Abstract
Context
McCune-Albright syndrome (MAS) is a rare disorder characterized by fibrous dysplasia of bone, café-au-lait macules, and hyperfunctioning endocrinopathies. It arises from somatic gain-of-function mutations in GNAS, which encodes the cAMP-regulating protein Gαs. Somatic GNAS mutations have been reported in intraductal papillary mucinous neoplasms (IPMNs) and various gastrointestinal (GI) tumors. The clinical spectrum and prevalence of MAS-associated GI disease is not well established.
Objective
Define the spectrum and prevalence of MAS-associated GI pathology in a large cohort of patients with MAS.
Design
Cross-sectional study.
Setting
National Institutes of Health Clinical Center and The Johns Hopkins Hospital.
Methods
Fifty-four consecutive subjects with MAS (28 males; age range, 7 to 67 years) were screened with magnetic resonance cholangiopancreatography (MRCP).
Results
Thirty of 54 subjects (56%) had radiographic GI abnormalities. Twenty-five (46%) of the screened subjects had IPMNs (mean age of 35.1 years). Fourteen of the 25 had IPMNs alone, and 11 had IPMNs and abnormal hepatobiliary imaging. The 30 patients with MAS-associated GI pathology had a higher prevalence of acute pancreatitis, diabetes mellitus, and skeletal disease burden of fibrous dysplasia than patients without GI disease.
Conclusions
A broad spectrum of GI pathology is associated with MAS. IPMNs are common and occur at a younger age than in the general population. Patients with MAS should be considered for screening with a focused GI history and baseline MRCP. Further determination of the natural history and malignant potential of IPMNs in MAS is needed.
GI abnormalities are common in McCune-Albright syndrome (MAS). MAS-associated pancreatic disease represents an excellent model for studying the pathophysiology of Gαs-related GI disease.
McCune-Albright syndrome (MAS) is a rare disorder characterized by fibrous dysplasia of bone, hyperfunctioning endocrinopathies, and café-au-lait macules resulting from a somatic gain-of-function mutation in GNAS, which encodes the cAMP-regulating protein Gαs. These mutations occur early in embryonic development, predominantly at codon R201, with arginine substituted by cysteine (R201C) or histidine (R201H), leading to ligand-independent constitutive activation of Gαs and dysregulated cAMP signaling (1). Gain-of-function GNAS mutations were previously described in pituitary, thyroid, appendiceal, and gonadal tumors and breast cancer (2–6). In recent years, the same activating mutations have been identified as somatic driver mutations in sporadic intraductal papillary mucinous neoplasms (IPMNs) and various gastrointestinal (GI) lesions, suggesting a potential role in pancreatic and GI tumorigenesis. Approximately 60% to 80% of sporadic IPMNs have activating GNAS mutations (7).
IPMNs are pancreatic mucin-producing cysts and are the most common type of neoplastic pancreatic cyst. There are three types of IPMNs: main-duct IPMN; branch-duct IPMN; and mixed-type IPMN, which involves both the main and branch ducts. IPMNs are incidentally noted in between 2% and 13% of MRI scans of the abdomen and are diagnosed with increasing frequency because of the widespread use of imaging modalities (8, 9). Gain-of-function mutations of GNAS have been detected in pancreatic cyst fluid samples, primary IPMN tissue, and duodenal collection of secretin-stimulated pancreatic juice (7, 10–17). IPMNs are a precursor lesion of invasive adenocarcinoma (18). In addition to their detection in IPMNs, the same GNAS-activating mutations have been increasingly reported in various other GI pathologies, including GI tumors, liver tumors, intraductal papillary neoplasms of the bile duct, and pyloric gland adenomas of the upper GI tract (19–24).
The precise timing of the development of GNAS mutations and the pathway(s) for IPMN development and tumorigenesis have not been established. In addition to GNAS mutations, activating KRAS and inactivating RNF43, TP53, p16/CDKN2A, and SMAD4 gene mutations are frequently reported in IPMNs; however, the timing and order of these mutations have not been fully elucidated (17, 25–29).
GI abnormalities have been reported in MAS, including IPMNs, hepatic and biliary lesions, and gastric and duodenal polyps; however, the clinical spectrum and prevalence of MAS-associated GI disease are not well established (30, 31). This study investigated the spectrum of GI manifestations in the largest cohort of patients with MAS studied to date to define the prevalence of GI pathology in MAS and to outline optimal care for these patients.
Subjects and Methods
Study subjects
The discovery of GNAS mutations as driver mutations in IPMNs (7) led us to give more significance to previous reports of sporadic, idiopathic pancreatitis by three of our patients in the cohort. At that time, we began screening patients with a history of pancreatitis using magnetic resonance cholangiopancreatography (MRCP); having found major pancreatic pathology in these patients, we began performing screening MRCPs on enrolled patients in a long-standing MAS natural history study at the National Institutes of Health (NIH). Thus, 54 consecutive subjects (26 females; age range, 7 to 67 years) at the NIH were evaluated between 2013 and 2016. This study was approved by the National Institute of Dental and Craniofacial Research Institutional Review Board. Informed written consent and/or assent was obtained from all participants, including parents and children. Studies were conducted at the NIH Clinical Center in Bethesda, Maryland, and The Johns Hopkins Hospital in Baltimore, Maryland. In the majority of cases, the diagnosis of MAS was made on a clinical basis according to previously published guidelines, including the presence of fibrous dysplasia, café-au-lait macules, and/or hyperfunctioning endocrinopathies (32). In cases with an unclear clinical diagnosis (three of 54 subjects), genetic testing for GNAS mutation was performed on the affected tissue.
The remaining 136 subjects who were also enrolled in the long-standing MAS natural history study at the NIH and who did not undergo any clinical GI screening or baseline MRCP served as the unscreened control group. Clinical features of MAS have been well documented in these subjects.
Study design
Subjects underwent baseline screening at the NIH Clinical Center, which encompassed a history and physical examination, including a detailed GI history, and radiographic evaluation with contrast-enhanced abdominal MRI and MRCP. Pancreatitis was diagnosed per routine criteria (33). All MRI/MRCP images were reviewed by a single radiologist (A.Z.), and any radiographic abnormalities in the liver, pancreas, bile ducts, or gallbladder were documented. High-risk or worrisome features were documented on the basis of the 2012 International Consensus Criteria (34) (Table 1).
Table 1.
Criteria for Worrisome Features and High-Risk Stigmata of the IPMNs [34]
| Worrisome Features | High-Risk Stigmata | |
|---|---|---|
| Clinical | Pancreatitis | Obstructive jaundice secondary to an IPMN |
| Imaging | Cyst ≥3 cm Thickened/enhancing cyst walls Main duct size 5–9 mm in diameter Nonenhancing mural nodule Abrupt change in caliber of pancreatic duct with pancreatic atrophy | Enhancing solid component within the cyst Main pancreatic duct ≥10 mm in diameter |
Thirty subjects in whom radiographic pancreatic abnormalities were identified were first categorized as having either high-risk or worrisome features (34). Ten subjects identified as having worrisome or high-risk features were then referred for further evaluation to the Pancreatic Cyst Clinic or Pancreatitis Clinic at The Johns Hopkins Hospital. Seven of these 10 subjects completed the testing. Patients with a history of acute pancreatitis or concerning features on MRI/MRCP underwent evaluation with endoscopic ultrasonography (EUS) and endoscopic retrograde cholangiopancreatography as indicated.
