumor spreading from pancreas to other single distant organ or to two distant organs was defined as mM2-stage. Tumor spreading to three or more distant organs was defined as mM3-stage.
Table 2.
Definition of M-stage classifications
| AJCC and ENTES M-stage classifications | Modified M-stage classifications |
|---|---|
| M0-stage, no distant metastasis M1-stage, distant metastasis |
mM0-stage, no distant metastasis mM1-stage, only liver metastasis mM2-stage, other single distant organ or two organs metastases mM3-stage, three or more organs metastases |
AJCC American Joint Committee on Cancer; ENETS European Neuroendocrine Tumor Society
To evaluate survival time among mM-stage classifications, survival curves were plotted using the Kaplan-Meier estimator and then compared with the log-rank test. We observed that all survival curves were well separated (Fig. 2d). Patients with advanced mM stages (mM1, mM2, mM3) had significantly (P < 0.001) shorter survival times than patients with mM0-stage (Fig. 2e). Moreover, the modified M-stage classification was an independent prognostic factor for pNENs, after adjusting for other clinical and pathological characteristics (Table 3).
Table 3.
Independent Prognostic Factors
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR and 95% CI | P-value | HR and 95% CI | P-value | |
| Age (years) | ||||
| ≤ 60 | Reference | Reference | ||
| > 60 | 1.875 (1.595–2.204) | < 0.001 | 1.744 (1.479–2.055) | < 0.001 |
| Sex | ||||
| Male | Reference | Reference | ||
| Female | 0.808 (0.691–0.945) | 0.008 | 0.850 (0.726–0.996) | 0.044 |
| Race | ||||
| White | Reference | Reference | ||
| Black | 1.345 (1.082–1.670) | 0.007 | 1.247 (0.998–1.558) | 0.052 |
| Other | 0.715 (0.527–0.970) | 0.031 | 0.767 (0.565–1.043) | 0.090 |
| Size (cm) | ||||
| ≤ 2 | Reference | Reference | ||
| > 2 | 2.889 (2.241–3.724) | < 0.001 | 1.322 (1.009–1.731) | 0.043 |
| Unclear | 6.799(5.110–9.047) | < 0.001 | 1.547(1.129–2.119) | 0.007 |
| Primary Site | a | |||
| Head | Reference | |||
| Body | 0.684(0.530–0.884) | 0.004 | ||
| Tail | 0.682(0.556–0.836) | < 0.001 | ||
| Other | 1.128(0.929–1.368) | 0.223 | ||
| Differentiation | ||||
| Well | Reference | Reference | ||
| Moderately | 1.557 (1.095–2.215) | 0.014 | 1.049 (0.735–1.498) | 0.791 |
| Poorly | 7.414 (5.608–9.803) | < 0.001 | 3.349 (2.498–4.489) | < 0.001 |
| Undifferentiated | 9.494 (6.113–14.743) | < 0.001 | 3.166 (2.000–5.011) | < 0.001 |
| Unclear | 5.136 (4.179–6.311) | < 0.001 | 1.626 (1.290–2.048) | < 0.001 |
| T-stage | a | |||
| T1 | Reference | |||
| T2 | 3.434(2.474–4.766) | < 0.001 | ||
| T3 | 3.353(2.399–4.688) | < 0.001 | ||
| T4 | 7.082(4.867–10.306) | < 0.001 | ||
| Tx | 9.288(6.696–12.882) | < 0.001 | ||
| N-stage | ||||
| N0 | Reference | Reference | ||
| N1 | 1.679 (1.415–1.993) | < 0.001 | 1.304 (1.092–1.557) | 0.003 |
| Nx | 3.732 (3.019–4.613) | < 0.001 | 1.452 (1.152–1.829) | 0.002 |
| Surgery | ||||
| Yes | Reference | Reference | ||
| No | 8.556 (6.941–10.548) | < 0.001 | 3.901 (3.013–5.050) | < 0.001 |
| Unclear | 1.991 (0.812–4.883) | 0.133 | 1.487 (0.601–3.680) | 0.391 |
| Radiation | a | |||
| Yes | Reference | |||
| No | 1.984(1.511–2.603) | < 0.001 | ||
| Unclear | 0.939(0.420–2.098) | 0.878 | ||
| mM-stage | ||||
| mM0-stage | Reference | Reference | ||
| mM1-stage | 4.520 (3.789–5.393) | < 0.001 | 1.643 (1.339–2.016) | < 0.001 |
| mM2-stage | 8.199(6.380–10.537) | < 0.001 | 2.249(1.704–2.968) | < 0.001 |
| mM3-stage | 16.356 (10.266–26.059) | < 0.001 | 5.034 (3.110–8.150) | < 0.001 |
avariables excluded by multivariate forward stepwise cox regression
To explore discriminatory capability of the modified M-stage classification, Harrell’s concordance index was calculated. The mM-stage classification had a better discriminatory capability (Harrell’s concordance index, 0.712; 95% CI, 0.692–0.732) than AJCC M-stage and ENETS M-stage (Harrell’s concordance index, 0.697; 95% CI, 0.678–0.717).
