Abstract
Context
Most cases of autosomal dominant isolated hypoparathyroidism are caused by gain-of-function mutations in CASR or GNA11 or dominant negative mutations in GCM2 or PTH.
Objective
To identify the genetic etiology for dominantly transmitted isolated hypoparathyroidism in two multigenerational families with 14 affected family members.
Methods
We performed whole exome sequencing of DNA from two families and examined the consequences of mutations by minigene splicing assay.
Results
We discovered disease-causing mutations in both families. A splice-altering mutation in TBX1 (c.1009+1G>C) leading to skipping of exon 8 (101 bp) was identified in 10 affected family members and five unaffected subjects of family A, indicating reduced penetrance for this point mutation. In a second family from France (family B), we identified another splice-altering mutation (c.1009+2T>C) adjacent to the mutation identified in family A that results in skipping of the same exon; two subjects in family B had isolated hypoparathyroidism, whereas a third subject manifested the clinical triad of the 22q11.2 deletion syndrome, indicative of variable expressivity.
Conclusions
We report evidence that heterozygous TBX1 mutations can cause isolated hypoparathyroidism. This study adds knowledge to the increasingly expanding list of causative and candidate genes in isolated hypoparathyroidism.
Heterozygous donor splice site mutations in TBX1 located in the 22q11.2 region can cause autosomal dominant isolated hypoparathyroidism as well as 22q11.2 deletion syndrome.
Hypoparathyroidism is characterized by hypocalcemia and hyperphosphatemia, both of which result from the absence or insufficient secretion of biologically active PTH. Injury or removal of parathyroid glands during neck surgery is the most common cause of hypoparathyroidism, but hypoparathyroidism can also result from autoimmune or genetic defects that impair synthesis or secretion of PTH or prevent normal development or maintenance of adequate parathyroid tissue (1, 2). Hypoparathyroidism can also occur as a component of a complex developmental, metabolic, or endocrine syndrome (1), such as autoimmune polyglandular syndrome type 1 due to mutations of AIRE (3), DiGeorge syndrome due to chromosomal microdeletions or small mutations that inactivate TBX1 (4), Kenny-Caffey syndrome due to mutations of TBCE and FAM111A (5, 6), CHARGE syndrome due to mutations in CHD7 (7), and hypoparathyroidism-deafness-renal dysplasia syndrome due to GATA3 mutations (8). By contrast, most cases of isolated or nonsyndromic hypoparathyroidism involve pathogenic gain-of-function mutations in CASR (9) or GNA11 (10–12) and dominant negative mutations in PTH (13–15) or GCM2 (16).
Using whole exome sequencing (WES), we recently identified AIRE mutations in patients with isolated hypoparathyroidism, highlighting the challenges of diagnosing complex disorders when a phenotype is restricted or partial (17). Here, we extend this work with the application of WES to identify splice-altering mutations in TBX1 in affected members of two extended families characterized by autosomal dominant isolated hypoparathyroidism. Subsequent Sanger sequencing revealed heterozygous splice-altering mutations in TBX1 in 13 affected subjects, including one with additional cardiac features, plus one obligate carrier and four unaffected individuals. Our analyses indicate that TBX1 mutations can have incomplete penetrance and variable expressivity, and provide evidence that TBX1 splice-altering mutations are the bases for hypoparathyroidism in patients with the common 22q11 deletion syndrome.