Statistical analysis
Statistical analyses were performed with GraphPad Prism 7 for Windows, version 7.01, using paired t tests, Mann-Whitney test, and Fisher exact test. Significance was established at P < 0.05. Data are presented as mean ± SD.
Results
Fifty-four consecutive subjects out of 190 total subjects in the cohort (28%) were screened with MRCP. There were no differences in age or sex between any of the groups (screened vs unscreened or within the screened cohort between those with and without GI pathology). Regarding features of MAS, the screened group had a higher prevalence of café-au-lait macules (88% vs 71%; P = 0.01) and GH excess (33% vs 19%; P = 0.004) than the unscreened group (Table 2). Within the screened group, those with GI pathology had more fibrous dysplasia than those without as assessed by the skeletal burden score, a validated measure of disease burden (35) (38.6% vs 23.0%; P = 0.01), but there were no differences in other features of MAS (Table 2).
Table 2.
Clinical Characteristics of Screened and Unscreened MAS Cohorts
|
|
Screened
(n = 54)
|
Unscreened
(n = 136)
|
|||
|---|---|---|---|---|---|
| GI Pathology (n = 30; 56%) | No GI Pathology (n = 24; 44%) | P a | P b | ||
| Female | 17 (57%) | 8 (33%) | NS | 82 (60%) | NS |
| Age range, mean ± SD, y | 13–67, 36.5 ± 16.2 | 7–31, 18.7 ± 5.8 | NS | 1–84, 25.1 ± 11.3 | NS |
| Features of MAS | |||||
| FD | 30 (100%) | 24 (100%) | NS | 132 (97%) | NS |
| Café-au-lait macules | 27 (90%) | 21 (88%) | NS | 96 (71%) | 0.01 |
| Precocious puberty | 18 (60%) | 8 (33%) | NS | 69 (51%) | NS |
| Hyperthyroidism | 12 (40%) | 4 (17%) | NS | 38 (28%) | NS |
| GH excess | 8 (27%) | 10 (42%) | NS | 19 (14%) | 0.004 |
| Hypophosphatemia | 9 (30%) | 3 (13%) | NS | 32 (24%) | NS |
| Neonatal Cushing | 2 (7%) | 0 | NS | 8 (6%) | NS |
| Skeletal burden score, mean ± SD | 38.56 ± 21.76 | 22.98 ± 18.7 | 0.01 | 26.12 ± 20.87 | NS |
| History of pancreatitis | 6 (20%) | 0 | 0.02 | 2 (1%) | 0.01 |
| History of GERD/gastritis | 6 (20%) | 0 | 0.02 | 3 (2%) | 0.02 |
| History of diabetes mellitus | 6 (20%) | 0 | 0.02 | 3 (2%) | 0.02 |
Data are presented as number of subjects (prevalence) unless otherwise indicated.
Abbreviations: FD, fibrous dysplasia; GERD, gastroesophageal reflux disease; NS, not significant; n, number of subjects; % refers to prevalence.
Refers to differences between the screened group with and without GI pathology (Fisher exact test).
Refers to differences between the screened group and unscreened group (Fisher exact test).
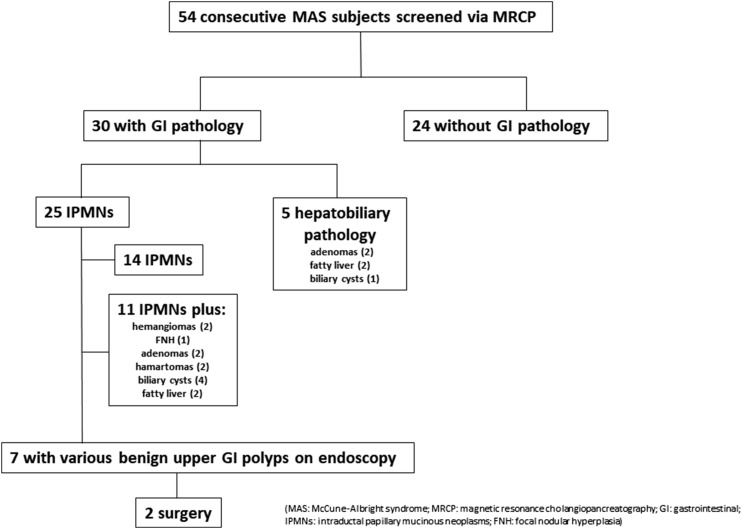
Thirty of the 54 subjects screened (56%) had radiographic GI abnormalities on MRI of the abdomen (Fig. 1; Table 3). Twenty-five subjects (46%) had IPMNs, 14 of whom had IPMNs alone, whereas 11 had IPMNs and a range of hepatobiliary findings including hepatic adenomas (2), hemangiomas (2), hamartomas (2), focal nodular hyperplasia (1), biliary cysts (4), and fatty liver (2), all diagnosed radiographically on MRI. Five subjects (17%) had hepatobiliary abnormalities alone, consisting of hepatic adenomas (2), fatty liver (2), and biliary cysts (1). Three of the four subjects who had fatty liver had a body mass index (BMI) in the obesity range (patients 7, 11, and 12 in Table 3), and patient 8 had a BMI in the overweight category. The mean age of the patients with an IPMN was 35.1 years (range, 18 to 67 years). In the screened cohort, a total of 10 subjects had either worrisome or high-risk features (Table 3; age and sex in boldface). Eight subjects had IPMNs with worrisome features. Seven had main-duct dilation between 5 and 9 mm, six had acute/acute recurrent pancreatitis, and two had pancreatic cysts >3 cm (Table 3). Two subjects (Table 3; subjects 23 and 30) had high-risk stigmata with main-duct dilation >10 mm. Subject 23 also had acute recurrent pancreatitis.
Figure 1.
Summary flow diagram describing the radiographic findings and clinical management of patients with MAS-associated pancreatic lesions. MRCP findings in 54 consecutively screened patients with MAS are shown. Thirty subjects were identified as having radiographic GI pathology. A total of 25 subjects had IPMNs; 14 had IPMNs only, and 11 had IPMNs and additional findings, most commonly benign liver lesions. Five of the 30 subjects had various GI abnormalities without IPMNs. Within the cohort who screened positive for GI pathology, seven subjects underwent detailed evaluation including esophagogastroduodenoscopy, EUS, and/or pancreatic cyst fluid analysis. All seven subjects were found to have benign upper GI polyps. Two subjects underwent pancreatic surgery.
Table 3.
