Abstract
Context
Radioactive iodine (RAI) has been epidemiologically associated with the development of hematologic malignancies. Clonal hematopoiesis (CH) is a precursor clonal state that confers increased risk of leukemia and occurs at an elevated rate in patients with thyroid cancer relative to other solid tumors.
Objective
We explore if the high prevalence of CH may be a result of RAI exposure and whether CH may be a surrogate in the association between RAI and leukemia.
Design
CH, CH-potential driver (CH-PD), and overall survival were evaluated in 279 patients with advanced thyroid carcinoma.
Results
The prevalence of CH in patients with thyroid cancer was 37%, and that of CH-PD was 5.2%. Age was the strongest predictor of CH and CH-PD. For every year increase in age, there was a 5% and 13% increase in the odds of CH and CH-PD, respectively. RAI dose was significantly associated with CH and CH-PD, even after adjustment for age, external beam radiation therapy, and chemotherapy. For every 10 mCi increase in the dose of RAI administered, there was a 2% and 4% increase in the odds of CH and CH-PD, respectively. Patients with CH-PD previously exposed to RAI had a significantly poorer survival, even when stratified by age (heart rate = 3.75, 95% CI = 1.23 to 11.5, P = 0.02).
Conclusions
RAI was associated with a high prevalence of CH, and CH is a precursor state of hematologic malignancies. The implications of this study may favor identification of CH in patients where the risks might outweigh the benefits of receiving RAI therapy for thyroid cancer.
Radioactive iodine dose was independently associated with clonal hematopoiesis (CH), a precursor clonal state that confers increased risk of leukemia. Patients with CH had decreased survival.
Radioactive iodine (RAI) administration, following total thyroidectomy, has been traditionally used as an adjuvant treatment of all stages of well-differentiated thyroid carcinoma. More recently, the benefits of such therapy have been questioned in low- and intermediate-risk patients with thyroid cancer, given reports of marginal or no improvement of recurrence or mortality rates in this setting (1–3). Based on these reports and others (4, 5), the American Thyroid Association and the National Comprehensive Cancer Network guidelines recommend a tailored administration of RAI based on a risk-adapted approach, where factors, such as risk of disease-specific mortality, recurrence, benefit in initial staging, and follow-up, are weighted against the potential side effects of RAI.
Complications from RAI range from minor short- and long-term sialadenitis, nasolacrimal duct obstruction, and reproductive disturbances to more serious but rare forms of leukemia, myelosuppression, and aplastic anemia (6–10). When controlling for other treatment exposures and taking into account dose-response effects, RAI appears to confer a modest increased risk of secondary hematologic malignancies, particularly at higher dose exposures, as evidenced in multiple cohorts worldwide (9–13). These studies have grouped all hematologic malignancies under one broad category, but a recent analysis from the Surveillance, Epidemiology, and End Results registries suggests that RAI is associated with an increased early risk of acute myeloid leukemia (AML) and chronic myeloid leukemia but not of leukemias of lymphoid origin or of multiple myeloma (14).
Clonal hematopoiesis (CH) refers to recurrent somatic mutations in leukemia-associated genes that are commonly acquired in aging human hematopoietic stem cells. Large-scale studies of asymptomatic individuals without known hematologic disease have identified somatic mutations in DNMT3, TET2, and ASXL1 that promote clonal expansion of progenitor cells in the absence of overt hematologic transformation (15–18), and these mutations persist over time. Healthy individuals with CH have been shown subsequently to develop hematologic cancers at a rate of ∼0.5% to 1.0% per year compared with <0.1% in non-CH controls, and CH has been linked to decreased overall survival (OS) (15, 16). Hence, CH is a precursor clonal state that confers increased risk of developing leukemia.
The prevalence of CH has been recently found to be 25% among patients with cancer, with 4.5% of these harboring presumptive leukemia driver mutations at higher clonal burden [CH-potential driver (CH-PD)] (19). The most common solid tumor associated with CH was thyroid carcinoma, with a prevalence of 36% (19). CH was associated with increased age, prior radiation therapy, and tobacco use. CH and CH-PD led to an increased incidence of subsequent hematologic malignancies, and CH-PD was associated with shorter patient survival (19). We designed the current study to explore why CH was enriched in patients with thyroid cancer. We hypothesized that the higher prevalence of CH among patients with thyroid cancer compared with other solid malignancies may be explained by the exposure of patients with thyroid cancer to RAI. If confirmed, CH may be a surrogate marker to validate the epidemiologic association seen between RAI and hematologic malignancies.
Methods
Human subjects
The study population included patients with thyroid carcinoma and no other concomitant hematologic malignancy treated at Memorial Sloan-Kettering Cancer Center (MSKCC) who underwent matched tumor and blood sequencing using the Memorial Sloan-Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT) cancer panel (20) on an institutional prospective tumor sequencing protocol (ClinicalTrials.gov NCT01775072). This study was approved by the MSKCC Institutional Review Board. Patients with thyroid carcinoma undergoing MSK-IMPACT testing typically harbored recurrent or metastatic disease and were considered potentially to benefit from targeted therapies in the near future. Associations with any systemic cancer treatments or radiation therapy received before genotyping were ascertained by review of electronic medical records. Patients were excluded from analysis if they had an active hematologic cancer or precursor condition, such as monoclonal gammopathy of uncertain significance or monoclonal B cell lymphocytosis at the time of blood sequencing. Clinical characteristics were analyzed in all patients with thyroid cancer for whom MSK-IMPACT testing was performed between 16 May 2014 and 20 July 2017 (n = 309). Patients were prospectively followed for development of hematologic cancers and for OS through 31 January 2018.