Subjects and Methods
Patients
Family A
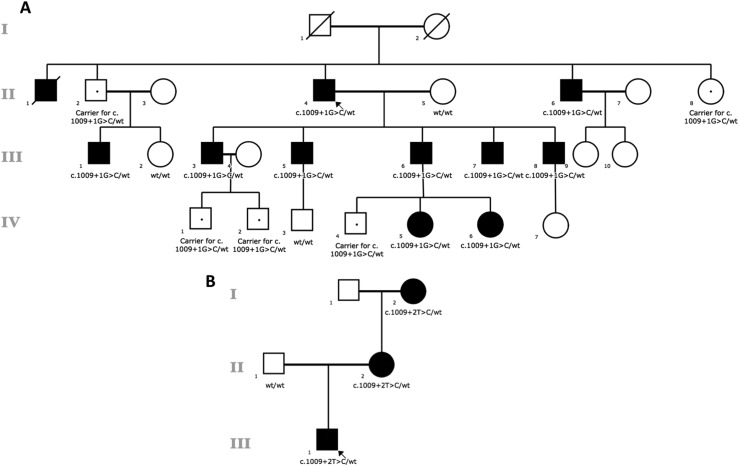
The proband, now aged 69 years, had begun to experience muscle cramping and tetany at age 8 years and was subsequently diagnosed with pseudoidiopathic hypoparathyroidism at age 20 years on the basis of severe hypocalcemia (6.9 mg/dL) with normal serum levels of magnesium and hyperphosphatemia (6.5 mg/dL), serum levels of PTH that were normal or elevated when measured using several assay formats, and a normal urinary cyclic AMP response to exogenous PTH (18). He had bilateral subcapsular cataracts. Over the next several years, his serum calcium level ranged from 5.3 to 6.9 mg/dL and serum inorganic phosphorus level ranged from 6.3 to 7.7 mg/dL when therapy with calcitriol and calcium was intermittently discontinued. He was reassessed at age 30 years and diagnosed with idiopathic hypoparathyroidism when he and one affected son were evaluated using radioimmunoassays that were specific for midmolecule or carboxyterminal PTH epitopes and was found to have markedly low serum levels of PTH when hypocalcemic (13). All five of his sons developed hypocalcemic symptoms and biochemical evidence of hypoparathyroidism, with low or inappropriately normal levels of intact PTH within the first two decades of life (Fig. 1A). Over the years, additional members of the proband’s extended family were evaluated and found to have isolated hypoparathyroidism as well (Fig. 1A). Affected members of this kindred have been treated with conventional therapy as calcium and calcitriol as well as with PTH 1-34 and recombinant human parathyroid hormone 1-84. There was no history of renal stones in any affected subjects. The youngest members of the kindred who carry the mutation have been tested annually and have not yet developed hypoparathyroidism.
Figure 1.
Pedigrees of the studied families and cosegregating pattern of the TBX1 mutations. The pedigree is drawn using open circles to denote unaffected female subjects and the open squares to denote unaffected male subjects; the solid figures indicate affected subjects. The Roman numerals denote generation. TBX1 genotypes are noted beneath the symbol for each subject from whom DNA was available for testing, and the dots indicate asymptomatic carriers.
Family B
The proband, a male, is the second child of nonconsanguineous parents originating from France. Echotomography at 20 weeks’ gestation identified a persistent truncus arteriosus. Amniocentesis was performed, and Agilent 60k CGH showed no copy number variation. He was term born with normal birth parameters. He subsequently developed hypocalcemia due to hypoparathyroidism and was treated with calcium and activated vitamin D. The cardiac defect was surgically repaired at 1 month of age, at which time no thymic tissue was observed. T-cell immunodeficiency remained asymptomatic with prophylactic antibiotherapy. Asymptomatic hypocalcemia had been diagnosed serendipitously in the proband’s mother and maternal grandmother during adulthood. Retrospectively, the grandmother had had chronic otitis media in childhood and paresthesia of the extremities and the peribuccal area from adolescence. They had neither cardiac defect nor lymphopenia.
These studies were approved by the institutional review boards of the participating hospitals, and all subjects provided written informed consent/assent for participation in the study.
