Abstract
Context
Bone health declines in the initial years after Roux-en-Y gastric bypass (RYGB), but long-term skeletal effects are unclear.
Objective
To document longitudinal changes in bone mineral density (BMD) and microarchitecture 5 years after RYGB.
Design, Setting, and Participants
Prospective 5-year observational study of 21 adults with severe obesity receiving RYGB at an academic medical center.
Main Outcome Measures
Spine and hip areal BMD were measured by dual-energy X-ray absorptiometry, and trabecular volumetric BMD (vBMD) of the spine was assessed by quantitative CT (QCT). We measured vBMD and microarchitecture of the distal radius and tibia by high-resolution peripheral QCT in a subset of subjects. Serum type I collagen C–terminal telopeptide (CTX) and procollagen type I N-terminal propeptide (P1NP) were also measured.
Results
Areal BMD declined by −7.8% ± 7.6% at the spine and −15.3% ± 6.3% at the total hip by 5 years after RYGB (P ≤ 0.001), although the rate of bone loss slowed in later years. Trabecular spine vBMD decreased by −12.1% ± 12.3% by 5 years (P ≤ 0.001). At peripheral sites, vBMD continued to decrease steadily throughout 5 years, with parallel declines in cortical and trabecular microarchitecture, leading to decreases in estimated failure load of −20% and −13% at the radius and tibia, respectively (P < 0.001). Five years after RYGB, CTX and P1NP were 150% and 34% above baseline (P < 0.001 and P = 0.017, respectively).
Conclusions
Sustained high-turnover bone loss and bone microarchitectural deterioration occur in the 5 years after RYGB. Adults receiving RYGB warrant assessment of bone health.
In this longitudinal 5-year study of adults with severe obesity, we documented sustained high-turnover bone loss and deterioration of bone microarchitecture in the 5 years after RYGB.
The clinical management of severe obesity [body mass index (BMI) ≥40 kg/m2] has been transformed by the emergence of bariatric surgery procedures, which lead to improvements in a wide range of obesity comorbidities and are associated with decreased mortality (1–3). Roux-en-Y gastric bypass (RYGB) has traditionally been one of the most commonly performed types of weight loss surgery (4) but is associated with negative skeletal effects. RYGB and other bariatric procedures with malabsorptive features have been shown to increase the risk of fractures at sites including the spine, hip, and upper extremity (5–7).
This RYGB-associated skeletal fragility is mediated by accelerated high-turnover bone loss and has been documented in the short term in multiple longitudinal studies (8–22). Collectively, these studies document that a decline in bone density up to 10% is common in the initial 1 to 2 years after RYGB. RYGB also leads to short-term declines in volumetric bone density of the axial and peripheral skeleton (18, 23) and weakening of peripheral bone microarchitecture (18–20, 23). However, the long-term skeletal consequences of RYGB have not been well characterized beyond these initial postsurgical years. In particular, no studies have looked at changes in volumetric bone density or skeletal microarchitecture beyond 2 years. Furthermore, the mechanisms of bone loss after RYGB remain unclear but may involve a combination of mechanical unloading of the skeleton, calcium and vitamin D malabsorption, and changes in metabolic hormones that may affect the entero-osseous axis (24, 25).
We previously reported substantial bone loss by dual-energy X-ray absorptiometry (DXA) and a deterioration in both cortical and trabecular microarchitecture 2 years after RYGB (22). To determine whether these skeletal changes progress, we assessed bone density and bone microarchitecture up to 5 years after RYGB. In addition, we assessed whether changes in weight, body composition, and calciotropic or entero-osseous hormones are associated with bone loss after RYGB.
Methods
Study subjects
We prospectively evaluated longitudinal data in adults with severe obesity (n = 21) in the 5 years after RYGB. We initially recruited 30 men and women with obesity (≥18 years old) who were planning RYGB with no history of medical disorders or use of medications known to affect bone metabolism (22). Subjects who weighed >204 kg were excluded due to weight limitations of the DXA and CT scanners. Nine subjects did not complete visits through 5 years: three developed medical conditions that could alter bone metabolism, three moved from the study area, and three chose not to continue participating or were lost to follow-up. These nine subjects had a younger baseline age (mean ± SD: 38 ± 12 years) and were more likely to be nonwhite (56%), but preoperative weight, BMI, serum 25-hydroxyvitamin D, and PTH were similar to the 21 subjects who completed the study. Data for the remaining 21 subjects were obtained at a baseline preoperative visit (within 6 weeks prior to RYGB) and at 2, 3.5, and 5 years after surgery. The study was approved by the Institutional Review Board at Massachusetts General Hospital, and all subjects provided written informed consent.
Study protocol
DXA bone density and body composition
Areal bone mineral density (aBMD, g/cm2) of the posterior-anterior (PA) lumbar spine (L1 to L4) and proximal femur was measured by DXA (Discovery A; Hologic, Bedford, MA) at baseline and 2, 3.5, and 5 years after RYGB. When necessary, manual retraction of pannus overlying the proximal femur was performed during hip measurements. For subjects with body dimensions that extended beyond the whole-body scan area, the left arm was incompletely imaged and the measurements from the right arm replaced the left arm values. In vivo scanning precision at our institution is 0.007, 0.008, and 0.012 g/cm2 for PA spine, total hip, and femoral neck, respectively. Body composition was measured by DXA to obtain assessments of subtotal (total body excluding head) measurements of fat mass (kg) and lean mass (kg).
Quantitative CT bone density
Volumetric bone mineral density (vBMD, mg/cm3) of the lumbar spine (L1 to L2) was assessed by quantitative CT (QCT) (General Electric LightSpeed Pro CT scanner; General Electric Healthcare, Waukesha, WI) at baseline, 2 years, and 5 years after RYGB. Scans were performed with a calibration phantom (Mindways Software, Austin, TX) using helical acquisition under the following settings: kVp 120, mA 100 (L1 to L2)/mA 200 (proximal femur), slice thickness 2.5 mm, field of view 500 mm, and table height 144 mm [2-year coefficient of variation (CV) ≤2%] (26). Analysis of vBMD of L1 to L2 and total hip was performed with QCTPro software (Mindways Software, Austin, TX), as previously described (23).