Next-generation sequencing assay
All patients underwent next-generation sequencing using MSK-IMPACT, a hybridization capture-based next-generation sequencing assay encompassing all protein-coding exons of 468 cancer-associated genes (20). MSK-IMPACT is validated and approved for clinical use by the New York State Department of Health Clinical Laboratory Evaluation Program and authorized by the Food and Drug Administration and is used to sequence patients with advance-stage cancer at MSKCC. DNA is extracted from deparaffinized formalin-fixed paraffin-embedded tumor tissue and patient-matched blood sample using the chemagic STAR instrument (Hamilton) with magnetic beads (PerkinElmer). Extracted DNA samples were normalized in Tris-EDTA buffer and sheared on the Covaris instrument. KAPA Biosystems library preparation kit was used to prepare barcoded DNA molecules on the Biomek FXP instrument. Libraries were pooled, and DNA fragments were captured using custom-designed biotinylated probes (NimbleGen). Further details have been previously described (20, 21).
Determination of CH events
We identified CH mutations in all genes captured in MSK-IMPACT through a two-tiered filtering schema, where the variant allele fraction (VAF) in the blood was greater than twice the VAF in the tumor after removing false positives and germline mutations. The following criteria were used to retain mutations:
Mutations present in one of the curated leukemia/lymphoma-related gene list (ASXL1, CBL, DNMT3A, GNAS, JAK2, NRAS, SF3B1, TP53, U2AF1, IDH2, BCOR, PPM1D, TET2, IDH1, IDH2, SRSF2, RUNX1, SH2B3, ZRSR2, STAT3, KRAS, MYD88, ATM, CALR, CEBPA, ETV6, EZH2, FLT3, KIT, MPL, NPM1, STAG2, WT1, SETD2, CREBBP), where VAF was ≥2%, and at least eight reads supported the alternate allele.
Mutations present in nonleukemia/lymphoma genes where VAF is ≥5%, and at least eight reads supported the alternate allele.
In samples where one CH mutation was identified, we also looked for the presence of additional mutations at lower variant frequencies, where mutations with VAF ≥1% in leukemia/lymphoma genes and VAF ≥3% for nonleukemia/lymphoma genes were retained.
For mutations in leukemia/lymphoma genes where the variant frequency was >35% in the blood sample but <35% in the tumor tissue, which could conceivably be considered as germline events with loss of allele in tumor tissue, we included only the mutations that were present in the Catalogue for Somatic Mutations in Cancer (COSMIC) database with >10 occurrences in the ‘‘hematopoietic and lymphoid’’ category into the pool of CH mutations.
Determination of CH-PD events
Variants were considered for CH-PD if they had a VAF ≥10% in the blood sample and satisfied at least one of the following criteria:
Any variant in Catalogue for Somatic Mutations in Cancer occurring in the ‘‘hematopoietic and lymphoid’’ category greater than or equal to five times
Any damaging variant in the DNMT3A gene within exons 7 to 23
Any damaging variant in the following genes: ASXL1, TET2, PPM1D, TP53, RAD21, STAG2, ATM, NF1
Any inactivating mutation in CALR exon 9
JAK2 V617F variant
CBL E366K, C384Y, C404S, and C416S variants
SETD2 R1625C variant
MPL W515S variant
Any oncogenic variant in the Papaemmanuil et al. study (22) that did not satisfy the above criteria
For detailed methods on determination of CH and CH-PD, refer to Coombs et al. (19) and Supplemental Table 1.
Survival determination
Survival data were obtained through internal MSKCC databases based on deaths while admitted to an MSKCC hospital or by Death Notification forms submitted from physicians’ offices. Last known follow-up information was also electronically updated for all patients with activity at any MSKCC site. Additionally, vital status was updated electronically by using the Social Security Death Index matched against the MSKCC patient database. The Social Security Death Index update is run on a monthly basis. If there is no update or activity on a patient’s account over a 15-month period, then the Omnipro Medicare Database is queried to determine vital status and/or data of last account activity.
Statistical analysis
χ 2 Tests, Student t tests, and Wilcoxon-rank sum tests were used to compare patient- and clinical-care characteristics among patients with and without CH. Logistic regression analysis was used to build multivariate models to examine the association between RAI and CH. The incidence of hematologic cancer, defined as the time from matched normal blood sampling to pathologically confirmed hematologic cancer development, was visually displayed using cumulative-incidence functions, whereas death in the absence of a hematologic malignancy was considered a competing event. OS, defined as the time from sample collection to death or last follow-up, was displayed using Kaplan-Meier curves. Cox-proportional hazards models were used to examine the association between CH and CH-PD and death. All statistical analyses were performed using STATA Statistical Software v12 (StataCorp).
Results
A total of 309 patients with thyroid carcinoma underwent MSK-IMPACT testing between 16 May 2014 and 20 July 2017. The overall prevalence of CH was 37% (115/309 patients) and that of CH-PD was 5.2% (16/309). Table 1 shows the prevalence of CH and CH-PD according to histologic subtype. There was no statistically significant difference in CH or CH-PD among the different subtypes of thyroid carcinoma. Ten percent of our patients (n = 30) had medullary thyroid carcinoma. Because patients with medullary thyroid carcinoma do not receive RAI as part of their treatment, they were excluded from our subsequent analyses. Out of 47 patients with anaplastic thyroid carcinoma, 10 (21.3%) had received RAI. The proportion of patients with anaplastic thyroid carcinoma who developed CH or CH-PD was comparable with the overall cohort, and therefore, they were included in our analyses. A total of 279 patients with follicular cell-derived thyroid carcinoma were analyzed.