Discussion
In agreement with previous studies [9–11], the present study also demonstrated that nearly one quarter of patients (37.02%, 987/2666) presenting metastasis at the time of pNEN diagnosis. In addition, liver metastasis was the majority metastatic pattern, followed by lung, bone and brain metastasis. The hematogenous mode of metastasis might contribute to the metastatic pattern, which we have observed in the present study. Unsually, carcinoma cells seed in the liver via the portal venous system. Then, these cells would spread to lung via the inferior vena cava and pulmonary arteries. Finally, the carcinoma cells from lung metastases would seed in other organs via arterial blood [12].
The present study found that with an increasing number of metastatic organs, there was a significant decrease in survival time. In addition, pNENs with liver metastasis had longer overall survival than other single-organ metastatic patterns. However, AJCC and ENETS classify both pNENs with liver metastasis and pNENs with the other metastasitic patterns as M1-stage. Our modified M-stage classification distinguishes that tumor spreading from pancreas only to liver should be separated from the other metastatic patterns, and that it is necessary to design individualized treatment and follow-up programs for patients with lung, bone, or brain metastasis.
Usually, pancreatic resection is not performed when the pancreatic malignant tumor has spread to other organs [13]. However, considering the indolent behavior of pNENs and the high frequency of liver metastasis, several clinicians suggested surgical management could give rise to benefit to pNENs with liver metastasis [4, 14]. Birnbaum et al. pancreatic resection could slow down tumor growth and reduce hormone production [14], possibly resulting in considerable benefit for patients with liver metastasis [4].
Consistent with previous studies, the tumor size, primary site, differentiation, AJCC T-stage and AJCC N-stage were identified as predictors of distant organ metastasis (Additional file 1: Table S1). Unfortunately, SEER database did not record Ki-67 status and graded the primary tumor only on the basis of morphological description (ICD-O-3) in the pathology report. Thus, we failed to evaluate the predictive role of Ki-67 status and WHO 2010 grading classification (NET G1, NET G2, NET G3 and NEC) in distant organ metastasis.
It seems the primary tumor site is a particularly useful predictor because it is available before any operation occurs. Hao et al. reported that compared to tumors located in the head and neck of the pancreas, tumors in the body and tail showed a decreased risk of liver metastasis in pancreatic adenocarcinoma [15]. In contrast, the present study showed that pNENs located in the pancreatic tail are actually 1.728 times more likely (P < 0.001) to develop metastasis, as compared to tumors located in the pancreatic head. This may be due to the fact that patients with pNENs, especially non-functioning pNENs, in the tail of the pancreas are less likely to experience obstructive signs and hormonal symptoms until tumors spread to the peritoneum, spleen, and distant organs [16, 17]. Thus, at the time of diagnosis, distant organ metastases exist in most of these patients.