Exome sequencing and bioinformatic analysis
We isolated genomic DNA from members of family A using samples of whole blood or saliva obtained from five family members in the second generation, seven in the third generation, and six in the fourth generation (Fig. 1A). In 2012 to 2013, the exome was captured for four affected subjects (II-4, III-5, III-6, and III-7) using the Agilent SureSelect Human All Exon version 3 kit (Agilent Technologies, Santa Clara, CA), guided by the manufacturer’s protocols. As a result of negative findings after the initial analysis of the four sequenced individuals, one more affected subject (III-3) was included in the study (Fig. 1A). His exome was captured in 2014 with the Agilent SureSelect Human All Exon version 5 kit (Agilent Technologies), guided by the manufacturer’s protocols. Sequencing reads passing the quality filter were aligned to the human reference genome (hg19) with Burrows-Wheeler Aligner (v.0.6.2; BWA) (19). PCR duplicates were removed using Picard (v.1.97). The Genome Analysis Toolkit (v.2.6-5; GATK) was used to generate variant calls (20). All variants were then annotated by ANNOVAR (21) and SnpEff (v.2.0.5) (22) to retrieve amino acid changes, protein functional effect, conservation score, minor allele frequency, and output from prediction programs (SIFT, Polyphen-2, LRT, and MutationTaster). We examined missense, nonsense, splice-altering, and coding indels fitting a dominant mode of inheritance in the exome data as suggested by the family pedigree. Results were filtered to exclude synonymous variants, variants with minor allele frequency >0.5% in public databases (i.e., 1000 Genomes Project and NHLBI ESP6500SI), and variants previously identified in controls in our in-house exome variant database (17, 23). Subsequent gene prioritization was based on deleterious variant predication and biological relevance according to the Online Mendelian Inheritance in Man database. Validation of candidate variants passing the above filters was performed by standard Sanger sequencing.
WES was performed following protocols similar to those previously published for family B (24). Briefly, libraries were prepared from 3 μg of sheared genomic DNA. Exome capture was performed with the 51 Mb Agilent SureSelect Human All Exon kit V5 (Agilent Technologies) followed by sequencing on a HiSeq 2500 (Illumina, San Diego, CA), generating 2 × 100 bp paired-end reads. Sequences were mapped to the reference human genome (GRCh37/hg19) using the Burrows-Wheeler Aligner and variant calling was performed using the GATK UnifiedGenotyper. Variants were filtered against public SNP databases (dbSNP, 1000 genomes, EVS, ExAC, and gnomAD) and >11,000 in-house exomes.
Synthesis of hybrid minigenes
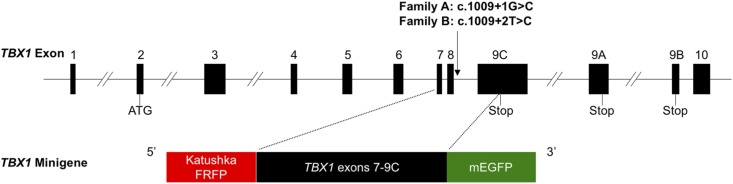
Because we were unable to detect TBX1 transcripts in transformed lymphoblastoid cells from the patients by RT-PCR, we synthesized hybrid TBX1 minigenes to determine the effects of the two mutations on pre-mRNA splicing efficiency. We created wild-type and mutant TBX1 minigenes in a specialized reporter vector (generous gift of Dr. Konstantin A. Lukyanov, Moscow, Russia) designed to quantitatively analyze the alternative splicing of a target cassette that has been subcloned between Katushka far-red mutant of red fluorescent protein (TurboFP635; FRFP) and enhanced green fluorescent protein (EGFP)‒encoding sequences (25). Briefly, the TBX1 sequences of interest (Fig. 2) were subcloned so that translation of the normally spliced TBX1 transcript resulted in expression of both red fluorescent protein and green fluorescent protein in a form of FRFP-TBX1-EGFP fusion protein, whereas alternative exon splicing resulted in the synthesis of red fluorescent protein only. We used PCR to amplify and assemble exons 7 to 9C plus intronic regions of TBX1 from genomic DNA from normal humans and affected patients and subcloned the cassettes with an order of FRFP, TBX1 exons 7 to 9C, and EGFP. All the sequences were confirmed by direct Sanger sequencing.
Figure 2.
Schematic representation of TBX1 minigene. Exons 7 to 9C plus intronic regions of TBX1 were amplified from genomic DNA from a healthy subject and affected patients, and the cassettes were subcloned in the following order (5′ to 3′): TurboFP635 (FRFP), TBX1 exons 7 to 9C, and monomeric enhanced green fluorescent protein (mEGFP). ATG denotes the start codon of TBX1 gene.
