Abstract
Background
Kawasaki disease (KD) is a serious disease characterized by systemic lesions of the skin and mucous membranes, as well as lymphomas and vascular inflammation. KD threatens the health and lives of children, especially young ones. Here, we compared the therapeutic effects of single intravenous immunoglobulin gamma (IVIG) vs. a combination of IVIG and infliximab in young children with Kawasaki disease (KD).
Material/Methods
A total of 154 children with KD, younger than 5 years old, were enrolled in the study from January 2013 to January 2017. The patients were randomly divided into an IVIG group and a combination of IVIG and infliximab treatment group. After systematic treatments, the therapeutic indicators of the 2 groups were compared. During the treatment process, body temperature and other important inflammatory indicators, including C-reactive protein (CRP), white blood cell count (WBC), and tumor necrosis factor alpha (TNF-α), were monitored in the first 4 days.
Results
There were fewer refractory KD patients in the combined treatment group than in the IVIG group (4 vs. 14, p<0.001). KD patients in the combined treatment group had better outcomes with shorter fever durations and hospital stays, as well as less coronary artery dilation. However, there was no obvious differences in the incidence rate of coronary artery aneurysms between the 2 groups (p>0.05). Costs of administration were similar between groups (p>0.05). Body temperature, CRP, WBC, and TNF-α in the combined therapy group all showed an earlier drop than in the IVIG group, indicating a more effective anti-inflammation effect.
Conclusion
The introduction of IVIG combined with infliximab in the treatment of young children with KD has more advantages than single IVIG therapy and can be considered as a preferred treatment for KD. However, it would be necessary to further investigate whether there is a significant difference in aneurysm frequency and long-term outcome between these 2 strategies among a larger number of patients.
MeSH Keywords: Antibodies, Monoclonal; Immunoglobulins, Intravenous; Mucocutaneous Lymph Node Syndrome
Background
Mucocutaneous lymph node syndrome was first proposed by a Japanese physician, Kawasaki, in 1967, which is consequently known as Kawasaki disease (KD) [1]. Despite 50 years of research, the mechanism of KD is still not well understood. Many studies have shown that KD may be related to infection and autoimmune allergy [2,3]. The clinical manifestations of KD are sustained high fever, severe skin mucosal damage, systemic vascular inflammatory lesions, and severe lesions involving the vital organs, especially the coronary arteries [4], greatly increasing the risk of myocardial infarction and threatening the lives of these children.
Intravenous immunoglobulin gamma (IVIG) is currently recognized as an effective treatment of KD in clinical practice [5]. However, 15–20% of children with KD show resistance to IVIG, resulting in poor prognosis [6]. Infliximab is a novel biologic drug that can produce anti-inflammatory effects through specific blocking of tumor necrosis factor alpha (TNF-α), which has been widely used in the treatment of spondylitis, arthritis, rheumatoid and autoimmune diseases like Crohn’s disease. Recently, it has been reported that infliximab exhibited a satisfactory effect in patients with IVIG-resistant KD or refractory KD [7,8]. However, infliximab is rarely used as the first-line drug for KD treatment, and there is no systematic comparison of the therapeutic effect between single IVIG and the combination of IVIG and infliximab in patients with KD, especially in young children (<5 years old).
Therefore, in the present study, KD children younger than 5 years old were treated with these 2 regimens and therapeutic effects were compared simultaneously. These valuable results provide reliable guidance for the clinical treatment of KD in young children.
Material and Methods
Patients
We enrolled 154 children diagnosed with KD at the Maternity and Child Care Centre of Baoji from January 2013 to January 2017. The patients had an average age of 2.20 years old (a minimum of 0.50 years old and a maximum of 4.70 years old). The average weight was 8.25 kg (from 4.12 kg to 22.35 kg). Sixty-two were boys, accounting for 40.26% of the total. The diagnosis of KD was determined by 2 physicians based on the presence of at least 5 days of fever and 4 of the 5 principal clinical features: 1) Changes in extremities, including erythema of palms and soles, edema of hands and feet (acute phase), and desquamation of fingers and toes (subacute phase); 2) Polymorphous exanthema; 3) Bilateral bulbar conjunctival injection; 4) Changes in lips and oral mucosa, including erythematous and cracked lips, strawberry tongue, and oral and pharyngeal hyperemia; and 5) Cervical lymphadenopathy (>1.5 cm diameter). Patients with at least 5 days of fever but less than 4 principal criteria can be diagnosed with KD when coronary artery abnormalities are detected. If IVIG-resistant KD or refractory KD were found in the process of research, the children would be excluded from the group to receive more complex integrated treatments. The parents of the children were informed of the details of this study, and corresponding medical treatments were conducted with the consent of patients and parents.