Table 1.
Prevalence of CH and CH-PD, According to Thyroid Carcinoma Histologic Subtype
| Histology | n (%) | CH/n (%) | CH-PD/n (%) |
|---|---|---|---|
| Papillary thyroid cancer | 115 (37) | 46/115 (40) | 6/115 (5) |
| Poorly differentiated thyroid cancer | 87 (28) | 37/87 (43) | 5/87 (6) |
| Anaplastic thyroid cancer | 47 (15) | 15/47 (32) | 2/47 (4) |
| Hurthle cell carcinoma | 26 (9) | 8/26 (31) | 2/26 (8) |
| Medullary thyroid cancer | 30 (10) | 7/30 (23) | 1/30 (3) |
| Follicular thyroid cancer | 4 (1) | 2/4 (50) | 0/4 |
| Total | 309 (100) | 115/309 (37) | 16/309 (5.18) |
The median age of our population was 64 years (median, interquartile range = 64, 57 to 71). Older age was significantly associated with the presence of CH and CH-PD (Table 2). CH occurred more often in black patients and less often in Asians (P = 0.023). We did not find a statistically significant association between smoking status and CH (P = 0.916). Seventy-five percent of our population was treated with RAI. A higher administered dose of RAI was significantly associated with CH and CH-PD. The median time from exposure to RAI to testing for CH or CH-PD was 1349 days (minimum 19 days; maximum 12,691 days). Other therapies, including external beam radiation therapy (EBRT) and chemotherapy, were not significantly associated with CH or with CH-PD (Table 2).
Table 2.
Associations Among CH and CH-PD and Clinical Variables
|
|
|
CH |
|
CH-PD |
|
||
|---|---|---|---|---|---|---|---|
| Overall |
No (n = 171) | Yes (n = 108) |
P Value |
No (n = 264) | Yes (n = 15) |
P Value |
|
| Females, n (%) | 131 (47) | 82 (46) | 49 (45.4) | 0.674 | 123 (47) | 8 (53) | 0.61 |
| Age (median, IQR) | 64 (57–71) | 62 (52–68) | 67 (60–74) | <0.0001 | 64 (56–70) | 76 (69–79) | 0.0004 |
| Race | 0.023 | 1 | |||||
| White | 223 (80%) | 139 (62%) | 84 (38%) | 210 (94%) | 13 (6%) | ||
| Asian | 24 (8.6%) | 19 (79%) | 5 (21%) | 23 (96%) | 1 (4%) | ||
| Black | 17 (6%) | 6 (35%) | 11 (65%) | 16 (94%) | 1 (6%) | ||
| Other | 15 (5.4%) | 7 (47%) | 8 (53%) | 15 (100%) | 0 (0%) | ||
| Smoking status | 0.916 | 0.418 | |||||
| Past or current | 110 (39%) | 67 (61%) | 43 (39%) | 106 (96%) | 4 (4%) | ||
| Never | 169 (61%) | 104 (62%) | 65 (38%) | 158 (93%) | 11 (7%) | ||
| Tumor type | 0.748 | 0.94 | |||||
| Anaplastic | 47 (17%) | 32 (18.7%) | 15 (14%) | 45 (17%) | 2 (13%) | ||
| Poorly differentiated | 87 (31%) | 50 (29%) | 37 (34%) | 82 (31%) | 5 (33%) | ||
| Papillary | 115 (41%) | 69 (40%) | 46 (43%) | 109 (41%) | 6 (40%) | ||
| Hurthle cell | 26 (9%) | 18 (11%) | 8 (7.4%) | 24 (9%) | 2 (13%) | ||
| Follicular | 4 (1.5%) | 2 (1%) | 2 (1.8%) | 4 (1.5%) | 0 | ||
| RAI before CH test | 209 (75%) | 126 (74%) | 83 (77%) | 0.552 | 197 (75%) | 12 (80%) | 0.64 |
| Dose RAI (median, IQR) | 151 (0–296) | 150 (0–250) | 192 (62–391) | 0.03 | 151 (0–292) | 205 (101–727) | 0.06 |
| EBRT before CH test | 113 (41%) | 65 (38%) | 48 (45%) | 0.258 | 104 (40%) | 9 (60%) | 0.117 |
| Dose EBRT (median, IQR) | 0 (0–5400) | 0 (0–4200) | 0 (0–5940) | 0.323 | 0 (0–5220) | 4800 (0–6200) | 0.11 |
| Chemo before CH test | 101 (36%) | 62 (36%) | 39 (36%) | 0.97 | 93 (35%) | 8 (53%) | 0.159 |
Abbreviations: Chemo, chemotherapy; IQR, interquartile range.
To explore further the association between administered dose of RAI and CH or CH-PD, we built multivariate logistic regression models (Table 3). We found that age and dose of RAI were significantly associated with CH and CH-PD when adjusted for EBRT and chemotherapy. Specifically, for every year increase in age, there was a 5% increase in the odds of having CH and a 13% increase in the odds of having CH-PD when adjusted for RAI administration, EBRT, and chemotherapy. RAI was also independently associated with CH and CH-PD. For every 10 mCi increase of RAI administered, there was a 2% increase in the odds of having CH and a 4% increase in the odds of having CH-PD when adjusted for age, EBRT, and chemotherapy. There was no significant association between EBRT or chemotherapy and CH or CH-PD (Table 3).