Detailed Radiographic and Endoscopic Findings in the Screened MAS Cohort
| Patient | Age (y) | Sex | Type of IPMN | No. of Cysts | Location of Cysts | Largest Cyst Size (mm) | Main Duct Dilation (mm) | Liver Findings | GI Symptoms | GI Lesions: Location, GNAS testing (if +) ( 35 ) | Other Findings | Surgical Histopathology |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 22 | F | Branch duct | >10 | H,B,T | 10 | 15.1- × 11.7-mm adenoma | Gastric hyperplastic polyps: Proximal stomach Gastric heterotopia/metaplasia: Duodenal bulb (R201C) Duodenum Ampulla | ||||
| 2 | 27 | M | Mixed | >10 | H,B,T | 34 | 7 (S) | Acute recurrent pancreatitis | Gastric heterotopia/metaplasia: Duodenum (R201H) | Pancreatic atrophy | IPMN with high-grade dysplasia | |
| 3 | 30 | F | None | 4.8-cm adenoma | ||||||||
| 4 | 47 | F | Branch duct | >10 | H,B,T | 5 | Hamartomas | |||||
| 5 | 15 | M | None | 5.2-cm adenoma | ||||||||
| 6 | 53 | F | Branch duct | 5 | H,B | 3 | Pancreas divisum | |||||
| 7 | 59 | M | Mixed | >10 | H,B,T | 13 | 8 (D) | Fatty liver Hamartomas Small cysts | Acute pancreatitis | |||
| 8 | 16 | M | Branch duct | 3 | H | 12 | Fatty liver | |||||
| 9 | 20 | M | Mixed | 6 | H,B | 30 | 6.5 (S) | Acute recurrent pancreatitis | Fundic gland polyp: Stomach body Gastric heterotopia/metaplasia: Proximal esophagus (R201C) Minor papilla Hamartomatous-type polyp: Major papilla | |||
| 10 | 70 | M | Mixed | >10 | H,B,T | 7 | 9 (D) | <1-cm biliary cyst | Acute pancreatitis | Pancreatic atrophy | ||
| 11 | 49 | F | None | Fatty liver | ||||||||
| 12 | 16 | F | Branch duct | >10 | H,B,T | 13 | ||||||
| 13 | 17 | F | Branch duct | 1 | H | 5 | ||||||
| 14 | 36 | F | Branch duct | 1 | H | 8 | ||||||
| 15 | 19 | M | Branch duct | 8 | H,B,T | 24 | Focal nodular hyperplasia | Acute recurrent pancreatitis | Gastric hyperplastic polyps: Proximal stomach Gastric heterotopia/metaplasia: Proximal esophagus (R201C) Proximal duodenum (R201C) Duodenum (R201C) Ampulla (R201C) | Santorinicele Pancreas divisum | ||
| 16 | 28 | F | Branch duct | 1 | H | 6 | <1-cm biliary cyst | |||||
| 17 | 18 | F | Branch duct | 2 | H | 10 | ||||||
| 18 | 33 | F | Branch duct | 2 | H,B | 12 | <1-cm biliary cyst | |||||
| 19 | 27 | F | None | <1-cm biliary cyst | ||||||||
| 20 | 31 | F | Branch duct | 3 | B | 10 | ||||||
| 21 | 42 | F | Branch duct | 6 | H,B,T | 4 | ||||||
| 22 | 23 | M | None | Fatty liver | ||||||||
| 23 | 55 | M | Mixed | >10 | H,B,T | 22 | 13 (D) | 2 Hemangiomas <1 cm biliary cyst | Acute recurrent pancreatitis | 5.5-cm polyp: GE junction, (R201C) Fundic gland polyps: Stomach Gastric heterotopia/metaplasia: Proximal esophagus Duodenum Ampulla (R201C) Foveolar hyperplasia: Stomach body (R201C) | Pancreatic atrophy | IPMN with low-grade dysplasia Gastric polyp with high-grade dysplasia |
| 24 | 46 | F | Mixed | >10 | H,B,T | 9 | 5 (S) | 1.3-cm hemangioma | Gastric hyperplastic polyp: GE junction (R201H) Gastric heterotopia/metaplasia: Duodenum (R201H) | Pancreatic atrophy | ||
| 25 | 24 | F | Branch duct | 2 | B | 3 | ||||||
| 26 | 58 | F | Mixed | >10 | H,B,T | 11 | ||||||
| 27 | 57 | M | Mixed | >10 | H,B,T | 16 | 6 (D) | Pancreatic atrophy | ||||
| 28 | 24 | M | Branch duct | 7 | B,T | 5 | ||||||
| 29 | 17 | M | Branch duct | 5 | H,B,T | 5 | 2.7-cm adenoma | |||||
| 30 | 50 | M | Mixed | >10 | H,B,T | 27 | 11 (S) | Gastric hyperplastic polyp: Distal esophagus Gastric heterotopia/metaplasia: Proximal esophagus (R201C) Duodenum (R201C) Intestinal metaplasia: GE junction | Pancreatic atrophy |
Boldface indicates subjects with either worrisome or high-risk features on MRCP.
Abbreviations: B, body; D, diffuse, GE, gastroesophageal; H, head; S, segmental; T, tail.
Sixteen subjects had branch-duct IPMNs, and nine had mixed-type IPMNs (Table 3). In subjects with a mixed-duct IPMN (defined as a main duct of 5 mm or larger), the main pancreatic duct diameter ranged from 5 to 13 mm (mean, 7.9 mm). Five subjects had segmental dilation of the main pancreatic duct, and four had diffuse involvement of the main pancreatic duct. Representative radiographic findings of GI pathology can be seen in Figs. 2 and 3, including IPMNs and biliary cysts (Fig. 2A) and hepatic lesions (Fig. 3). The screened cohort was more likely to have acute pancreatitis, gastroesophageal reflux disease, and diabetes mellitus than was the unscreened cohort (P < 0.05) (Table 2).
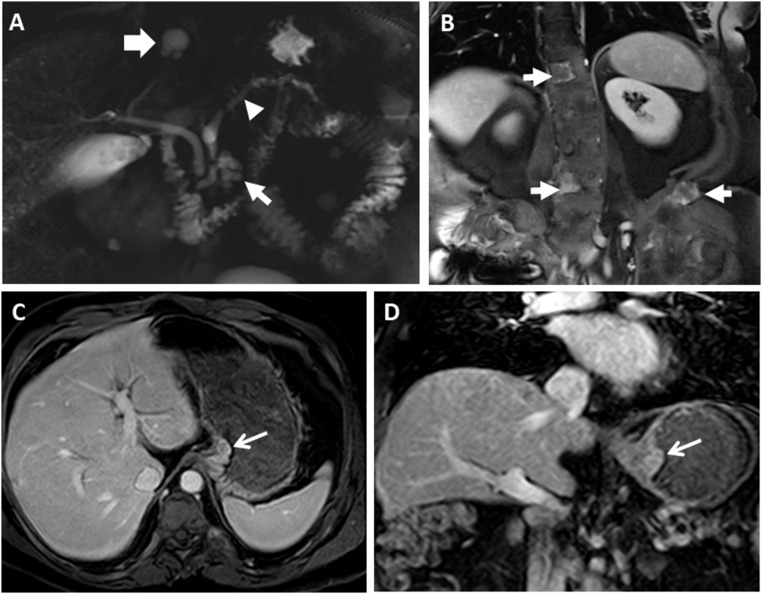
Figure 2.