Methods
The KD patients were randomly divided into 2 groups with 77 patients in each group. They were treated with IVIG or the combination of IVIG and infliximab. The age, sex, weight, body temperature at admission, time from fever onset to diagnosis, hematologic examination, and coronary artery status were compared between the 2 groups.
Patients administrations in IVIG group
A single intravenous infusion of 1 g/kg gammaglobulin on the first day of treatment was followed by slow-infusion pulse therapy for 10–11 h. Large doses of aspirin were given at a dose of 80 mg/kg/d through 4 oral administrations (20 mg/kg every time) until they were afebrile for 48 h, at which point the aspirin dose was reduced to 5 mg/kg/d.
Patients administrations in infliximab group
An intravenous infusion of 1 g/kg gamma globulin and 5 mg/kg infliximab (in that order) on the first day of treatment were administered. Large doses of aspirin were given at a dose of 80 mg/kg/d through 4 oral administrations (20 mg/kg every time) until they were afebrile for 48 h, at which point the aspirin dose was reduced to 5 mg/kg/d.
Monitoring of therapeutic effect
During the different treatments, the patients’ conditions and physical recovery were closely monitored. The related indicators were assessed and recorded by medical examinations: 1) Therapeutic effect indicators: C-reactive protein (CRP), white blood cell count (WBC), serum TNF-α level, duration of fever, and length of hospital stay; 2) Complications: incident rate of coronary artery dilation and aneurysms; and 3) Economic indicator: hospitalization expenses.
Statistical analysis
All quantitative data are presented as means ±SEM. For pairwise comparison of 2 or more groups of quantitative data, p values were calculated by t test, analysis of variance (ANOVA), and chi-square, as appropriate. P<0.05 was regarded as having statistical significance.
Results
KD patients had intense inflammatory responses and most of them had high fever and remarkably increased inflammatory indicators like white blood cell count (WBC), absolute neutrophil count (ANC), C-reactive protein (CRP), and erythrocyte sedimentation rate (ESR). Demographic and clinical characteristics of KD patients are shown in Table 1, and there were no significant differences in baseline characteristics between the IVIG group and the combined treatment group.
Table 1.
Demographic and clinical characteristics of patient populations.
| IVIG (n=77) | IVIG and infliximab (n=77) | |
|---|---|---|
| Median age, years (range) | 2.3 (0.5–4.5) | 2.1 (0.5–4.7) |
| Males (%) | 28 (18.18%) | 34 (22.08%) |
| Weight, kg (range) | 8.34 (4.12–22.35) | 8.16 (4.18–22.10) |
| Body temperature at admission, °C | 38.7 (37.5–39.4) | 38.9 (37.8–40.2) |
| Time from fever onset to diagnosis, days | 3.2 (1–7) | 3.5 (2–8) |
| Hematologic examination | ||
| WBC (×103/μL) | 12.7 (8.9–18.9) | 14.1 (9.1–19.2) |
| ANC (/μL) | 8554 (4758–17568) | 10286 (5472–18754) |
| ESR (mm/h) | 61 (30–138) | 65 (33–118) |
| CRP (mg/dl) | 8.0 (4.9–29.8) | 8.5 (4.3–33.5) |
| CA status | ||
| Aneurysm | 2 | 1 |
| Dilated | 13 | 11 |
| Normal | 62 | 65 |
ANC – absolute neutrophil count; CA – coronary artery; CRP – C-reactive protein; ESR – erythrocyte sedimentation rate; IVIG – intravenous immunoglobulin; WBC – white blood cell count.