Table 3.
Multivariate Models Examining the Association Among RAI and CH and CH-PD
| Odds Ratio | P Value | 95% CI |
||
|---|---|---|---|---|
| CH | ||||
| Age | 1.053174 | 0.000 | 1.028548 | 1.078389 |
| Dose of RAI | 1.001879 | 0.006 | 1.000544 | 1.003216 |
| EBRT | 1.080751 | 0.779 | 0.6284786 | 1.858492 |
| Chemo | 1.124853 | 0.677 | 0.6462175 | 1.958 |
| _cons | 0.0145339 | 0.000 | 0.002879 | 0.0733723 |
| CH-PD | ||||
| Age | 1.136906 | 0.000 | 1.058503 | 1.221116 |
| Dose of RAI | 1.003603 | 0.003 | 1.001245 | 1.005967 |
| EBRT | 1.306661 | 0.668 | 0.384898 | 4.435881 |
| Chemo | 2.942447 | 0.076 | 0.893934 | 9.685271 |
| _cons | 1.75e-06 | 0.000 | 6.01e-09 | 0.0005117 |
Abbreviation: _cons, constant.
We next sought to identify any potential associations among RAI, CH, and leukemia. We only had one CH-positive case of myelodysplastic syndrome, one CH-negative case of myelofibrosis, and two of monoclonal gammopathy of uncertain significance in the follow-up period (one CH positive; one CH negative). We found higher median doses of RAI administered to patients who subsequently developed these hematologic disorders compared with those who did not (316 vs 151 mCi), but this association was not statistically significant (P = 0.27). This may be, in part, a result of the short follow-up period of our cohort.
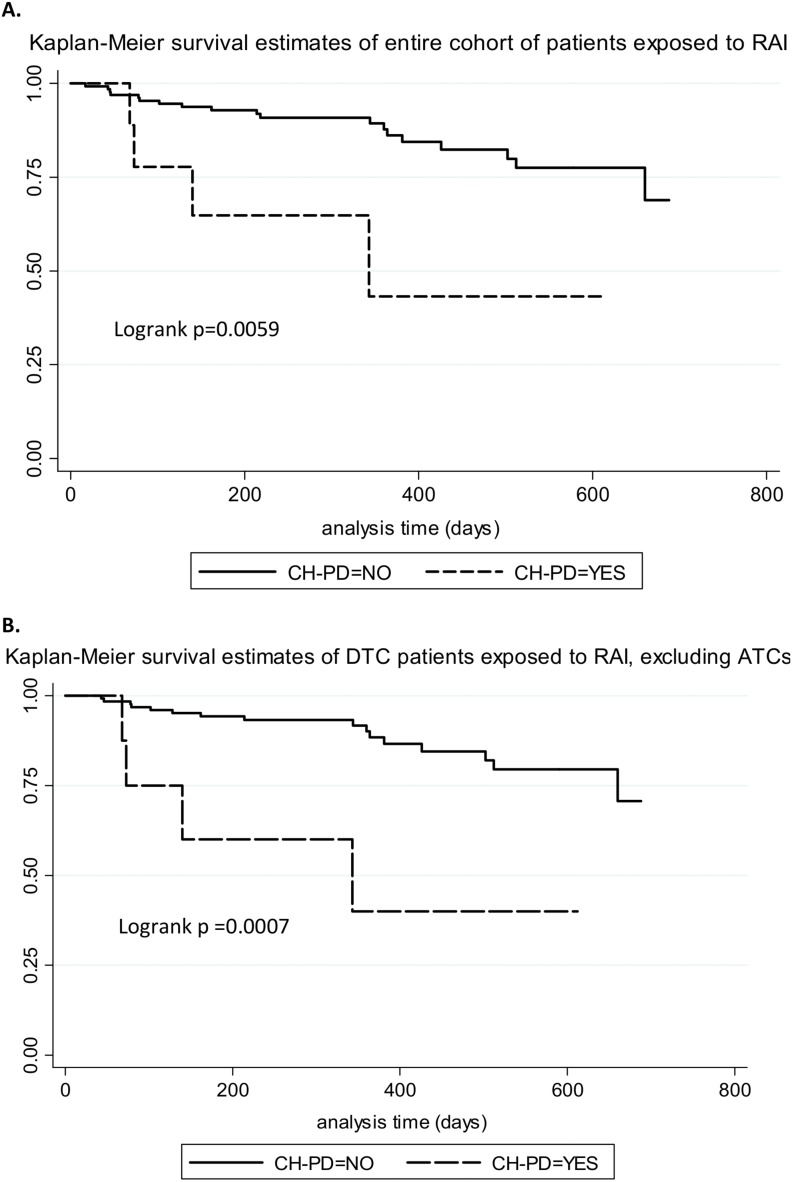
We assessed whether the presence of CH had an effect on OS. With the consideration of the entire population, we did not find a statistically significant association between CH and OS [heart rate (HR) = 1.3, 95% CI = 0.76 to 2.32, P = 0.319]. However, among patients who had been exposed to any dose of RAI, CH conferred a borderline, significantly poorer survival (HR = 2.16, 95% CI = 0.95 to 4.94, P = 0.06). This weak association did not persist after stratifying by age (P = 0.11). CH-PD had a borderline-significant association with decreased OS in the overall cohort (HR = 2.4, 95% CI = 0.96 to 6.2, P = 0.06), but when considered only for patients exposed to RAI, CH-PD had a strong association with adverse survival (HR = 4.1, 95% CI = 1.4 to 12.1, P = 0.01), which persisted after stratifying by age (HR = 3.75, 95% CI = 1.23 to 11.5, P = 0.02). Figure 1 shows Kaplan-Meier survival estimates of patients exposed to RAI with and without CH-PD. Figure 1A shows the entire cohort of patients, and Fig. 1B shows patients with differentiated thyroid carcinoma, excluding those with anaplastic thyroid cancer.