Some limitations of the present study should be noted. First, the SEER database only provides information on pNEN metastasis to liver, lung, bone, and brain. The frequency of pNEN metastasis might be underestimated. Second, Hlatky et al. noted that multiple metastatic lesions may be related to a short survival time [18]. However, the SEER database did not collect data on the number of metastatic lesions in each distant organ.
Conclusions
In conclusion, this is the first population-based study to investigate the metastatic patterns and predictors in advanced pNENs. We found significant differences in survival time across different metastatic patterns. Thus, the modified M-stage classification show a better discriminatory capability than the AJCC and ENETS M-stage classifications. In the future, clinicians should determine individualized treatment and follow-up programs for pNENs with different metastatic patterns.
Additional file
Table S1. Clinicopathological characters associated with metastasis. (DOCX 22 kb)
Acknowledgments
We thank the Surveillance, Epidemiology, and End Results (SEER) program providing the original data. We also thank Prof. Wenli Zhang and Prof. Houli Zhang gave us critical comments during the revision of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China [grant number 81473504, 81200989]; China Scholarship Council [grant number 201608080195]. The funders had no any role in the manuscript.
Availability of data and materials
The datasets generated and analyzed during the current study are available in the SEER database (https://seer.cancer.gov/).
Abbreviations
- AJCC
American Joint Committee on Cancer
- ENETS
European Neuroendocrine Tumor Society
- OS
Overall survival
- pNENs
Pancreatic neuroendocrine neoplasms
- SD
Standard deviation
- SEER
Surveillance, Epidemiology, and End Result
Authors’ contributions
XZ identified the pNENs from SEER database, designed the study and wrote the manuscript; XZ, JS, MM, PL, LL, YS analyzed and interpreted the data; YW is responsible for the statistical analyses; PG and LM contributed to conception, design and funding. All authors have been involved in revising and proofreading of the manuscript. All authors listed have approved the manuscript.
Ethics approval and consent to participate
The original data of our study were provided by SEER database (research data agreement to Xianbin Zhang) and the study protocol was approved by the Ethics Committee of the First Affiliated Hospital of Dalian Medical University (approval number: YJ-KY-FB-2017-05).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xianbin Zhang, Phone: +86-411-86110139, Email: zhangxianbin@hotmail.com.
Jiaxin Song, Email: jiaxian-song@hotmail.com.
Peng Liu, Email: surgeonliupeng@126.com.
Mohammad Abdul Mazid, Email: dr_mazid14@hotmail.com.
Lili Lu, Email: lulili_92@sina.com.
Yuru Shang, Email: shangyuru@hotmail.com.
Yushan Wei, Email: weiyushan@outlook.com.
Peng Gong, Email: gongpengdalian@163.com.
Li Ma, Phone: +86-411-86110332, Email: mali_lele@sina.com.
References
- 1.Dasari A, Shen C, Halperin D, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017;3:1335–1342. doi: 10.1001/jamaoncol.2017.0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Panzuto F, Boninsegna L, Fazio N, et al. Metastatic and locally advanced pancreatic endocrine carcinomas: analysis of factors associated with disease progression. J Clin Oncol. 2011;29:2372–2377. doi: 10.1200/JCO.2010.33.0688. [DOI] [PubMed] [Google Scholar]
- 3.Zhang X, Lu L, Shang Y, et al. The number of positive lymph node is a better predictor of survival than the lymph node metastasis status for pancreatic neuroendocrine neoplasms: a retrospective cohort study. Int J Surg. 2017;48:142–148. doi: 10.1016/j.ijsu.2017.10.064. [DOI] [PubMed] [Google Scholar]