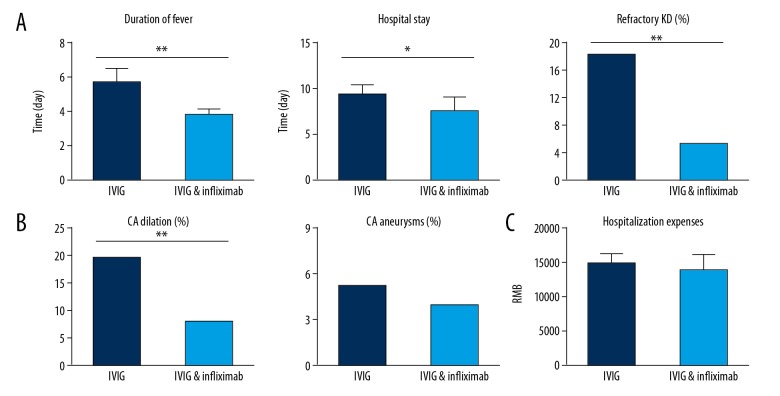
During the therapeutic process, 18 patients with refractory KD were excluded from the 2 groups and received other comprehensive treatments for the sake of security. There were significantly fewer refractory KD patients in the combined treatment group than in the IVIG group (4 vs. 14, p<0.001). The comparisons of other therapeutic indictors between the 2 groups were also carried out. As shown in Table 2 and Figures 1 and 2, KD patients in the combined treatment group had better outcomes with shorter fever duration and hospital stay, as well as less coronary artery dilation. However, there was no obvious difference in the occurrence rate of coronary artery aneurysms between the 2 groups (p>0.05). The 2 groups had similar costs of administration (p>0.05).
Table 2.
Comparisons of clinical symptoms before and after IVIG or combination of IVIG and infliximab treatments.
| Symptoms | IVIG | IVGI & infliximab | ||
|---|---|---|---|---|
| Before | After (48 h) | Before | After (48 h) | |
| Cases (%) | Cases (%) | Cases (%) | Cases (%) | |
| Fever | 75 (97.40%) | 41 (53.25%) | 77 (100%) | 19 (24.68%) |
| Conjunctival injection | 66 (85.71%) | 29 (37.66%) | 61 (79.22%) | 22 (28.57%) |
| Changes in lip and oral cavity | 69 (89.61) | 38 (49.35%) | 66 (85.71%) | 21 (27.27%) |
| Cervical lymphadenopathy | 57 (74.03%) | 28 (36.36%) | 62 (80.52%) | 16 (20.78%) |
| Polymorphous exanthema | 43 (55.84%) | 18 (23.38%) | 35 (45.45%) | 9 (11.69) |
Figure 1.
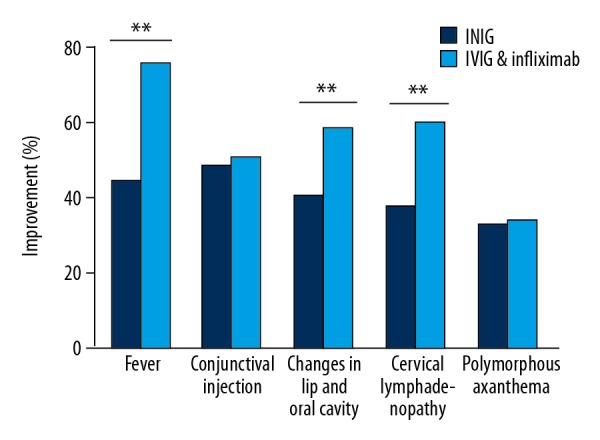
Symptom improvements after different treatments (** p<0.001).
Figure 2.
Comparisons of indicators after IVIG or combination of IVIG and infliximab treatments. (A) Therapeutic effect indicator: duration of fever, length of hospital stay, and occurrence rate of refractory KD. (B) Complications indictors: coronary artery (CA) dilation and CA aneurysms. (C) Economic indicator: hospitalization expenses.