Characterization of TBX1 expression
Wild-type or mutant TBX1 minigenes were transiently transfected into HEK293T cells using FuGENE 6 Transfection Reagent (Roche Applied Science, Indianapolis, IN) according to the manufacturer’s recommendation. Approximately 48 hours after transfection, we harvested cells (5 million cells per sample) for extraction of total RNA using TRIzol reagent (Thermo Fisher, Waltham, MA) and purified with the RNeasy Mini kit (Qiagen Inc., Valencia, CA). We determined RNA concentrations using a NanoDrop ND-1000 spectrophotometer (Thermo Scientific, Wilmington, DE), and RNA integrity was verified by agarose gel electrophoresis. For each sample, 500 ng of total RNA was used to generate cDNA with a High-Capacity cDNA Reverse Transcription Kit (#4368814; Applied Biosystems) using random primers. After first-strand cDNA synthesis, the reaction was diluted 1:10 for RT-PCR.
We performed confocal fluorescence microscopy using an Olympus imaging system equipped with an HQ CCD camera and a 120W HXP short arc lamp (Osram). Green and red fluorescent images were acquired using 10× objective and standard filter sets. We also quantified FRFP and EGFP expression by flow cytometry with a FACSCalibur (BD Biosciences, San Jose, CA) cytometer equipped with blue and red lasers in the Flow Cytometry Core Laboratory at The Children’s Hospital of the Philadelphia Research Institute. Control cells and cells transfected with empty vector or TBX1 minigenes were dissociated using trypsin 0.25%, washed, and resuspended in PBS containing 0.2% bovine serum albumin. EGFP was detected in the FL-1 channel (excitation 488 nm; emission 530/30-nm bandpass filter), and Katushka FRFP was detected in the FL-3 channel (excitation 488 nm; emission 670-nm longpass filter). At least 10,000 events were recorded for each sample. FlowJo v10 (BD Biosciences, San Jose, CA) was used for data analysis. The main population of cells was gated on a forward scatter‒side scatter plot. Quadrant gates were defined according to the signal recorded in control cells to determine the percentage of EGFP-positive, Katushka-positive, and double-positive cells in each sample.
Statistics
The data presented represent the results of six separate experiments. All results are presented as the mean ± SD, and comparisons between three groups were analyzed by ANOVA using Tukey-Kramer multiple comparison tests using GraphPad InStat.
Results
Genetic analyses
We sequenced the coding exons and intron-exon boundaries of the candidate genes CASR, PTH, GCM2, and GNA11, which have been associated with isolated hypoparathyroidism, but failed to identify any disease-causing mutations in family A. Therefore, we performed WES on DNA from subjects II-4, III-5, III-6, and III-7 (Fig. 1A); analysis of the data under a dominant mode of inheritance was unrevealing as well. When we sequenced another affected subject (III-3) with an improved version of the exome capture kit, we observed a potential splice-altering mutation, c.1009+1G>C in intron 8 of TBX1 (GenBank: NM_080647.1), with five reads covering the alternative allele and nine reads on the wild-type allele. This led us to examine the previous WES data on II-4, III-5, III-6, and III-7, which revealed that these libraries had very limited coverage for this variant (zero to six reads). We therefore performed Sanger sequencing on DNA samples and confirmed that all eight affected subjects in the second and third generations and one obligate carrier (II-2) have the same mutation in TBX1. Interestingly, six subjects, II-8 and five young grandchildren, all carry the mutation (Fig. 1A) but had previously refused to undergo biochemical screening. Subsequent to molecular testing, these subjects underwent biochemical evaluation, and two of these subjects (IV-5 and IV-6) were diagnosed with hypoparathyroidism; reevaluation indicated that these two subjects had experienced past episodes of tingling/cramping and seizures.
We performed WES of the family B proband and his parents (Fig. 1B) and found a second splice-altering mutation, c.1009+2T>C, in TBX1 in the proband and his mother, adjacent to the mutation identified in family A. Subsequent segregation analysis determined that the mutation was inherited from the maternal grandmother who is affected with isolated hypoparathyroidism. Both TBX1 mutations were absent from the 1000 Genomes Project, ESP6500SI, gnomAD, and additional exome-sequence data from >4000 samples (17, 23) that we had previously sequenced in our in-house database.