High-resolution peripheral QCT bone density and microarchitecture
vBMD (mg/cm3) and bone microarchitecture of the ultradistal radius and tibia were assessed at baseline and at 2, 3.5, and 5 years after surgery using high-resolution peripheral QCT (HR-pQCT) (XtremeCT; Scanco Medical AG, Brüttisellen, Switzerland). Cortical and trabecular geometry, density, and microarchitecture were calculated using standard analysis software (Scanco software V6.0; Scanco Medical AG, Brüttisellen, Switzerland) (22). To characterize cortical microarchitecture in greater detail, HR-pQCT images were processed by a semiautomated cortical bone segmentation technique to allow measurement of cortical geometry, density, and porosity (27). Failure load, a measurement of bone strength, was estimated using linear microfinite element analysis in response to simulated uniaxial compression (28). Longitudinal HR-pQCT data were not available in nine subjects because of motion artifact (n = 3) or unavailability of HR-pQCT equipment at baseline or follow-up visits (n = 6).
Biochemical measurements
Fasting serum type I collagen C–terminal telopeptide (CTX), a measure of bone resorption, and procollagen type I N-terminal propeptide (P1NP), a measure of bone formation, were assessed at each study visit. CTX was measured using an ELISA (Serum Crosslaps; Immunodiagnostic Systems, Tyne & Wear, UK) with intra-assay and interassay CVs of 1.8% to 3.0% and 2.5% to 10.9%, respectively. P1NP was measured using a radioimmunoassay (UniQ P1NP RIA; Orion Diagnostica, Espoo, Finland) with intra-assay and interassay CVs of 5.4% to 9.6% and 5.5% to 8.9%, respectively. We also measured serum calcium, 25-hydroxyvitamin D (DiaSorin LIAISON, Saluggia, Italy), and bioactive PTH (PTH 1–84) (ELISA; Immutopics, San Clemente, CA). Insulin was measured by chemiluminescent immunoassay (Beckman Coulter, Fullerton, CA) with an intra-assay CV of 2.0% to 4.2% and an interassay CV of 3.1% to 5.6%. Serum peptide YY (PYY) levels were measured by ELISA [Millipore Corporation (Linco Research), Billerica, MA], with an intra-assay CV of 17% to 18% and interassay CV of 12% to 18%.
Bionutrition measurements
Height and weight were measured in triplicate using a wall-mounted stadiometer (Harpenden; Seritex) and digital scale (Tanita BWB-800; Tanita Corporation of America, Arlington Heights, IL), respectively. To measure leisure and occupational physical activity, the modifiable activity questionnaire (29) was administered by a registered dietitian. Throughout the study, subjects were counseled to maintain a calcium intake of 1200 to 1500 mg/d and vitamin D intake of 3000 IU/d through diet and supplements. Dietary calcium and vitamin D were assessed through self-reported food diaries and questionnaires administered by a registered dietitian.
Statistical analysis
Longitudinal values are reported as mean ± SD unless otherwise noted. Mean percentage changes over time were analyzed with linear mixed models using repeated measures with random subject intercepts and fixed effect of time, as well as a compound symmetry covariance structure. Adjustment of P values for multiple comparisons was performed using the Dunnett-Hsu method, with the baseline visit as the control. In addition, we used linear contrasts to compare percent change from baseline between the 2-year and 5-year time points. Exploratory assessments of predictors and mediators of 5-year bone loss were evaluated using Spearman correlations. Effect modification by sex/menopause status was investigated using longitudinal regression analysis with interaction terms. Sensitivity analyses were performed to (1) exclude two premenopausal women who became menopausal during the course of this study and (2) examine the characteristics of the subset of study subjects who had interpretable HR-pQCT scans. All analyses were performed using SAS 9.4 software (SAS Institute, Cary, NC). Adjusted P values <0.05 were considered significant.
Results
Clinical characteristics
The clinical characteristics of the 21 adults who received RYGB are shown in Table 1. At the preoperative baseline visit, the cohort had a mean age of 51 ± 14 years, and there was a mix of premenopausal (n = 9) and postmenopausal women (n = 8) and men (n = 4). Two women became menopausal during the course of this 5-year longitudinal study. Average preoperative weight was 121 ± 17 kg with a BMI of 45 ± 7 kg/m2. By 2 years after RYGB, the subjects had experienced an average weight loss of −29 ± 10%, with parallel declines in lean mass and fat mass (P < 0.001 for all comparisons vs baseline). There was no further change in weight or body composition between 2 and 5 years. Total calcium intake peaked at 2 years and then returned to preoperative baseline at 3.5 and 5 years, whereas vitamin D intake was increased at 5 years. Average serum calcium and vitamin D levels remained unchanged and in the normal range throughout the 5-year study. There was no significant change in PTH in the initial 2 years, but average levels increased to the upper portion of the normal range by 3.5 and 5 years after RYGB (P = 0.023 for comparison of 2 vs 5 years). As expected, RYGB fasting glucose and insulin were significantly lower 2 years after RYGB than at baseline and remained low throughout the 5-year postoperative period. Fasting serum PYY levels at 3.5 and 5 years after RYGB were significantly higher than at baseline.
Table 1.