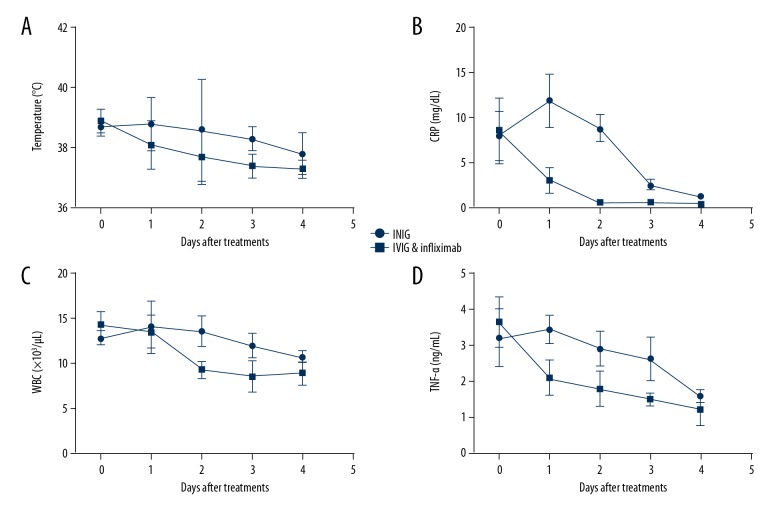
We monitored the inflammatory indictors (body temperature, CRP, WBC and TNF-α) in the first 4 days in the 2 groups. As shown in Figure 3, body temperature, CRP, WBC, and TNF-α in the combined therapy group all showed an earlier drop than in the IVIG group, and the inflammatory responses in many patients were relieved only at day 1 after combined therapy, suggesting that early administration of IVIG together with infliximab has a stronger anti-inflammation effect.
Figure 3.
Monitoring of inflammatory indicators: temperature (A), CRP (B), WBC (C), and TNF-α (D) in KD children with different treatments in the first 4 days (CRP, C-reactive protein; WBC, white blood cell count).
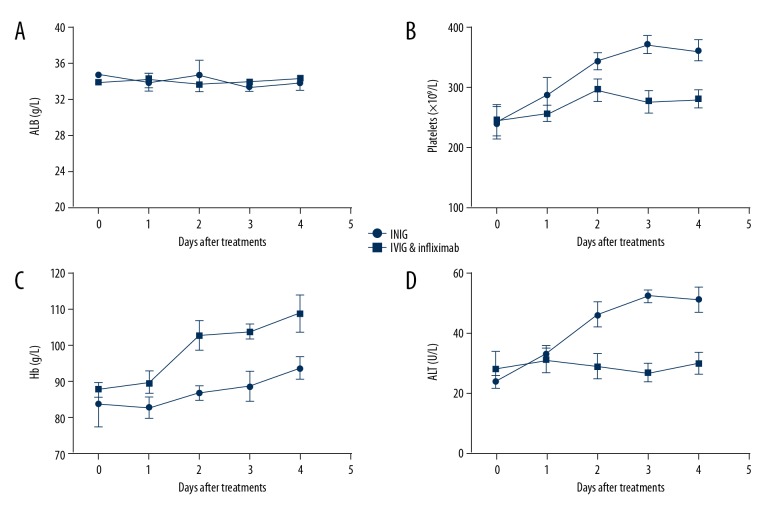
We also monitored several other important indicators related to the KD process, such as albumin (ALB), platelets, hemoglobin (Hb), and alanine aminotransferase (ALT). As shown in Figure 4A, the ALB level did not show an obvious difference between the IVIG group and IVIG plus Infliximab group. The platelets level in the IVIG group had a more remarkable rise when compared with the IVIG plus Infliximab group (Figure 4B), indicating a higher risk for coronary complications. Hb in the IVIG plus Infliximab group exhibited a faster recovery than in the IVIG group (Figure 4C). ALT level in the IVIG plus Infliximab group was more stable (Figure 4D), while the ALT level in the IVIG group showed an upward tendency, indicating potential liver function impairments.
Figure 4.
Monitoring of other indicators containing ALB (A), platelets (B), Hb (C), and ALT (D) in KD children with different treatments in the first 4 days.