Figure 1.
Kaplan-Meier survival estimates of patients exposed to RAI, with and without CH-PD. (A) Entire cohort; (B) excluding patients with anaplastic thyroid carcinoma (ATC). DTC, differentiated thyroid carcinoma.
Discussion
In this study, we found that the prevalence of CH in patients with thyroid cancer was 37%, and that of potential drivers of hematologic malignancies (CH-PD) was 5.2%. Age was the strongest predictor of CH and CH-PD. For every year increase in age, there was a 5% and 13% increase in the odds of CH and CH-PD, respectively. Dose of RAI was significantly associated with CH and CH-PD, even after adjustment for age, EBRT, and chemotherapy. For every 10 mCi increase in the dose of RAI administered, there was a 2% and 4% increase in the odds of CH and CH-PD, respectively. Among patients who had received RAI, those with CH-PD had a significantly shorter OS, even when stratified by age.
With a rising incidence of thyroid carcinoma and the consequent large pool of long-term survivors who received RAI therapy, there is an understandable concern about the risk of secondary malignancies in general and hematologic malignancies in particular. Previous studies described the association between RAI and hematologic malignancies (9, 14, 23–25). This report demonstrates a dose-dependent association between RAI and clonal events that have a low probability of leading to the development of hematologic malignancies.
Ionizing radiation induces chromosomal aberrations frequently found in secondary leukemias, and its causal contribution to leukemogenesis has generally been established (26). Nevertheless, the link between RAI and the evolution to leukemia has long been a matter of debate, as early epidemiological studies yielded conflicting results (27–29). In a comprehensive review and meta-analysis of the literature covering 16,502 patients with thyroid cancer, the relative risk for the development of leukemia increased 2.5-fold in patients treated with radioiodine (30). In line with this, thyroid cancer was also identified as the second-most common primary neoplasm arising in patients who developed AML following treatment of solid cancers (31) and as the most common primary malignancy associated with CH (19). Based on current evidence, CH is a precursor clonal state conferring a five- to 10-fold greater risk for development of hematological neoplasms compared with non-CH controls (15, 16, 32, 33). Therefore, the signal for association of CH with RAI exposure in our retrospective series justifies the development of a more rigorously designed study to ascertain dose response, time course, persistence of CH, and longitudinal risk for development of hematologic malignancies to confirm this association.
Besides assessing for risk, there is intriguing preclinical evidence suggesting that patients with CH harboring mutations of TET2 may benefit from treatment with vitamin C. Loss-of-function heterozygous mutations in TET2 occur frequently in patients with CH, myelodysplastic syndrome, and AML and are associated with DNA hypermethylation. Treatment with vitamin C, a cofactor of Fe2+ and α-ketoglutarate-dependent dioxygenases, can reverse the hypermethylation state by enhancing 5-hydroxymethylcytosine formation. In mouse models, this has been shown to suppress human leukemic colony formation and leukemia progression (34, 35).
CH and particularly CH-PD were associated with poorer survival. In contrast to CH, in a general population without a diagnosis of advanced cancer, where the primary cause of death is attributed to cardiovascular events, the main reason for this cohort’s demise was progression of its primary thyroid carcinoma. The association between CH and cancer progression could be a result of cell–cell interactions among mutant myeloid clones and cancer cells (36), impact of CH on immune surveillance, or other factors that will require further clinical and functional investigation.
The findings of our study are limited by the fact that our cohort was composed of thyroid cancer patients with advanced disease stage and older age. The high prevalence of CH may be related to the increased age of our population compared with that of subjects with other solid tumors. In addition, the low incidence of hematologic malignancies prevented us from exploring the association among RAI, CH, and leukemia, which may have been a result of the short follow-up time after sequencing of their blood, compounded by the fact that mortality was high in this population with advanced thyroid cancer. Furthermore, the association between CH-PD and decreased survival among patients exposed to RAI may be related to the advanced stage of the tumors genotyped. It remains unclear if the association of CH with poor prognosis would also be seen in younger patients with more indolent disease.
In conclusion, the model that has been proposed (19) and further validated here is that age-dependent mutations are constantly acquired in hematopoietic stem cells, which are then selected for by external perturbations, such as RAI, which allow clones to expand. These mutant hematopoietic clones may impact the biology and therapeutic response of primary tumors, besides conferring an increased risk of hematologic malignancies. RAI is associated with a high prevalence of CH, and CH is a precursor state of hematologic malignancies. The extent to which the presence of CH should be considered before administration of RAI is unclear. Furthermore, identification of CH and particularly of CH-PD may inform lifestyle interventions and the use of molecularly targeted therapies to prevent clonal expansion and subsequent hematologic malignancies.