Representative radiographic GI findings in MAS. (A) MRCP imaging from a 55-year-old man shows diffuse dilation of the main pancreatic duct (arrowhead) along with a cystic lesion in the pancreatic head (thin arrow). A biliary cyst is also seen (thick white arrow). (B) Coronal postcontrast image demonstrates heterogeneously enhancing lesions in the spine and left iliac wing (arrows) in the same patient, consistent with fibrous dysplasia of the bone. (C and D) MRCP images in the same patient who underwent distal pancreatectomy and removal of a gastroesophageal junction polyp are shown. (C) Axial and (D) coronal postcontrast T1-weighted images show the gastroesophageal junction polyp (arrows).
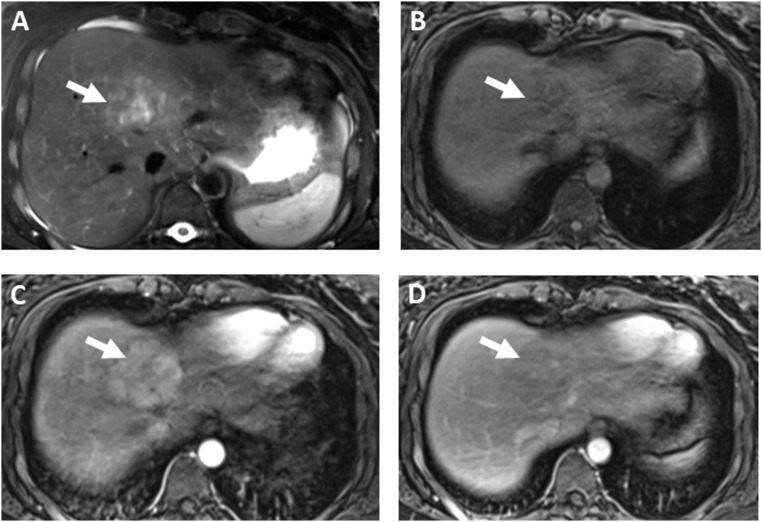
Figure 3.
Representative hepatic lesions. (A‒D) MRCP imaging from a 22-year-old woman with MAS shows (A) a heterogeneous hyperintense mass in the dome of the liver on axial T2-weighted image (arrow). (B) The mass is hypointense on T1-precontrast image (arrow), (C) shows arterial enhancement on the T1-postcontrast arterial phase image (arrow), and (D) washout on the venous phase postcontrast image (arrow). The signal and enhancement characteristics are suggestive of an adenoma.
Seven subjects with identified radiographic pancreatic abnormalities (highlighted in Table 3) underwent additional GI testing. Descriptions of the gastric lesions and their histopathology have been reported in detail (36). All were found to have polyps of the upper GI tract on endoscopy; representative images are shown in Fig. 4. Surgery was indicated in two subjects. The first was a 27-year-old man (Table 3; patient 2) with idiopathic acute recurrent pancreatitis who had had five episodes of pancreatitis during the prior 8 years, for which he had no risk factors other than his IPMNs. On MRCP, he had a mixed-type IPMN, with the main pancreatic duct measuring 7 mm. Multiple pancreatic cysts were located in the head, body, and tail of the pancreas, with the largest cyst having a diameter of 34 mm. A pancreaticoduodenectomy was performed on the basis of his main pancreatic duct involvement and acute recurrent pancreatitis, which was thought to be secondary to his IPMNs. Surgical pathology confirmed a mixed-type IPMN with high-grade dysplasia. The second was a 55-year-old man (Table 3; patient 23) with a history of idiopathic acute recurrent pancreatitis. MRCP revealed a mixed-type IPMN with the main pancreatic duct measuring 13 mm and multiple pancreatic cysts, the largest of which measured 22 mm (Fig. 2A). In addition, a 5.5-cm polyp was identified at the gastroesophageal junction. Surgical pathology of the pancreas confirmed the presence of a mixed-type IPMN with low-grade dysplasia, whereas the gastric polyp was consistent with a gastric adenoma with high-grade dysplasia (36).
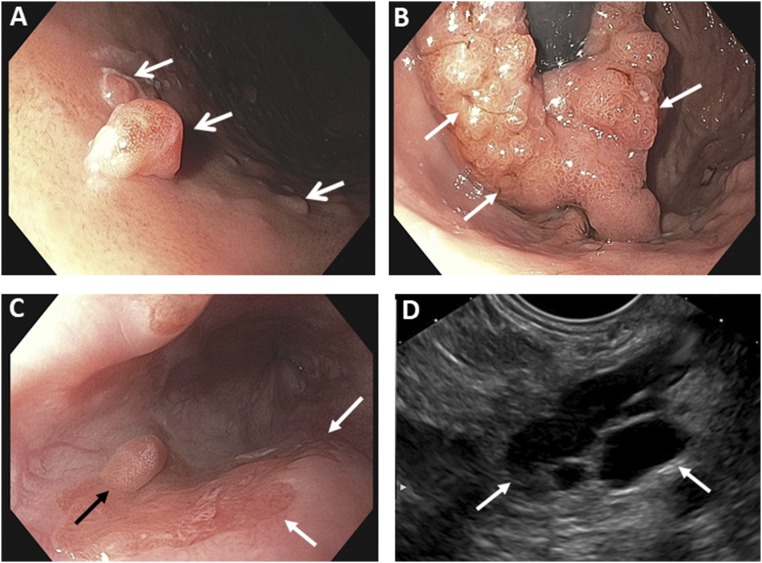
Figure 4.
Representative endoscopic findings. (A) Several gastric polyps (arrows) are shown. (B) A retroflexed view shows the gastric cardia. The endoscope is seen as the black tube in the center of the image and is surrounded by a large mass (arrows), consistent with a gastric adenoma with high-grade dysplasia. (C) Endoscopic view of the distal esophagus shows a polypoid lesion (black arrow) and several flat lesions (white arrows). (D) Endoscopic ultrasonographic image of a large cyst (highlighted by arrows) is consistent with an IPMN in the pancreas.
Discussion
In this cross-sectional study of the largest cohort of patients with MAS studied to date, we describe a spectrum of hepatobiliary and pancreatic pathology, including IPMNs, hepatic adenomas, and biliary cysts. In the general population, IPMNs typically occur at a mean age of 63 years (37, 38). IPMNs in MAS were found at an earlier age (mean age, 35.1 years). The finding of more fibrous dysplasia in the group with GI pathology has at least two possible explanations. The first is possibly the fact that the group with GI abnormalities was older, although the difference in ages was not statistically significant. We also hypothesize that GI involvement may be the reflection of the mutation arising earlier in development, suggesting that an earlier mutational event could result in more fibrous dysplasia and more tissues being involved, including the GI tract. The early presentation in MAS and the fact that the group with GI pathology was not older than the group without suggest that pancreatic disease may be present from a very early age in MAS. This is consistent with the hypothesis that in MAS the Gαs mutation arises early in development, before gastrulation. As such, MAS-associated pancreatic disease, especially IPMNs, represents an excellent model for studying the natural history and pathophysiology of Gαs/IPMN-related GI disease.