Clinical Characteristics and Metabolic Bone Laboratories of RYGB Study Subjects Over 5 Years
| Characteristic | Baseline | 2 y | 3.5 y | 5 y |
|---|---|---|---|---|
| Demographics | ||||
| Age, y | 51 ± 14 | |||
| Sex/menopause status | ||||
| Premenopausal womena | 9 | |||
| Postmenopausal women | 8 | |||
| Men | 4 | |||
| Race/ethnicity | ||||
| White | 15 | |||
| Black | 2 | |||
| Hispanic | 4 | |||
| Body composition | ||||
| Weight, kg | 121 ± 17 | 86 ± 14b | 88 ± 14b | 88 ± 16b |
| BMI, kg/m2 | 45 ± 7 | 32 ± 5b | 33 ± 4b | 33 ± 6b |
| Lean mass, kg | 59 ± 9 | 47 ± 9b | 47 ± 8b | 47 ± 8b |
| Fat mass, kg | 53 ± 11 | 32 ± 8b | 33 ± 9b | 35 ± 10b |
| Bionutrition data | ||||
| Physical activity, h/wk | 17 ± 20 | 18 ± 14 | 25 ± 22 | 26 ± 23 |
| Calcium intake, mg/d | 1327 ± 679 | 1897 ± 1394b | 1605 ± 894 | 1504 ± 855 |
| Vitamin D intake, IU/d | 942 ± 1107 | 1406 ± 1446 | 2519 ± 2177 | 2003 ± 3069b |
| Laboratory data | ||||
| Calcium, mg/dL | 9.4 ± 0.4 | 9.3 ± 0.4 | 9.3 ± 0.5 | 9.2 ± 0.5 |
| 25-Hydoxyvitamin D, ng/mL | 30 ± 12 | 30 ± 9 | 31 ± 11 | 31 ± 11 |
| PTH, pg/mL | 37 ± 24 | 39 ± 21 | 53 ± 27b | 54 ± 26b,c |
| Fasting glucose, mg/dL | 117 ± 47 | 94 ± 18b | 95 ± 19b | 94 ± 19b |
| Fasting insulin, uIU/mL | 22 ± 20 | 8 ± 7b | 6 ± 5b | 11 ± 15b |
| Fasting PYY, ng/mL | 110 ± 65 | 131 ± 53 | 158 ± 83b | 170 ± 106b |
Data are presented as mean ± SD or number.
Note that two women became menopausal during the course of the 5-y study.
Dunnett-adjusted P < 0.05 for comparison vs baseline visit.
P < 0.05 for comparison of 5-y vs 2-y visit.
Changes in spine and total hip bone mineral density
Longitudinal changes in bone density are shown in Fig. 1. By 5 years after RYGB, spine aBMD declined by −7.8% ± 7.6%, as assessed by DXA, and trabecular spine vBMD by −12.1% ± 12.3%, as assessed by QCT (P ≤ 0.001 for all comparisons vs baseline). However, the rate of spine bone mineral density (BMD) decline appeared to have slowed, with the majority of bone loss having occurred within the initial 2 years. At the total hip and femoral neck, aBMD declined by −15.3% ± 6.3% and −14.1% ± 8.0%, respectively, in the 5 years after surgery (P < 0.001 for all comparisons vs baseline). Although continued femoral bone loss was detected in later years (P < 0.025 for comparisons of 2 vs 5 years), the majority of the cumulative decline at the proximal femur occurred in the first 2 years after RYGB.
Figure 1.
Longitudinal changes in spine and hip BMD by DXA and QCT after RYGB. Mean ± SEM percentage change vs baseline over 5 y is shown for (A) PA spine aBMD, (B) trabecular spine vBMD, (C) total hip aBMD, and (D) femoral neck aBMD. *Dunnett-adjusted P < 0.05 for comparison vs baseline visit. #P < 0.05 for comparison vs 2-y visit.
Changes in radius and tibia BMD, microarchitecture, and estimated strength
Peripheral vBMD and microarchitecture continued to decline at a steady rate between 2 and 5 years after RYGB (Table 2). Cumulative declines in total vBMD were −18.7% ± 5.3% at the radius and −14.4% ± 4.9% at the tibia, and they were accompanied by decreases in both trabecular and cortical vBMD. In contrast to axial sites, the rates of peripheral vBMD loss in later years mirrored the accelerated bone loss observed in the initial 2 years after surgery (P < 0.005 for all comparisons of 2 vs 5 years). Cortical and trabecular microarchitecture also continued to deteriorate at the radius, with decreased cortical thickness, increased cortical porosity, decreased trabecular number, and increased trabecular separation and heterogeneity (P ≤ 0.01 for all comparisons of 2 vs 5 years; Fig. 2). In contrast, there was relatively less change in cortical and trabecular microarchitecture at the tibia in later years after RYGB. Nevertheless, cumulative densitometric and microarchitectural declines contributed to a continued worsening of estimated failure load at both the radius and tibia between 2 and 5 years after RYGB (P < 0.001 for both).
Table 2.