Supplementary Material
Acknowledgments
Financial Support: This work was supported by the National Cancer Institute, US National Institutes of Health (Grants P30-CA008748 and P50-CA72012; to J.A.F.).
Clinical Trial Information: ClinicalTrials.gov no. NCT01775072 (registered 24 January 2013).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- AML
acute myeloid leukemia
- CH
clonal hematopoiesis
- CH-PD
clonal hematopoiesis-potential driver
- EBRT
external beam radiation therapy
- HR
heart rate
- MSKCC
Memorial Sloan-Kettering Cancer Center
- MSK-IMPACT
Memorial Sloan-Kettering-Integrated Mutation Profiling of Actionable Cancer Targets
- OS
overall survival
- RAI
radioactive iodine
- VAF
variant allele fraction
References
- 1. Hay ID, Thompson GB, Grant CS, Bergstralh EJ, Dvorak CE, Gorman CA, Maurer MS, McIver B, Mullan BP, Oberg AL, Powell CC, van Heerden JA, Goellner JR. Papillary thyroid carcinoma managed at the Mayo Clinic during six decades (1940–1999): temporal trends in initial therapy and long-term outcome in 2444 consecutively treated patients. World J Surg. 2002;26(8):879–885. [DOI] [PubMed] [Google Scholar]
- 2. Jonklaas J, Sarlis NJ, Litofsky D, Ain KB, Bigos ST, Brierley JD, Cooper DS, Haugen BR, Ladenson PW, Magner J, Robbins J, Ross DS, Skarulis M, Maxon HR, Sherman SI. Outcomes of patients with differentiated thyroid carcinoma following initial therapy. Thyroid. 2006;16:1229–1242. [DOI] [PubMed] [Google Scholar]
- 3. Lamartina L, Durante C, Filetti S, Cooper DS. Low-risk differentiated thyroid cancer and radioiodine remnant ablation: a systematic review of the literature. J Clin Endocrinol Metab. 2015;100(5):1748–1761. [DOI] [PubMed] [Google Scholar]
- 4. Nixon IJ, Ganly I, Patel SG, Palmer FL, Di Lorenzo MM, Grewal RK, Larson SM, Tuttle RM, Shaha A, Shah JP. The results of selective use of radioactive iodine on survival and on recurrence in the management of papillary thyroid cancer, based on Memorial Sloan-Kettering Cancer Center risk group stratification. Thyroid. 2013;23(6):83–694. [DOI] [PubMed] [Google Scholar]
- 5. Haymart MR, Banerjee M, Stewart AK, Koenig RJ, Birkmeyer JD, Griggs JJ. Use of radioactive iodine for thyroid cancer. JAMA. 2011;306(7):721–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alexander C, Bader JB, Schaefer A, Finke C, Kirsch CM. Intermediate and long-term side effects of high-dose radioiodine therapy for thyroid carcinoma. J Nucl Med. 1998;39(9):1551–1554. [PubMed] [Google Scholar]
- 7. Kloos RT, Duvuuri V, Jhiang SM, Cahill KV, Foster JA, Burns JA. Nasolacrimal drainage system obstruction from radioactive iodine therapy for thyroid carcinoma. J Clin Endocrinol Metab. 2002;87(12):5817–5820. [DOI] [PubMed] [Google Scholar]
- 8. Wichers M, Benz E, Palmedo H, Biersack HJ, Grünwald F, Klingmüller D. Testicular function after radioiodine therapy for thyroid carcinoma. Eur J Nucl Med. 2000;27(5):503–507. [DOI] [PubMed] [Google Scholar]
- 9. Rubino C, de Vathaire F, Dottorini ME, Hall P, Schvartz C, Couette JE, Dondon MG, Abbas MT, Langlois C, Schlumberger M. Second primary malignancies in thyroid cancer patients. Br J Cancer. 2003;89(9):1638–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brown AP, Chen J, Hitchcock YJ, Szabo A, Shrieve DC, Tward JD. The risk of second primary malignancies up to three decades after the treatment of differentiated thyroid cancer. J Clin Endocrinol Metab. 2008;93(2):504–515. [DOI] [PubMed] [Google Scholar]
- 11. Sandeep TC, Strachan MW, Reynolds RM, Brewster DH, Scélo G, Pukkala E, Hemminki K, Anderson A, Tracey E, Friis S, McBride ML, Kee-Seng C, Pompe-Kirn V, Kliewer EV, Tonita JM, Jonasson JG, Martos C, Boffetta P, Brennan P. Second primary cancers in thyroid cancer patients: a multinational record linkage study. J Clin Endocrinol Metab. 2006;91(5):1819–1825. [DOI] [PubMed] [Google Scholar]
- 12. Lu CH, Lee KD, Chen PT, Chen CC, Kuan FC, Huang CE, Chen MF, Chen MC. Second primary malignancies following thyroid cancer: a population-based study in Taiwan. Eur J Endocrinol. 2013;169(5):577–585. [DOI] [PubMed] [Google Scholar]
- 13. Teng CJ, Hu YW, Chen SC, Yeh CM, Chiang HL, Chen TJ, Liu CJ. Use of radioactive iodine for thyroid cancer and risk of second primary malignancy: a nationwide population-based study. J Natl Cancer Inst. 2015;108(2):108. [DOI] [PubMed] [Google Scholar]