Discussion
KD is a serious disease characterized by systemic skin, mucous membranes, lymphomas, and vascular inflammatory lesions, threatening the health and life of children, especially young ones [9]. Statistically, the disease has surpassed rheumatic fever and has become the first major cause of acquired heart disease in children [10,11]. Therefore, developing effective treatments to improve clinical symptoms of KD and protect children from heart injury is of great significance.
Although many studies have been focused on the pathogenesis of KD, the mechanism is not yet fully understood. At present, the dominant view is that the occurrence of KD may be related to external infection and internal immune dysfunction [12]. Therefore, modulating the immune function and reducing the inflammation damage are important in treating KD. Studies have shown that gamma globulin treatment of KD has a variety of immune system regulatory effects: 1) Gamma globulin inhibits the secretion of endotoxin from streptococcus and staphylococcus, improving the degree of infection in KD patients [13]. 2) In response to the autoimmune disorders in the body, gamma globulin can promote lymphocyte apoptosis, reduce the secretion of self-attacking antibodies from B cells, and inhibit inflammatory response [14]. 3) Gamma globulin can increase the expression of CD25+ and CD4+ through negative regulation, thereby improving the immune dysfunction due to the decrease of CD25+ and CD4+ [15]. 4) Gamma globulin reduces damage to the endovascular barrier by increasing metalloproteinase-9 and upregulating the expression of CD154 [16]. 5) Gamma globulin can reduce the immune damage in KD by blocking the LTB4-leukotriene B4 pathway [17]. 6) Gamma globulin can reduce coronary artery damage by sealing iron receptors on the mononuclear macrophages and endothelial cells [18]. 7) Gamma globulin can down-regulate the expression of adhesion molecules on the multinuclear cell surface, thereby reducing coronary artery injury caused by cytokines in the serum [19].
In contrast to the therapeutic mechanism of gamma globulin, infliximab has more targeting ability and specificity in the fight against KD [20]. It is well known that the release and aggregation of a large number of vascular endothelial activating factors and cytokines lead to intense activation of immune system, resulting in the immune disorder [21]. Studies have shown that blood levels of TNF-α increased significantly in children with acute KD, while children with coronary artery lesions have a higher level of TNF-α. Increased levels of TNF-α can activate monocyte chemotactic factor-1 and up-regulate the expression of intercellular adhesion molecule-1 in vascular endothelial cells, leading to the aggregation and infiltration of monocytes and neutrophils, inducing vascular and coronary artery lesions [22,23]. Theoretically, reducing the level of TNF-α or blocking the binding of TNF-α to its receptor can relieve inflammatory injury. Infliximab is a human-mouse chimeric monoclonal antibody that specifically binds tightly to TNF-α and inhibits TNF-α from binding to its receptor, reducing the subsequent vascular inflammatory damage [24,25]. Compared with gamma globulin, more is known about the mechanism of infliximab. Therefore, the combination of IVIG and infliximab is expected to produce better outcomes.
Our study investigated the treatment efficacy of traditional gamma globulin vs. combination therapy of IVIG and infliximab. According to the disease symptoms of KD, infliximab specifically inhibits the sharply increased TNF-α level in circulation caused by the over-reactive immune system. It plays a blocking role in reducing inflammatory damage in blood vessels and coronary arteries. During the therapeutic process, body temperature, CRP, WBC, and TNF-α in combined therapy-treated patients all showed an earlier and more obvious drop than in the IVIG group, indicating a more intense anti-inflammation effect than with single IVIG. The reasons for the significant difference between the 2 treatments may be related to the fact that IVIG non-responsive KD and refractory KD actually exist in the younger patients, and nearly 20% patients are not sensitive to IVIG therapy [26–28]. Infliximab produces better outcomes and markedly reduces the incidence of associated coronary complications compared to traditional IVIG treatments.
We demonstrated that the early introduction of both IVIG and infliximab in the treatment of children with KD at a younger age has advantages over the traditional single IVIG treatment and can be considered as a preferred choice for KD.
Conclusions
Our study has certain limitations that need to be considered. The therapeutic effect of IVIG and IVIG plus Infliximab treatments on atypical or incomplete KD patients has not been investigated. Other therapeutic regimens should also be considered and compared. It is necessary to further investigate whether there is a significant difference in aneurysm frequency and long-term outcomes between these 2 strategies among a larger number of patients, which we plan to do in future research.