Longitudinal Changes in vBMD and Microarchitecture at the Distal Radius and Distal Tibia Assessed by HR-pQCT at 2 and 5 Years After RYGB
| Characteristic |
% Change at Radius, Mean ± SD
|
% Change at Tibia, Mean ± SD
|
||
|---|---|---|---|---|
| 2 y | 5 y | 2 y | 5 y | |
| Density | ||||
| Total vBMD | −6.8 ± 4.9a | −18.7 ± 5.3a,b | −6.7 ± 2.9a | −14.4 ± 4.9a,b |
| Trabecular vBMD | −7.4 ± 4.4a | −19.5 ± 9.1a,b | −3.1 ± 4.5 | −10.4 ± 12.1a,b |
| Cortical vBMD | −1.7 ± 2.3 | −6.8 ± 5.4a,b | −4.3 ± 4.4a | −10.7 ± 6.9a,b |
| Morphology | ||||
| Total area | −0.11 ± 0.49 | 0.01 ± 0.34 | 0.00 ± 0.06 | 0.02 ± 0.06 |
| Trabecular area | 1.1 ± 3.0 | 2.6 ± 3.2a | 1.5 ± 3.8 | 1.1 ± 3.3 |
| Cortical area | −3.2 ± 9.6 | −9.6 ± 9.4a,b | −4.4 ± 12.4 | −3.9 ± 12.3 |
| Microarchitecture | ||||
| Trabecular thickness | −1.0 ± 9.1 | 1.7 ± 10.9 | −0.6 ± 15.9 | −3.5 ± 15.9 |
| Trabecular number | −5.7 ± 11.3 | −20.2 ± 10.1a,b | 0.3 ± 16.8 | −6.1 ± 12.7 |
| Trabecular separation | 8.5 ± 11.6 | 32.0 ± 21.6a,b | 3.4 ± 17.5 | 10.4 ± 17.2 |
| Trabecular heterogeneity | 20.9 ± 31.2 | 89.3 ± 116.8a,b | 4.7 ± 22.0 | 17.1 ± 27.9a |
| Cortical thickness | −2.1 ± 8.2 | −11.3 ± 9.0a,b | −4.3 ± 9.7 | −6.1 ± 9.9a |
| Cortical porosity | 50.3 ± 58.8 | 269.1 ± 364.6a,b | 66.4 ± 75.5a | 107.0 ± 88.0a |
| µFEA | ||||
| Failure load | −10.5 ± 6.4a | −19.6 ± 5.3a,b | −6.4 ± 4.3a | −13.4 ± 6.1a,b |
Abbreviation: µFEA, microfinite element analysis.
Dunnett-adjusted P < 0.05 for comparison vs baseline visit.
P < 0.05 for comparison vs 2-y visit.
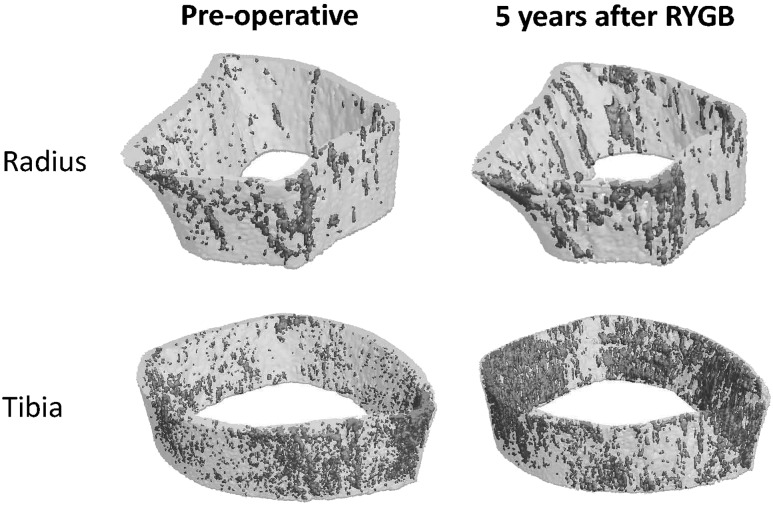
Figure 2.
Cortical porosity at the distal radius and tibia at preoperative baseline and 5 y after RYGB. Cortical pores are denoted by gray shading.
Change in bone turnover markers
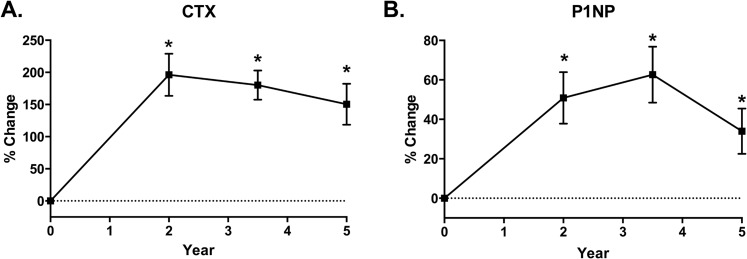
Bone turnover markers increased dramatically after RYGB. By 2 years, serum CTX was 196% ± 143% elevated above baseline levels (Fig. 3) and remained 150% ± 146% above baseline at 5 years (P < 0.001 for comparisons vs baseline). Serum P1NP also increased, peaking at 63% ± 60% at 3.5 years after RYGB before declining somewhat to remain 34% ± 53% above baseline levels at 5 years (P = 0.017 for comparisons vs baseline).
Figure 3.
Longitudinal changes in serum CTX and P1NP after RYGB. Mean ± SEM percentage change over 5 y is shown for serum (A) CTX and (B) serum P1NP. *Dunnett-adjusted P < 0.05 for comparison vs baseline visit.
Predictors and mediators of RYGB-associated skeletal changes
Baseline age and weight did not predict changes in bone density in the 5 years after RYGB. Although postmenopausal women tended to have numerically greater declines in bone density and microarchitecture than either premenopausal women or men, there were no statistically significant interactions between sex/menopause status and skeletal changes.
Weight loss in the 5 years after RYGB did not correlate with axial bone loss. In contrast, 5-year decline in lean mass was associated with the decline in spine aBMD (r = 0.47, P = 0.041), and there was a similar trend for an association between change in lean mass and change in total hip aBMD (r = 0.47, P = 0.057). Increases in CTX at 5 years were correlated with declines in spine and total hip aBMD as well as spine vBMD (r = −0.50 to −0.61, P < 0.030 for all). Increases in PTH were also associated with decreases in spine vBMD (r = −0.51, P = 0.023), but there was no correlation between PTH and bone turnover marker CTX or P1NP. In addition, decreases in fasting insulin were correlated with declines in spine aBMD (r = 0.56, P = 0.008; Fig. 4). We did not find evidence of associations between changes in DXA, QCT, or HR-pQCT BMD or microarchitecture with increases in fasting PYY.
Figure 4.
Scatterplot of 5-y percentage changes in spine aBMD vs insulin after RYGB.