The majority of the subjects in this screened cohort, who were found to have IPMNs, were asymptomatic. Those subjects with a history of acute or acute recurrent pancreatitis did not have any of the common risk factors associated with pancreatitis and had no history of alcohol excess, smoking, hypertriglyceridemia, or choledocholithiasis. Pancreatitis has not been reported as a common feature of MAS. In this large MAS cohort, a total of eight patients out of 190 had a clinical history of pancreatitis. Among these eight patients, six were screened with an MRCP, and all six had IPMNs. In three of the subjects (patients 2, 9, and 23 in Table 3), acute pancreatitis was thought to be due to their mixed-type IPMNs. A comprehensive description of the histologic findings and tissue mutation testing results from seven subjects in the cohort with IPMNs who underwent EUS was previously reported (36). In these subjects, hotspot GNAS mutations (R201C or R201H) were detected in the upper GI polyps, in the IPMN tissue of the two patients who underwent pancreatic surgery, and in the adenomatous gastroesophageal junction polyp that was resected (36). In addition, hotspot mutations in KRAS (Q61H) and PIK3CA (Q546R) were detected in pancreatic cyst fluid of the 55-year-old patient (Table 3; patient 23), who underwent a distal pancreatectomy (36). A GNAS mutation (R201C) and an inactivating mutation in CDKN2A (V115fs) were detected in the cyst fluid of a 50-year-old patient with MAS (Table 3; patient 30) (36).
GI polyps were previously reported in patients who had MAS, and activating GNAS mutations were identified in these GI polyps (31, 36). Results of this study confirm these findings. The most important relationship between IPMNs and polyps of the upper GI tract is the detection of the same activating GNAS mutation in both. We hypothesize that these manifestations are direct effects of mosaic Gαs involvement in the upper GI and pancreatic tissues, respectively. We suspect that diffuse involvement of the GI tract, which includes both luminal and pancreatic involvement, reflects the possibility that the mutation arose in an early GI progenitor cell; this is similar to what is observed in the skeleton, wherein multiple skeletal compartments can be involved (craniofacial, axial, and appendicular).
The pathway of progression from an IPMN to invasive adenocarcinoma has not been defined yet. In a recent transgenic mouse model that mimicked human IPMNs, GNASR201H and KrasG12D were shown to cooperatively promote murine pancreatic tumorigenesis (39). Identification of distinct genetic profiles of IPMNs can help to elucidate potential mechanisms of pancreatic tumorigenesis. GNAS is an early driver mutation in IPMN formation; however, its exact role in tumorigenesis remains unknown (7, 10, 15, 16). Additional genes potentially contribute to pancreatic disease progression, including mutations in KRAS, TP53, p16/CDKN2A, and RNF43; these genes are commonly associated with pancreatic neoplasia and were previously identified in sporadic IPMNs (10, 40). In addition to serving as diagnostic markers for IPMNs, GNAS and KRAS are postulated to have independent roles in tumorigenesis (10, 41). The timeline of mutation accrual and the potential role of additional mutations on pancreatic tumorigenesis remain unknown. MAS can be a potential model to study pancreatic tumorigenesis because the onset of the GNAS mutation in utero is precisely defined, which can provide a model to study the progression of IPMNs over time. Further studies on animal models and through screening and surveillance of patients with MAS can provide useful insights into potential pathways of progression from an IPMN to pancreatic cancer.
As described previously in the literature, the presence of hepatic lesions, including adenomas, was confirmed in this MAS cohort as well. It is important to note that hemangiomas, hepatic cysts, and focal nodular hyperplasia are commonly seen in normal individuals and may not be indicative of clinically significant, actual GI pathology in MAS (42). Activating mutations of Gαs have been reported in sporadic benign and malignant liver tumors, acting through cAMP and JAK/STAT pathways (16). Hepatocellular adenomas are rare benign tumors with hepatocellular differentiation, frequently associated with oral contraceptive use. They are linked to mutations in the HNF1A gene, CTNNB1 gene, and several genes that promote the constitutive activation of the STAT3 pathway, including IL-6 signal transducer (IL6ST), Fyn-related kinase (FRK), STAT3, Janus kinase 1 (JAK1), and GNAS complex locus (43). Hepatic tumors with activating mutations in GNAS demonstrate an inflammatory type, as would be expected in STAT3 activation (16). Thus, the association of MAS and hepatocellular adenoma, both rare diseases, could potentially be explained by STAT3 activation induced by GNAS activation (16). It is possible that a similar cross-talk between GNAS activation and a distinct signaling pathway plays a role in IPMN development and pancreatic tumorigenesis.
An important goal in defining the spectrum of GI pathology in MAS is to define optimal screening and clinical management. IPMNs are one of three precursors to pancreatic adenocarcinoma, which has a very poor survival rate, with only 8% of patients alive at 5 years (18, 44). Despite the presence of a GNAS mutation since in utero, not all patients with MAS develop IPMNs. In this MAS cohort, 46% developed IPMNs, a much higher number than anticipated. Pancreatic adenocarcinoma is an extremely uncommon feature in MAS, with only one case reported to date (45). A retrospective review in a cohort of 272 patients who underwent surgery for IPMNs demonstrated that one patient (0.4%), a 62-year-old man who had been operated on for a large invasive colloid adenocarcinoma, had polyostotic fibrous dysplasia and café-au-lait macules, consistent with a clinical diagnosis of MAS (45). The low prevalence of pancreatic adenocarcinoma in MAS is reassuring, yet makes it difficult to determine how best to follow up patients with MAS and whether the same criteria used in sporadic IPMNs to determine the need for surgical resection should be applied to MAS.
When this collaboration started, there were no reports of pancreatic adenocarcinoma in MAS. Ten of the 54 patients screened were found to have high-risk or worrisome features, and seven had completed a detailed GI evaluation. The options of surveillance vs surgical resection were discussed in detail with these seven patients. Two patients opted for surgical resection. The indication for surgical resection was the presence of acute recurrent pancreatitis in the 27-year-old male (Table 3; patient 23), who was found to have a mixed-type IPMN with high-grade dysplasia. The second patient was a 55-year-old male (Table 3; patient 30) with history of acute recurrent pancreatitis who had a mixed-type IPMN that was confirmed on surgical pathology as a mixed-type IPMN with low-grade dysplasia. The presence of high-grade dysplasia in the younger patient with MAS but not in the older patient with MAS highlights the difficulties in caring for these patients and the need for better markers to identify those at highest risk for progression to high-grade dysplasia or invasive adenocarcinoma. There does not appear to be a clear correlation between age and degree of dysplasia because we had only two subjects who underwent surgery with pathology results. However, the finding of high-grade dysplasia in the 27-year-old patient suggests that the prior hypothesis that MAS patients with IPMNs have minimal or no risk of progression may be incorrect.