Functional evaluation of the splice-altering mutations
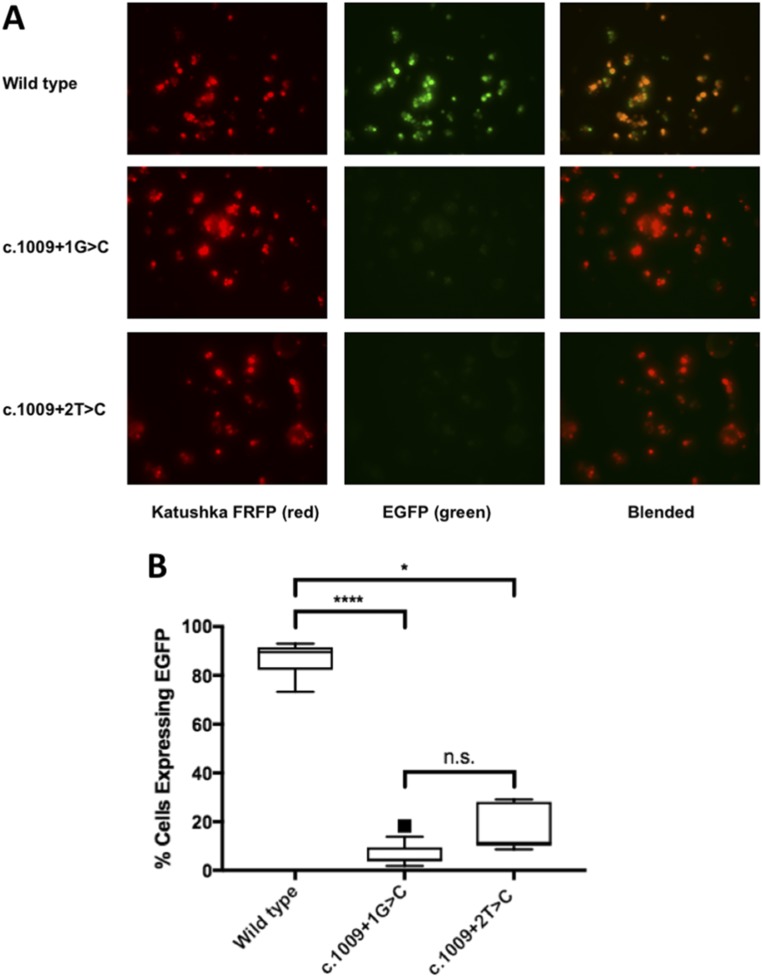
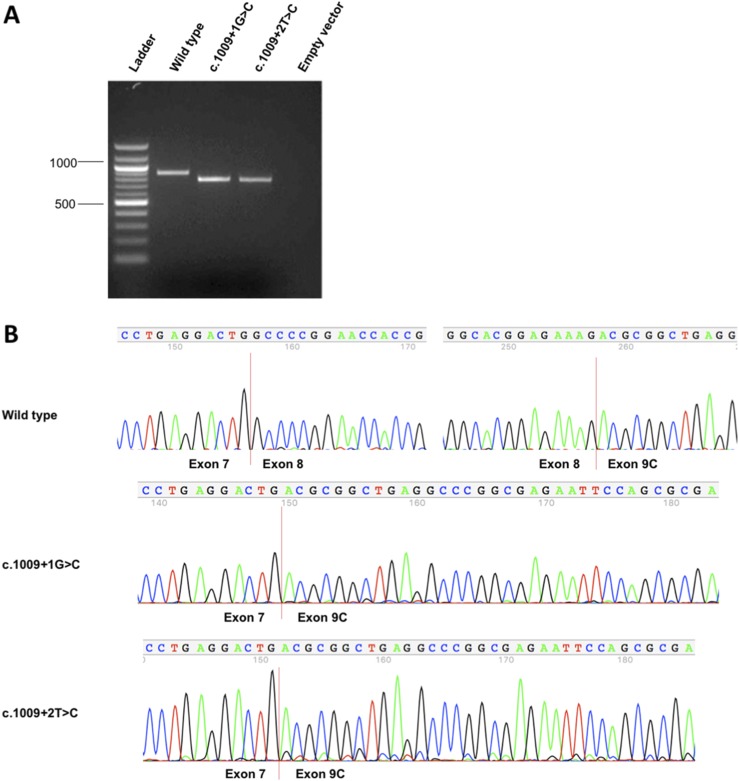
Both mutations alter the canonical splice donor motif of intron 8 affecting TBX1 isoforms A, B, and C that share the first eight exons but differ in the terminal coding exon(s). However, only isoform C is highly conserved between mouse and human transcriptomes and has been shown to be the predominant human transcript (4). Accordingly, we made minigene constructs in which cassettes containing exons 7 to 9C plus appropriate intronic regions of TBX1 were inserted downstream of the Katushka FRFP and upstream of the EGFP (Fig. 2). We anticipated that the wild-type minigene would express both fluorescent proteins because proper splicing of the TBX1 cassette would generate an in-frame transcript, whereas both mutants would express only the Katushka FRFP because aberrant splicing would place the EGFP out of frame. Indeed, we observed expression of both red and green reporters in HEK293T cells that had been transfected with the wild-type construct but very low EGFP expression in both mutants (Fig. 3A). Fluorescent flow cytometry of transfected HEK293T cells confirmed these qualitative results and showed significant quantitative differences in the percentage of transfected cells (i.e., FRFP-expressing cells) that expressed EGFP [median (lower to upper quartile range): 89.5% (85.4% to 90.9%) of wild-type vs 4.5% (3.8% to 7.8%) and 11.4% (10.9% to 23.2%) for c.1009+1G>C and c.1009+2T>C, respectively; P < 0.0001 for wild-type vs c.1009+1G>C, P < 0.05 for wild-type vs c.1009+2T>C, and no significant difference between the two mutants] (Fig. 3B). Interestingly, RT-PCR showed that the TBX1 mutant minigenes produced alternative transcripts different from the wild-type minigene (Fig. 4A). Direct sequencing confirmed that both mutations induced skipping of exon 8 (101 bp) (Fig. 4B).
Figure 3.
Minigene splicing assay confirmed the pathogenicity of the mutations. (A) HEK293T cells were transfected with wild-type or mutant minigene plasmids. The wild-type minigene expressed both fluorescent proteins, whereas both mutants had limited EGFP expression, indicative of aberrant gene splicing. (B) Flow cytometry analysis of transfected HEK293T cells showed significant quantitative differences in the percentage of cells expressing EGFP. Tukey box and whisker plot, where center line represents median, box limits represent interquartile ranges, and whiskers represent minimum to maximum data ranges; black square, outlier; the mean values for the mutants overlap the lower line in the boxes. *P < 0.05; ****P < 0.0001; n.s., not significant.