Sensitivity analyses
Sensitivity analyses were performed after excluding the two women who transitioned to menopause during the course of the study. Changes in aBMD, vBMD, bone microarchitecture, and bone turnover markers were not significantly changed after exclusion of these study subjects. In addition, examination of the subset of subjects who contributed HR-pQCT data demonstrated that they were similar to the overall cohort in their baseline characteristics and patterns of 5-year decline in weight and bone density as assessed by DXA and QCT (data not shown).
Discussion
This prospective study provides the longest longitudinal assessment of changes in areal and volumetric bone density and microarchitecture after RYGB. We documented sustained high-turnover bone loss and bone microarchitectural deterioration in the first 5 years after RYGB. Axial bone loss was greatest in the first 2 years after surgery and then continued at a slower rate up to 5 years, with overall declines in BMD of 7% to 12% at the spine and 14% to 15% at the hip. In contrast, peripheral bone loss and deterioration in bone microarchitecture continued steadily throughout the 5 years following RYGB, leading to a 15% to 20% decrease in the estimated bone strength at the radius and tibia. Bone resorption remained elevated 5 years after surgery, with average serum CTX levels plateauing at 150% above baseline levels.
The cumulative magnitude of long-term bone loss after RYGB is large. Only a few prospective studies have evaluated areal bone density or bone markers for >2 years after RYGB. Vilarrasa et al. (21) reported declines of ∼6% and 13% in spine and total hip aBMD in the 3 years after RYGB, with concurrent increases in PTH. These findings are consistent in magnitude with our DXA-based measurements of axial bone loss at 3.5 years and are further corroborated by our QCT-based measurements of vBMD. Our confirmation of RYGB-induced bone loss by QCT is important given potential concerns about the accuracy of DXA-based bone density measurements in the setting of substantial weight loss (30). In addition, the persistent elevation in CTX in our subjects is similar in magnitude to a study by Crawford et al. (31), which noted a 137% increase in serum CTX 5 years after RYGB in subjects with type 2 diabetes. Our 5-year bone density results, however, were more modest than observed in a study by Raoof et al. (32) that documented dramatic declines of 19% and 25% in spine and femoral neck aBMD by 5 years after surgery. The latter study did not administer calcium and vitamin D, and most of the subjects developed secondary hyperparathyroidism, which may have magnified the negative skeletal effects of RYGB. In our subjects, all of whom received calcium and vitamin D supplementation, PTH levels only increased modestly at 3.5 and 5 years, although mean PTH levels remained within the normal range.
Decreases in peripheral vBMD, cortical thickness, and failure load, as well as increases in cortical porosity in the first 2 years after RYGB, have been reported in several studies (18–20, 22), but no information is available regarding microarchitectural changes beyond 2 years. Our data demonstrate that whereas declines in BMD in the axial skeleton slowed considerably 2 years after RYGB, deterioration of the peripheral skeleton was largely unabated throughout the 5-year study period. The mechanism(s) responsible for the long-term differences in the patterns of bone loss after RYGB in the axial and peripheral skeleton is unknown. It is possible that late increases in PTH may preferentially aggravate peripheral bone loss, although we did not find significant associations between 5-year changes in PTH and peripheral vBMD or CTX in our exploratory analyses. Although peripheral sites may be less susceptible to imaging artifact from weight loss than axial sites (30), weight is stable after the first postoperative year, and therefore it is unlikely that artifact can explain discordant bone loss in later years. In addition to densitometric declines, we also found that RYGB affects both the trabecular and cortical microarchitecture at the non-weight–bearing radius. The tibia, however, was less affected by microarchitectural changes, which we hypothesize may be due to mitigation from weight-bearing and exercise. Nevertheless, cumulative declines in cortical and trabecular bone led to significant deficits in estimated bone strength via microfinite element modeling at both the radius and tibia. These 5-year findings greatly extend prior work showing short-term deleterious changes in peripheral vBMD and bone microarchitecture after RYGB.
The underlying mechanisms of RYGB-induced bone loss remain unclear but are likely multifactorial. Although we did not find a correlation between bone loss and weight loss, our exploratory analyses did suggest an association between lean mass loss and declines in spine and possibly hip bone density. We had not observed this association in our earlier studies of shorter duration (22, 30), but a similar connection has been reported in other studies (9, 12, 19–21, 33). Furthermore, published studies have demonstrated that strategies to promote maintenance of lean mass during weight loss can partially mitigate bone loss at the spine and hip after RYGB (34, 35) or nonsurgical weight loss (36), lending further credence to the idea that direct effects of skeletal muscle on static and dynamic loading may have beneficial effects on bone. We also found a modest correlation between improvement in insulin levels and decline in spine bone density, which is consistent with a prior study that found that improvements in hemoglobin A1c were correlated with increases in CTX after RYGB (37). Insulin signaling within the osteoblast promotes osteoblast differentiation (38), and hyperinsulinism is associated with higher bone mass, independent of BMI (39, 40). However, insulin is not known to have direct osteoclastic effects, and therefore it is unclear whether lowering insulin after RYGB is directly responsible for the observed high-turnover bone loss or whether the same factors that mediate metabolic improvements after RYGB may also be implicated in skeletal pathways of bone loss.
Other factors have been postulated to contribute to bone loss after RYGB. A recently published study found that postmenopausal women had greater rates of bone loss at 1 year compared with younger women or with men (18). In our smaller study, there was a suggestion that postmenopausal women had numerically greater declines in bone density and bone microarchitecture, but we were not powered to detect statistical differences by menopause status. As noted earlier, we detected minor increases in PTH in later years after RYGB that were negatively associated with bone loss at the trabecular spine and coincided with waning adherence with calcium supplements despite one-on-one nutritional counseling. In light of prior studies documenting decreased calcium absorption after RYGB (41, 42), it is possible that PTH elevations contribute to bone loss in later years. As expected, we found increases in fasting PYY after RYGB, a neurohormonal gut peptide that is thought to have direct catabolic effects on bone (43). In contrast to our prior work, which noted associations between fasting PYY and CTX after RYGB (37), we did not find any correlations between fasting PYY and bone density, bone markers, or bone microarchitecture in the current study. Future studies using either animal models or larger clinical cohorts will be required to more precisely determine the various pathways that underlie skeletal deterioration after RYGB.