In any screening program, the goal should be to minimize harm while maximizing benefit to the patient (46). Screening asymptomatic patients with MAS for GI lesions has potential benefits, such as early detection and removal of lesions with high-grade dysplasia in a young patient in his or her 20s, before development of an invasive adenocarcinoma. It also has potential risks, as we would discover findings that may not have clinical significance but could result in unnecessary surgery as well as costs associated with both screening and interventions. Given the prevalence of IPMNs in patients with MAS, though reflecting some degree of ascertainment bias in this cohort, evaluation with MRI/MRCP for patients with MAS and a history of acute pancreatitis may be considered. Those with evidence of IPMNs should undergo surveillance, with patients having symptoms and/or high-risk or worrisome features reviewed by a multidisciplinary pancreas group. Given the finding of a gastric adenoma with high-grade dysplasia, esophagogastroduodenoscopy should be considered in patients with MAS undergoing EUS. We propose that patients with MAS should be clinically evaluated, with careful attention to reports of GI symptoms that may prompt consideration of imaging and further detailed GI evaluation.
Diabetes mellitus is not an established feature of MAS and has been described in only two case reports (47, 48). In this screened MAS cohort who also had IPMNs, we identified seven patients with diabetes of unclear etiology, raising the possibility of a relationship between pancreatic cysts and diabetes in these patients. These seven patients did not have a family history of diabetes. Only one patient, a 29-year-old female, had a normal BMI. Other patients were in the overweight category, which is a risk factor for type 2 diabetes. The etiology and pathogenesis of MAS-associated diabetes warrant further investigation. A combination of insulin deficiency secondary to IPMNs and insulin resistance may contribute to the development of diabetes. Examples to support such a hypothesis are seen in various forms of pancreatogenic diabetes, which demonstrate differing mechanisms of hyperglycemia, but in which both inadequate pancreatic β-cell function and insulin resistance contribute differentially to hyperglycemia (49). In-depth study of patients with diabetes is needed to understand the pathophysiology of this intriguing finding in MAS. Further studies are required to better understand the natural history and spectrum of the GI phenotype in MAS and to determine the optimal treatment of these patients. Specifically, further studies are required to determine the true risk of progression to high-grade dysplasia or invasive adenocarcinoma in patients with MAS and IPMNs and the indications for surgical resection in this cohort.
In conclusion, we describe clinical and radiographic GI abnormalities in the largest MAS cohort screened to date. The results of this study indicate that GI manifestations in MAS are common and that patients with MAS may benefit from evaluation for GI pathology. The optimal care for GI findings in MAS remains to be defined; however, at a minimum, a thorough GI history at every visit and consideration of imaging with abdominal MRI/MRCP are warranted.
Acknowledgments
Study subjects were evaluated at the NIH under institutional review board‒approved protocol 98-D-0145, with written informed consent.
Financial Support: This research was supported in part by the Division of Intramural Research of the National Institutes of Health, NIH 1 Z01 DE000649 (to M.T.C.), the National Institute of Dental and Craniofacial Research, the Lustgarten Foundation for Pancreatic Cancer Research, The Sol Goldman Center for Pancreatic Cancer Research, R01CA176828, P50CA062924. The Benjamin Baker Scholarship, and a GI SPORE grant (CA 62924) from the National Cancer Institute.
Clinical Trial Information: ClinicalTrials.gov no. NCT00001727 (registered 25 August 1998).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- BMI
body mass index
- EUS
endoscopic ultrasonography
- IPMN
intraductal papillary mucinous neoplasm
- GI
gastrointestinal
- MAS
McCune-Albright syndrome
- MRCP
magnetic resonance cholangiopancreatography
- NIH
National Institutes of Health
References
- 1. Weinstein LS. Gsα mutations in fibrous dysplasia and McCune-Albright syndrome. J Bone Miner Res. 2006;21(Suppl 2):120–124. [DOI] [PubMed] [Google Scholar]
- 2. Majoor BC, Boyce AM, Bovée JV, Smit VT, Collins MT, Cleton-Jansen AM, Dekkers OM, Hamdy NA, Dijkstra PS, Appelman-Dijkstra NM. Increased risk of breast cancer at a young age in women with fibrous dysplasia. J Bone Miner Res. 2018;33(1):84–90. [DOI] [PubMed] [Google Scholar]
- 3. Vortmeyer AO, Gläsker S, Mehta GU, Abu-Asab MS, Smith JH, Zhuang Z, Collins MT, Oldfield EH. Somatic GNAS mutation causes widespread and diffuse pituitary disease in acromegalic patients with McCune-Albright syndrome. J Clin Endocrinol Metab. 2012;97(7):2404–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Collins MT, Sarlis NJ, Merino MJ, Monroe J, Crawford SE, Krakoff JA, Guthrie LC, Bonat S, Robey PG, Shenker A. Thyroid carcinoma in the McCune-Albright syndrome: contributory role of activating Gsα mutations. J Clin Endocrinol Metab. 2003;88(9):4413–4417. [DOI] [PubMed] [Google Scholar]
- 5. Nishikawa G, Sekine S, Ogawa R, Matsubara A, Mori T, Taniguchi H, Kushima R, Hiraoka N, Tsuta K, Tsuda H, Kanai Y. Frequent GNAS mutations in low-grade appendiceal mucinous neoplasms. Br J Cancer. 2013;108(4):951–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fragoso MC, Latronico AC, Carvalho FM, Zerbini MC, Marcondes JA, Araujo LM, Lando VS, Frazzatto ET, Mendonca BB, Villares SM. Activating mutation of the stimulatory G protein (gsp) as a putative cause of ovarian and testicular human stromal Leydig cell tumors. J Clin Endocrinol Metab. 1998;83(6):2074–2078. [DOI] [PubMed] [Google Scholar]
- 7. Wu J, Matthaei H, Maitra A, Dal Molin M, Wood LD, Eshleman JR, Goggins M, Canto MI, Schulick RD, Edil BH, Wolfgang CL, Klein AP, Diaz LA Jr, Allen PJ, Schmidt CM, Kinzler KW, Papadopoulos N, Hruban RH, Vogelstein B. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sci Transl Med. 2011;3(92):92ra66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee KS, Sekhar A, Rofsky NM, Pedrosa I. Prevalence of incidental pancreatic cysts in the adult population on MR imaging. Am J Gastroenterol. 2010;105(9):2079–2084. [DOI] [PubMed] [Google Scholar]