Figure 4.
RT-PCR and Sanger sequencing confirmed aberrant TBX1 splicing induced by the mutations. (A) Agarose gel electrophoresis shows RT-PCR products produced by TBX1 minigenes. (B) Direct Sanger sequencing confirmed that c.1009+1G>C and c.1009+2T>C both induced skipping of exon 8 (101 bp).
Discussion
We describe here two multigenerational families in which multiple members who had been diagnosed with autosomal dominant isolated hypoparathyroidism were found to have heterozygous TBX1 mutations that alter gene splicing. Moreover, there was no evidence for any other deletions or mutations that affect the TBX1 locus. The two point mutations affect the same canonical splice donor motif of intron 8, c.1009+1G>C and c.1009+2T>C, and lead to the same skipping of exon 8 on the normal splicing mechanism. Both mutations are predicted to result in truncated proteins (p.W303*) that lack the nuclear localization signal and the transactivation domain of TBX1 (26) but retain the T-box DNA-binding domain. Our splicing assays suggest both mutations can escape from nonsense-mediated decay as shown by RT-PCR (Fig. 4), which may produce a stable, truncated protein with altered activity. Therefore, it is conceivable that the mutations that we have identified do not exert their pathogenic effects through simple TBX1 haploinsufficiency; in addition, given that T-box transcription factors bind DNA as dimers, it is possible that these mutations result in a more complex dysregulation of TBX1-dependent gene transcription and lead to the limited phenotype of isolated hypoparathyroidism. Moreover, the very limited phenotype of isolated hypoparathyroidism in most affected subjects suggests that the effect(s) of the truncated TBX1 protein may be greatest in parathyroid cells, although the additional DiGeorge sequencelike features in the proband in family B suggest that cardiac development in this one subject may also be affected. Our results thereby confirm the pathogenic roles of both TBX1 point mutations and demonstrate that heterozygous TBX1 splice-altering mutations can cause isolated hypoparathyroidism as well as the syndromic DiGeorge phenotype.