Persistent long-term declines in bone density have important implications in the clinical care of RYGB patients. Multiple studies have shown that fracture risk increases after bariatric surgery (6, 44) and more specifically after RYGB (5, 7, 45). Importantly, this increased risk appears to be delayed, with fracture risk becoming more apparent several years after surgery (7, 46). Our study raises the possibility that this long-term risk is mediated by the cumulative effects of persistent high-turnover bone loss and highlights the importance of current bariatric guidelines that recommend selected preoperative and all postoperative RYGB patients undergo bone density screening (47). It is conceivable that treatment to prevent RYGB-induced accelerated bone turnover could mitigate this increased fracture risk, but bariatric surgery patients may be at increased risk for hypocalcemia with potent antiresorptive medications, and therefore additional studies are required to determine whether these medications are safe and effective to minimize RYGB-induced bone loss. Furthermore, encouragement of exercise to maintain lean mass and adherence to calcium and vitamin D supplementation must be emphasized as lifelong requirements after RYGB.
This study has certain limitations. We were unable to retain a nonsurgical control group for this lengthy study. Although our sample size was relatively small, this study is nevertheless the first, to our knowledge, to provide comprehensive skeletal characterization and long-term follow-up of RYGB patients over 5 years. Unfortunately, we were only able to obtain HR-pQCT measurements on a subset of the subjects, but this subset appeared to be representative of the whole cohort. Although we did examine potential mediators of bone loss, these associations should be viewed as preliminary in light of our small sample size and the borderline significance of some of these findings. Ultimately, the mechanism of RYGB-induced bone loss needs to be further elucidated in future studies that also examine other pathways within the entero-osseus axis.
In summary, we found that areal and volumetric bone density and skeletal microarchitecture continue to deteriorate through 5 years after RYGB surgery, leading to substantial cumulative bone loss. Adults undergoing RYGB surgery warrant close follow-up to detect changes in bone density as well as to prevent secondary hyperparathyroidism and promote physical activity. Further studies are needed to determine the mechanisms of bone loss, as well as to investigate therapeutic strategies to preserve skeletal health in patients receiving RYGB.
Acknowledgments
We thank the MGH Bone Density Center for DXA measurements, the MGH XtremeCT Core for HRpQCT measurements, the MGH Musculoskeletal Imaging Research Core for QCT measurements, the Brigham Research Assay Core for batch laboratory testing, and the nursing and dietary staff of the MGH Translational and Clinical Research Center for their dedicated care of the study participants.
Financial Support: This work was supported by the Clinical Scientist Development Award from the Doris Duke Charitable Foundation (to E.W.Y.), as well as National Institutes of Health Grants K23 DK093713-01 (to E.W.Y.), R03DK107869 (to E.W.Y.), P30 DK040561 (to E.W.Y.), S10 RR023405 (to M.L.B.), 8 UL1 TR000170, 1UL1TR001102, and 1 UL1 RR025758.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- aBMD
areal bone mineral density
- BMD
bone mineral density
- BMI
body mass index
- CTX
collagen C–terminal telopeptide
- CV
coefficient of variation
- DXA
dual-energy X-ray absorptiometry
- HR-pQCT
high-resolution peripheral quantitative CT
- P1NP
procollagen type I N-terminal propeptide
- PA
posterior-anterior
- PYY
peptide YY
- QCT
quantitative CT
- RYGB
Roux-en-Y gastric bypass
- vBMD
volumetric bone mineral density
References
- 1. Adams TD, Gress RE, Smith SC, Halverson RC, Simper SC, Rosamond WD, Lamonte MJ, Stroup AM, Hunt SC. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357(8):753–761. [DOI] [PubMed] [Google Scholar]
- 2. Arterburn DE, Olsen MK, Smith VA, Livingston EH, Van Scoyoc L, Yancy WS Jr, Eid G, Weidenbacher H, Maciejewski ML. Association between bariatric surgery and long-term survival. JAMA. 2015;313(1):62–70. [DOI] [PubMed] [Google Scholar]
- 3. Sjöström L, Narbro K, Sjöström CD, Karason K, Larsson B, Wedel H, Lystig T, Sullivan M, Bouchard C, Carlsson B, Bengtsson C, Dahlgren S, Gummesson A, Jacobson P, Karlsson J, Lindroos AK, Lönroth H, Näslund I, Olbers T, Stenlöf K, Torgerson J, Agren G, Carlsson LM; Swedish Obese Subjects Study . Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357(8):741–752. [DOI] [PubMed] [Google Scholar]
- 4. Angrisani L, Santonicola A, Iovino P, Vitiello A, Zundel N, Buchwald H, Scopinaro N.. Bariatric surgery and endoluminal procedures: IFSO Worldwide Survey 2014. Obes Surg. 2017;27(9):2279–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nakamura KM, Haglind EGC, Clowes JA, Achenbach SJ, Atkinson EJ, Melton LJ III, Kennel KA. Fracture risk following bariatric surgery: a population-based study. Osteoporos Int. 2013;25(1):151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rousseau C, Jean S, Gamache P, Lebel S, Mac-Way F, Biertho L, Michou L, Gagnon C. Change in fracture risk and fracture pattern after bariatric surgery: nested case-control study. BMJ. 2016;354:i3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yu EW, Lee MP, Landon JE, Lindeman KG, Kim SC. Fracture risk after bariatric surgery: Roux-en-Y gastric bypass versus adjustable gastric banding. J Bone Miner Res. 2017;32(6):1229–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carlin AM, Rao DS, Yager KM, Parikh NJ, Kapke A. Treatment of vitamin D depletion after Roux-en-Y gastric bypass: a randomized prospective clinical trial. Surg Obes Relat Dis. 2009;5(4):444–449. [DOI] [PubMed] [Google Scholar]