- 4.Jin K, Xu J, Chen J, et al. Surgical management for non-functional pancreatic neuroendocrine neoplasms with synchronous liver metastasis: a consensus from the Chinese study Group for Neuroendocrine Tumors (CSNET) Int J Oncol. 2016;49:1991–2000. doi: 10.3892/ijo.2016.3711. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Carbonero R, Rinke A, Valle JW, et al. ENETS consensus guidelines for the standards of care in neuroendocrine neoplasms. Systemic therapy 2: chemotherapy. Neuroendocrinology. 2017;105:281–294. doi: 10.1159/000473892. [DOI] [PubMed] [Google Scholar]
- 6.Chamberlain RS, Canes D, Brown KT, et al. Hepatic neuroendocrine metastases: does intervention alter outcomes? Am Coll Surg. 2000;190:432–445. doi: 10.1016/S1072-7515(00)00222-2. [DOI] [PubMed] [Google Scholar]
- 7.Vatandoust S, Price TJ, Karapetis CS. Colorectal cancer: metastases to a single organ. World J Gastroenterol. 2015;21:11767–11776. doi: 10.3748/wjg.v21.i41.11767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bando E, Makuuchi R, Tokunaga M, et al. Impact of clinical tumor–node–metastasis staging on survival in gastric carcinoma patients receiving surgery. Gastric Cancer. 2017;20:448–456. doi: 10.1007/s10120-016-0637-x. [DOI] [PubMed] [Google Scholar]
- 9.Niederle MB, Hackl M, Kaserer K, et al. Gastroenteropancreatic neuroendocrine tumours: the current incidence and staging based on the WHO and European neuroendocrine tumour society classification: an analysis based on prospectively collected parameters. Endocr Relat Cancer. 2010;17:909–918. doi: 10.1677/ERC-10-0152. [DOI] [PubMed] [Google Scholar]
- 10.Lawrence B, Gustafsson BI, Chan A, et al. The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin N Am. 2011;40:1–18. doi: 10.1016/j.ecl.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Pavel M, Costa F, Capdevila J, et al. ENETS consensus guidelines update for the management of distant metastatic disease of intestinal, pancreatic, bronchial neuroendocrine neoplasms (NEN) and NEN of unknown primary site. Neuroendocrinology. 2016;103:172–185. doi: 10.1159/000443167. [DOI] [PubMed] [Google Scholar]
- 12.Weiss L, Grundmann E, Torhorst J, et al. Haematogenous metastatic patterns in colonic carcinoma: an analysis of 1541 necropsies. J Pathol. 1986;150:195–203. doi: 10.1002/path.1711500308. [DOI] [PubMed] [Google Scholar]
- 13.Partelli S, Bartsch DK, Capdevila J, et al. ENETS consensus guidelines for standard of care in neuroendocrine tumours: surgery for small intestinal and pancreatic neuroendocrine tumours. Neuroendocrinology. 2017;105:255–265. doi: 10.1159/000464292. [DOI] [PubMed] [Google Scholar]
- 14.Birnbaum DJ, Turrini O, Vigano L, et al. Surgical management of advanced pancreatic neuroendocrine tumors: short-term and long-term results from an international multi-institutional study. Ann Surg Onco. 2015;22:1000–1007. doi: 10.1245/s10434-014-4016-8. [DOI] [PubMed] [Google Scholar]
- 15.S D LW, GY B, et al. Risk factors of liver metastasis from advanced pancreatic adenocarcinoma: a large multicenter cohort study. World J Surg Oncol. 2017;15:120. doi: 10.1186/s12957-017-1175-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freelove R, Walling AD. Pancreatic cancer: diagnosis and management. Am Fam Physician. 2006;73:485–492. [PubMed] [Google Scholar]
- 17.Zhang X, Ma L, Bao H, et al. Clinical, pathological and prognostic characteristics of gastroenteropancreatic neuroendocrine neoplasms in China: a retrospective study. BMC Endocr Disord. 2014;14:54. doi: 10.1186/1472-6823-14-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hlatky R, Suki D, Sawaya R. Carcinoid metastasis to the brain. Cancer. 2004;101:2605–2613. doi: 10.1002/cncr.20659. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Clinicopathological characters associated with metastasis. (DOCX 22 kb)
Data Availability Statement
The datasets generated and analyzed during the current study are available in the SEER database (https://seer.cancer.gov/).