TBX1 is located in the low-copy repeats on chromosome 22 (LCR22) between LCR22A and LCR22B, a region that contains some 30 to 40 genes and comprises the most common contiguous gene microdeletion in humans, with a frequency of ∼1:4000 live births (27). Deletions in this region are the basis for 22q11.2 deletion syndrome, a well-known disorder that is associated with a variety of clinical features that are alternatively associated with DiGeorge sequence (MIM 188400) and Shprintzen (velocardiofacial) syndrome (MIM 192430) (27, 28) and include cardiac outflow tract anomalies, hypoplasia of the thymus and parathyroid glands, cleft palate, and facial dysmorphogenesis that has been attributed to abnormal development of the pharyngeal arches and pouches. The majority of affected patients carry a chromosome 22 with a deletion of either 3 or 1.5 Mb, both of which include the TBX1 gene. Despite harboring of a similar genetic defect, there is considerable variability among the phenotypic features present in subjects with 22q11.2 deletion syndrome, and intrafamilial and interfamilial variabilities, even among monozygotic twins carrying identical deletions, are common (27, 29, 30). The most recent review of 22q11.2 deletion syndrome (27) indicated that ∼75% of pediatric patients have immunodeficiency, 50% to 65% have hypoparathyroidism, and ∼75% have congenital cardiac anomalies, which comprise the conventional clinical triad. Other complications involved include palatal defects in ∼75% of pediatric patients, feeding difficulties in ∼30% of pediatric patients, and renal anomalies in ∼31% of patients.
The 22q11 deletion region contains some 30 to 40 genes, but loss of TBX1 has been proposed as being responsible for most of the syndrome’s characteristic signs (such as heart defects, a cleft palate, distinctive facial features, hearing loss, and hypoparathyroidism). TBX1 encodes a dosage-sensitive transcription factor that regulates morphogenesis and organogenesis at an early stage during embryo development (31–33), and both loss-of-function and gain-of-function missense mutations have been identified in patients with overlapping but distinct 22q11.2‒like syndromes (34, 35). Moreover, the heterozygous frameshift mutation c.1253delA (p.Y418fsX459) has been identified in patients with craniofacial features with or without hypocalcemia in a Japanese family (36). A number of studies have been undertaken in knockout mice to determine the role of Tbx1 in development of the pharyngeal apparatus and how loss of Tbx1 leads to the characteristic malformations of 22q11.2 deletion syndrome, with varying success. TBX1 participates within a network of transcription factors that act in a spatiotemporal manner to control development and maintenance of the parathyroid glands (2, 37, 38). In humans the parathyroid glands are derived from the endoderm of the third and fourth pharyngeal pouches, whereas in mice the glands develop together with the thymus from the endoderm of the third pharyngeal pouch. TBX1 is required for the development of the third pharyngeal pouch, whereas GATA3, which is expressed later than TBX1 in the common parathyroid-thymus primordia, mediates the differentiation and survival of parathyroid and thymus progenitor cells. Moreover, GATA3 regulates the expression of GCM2, which is expressed in the parathyroid domain of the common primordia and is required for differentiation and survival of parathyroid cells (38). Tbx1 heterozygous mutant mice survive in relatively normal Mendelian ratios, with mild cardiovascular defects and ectopic but not absent parathyroid glands (39–41). By contrast, Tbx1 homozygous mutant mice die in the perinatal period and have a severe pharyngeal phenotype characterized by cleft palate, an absent outer and middle ear, thymus and parathyroid gland aplasia, aortic arch defects, a single cardiac outflow tract, and ventricular septal defects (39–43). Our study provides evidence that heterozygous TBX1 splice-altering mutations leading to exon skipping in vitro that may produce a truncated protein can cause isolated hypoparathyroidism.