- 9. Carrasco F, Ruz M, Rojas P, Csendes A, Rebolledo A, Codoceo J, Inostroza J, Basfi-Fer K, Papapietro K, Rojas J, Pizarro F, Olivares M. Changes in bone mineral density, body composition and adiponectin levels in morbidly obese patients after bariatric surgery. Obes Surg. 2008;19(1):41–46. [DOI] [PubMed] [Google Scholar]
- 10. Casagrande DS, Repetto G, Mottin CC, Shah J, Pietrobon R, Worni M, Schaan BD. Changes in bone mineral density in women following 1-year gastric bypass surgery. Obes Surg. 2012;22(8):1287–1292. [DOI] [PubMed] [Google Scholar]
- 11. Coates PS, Fernstrom JD, Fernstrom MH, Schauer PR, Greenspan SL. Gastric bypass surgery for morbid obesity leads to an increase in bone turnover and a decrease in bone mass. J Clin Endocrinol Metab. 2004;89(3):1061–1065. [DOI] [PubMed] [Google Scholar]
- 12. Fleischer J, Stein EM, Bessler M, Della Badia M, Restuccia N, Olivero-Rivera L, McMahon DJ, Silverberg SJ. The decline in hip bone density after gastric bypass surgery is associated with extent of weight loss. J Clin Endocrinol Metab. 2008;93(10):3735–3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Johnson JM, Maher JW, Samuel I, Heitshusen D, Doherty C, Downs RW. Effects of gastric bypass procedures on bone mineral density, calcium, parathyroid hormone, and vitamin D. J Gastrointest Surg. 2005;9(8):1106–1111, discussion 1110–1111. [DOI] [PubMed] [Google Scholar]
- 14. Kaulfers A-MD, Bean JA, Inge TH, Dolan LM, Kalkwarf HJ. Bone loss in adolescents after bariatric surgery. Pediatrics. 2011;127(4):e956–e961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mahdy T, Atia S, Farid M, Adulatif A. Effect of Roux-en Y gastric bypass on bone metabolism in patients with morbid obesity: Mansoura experiences. Obes Surg. 2008;18(12):1526–1531. [DOI] [PubMed] [Google Scholar]
- 16. Nogués X, Goday A, Peña MJ, Benaiges D, de Ramón M, Crous X, Vial M, Pera M, Grande L, Díez-Pérez A, Ramón JM. Bone mass loss after sleeve gastrectomy: a prospective comparative study with gastric bypass. Cir Esp. 2010;88(2):103–109. [DOI] [PubMed] [Google Scholar]
- 17. Olbers T, Björkman S, Lindroos A, Maleckas A, Lönn L, Sjöström L, Lönroth H. Body composition, dietary intake, and energy expenditure after laparoscopic Roux-en-Y gastric bypass and laparoscopic vertical banded gastroplasty: a randomized clinical trial. Ann Surg. 2006;244(5):715–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schafer AL, Kazakia GJ, Vittinghoff E, Stewart L, Rogers SJ, Kim TY, Carter JT, Posselt AM, Pasco C, Shoback DM, Black DM. Effects of gastric bypass surgery on bone mass and microarchitecture occur early and particularly impact postmenopausal women. J Bone Miner Res. 2018;33(6):975–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shanbhogue VV, Støving RK, Frederiksen KH, Hanson S, Brixen K, Gram J, Jørgensen NR, Hansen S. Bone structural changes after gastric bypass surgery evaluated by HR-pQCT: a two-year longitudinal study. Eur J Endocrinol. 2017;176(6):685–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stein EM, Carrelli A, Young P, Bucovsky M, Zhang C, Schrope B, Bessler M, Zhou B, Wang J, Guo XE, McMahon DJ, Silverberg SJ. Bariatric surgery results in cortical bone loss. J Clin Endocrinol Metab. 2013;98(2):541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vilarrasa N, San José P, García I, Gómez-Vaquero C, Miras PM, de Gordejuela AG, Masdevall C, Pujol J, Soler J, Gómez JM. Evaluation of bone mineral density loss in morbidly obese women after gastric bypass: 3-year follow-up. Obes Surg. 2010;21(4):465–472. [DOI] [PubMed] [Google Scholar]
- 22. Yu EW, Bouxsein ML, Putman MS, Monis EL, Roy AE, Pratt JS, Butsch WS, Finkelstein JS. Two-year changes in bone density after Roux-en-Y gastric bypass surgery. J Clin Endocrinol Metab. 2015;100(4):1452–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yu EW, Bouxsein ML, Roy AE, Baldwin C, Cange A, Neer RM, Kaplan LM, Finkelstein JS. Bone loss after bariatric surgery: discordant results between DXA and QCT bone density. J Bone Miner Res. 2014;29(3):542–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gagnon C, Schafer AL. Bone health after bariatric surgery. JBMR Plus. 2018;2(3):121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brzozowska MM, Sainsbury A, Eisman JA, Baldock PA, Center JR. Bariatric surgery, bone loss, obesity and possible mechanisms. Obes Rev. 2012;14(1):52–67. [DOI] [PubMed] [Google Scholar]
- 26. Cann CE, Adams JE, Brown JK, Brett AD. CTXA hip—an extension of classical DXA measurements using quantitative CT. PLoS One. 2014;9(3):e91904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Burghardt AJ, Buie HR, Laib A, Majumdar S, Boyd SK. Reproducibility of direct quantitative measures of cortical bone microarchitecture of the distal radius and tibia by HR-pQCT. Bone. 2010;47(3):519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Boutroy S, Van Rietbergen B, Sornay-Rendu E, Munoz F, Bouxsein ML, Delmas PD. Finite element analysis based on in vivo HR-pQCT images of the distal radius is associated with wrist fracture in postmenopausal women. J Bone Miner Res. 2007;23(3):392–399. [DOI] [PubMed] [Google Scholar]