- 9. de Jong K, Nio CY, Hermans JJ, Dijkgraaf MG, Gouma DJ, van Eijck CH, van Heel E, Klass G, Fockens P, Bruno MJ. High prevalence of pancreatic cysts detected by screening magnetic resonance imaging examinations. Clin Gastroenterol Hepatol. 2010;8(9):806–811. [DOI] [PubMed] [Google Scholar]
- 10. Tan MC, Basturk O, Brannon AR, Bhanot U, Scott SN, Bouvier N, LaFemina J, Jarnagin WR, Berger MF, Klimstra D, Allen PJ. GNAS and KRAS mutations define separate progression pathways in intraductal papillary mucinous neoplasm-associated carcinoma. J Am Coll Surg. 2015;220(5):845–854.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Furukawa T, Kuboki Y, Tanji E, Yoshida S, Hatori T, Yamamoto M, Shibata N, Shimizu K, Kamatani N, Shiratori K. Whole-exome sequencing uncovers frequent GNAS mutations in intraductal papillary mucinous neoplasms of the pancreas. Sci Rep. 2011;1(1):161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ideno N, Ohtsuka T, Matsunaga T, Kimura H, Watanabe Y, Tamura K, Aso T, Aishima S, Miyasaka Y, Ohuchida K, Ueda J, Takahata S, Oda Y, Mizumoto K, Tanaka M. Clinical significance of GNAS mutation in intraductal papillary mucinous neoplasm of the pancreas with concomitant pancreatic ductal adenocarcinoma. Pancreas. 2015;44(2):311–320. [DOI] [PubMed] [Google Scholar]
- 13. Tamura K, Ohtsuka T, Matsunaga T, Kimura H, Watanabe Y, Ideno N, Aso T, Miyazaki T, Ohuchida K, Takahata S, Ito T, Ushijima Y, Oda Y, Mizumoto K, Tanaka M. Assessment of clonality of multisegmental main duct intraductal papillary mucinous neoplasms of the pancreas based on GNAS mutation analysis. Surgery. 2015;157(2):277–284. [DOI] [PubMed] [Google Scholar]
- 14. Kanda M, Knight S, Topazian M, Syngal S, Farrell J, Lee J, Kamel I, Lennon AM, Borges M, Young A, Fujiwara S, Seike J, Eshleman J, Hruban RH, Canto MI, Goggins M. Mutant GNAS detected in duodenal collections of secretin-stimulated pancreatic juice indicates the presence or emergence of pancreatic cysts. Gut. 2013;62(7):1024–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hosoda W, Sasaki E, Murakami Y, Yamao K, Shimizu Y, Yatabe Y. GNAS mutation is a frequent event in pancreatic intraductal papillary mucinous neoplasms and associated adenocarcinomas. Virchows Arch. 2015;466(6):665–674. [DOI] [PubMed] [Google Scholar]
- 16. Lee LS, Doyle LA, Houghton J, Sah S, Bellizzi AM, Szafranska-Schwarzbach AE, Conner JR, Kadiyala V, Suleiman SL, Banks PA, Andruss BF, Conwell DL. Differential expression of GNAS and KRAS mutations in pancreatic cysts. JOP. 2014;15(6):581–586. [DOI] [PubMed] [Google Scholar]
- 17. Springer S, Wang Y, Dal Molin M, Masica DL, Jiao Y, Kinde I, Blackford A, Raman SP, Wolfgang CL, Tomita T, Niknafs N, Douville C, Ptak J, Dobbyn L, Allen PJ, Klimstra DS, Schattner MA, Schmidt CM, Yip-Schneider M, Cummings OW, Brand RE, Zeh HJ, Singhi AD, Scarpa A, Salvia R, Malleo G, Zamboni G, Falconi M, Jang JY, Kim SW, Kwon W, Hong SM, Song KB, Kim SC, Swan N, Murphy J, Geoghegan J, Brugge W, Fernandez-Del Castillo C, Mino-Kenudson M, Schulick R, Edil BH, Adsay V, Paulino J, van Hooft J, Yachida S, Nara S, Hiraoka N, Yamao K, Hijioka S, van der Merwe S, Goggins M, Canto MI, Ahuja N, Hirose K, Makary M, Weiss MJ, Cameron J, Pittman M, Eshleman JR, Diaz LA Jr, Papadopoulos N, Kinzler KW, Karchin R, Hruban RH, Vogelstein B, Lennon AM. A combination of molecular markers and clinical features improve the classification of pancreatic cysts. Gastroenterology. 2015;149(6):1501–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lennon AM, Wolfgang CL, Canto MI, Klein AP, Herman JM, Goggins M, Fishman EK, Kamel I, Weiss MJ, Diaz LA, Papadopoulos N, Kinzler KW, Vogelstein B, Hruban RH. The early detection of pancreatic cancer: what will it take to diagnose and treat curable pancreatic neoplasia? Cancer Res. 2014;74(13):3381–3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hashimoto T, Ogawa R, Matsubara A, Taniguchi H, Sugano K, Ushiama M, Yoshida T, Kanai Y, Sekine S. Familial adenomatous polyposis-associated and sporadic pyloric gland adenomas of the upper gastrointestinal tract share common genetic features. Histopathology. 2015;67(5):689–698. [DOI] [PubMed] [Google Scholar]
- 20. Yamada M, Sekine S, Ogawa R, Taniguchi H, Kushima R, Tsuda H, Kanai Y. Frequent activating GNAS mutations in villous adenoma of the colorectum. J Pathol. 2012;228(1):113–118. [DOI] [PubMed] [Google Scholar]
- 21. Walther BM, Walther I, Chen Y, Petersen I. GNAS1 mutation analysis in gastrointestinal tumors. Folia Histochem Cytobiol. 2014;52(2):90–95. [DOI] [PubMed] [Google Scholar]
- 22. Nault JC, Fabre M, Couchy G, Pilati C, Jeannot E, Tran Van Nhieu J, Saint-Paul MC, De Muret A, Redon MJ, Buffet C, Salenave S, Balabaud C, Prevot S, Labrune P, Bioulac-Sage P, Scoazec JY, Chanson P, Zucman-Rossi J. GNAS-activating mutations define a rare subgroup of inflammatory liver tumors characterized by STAT3 activation. J Hepatol. 2012;56(1):184–191. [DOI] [PubMed] [Google Scholar]
- 23. Date K, Ohtsuka T, Fujimoto T, Gotoh Y, Nakashima Y, Kimura H, Matsunaga T, Mori Y, Mochidome N, Miyazaki T, Oda Y, Tanaka M, Nakamura M. GNAS and KRAS mutational analyses of intraductal papillary neoplasms of the pancreas and bile duct developing in the same individual: a case report. Pancreatology. 2015;15(6):713–716. [DOI] [PubMed] [Google Scholar]