- 14. Molenaar RJ, Sidana S, Radivoyevitch T, Advani AS, Gerds AT, Carraway HE, Angelini D, Kalaycio M, Nazha A, Adelstein DJ, Nasr C, Maciejewski JP, Majhail NS, Sekeres MA, Mukherjee S. Risk of hematologic malignancies after radioiodine treatment of well-differentiated thyroid cancer. J Clin Oncol. 2018;36(18):1831–1839. JCO2017750232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Genovese G, Kähler AK, Handsaker RE, Lindberg J, Rose SA, Bakhoum SF, Chambert K, Mick E, Neale BM, Fromer M, Purcell SM, Svantesson O, Landén M, Höglund M, Lehmann S, Gabriel SB, Moran JL, Lander ES, Sullivan PF, Sklar P, Grönberg H, Hultman CM, McCarroll SA. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med. 2014;371(26):2477–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, Lindsley RC, Mermel CH, Burtt N, Chavez A, Higgins JM, Moltchanov V, Kuo FC, Kluk MJ, Henderson B, Kinnunen L, Koistinen HA, Ladenvall C, Getz G, Correa A, Banahan BF, Gabriel S, Kathiresan S, Stringham HM, McCarthy MI, Boehnke M, Tuomilehto J, Haiman C, Groop L, Atzmon G, Wilson JG, Neuberg D, Altshuler D, Ebert BL. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371(26):2488–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McKerrell T, Park N, Moreno T, Grove CS, Ponstingl H, Stephens J, Understanding Society Scientific Group, Crawley C, Craig J, Scott MA, Hodkinson C, Baxter J, Rad R, Forsyth DR, Quail MA, Zeggini E, Ouwehand W, Varela I, Vassiliou GS. Leukemia-associated somatic mutations drive distinct patterns of age-related clonal hemopoiesis. Cell Reports. 2015;10(8):1239–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xie M, Lu C, Wang J, McLellan MD, Johnson KJ, Wendl MC, McMichael JF, Schmidt HK, Yellapantula V, Miller CA, Ozenberger BA, Welch JS, Link DC, Walter MJ, Mardis ER, Dipersio JF, Chen F, Wilson RK, Ley TJ, Ding L. Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nat Med. 2014;20(12):1472–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Coombs CC, Zehir A, Devlin SM, Kishtagari A, Syed A, Jonsson P, Hyman DM, Solit DB, Robson ME, Baselga J, Arcila ME, Ladanyi M, Tallman MS, Levine RL, Berger MF. Therapy-related clonal hematopoiesis in patients with non-hematologic cancers is common and associated with adverse clinical outcomes. Cell Stem Cell. 2017;21(3):374–382.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zehir A, Benayed R, Shah RH, Syed A, Middha S, Kim HR, Srinivasan P, Gao J, Chakravarty D, Devlin SM, Hellmann MD, Barron DA, Schram AM, Hameed M, Dogan S, Ross DS, Hechtman JF, DeLair DF, Yao J, Mandelker DL, Cheng DT, Chandramohan R, Mohanty AS, Ptashkin RN, Jayakumaran G, Prasad M, Syed MH, Rema AB, Liu ZY, Nafa K, Borsu L, Sadowska J, Casanova J, Bacares R, Kiecka IJ, Razumova A, Son JB, Stewart L, Baldi T, Mullaney KA, Al-Ahmadie H, Vakiani E, Abeshouse AA, Penson AV, Jonsson P, Camacho N, Chang MT, Won HH, Gross BE, Kundra R, Heins ZJ, Chen HW, Phillips S, Zhang H, Wang J, Ochoa A, Wills J, Eubank M, Thomas SB, Gardos SM, Reales DN, Galle J, Durany R, Cambria R, Abida W, Cercek A, Feldman DR, Gounder MM, Hakimi AA, Harding JJ, Iyer G, Janjigian YY, Jordan EJ, Kelly CM, Lowery MA, Morris LGT, Omuro AM, Raj N, Razavi P, Shoushtari AN, Shukla N, Soumerai TE, Varghese AM, Yaeger R, Coleman J, Bochner B, Riely GJ, Saltz LB, Scher HI, Sabbatini PJ, Robson ME, Klimstra DS, Taylor BS, Baselga J, Schultz N, Hyman DM, Arcila ME, Solit DB, Ladanyi M, Berger MF. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med. 2017;23(6):703–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cheng DT, Mitchell TN, Zehir A, Shah RH, Benayed R, Syed A, Chandramohan R, Liu ZY, Won HH, Scott SN, Brannon AR, O’Reilly C, Sadowska J, Casanova J, Yannes A, Hechtman JF, Yao J, Song W, Ross DS, Oultache A, Dogan S, Borsu L, Hameed M, Nafa K, Arcila ME, Ladanyi M, Berger MF. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn. 2015;17(3):251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Papaemmanuil E, Gerstung M, Bullinger L, Gaidzik VI, Paschka P, Roberts ND, Potter NE, Heuser M, Thol F, Bolli N, Gundem G, Van Loo P, Martincorena I, Ganly P, Mudie L, McLaren S, O’Meara S, Raine K, Jones DR, Teague JW, Butler AP, Greaves MF, Ganser A, Döhner K, Schlenk RF, Döhner H, Campbell PJ. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. 2016;374(23):2209–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Seidlin SM, Siegal E, Yalow AA, Melamed S. Acute myeloid leukemia following prolonged iodine-131 therapy for metastatic thyroid carcinoma. Science. 1956;123(3201):800–801. [DOI] [PubMed] [Google Scholar]