- 29. Kriska AM, Knowler WC, LaPorte RE, Drash AL, Wing RR, Blair SN, Bennett PH, Kuller LH. Development of questionnaire to examine relationship of physical activity and diabetes in Pima Indians. Diabetes Care. 1990;13(4):401–411. [DOI] [PubMed] [Google Scholar]
- 30. Yu EW, Thomas BJ, Brown JK, Finkelstein JS. Simulated increases in body fat and errors in bone mineral density measurements by DXA and QCT. J Bone Miner Res. 2011;27(1):119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Crawford MR, Pham N, Khan L, Bena JF, Schauer PR, Kashyap SR. Increased bone turnover in type 2 diabetes patients randomized to bariatric surgery vs. medical therapy at 5 years. Endocr Pract. 2018;24(3):256–264. [DOI] [PubMed] [Google Scholar]
- 32. Raoof M, Näslund I, Rask E, Szabo E. Effect of gastric bypass on bone mineral density, parathyroid hormone and vitamin D: 5 years follow-up. Obes Surg. 2016;26(5):1141–1145. [DOI] [PubMed] [Google Scholar]
- 33. Brzozowska MM, Sainsbury A, Eisman JA, Baldock PA, Center JR. Bariatric surgery and bone loss: do we need to be concerned? Clin Rev Bone Miner Metab. 2014;12(4):207–227. [Google Scholar]
- 34. Campanha-Versiani L, Pereira DAG, Ribeiro-Samora GA, Ramos AV, de Sander Diniz MFH, De Marco LA, Soares MMS. The effect of a muscle weight-bearing and aerobic exercise program on the body composition, muscular strength, biochemical markers, and bone mass of obese patients who have undergone gastric bypass surgery. Obes Surg. 2017;27(8):2129–2137. [DOI] [PubMed] [Google Scholar]
- 35. Muschitz C, Kocijan R, Haschka J, Zendeli A, Pirker T, Geiger C, Müller A, Tschinder B, Kocijan A, Marterer C, Nia A, Muschitz GK, Resch H, Pietschmann P. The impact of vitamin D, calcium, protein supplementation, and physical exercise on bone metabolism after bariatric surgery: the BABS study. J Bone Miner Res. 2015;31(3):672–682. [DOI] [PubMed] [Google Scholar]
- 36. Villareal DT, Aguirre L, Gurney AB, Waters DL, Sinacore DR, Colombo E, Armamento-Villareal R, Qualls C. Aerobic or resistance exercise, or both, in dieting obese older adults. N Engl J Med. 2017;376(20):1943–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yu EW, Wewalka M, Ding S-A, Simonson DC, Foster K, Holst JJ, Vernon A, Goldfine AB, Halperin F. Effects of gastric bypass and gastric banding on bone remodeling in obese patients with type 2 diabetes. J Clin Endocrinol Metab. 2016;101(2):714–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fulzele K, Riddle RC, DiGirolamo DJ, Cao X, Wan C, Chen D, Faugere MC, Aja S, Hussain MA, Brüning JC, Clemens TL. Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell. 2010;142(2):309–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Haffner SM, Bauer RL. The association of obesity and glucose and insulin concentrations with bone density in premenopausal and postmenopausal women. Metabolism. 1993;42(6):735–738. [DOI] [PubMed] [Google Scholar]
- 40. Shanbhogue VV, Finkelstein JS, Bouxsein ML, Yu EW. Association between insulin resistance and bone structure in nondiabetic postmenopausal women. J Clin Endocrinol Metab. 2016;101(8):3114–3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Riedt CS, Brolin RE, Sherrell RM, Field MP, Shapses SA True fractional calcium absorption is decreased after Roux-en-Y gastric bypass surgery. Obesity (Silver Spring) 2006;14(11):1940–1948. [DOI] [PMC free article] [PubMed]
- 42. Schafer AL, Weaver CM, Black DM, Wheeler AL, Chang H, Szefc GV, Stewart L, Rogers SJ, Carter JT, Posselt AM, Shoback DM, Sellmeyer DE. Intestinal calcium absorption decreases dramatically after gastric bypass surgery despite optimization of vitamin D status. J Bone Miner Res. 2015;30(8):1377–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wong IPL, Driessler F, Khor EC, Shi YC, Hörmer B, Nguyen AD, Enriquez RF, Eisman JA, Sainsbury A, Herzog H, Baldock PA. Peptide YY regulates bone remodeling in mice: a link between gut and skeletal biology. PLoS One. 2012;7(7):e40038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lu C-W, Chang Y-K, Chang H-H, Kuo CS, Huang CT, Hsu CC, Huang KC. Fracture risk after bariatric surgery: a 12-year nationwide cohort study. Medicine (Baltimore). 2015;94(48):e2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fashandi AZ, Mehaffey JH, Hawkins RB, Schirmer B, Hallowell PT. Bariatric surgery increases risk of bone fracture. Surg Endosc. 2018;32(6):2650–2655. [DOI] [PubMed] [Google Scholar]
- 46. Lalmohamed A, de Vries F, Bazelier MT, Cooper A, van Staa TP, Cooper C, Harvey NC. Risk of fracture after bariatric surgery in the United Kingdom: population based, retrospective cohort study. BMJ. 2012;345:e5085–e5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mechanick JI, Youdim A, Jones DB, Timothy Garvey W, Hurley DL, Molly McMahon M, Heinberg LJ, Kushner R, Adams TD, Shikora S, Dixon JB, Brethauer S. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient—2013 update: cosponsored by American Association of Clinical Endocrinologists, the Obesity Society, and American Society for Metabolic & Bariatric Surgery. Surg Obes Relat Dis. 2013;9(2):159–191. [DOI] [PubMed] [Google Scholar]