Footnotes
Conflict of interest
None.
Source of support: This work was supported by the Maternity and Child Care Centre of Baoji
References
- 1.Accomando S, Liotta A, Maggio MC, et al. Infliximab administration effective in the treatment of refractory Kawasaki disease. Pediatr Allergy Immunol. 2010;21:1091–92. doi: 10.1111/j.1399-3038.2010.01029.x. [DOI] [PubMed] [Google Scholar]
- 2.Blaisdell LL, Hayman JA, Moran AM. Infliximab treatment for pediatric refractory Kawasaki disease. Pediatr Cardiol. 2011;32:1023–27. doi: 10.1007/s00246-011-0045-2. [DOI] [PubMed] [Google Scholar]
- 3.Brogan RJ, Eleftheriou D, Gnanapragasam J, et al. Infliximab for the treatment of intravenous immunoglobulin-resistant Kawasaki disease complicated by coronary artery aneurysms: A case report. Pediatr Rheumatol Online J. 2009;7:3. doi: 10.1186/1546-0096-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burns JC, Best BM, Mejias A, et al. Infliximab treatment of intravenous immunoglobulin-resistant Kawasaki disease. J Pediatr. 2008;153:833–38. doi: 10.1016/j.jpeds.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burns JC, Song Y, Bujold M, et al. Immune-monitoring in Kawasaki disease patients treated with infliximab and intravenous immunoglobulin. Clin Exp Immunol. 2013;174:337–44. doi: 10.1111/cei.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies S, Gold-von Simson G. Should infliximab be used as an adjuvant to IVIG in the treatment of children with Kawasaki disease who are at high risk for resistance to conventional therapy? Pediatr Cardiol. 2013;34:1756. doi: 10.1007/s00246-013-0731-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirono K, Kemmotsu Y, Wittkowski H, et al. Infliximab reduces the cytokine-mediated inflammation but does not suppress cellular infiltration of the vessel wall in refractory Kawasaki disease. Pediatr Res. 2009;65:696–701. doi: 10.1203/PDR.0b013e31819ed68d. [DOI] [PubMed] [Google Scholar]
- 8.Jimenez-Fernandez SG, Tremoulet AH. Infliximab treatment of pancreatitis complicating acute kawasaki disease. Pediatr Infect Dis J. 2012;31:1087–89. doi: 10.1097/INF.0b013e31826108c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kishimoto S, Muneuchi J, Takahashi Y, et al. Psoriasiform skin lesion and supprative acrodermatitis associated with Kawasaki disease followed by the treatment with infliximab: A case report. Acta Paediatr. 2010;99:1102–4. doi: 10.1111/j.1651-2227.2010.01745.x. [DOI] [PubMed] [Google Scholar]
- 10.Kurokawa Y, Masuda H, Kobayashi T, et al. Effective therapy with infliximab for clinically mild encephalitis/encephalopathy with a reversible splenial lesion in an infant with Kawasaki disease. Nihon Rinsho Meneki Gakkai Kaishi. 2017;40:190–95. doi: 10.2177/jsci.40.190. [DOI] [PubMed] [Google Scholar]
- 11.Lee AM, Burns JC, Tremoulet AH. Safety of infliximab following live virus vaccination in Kawasaki disease patients. Pediatr Infect Dis J. 2017;36:435–37. doi: 10.1097/INF.0000000000001447. [DOI] [PubMed] [Google Scholar]
- 12.Mori M, Imagawa T, Hara R, et al. Efficacy and limitation of infliximab treatment for children with Kawasaki disease intractable to intravenous immunoglobulin therapy: Report of an open-label case series. J Rheumatol. 2012;39:864–67. doi: 10.3899/jrheum.110877. [DOI] [PubMed] [Google Scholar]
- 13.O’Connor MJ, Saulsbury FT. Incomplete and atypical Kawasaki disease in a young infant: Severe, recalcitrant disease responsive to infliximab. Clin Pediatr (Phila) 2007;46:345–48. doi: 10.1177/0009922806294842. [DOI] [PubMed] [Google Scholar]