- 24. Matthaei H, Wu J, Dal Molin M, Debeljak M, Lingohr P, Katabi N, Klimstra DS, Adsay NV, Eshleman JR, Schulick RD, Kinzler KW, Vogelstein B, Hruban RH, Maitra A. GNAS codon 201 mutations are uncommon in intraductal papillary neoplasms of the bile duct. HPB. 2012;14(10):677–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wu J, Jiao Y, Dal Molin M, Maitra A, de Wilde RF, Wood LD, Eshleman JR, Goggins MG, Wolfgang CL, Canto MI, Schulick RD, Edil BH, Choti MA, Adsay V, Klimstra DS, Offerhaus GJ, Klein AP, Kopelovich L, Carter H, Karchin R, Allen PJ, Schmidt CM, Naito Y, Diaz LA Jr, Kinzler KW, Papadopoulos N, Hruban RH, Vogelstein B. Whole-exome sequencing of neoplastic cysts of the pancreas reveals recurrent mutations in components of ubiquitin-dependent pathways. Proc Natl Acad Sci USA. 2011;108(52):21188–21193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Amato E, Molin MD, Mafficini A, Yu J, Malleo G, Rusev B, Fassan M, Antonello D, Sadakari Y, Castelli P, Zamboni G, Maitra A, Salvia R, Hruban RH, Bassi C, Capelli P, Lawlor RT, Goggins M, Scarpa A. Targeted next-generation sequencing of cancer genes dissects the molecular profiles of intraductal papillary neoplasms of the pancreas. J Pathol. 2014;233(3):217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Takano S, Fukasawa M, Maekawa S, Kadokura M, Miura M, Shindo H, Takahashi E, Sato T, Enomoto N. Deep sequencing of cancer-related genes revealed GNAS mutations to be associated with intraductal papillary mucinous neoplasms and its main pancreatic duct dilation. PLoS One. 2014;9(6):e98718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tamura K, Ohtsuka T, Date K, Fujimoto T, Matsunaga T, Kimura H, Watanabe Y, Miyazaki T, Ohuchida K, Takahata S, Ishigami K, Oda Y, Mizumoto K, Nakamura M, Tanaka M. Distinction of invasive carcinoma derived from intraductal papillary mucinous neoplasms from concomitant ductal adenocarcinoma of the pancreas using molecular biomarkers. Pancreas. 2016;45(6):826–835. [DOI] [PubMed] [Google Scholar]
- 29. Matsuzaka S, Karasaki H, Ono Y, Ogata M, Oikawa K, Tamakawa S, Chiba S, Muraki M, Yokochi T, Funakoshi H, Kono T, Nagashima K, Mizukami Y. Tracking the clonal evolution of adenosquamous carcinoma, a rare variant of intraductal papillary mucinous neoplasm of the pancreas. Pancreas. 2016;45(6):915–918. [DOI] [PubMed] [Google Scholar]
- 30. Gaujoux S, Salenave S, Ronot M, Rangheard AS, Cros J, Belghiti J, Sauvanet A, Ruszniewski P, Chanson P. Hepatobiliary and pancreatic neoplasms in patients with McCune-Albright syndrome. J Clin Endocrinol Metab. 2014;99(1):E97–E101. [DOI] [PubMed] [Google Scholar]
- 31. Zacharin M, Bajpai A, Chow CW, Catto-Smith A, Stratakis C, Wong MW, Scott R. Gastrointestinal polyps in McCune Albright syndrome. J Med Genet. 2011;48(7):458–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Boyce AM, Collins MT. Fibrous dysplasia/McCune-Albright syndrome. In: Adams MP, Ardinger HH, Pagon RA, eds. GeneReviews. Seattle, WA: University of Washington, Seattle; 2015.
- 33. Tenner S, Baillie J, DeWitt J, Vege SS.. American College of Gastroenterology guideline: management of acute pancreatitis [published correction appears in Am J Gastroenterol. 2014;109(2):302]. Am J Gastroenterol. 2013;108(9):1400–1415, 1416. [DOI] [PubMed]
- 34. Tanaka M, Fernández-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, Kimura W, Levy P, Pitman MB, Schmidt CM, Shimizu M, Wolfgang CL, Yamaguchi K, Yamao K; International Association of Pancreatology . International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12(3):183–197. [DOI] [PubMed] [Google Scholar]
- 35. Collins MT, Kushner H, Reynolds JC, Chebli C, Kelly MH, Gupta A, Brillante B, Leet AI, Riminucci M, Robey PG, Bianco P, Wientroub S, Chen CC. An instrument to measure skeletal burden and predict functional outcome in fibrous dysplasia of bone. J Bone Miner Res. 2005;20(2):219–226. [DOI] [PubMed] [Google Scholar]
- 36. Wood LD, Noë M, Hackeng W, Brosens LA, Bhaijee F, Debeljak M, Yu J, Suenaga M, Singhi AD, Zaheer A, Boyce A, Robinson C, Eshleman JR, Goggins MG, Hruban RH, Collins MT, Lennon AM, Montgomery EA. Patients with McCune-Albright syndrome have a broad spectrum of abnormalities in the gastrointestinal tract and pancreas. Virchows Arch. 2017;470(4):391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zamboni G, Hirabayashi K, Castelli P, Lennon AM. Precancerous lesions of the pancreas. Best Pract Res Clin Gastroenterol. 2013;27(2):299–322. [DOI] [PubMed] [Google Scholar]
- 38. Hruban RH, Pitman MB, Klimstra DS. Tumors of the Pancreas: Atlas of Tumor Pathology, 4th Series. Washington, DC: American Registry of Pathology; 2007. [Google Scholar]
- 39. Taki K, Ohmuraya M, Tanji E, Komatsu H, Hashimoto D, Semba K, Araki K, Kawaguchi Y, Baba H, Furukawa T. GNASR201H and KrasG12D cooperate to promote murine pancreatic tumorigenesis recapitulating human intraductal papillary mucinous neoplasm. Oncogene. 2016;35(18):2407–2412. [DOI] [PubMed] [Google Scholar]
- 40. Jones M, Zheng Z, Wang J, Dudley J, Albanese E, Kadayifci A, Dias-Santagata D, Le L, Brugge WR, Fernandez-del Castillo C, Mino-Kenudson M, Iafrate AJ, Pitman MB. Impact of next-generation sequencing on the clinical diagnosis of pancreatic cysts. Gastrointest Endosc. 2016;83(1):140–148. [DOI] [PubMed] [Google Scholar]
- 41. Lee JH, Kim Y, Choi JW, Kim YS. KRAS, GNAS, and RNF43 mutations in intraductal papillary mucinous neoplasm of the pancreas: a meta-analysis. Springerplus. 2016;5(1):1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Assy N, Nasser G, Djibre A, Beniashvili Z, Elias S, Zidan J. Characteristics of common solid liver lesions and recommendations for diagnostic workup. World J Gastroenterol. 2009;15(26):3217–3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bioulac-Sage P, Sempoux C, Balabaud C. Hepatocellular adenomas: morphology and genomics. Gastroenterol Clin North Am. 2017;46(2):253–272. [DOI] [PubMed] [Google Scholar]
- 44. Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362(17):1605–1617. [DOI] [PubMed] [Google Scholar]
- 45. Parvanescu A, Cros J, Ronot M, Hentic O, Grybek V, Couvelard A, Levy P, Chanson P, Ruszniewski P, Sauvanet A, Gaujoux S. Lessons from McCune-Albright syndrome-associated intraductal papillary mucinous neoplasms: GNAS-activating mutations in pancreatic carcinogenesis. JAMA Surg. 2014;149(8):858–862. [DOI] [PubMed] [Google Scholar]
- 46. Gray JA, Patnick J, Blanks RG. Maximising benefit and minimising harm of screening. BMJ. 2008;336(7642):480–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sung SH, Yoon HD, Shon HS, Kim HT, Choi WY, Seo CJ, Lee JH. A case of McCune-Albright syndrome with associated multiple endocrinopathies. Korean J Intern Med (Korean Assoc Intern Med). 2007;22(1):45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chihaoui M, Hamza N, Lamine F, Jabeur S, Yazidi M, Ftouhi B, Slimane H. McCune-Albright syndrome associated with diabetes mellitus [in French] Arch Pediatr. 2012;19(3):282–284. [DOI] [PubMed] [Google Scholar]
- 49. Pedersen HK, Gudmundsdottir V, Brunak S. Pancreatic islet protein complexes and their dysregulation in type 2 diabetes. Front Genet. 2017;8:43. [DOI] [PMC free article] [PubMed] [Google Scholar]