- 24. Schroeder T, Kuendgen A, Kayser S, Kröger N, Braulke F, Platzbecker U, Klärner V, Zohren F, Haase D, Stadler M, Schlenk R, Czibere AG, Bruns I, Fenk R, Gattermann N, Haas R, Kobbe G, Germing U. Therapy-related myeloid neoplasms following treatment with radioiodine. Haematologica. 2012;97(2):206–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Oluwasanjo A, Pathak R, Ukaigwe A, Alese O. Therapy-related acute myeloid leukemia following radioactive iodine treatment for thyroid cancer. Cancer Causes Control. 2016;27(1):143–146. [DOI] [PubMed] [Google Scholar]
- 26. Sill H, Olipitz W, Zebisch A, Schulz E, Wölfler A. Therapy-related myeloid neoplasms: pathobiology and clinical characteristics. Br J Pharmacol. 2011;162(4):792–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Edmonds CJ, Smith T. The long-term hazards of the treatment of thyroid cancer with radioiodine. Br J Radiol. 1986;59(697):45–51. [DOI] [PubMed] [Google Scholar]
- 28. Hall P, Holm LE. Late consequences of radioiodine for diagnosis and therapy in Sweden. Thyroid. 1997;7(2):205–208. [DOI] [PubMed] [Google Scholar]
- 29. de Vathaire F. The carcinogenic effects of radioiodine therapy for thyroid carcinoma. Nat Clin Pract Endocrinol Metab. 2008;4(4):180–181. [DOI] [PubMed] [Google Scholar]
- 30. Sawka AM, Thabane L, Parlea L, Ibrahim-Zada I, Tsang RW, Brierley JD, Straus S, Ezzat S, Goldstein DP. Second primary malignancy risk after radioactive iodine treatment for thyroid cancer: a systematic review and meta-analysis. Thyroid. 2009;19(5):451–457. [DOI] [PubMed] [Google Scholar]
- 31. Kayser S, Döhner K, Krauter J, Köhne CH, Horst HA, Held G, von Lilienfeld-Toal M, Wilhelm S, Kündgen A, Götze K, Rummel M, Nachbaur D, Schlegelberger B, Göhring G, Späth D, Morlok C, Zucknick M, Ganser A, Döhner H, Schlenk RF, German-Austrian A; German-Austrian AMLSG . The impact of therapy-related acute myeloid leukemia (AML) on outcome in 2853 adult patients with newly diagnosed AML. Blood. 2011;117(7):2137–2145. [DOI] [PubMed] [Google Scholar]
- 32. Abelson S, Collord G, Ng SWK, Weissbrod O, Mendelson Cohen N, Niemeyer E, Barda N, Zuzarte PC, Heisler L, Sundaravadanam Y, Luben R, Hayat S, Wang TT, Zhao Z, Cirlan I, Pugh TJ, Soave D, Ng K, Latimer C, Hardy C, Raine K, Jones D, Hoult D, Britten A, McPherson JD, Johansson M, Mbabaali F, Eagles J, Miller JK, Pasternack D, Timms L, Krzyzanowski P, Awadalla P, Costa R, Segal E, Bratman SV, Beer P, Behjati S, Martincorena I, Wang JCY, Bowles KM, Quirós JR, Karakatsani A, La Vecchia C, Trichopoulou A, Salamanca-Fernández E, Huerta JM, Barricarte A, Travis RC, Tumino R, Masala G, Boeing H, Panico S, Kaaks R, Krämer A, Sieri S, Riboli E, Vineis P, Foll M, McKay J, Polidoro S, Sala N, Khaw KT, Vermeulen R, Campbell PJ, Papaemmanuil E, Minden MD, Tanay A, Balicer RD, Wareham NJ, Gerstung M, Dick JE, Brennan P, Vassiliou GS, Shlush LI. Prediction of acute myeloid leukaemia risk in healthy individuals. Nature. 2018;559(7714):400–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Desai P, Mencia-Trinchant N, Savenkov O, Simon MS, Cheang G, Lee S, Samuel M, Ritchie EK, Guzman ML, Ballman KV, Roboz GJ, Hassane DC. Somatic mutations precede acute myeloid leukemia years before diagnosis. Nat Med. 2018;24(7):1015–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cimmino L, Dolgalev I, Wang Y, Yoshimi A, Martin GH, Wang J, Ng V, Xia B, Witkowski MT, Mitchell-Flack M, Grillo I, Bakogianni S, Ndiaye-Lobry D, Martin MT, Guillamot M, Banh RS, Xu M, Figueroa ME, Dickins RA, Abdel-Wahab O, Park CY, Tsirigos A, Neel BG, Aifantis I. Restoration of TET2 function blocks aberrant self-renewal and leukemia progression. Cell. 2017;170(6):P1079–1095.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Agathocleous M, Meacham CE, Burgess RJ, Piskounova E, Zhao Z, Crane GM, Cowin BL, Bruner E, Murphy MM, Chen W, Spangrude GJ, Hu Z, DeBerardinis RJ, Morrison SJ. Ascorbate regulates haematopoietic stem cell function and leukaemogenesis. Nature. 2017;549(7673):476–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kleppe M, Comen E, Wen HY, Bastian L, Blum B, Rapaport FT, Keller M, Granot Z, Socci N, Viale A, You D, Benezra R, Weigelt B, Brogi E, Berger MF, Reis-Filho JS, Levine RL, Norton L. Somatic mutations in leukocytes infiltrating primary breast cancers. NPJ Breast Cancer. 2015;1(1):15005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.