- 14.Ogihara Y, Ogata S, Nomoto K, et al. Transcriptional regulation by infliximab therapy in Kawasaki disease patients with immunoglobulin resistance. Pediatr Res. 2014;76:287–93. doi: 10.1038/pr.2014.92. [DOI] [PubMed] [Google Scholar]
- 15.Oishi T, Fujieda M, Shiraishi T, et al. Infliximab treatment for refractory Kawasaki disease with coronary artery aneurysm. Circ J. 2008;72:850–52. doi: 10.1253/circj.72.850. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez-Gonzalez M, Matamala-Morillo MA, Segado-Arenas A. Infliximab as rescue therapy in refractory Kawasaki disease. Ann Pediatr Cardiol. 2014;7:74–75. doi: 10.4103/0974-2069.126578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saji T, Takatsuki S, Kobayashi T. [Anti TNF-alpha (infliximab) treatment for intravenous immunoglobulin (IVIG) resistance patients with acute Kawasaki disease the effects of anticytokine therapy]. Nihon Rinsho. 2014;72:1641–49. [in Japanese] [PubMed] [Google Scholar]
- 18.Salguero JS, Duran DG, Peracaula CS, et al. [Refractory Kawasaki disease with coronary aneurysms treated with infliximab]. An Pediatr (Barc) 2010;73:268–71. doi: 10.1016/j.anpedi.2010.06.006. [in Spanish] [DOI] [PubMed] [Google Scholar]
- 19.Shin JI, Lee JS, Choi JY, et al. Refractory Kawasaki disease: Infliximab or methotrexate therapy? Indian J Pediatr. 2009;76:1184. doi: 10.1007/s12098-009-0285-9. author reply 1184. [DOI] [PubMed] [Google Scholar]
- 20.Shirley DA, Stephens I. Primary treatment of incomplete Kawasaki disease with infliximab and methylprednisolone in a patient with a contraindication to intravenous immune globulin. Pediatr Infect Dis J. 2010;29:978–79. doi: 10.1097/INF.0b013e3181e05564. [DOI] [PubMed] [Google Scholar]
- 21.Singh S, Sharma D, Suri D, et al. Infliximab is the new kid on the block in Kawasaki disease: A single-centre study over 8 years from North India. Clin Exp Rheumatol. 2016;34:S134–38. [PubMed] [Google Scholar]
- 22.Son MB, Gauvreau K, Burns JC, et al. Infliximab for intravenous immunoglobulin resistance in Kawasaki disease: A retrospective study. J Pediatr. 2011;158:644–49.e1. doi: 10.1016/j.jpeds.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 23.Song MS, Lee SB, Sohn S, et al. Infliximab treatment for refractory Kawasaki disease in Korean children. Korean Circ J. 2010;40:334–38. doi: 10.4070/kcj.2010.40.7.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sonoda K, Mori M, Hokosaki T, Yokota S. Infliximab plus plasma exchange rescue therapy in Kawasaki disease. J Pediatr. 2014;164:1128–32.e1. doi: 10.1016/j.jpeds.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 25.Tremoulet AH, Jain S, Jaggi P, et al. Infliximab for intensification of primary therapy for Kawasaki disease: A phase 3 randomised, double-blind, placebo-controlled trial. Lancet. 2014;383:1731–38. doi: 10.1016/S0140-6736(13)62298-9. [DOI] [PubMed] [Google Scholar]
- 26.Weiss JE, Eberhard BA, Chowdhury D, Gottlieb BS. Infliximab as a novel therapy for refractory Kawasaki disease. J Rheumatol. 2004;31:808–10. [PubMed] [Google Scholar]
- 27.Youn Y, Kim J, Hong YM, Sohn S. Infliximab as the first retreatment in patients with Kawasaki disease resistant to initial intravenous immunoglobulin. Pediatr Infect Dis J. 2016;35:457–59. doi: 10.1097/INF.0000000000001039. [DOI] [PubMed] [Google Scholar]
- 28.Zulian F, Zanon G, Martini G, et al. Efficacy of infliximab in long-lasting refractory Kawasaki disease. Clin Exp Rheumatol. 2006;24:453. [PubMed] [Google Scholar]