Our study provides evidence that heterozygous TBX1 splice-altering mutations leading to exon skipping in vitro may produce a truncated protein that can cause isolated hypoparathyroidism, which represents a markedly restricted phenotype for the DiGeorge sequence. Remarkably, 22q11.2 deletions have also been associated with limited clinical manifestations, account for ∼5% of all cases of nonsyndromic congenital heart disease (26, 44), and are also the second-most common causative diagnosis in mental retardation, accounting for ∼2.4% of patients with developmental delay. Therefore, our work extends the variable expressivity of TBX1 mutations, as 12 of 13 patients that we describe in this report had isolated hypoparathyroidism as the sole clinical manifestation of a TBX1 mutation. Family A is most revealing; all 10 patients with the c.1009+1G>C mutation had isolated hypoparathyroidism at presentation, and despite years of monitoring by us and/or their local physicians, all 10 subjects have failed to evidence recurrent infections, developmental deficits, hearing loss, speech defects, or facial or neurocognitive features that are typical of 22q11.2 deletion syndrome or DiGeorge sequence. Moreover, five phenotypically normal subjects carry the same pathogenic mutation, which suggests that this point mutation has reduced penetrance or impairs parathyroid development in a manner that leads to later onset of hypoparathyroidism than is typical of larger 22q11 deletions. Certainly, these other carriers will have to be monitored closely as they age. Although we have no explanation for these effects, there are other examples of reduced penetrance of typical 22q11.2 deletions and duplications and other TBX1 mutations (4, 29, 30, 45), suggesting that other modifying genetic and/or environmental factors are at play. Most recently, several genome-wide studies have begun to discover modifiers and/or risk factors for congenital heart disease and psychiatric features in 22q11.2 deletion syndrome (46–48). Family B is equally instructive, as the proband, III-1 (Fig. 1B), manifested the clinical triad of 22q11.2 deletion syndrome, including athymia, truncus arteriosis, and hypoparathyroidism, whereas his mother and grandmother had only isolated hypoparathyroidism.
Our study has several important strengths. First, many of the patients that we describe with isolated hypoparathyroidism are in their fifth or sixth decade of life and have been monitored by endocrinologists for many years, which reduces the likelihood that obvious syndromic features such as recurrent infections have been overlooked. In addition, the proband of family A has been examined annually by one of us (M.A.L.). Second, in several cases, TBX1 mutation carriers are parents of children with isolated hypoparathyroidism, which confirms the pathogenicity of the mutation and provides clear evidence for reduced penetrance. And third, we describe two different TBX1 mutations that are predicted to produce identical truncated TBX1 proteins, which suggests that specific point mutations in TBX1 can cause either DiGeorge sequence or isolated hypoparathyroidism.
Our study also has some weaknesses. First, we were unable to obtain biochemical data or perform genotyping on all members of these kindreds. Nevertheless, we were able to obtain critical biochemical data on all patients who carried the TBX1 mutations. Second, given the limited number of families that we studied, we are unable to predict how common TBX1 mutations are as a cause of isolated hypoparathyroidism or whether other types of mutations (e.g., those that cause TBX1 haploinsufficiency) can also cause such a restricted phenotype. Third, we were unable to identify TBX1 proteins or transcripts in lymphoblastoid cells from normal or affected subjects and therefore are unable to provide evidence that the TBX1 mutations we describe in fact result in truncated TBX1 proteins. And fourth, an important weakness of our work is that we have not been able to personally reassess the affected members of family A over the past 2 years. The proband of family A has been examined annually by one of us (M.A.L.), but it is certainly possible that subtle neurocognitive deficiencies have been overlooked in at least some of the affected subjects. However, the developmental defects of DiGeorge sequence are usually present at birth and do not evolve over time. Although slow declines in T-cell populations have been described in patients with the classic 22q11.2 deletion syndrome, none of the clinical manifestations of immunodeficiency, such as recurrent infection and autoimmune disease, have been reported to us by even the oldest affected members of these two kindreds with isolated hypoparathyroidism. Although it is possible that some affected subjects with TBX1 mutations may later develop evidence of immunodeficiency, our results demonstrate that in at least some subjects, hypoparathyroidism can be the principle if not the only feature of a TBX1 mutation.
In summary, we report splice site mutations in TBX1 and provide evidence that defects in TBX1 are a cause of isolated hypoparathyroidism as well as the more common DiGeorge sequence. In addition, our results provide evidence that TBX1 is the causative gene for hypoparathyroidism in the very common 22q11.2 deletion syndrome. This report expands the phenotypic spectrum of endocrine defects ascribed to TBX1 mutations and extends the number of candidate genes that can cause isolated hypoparathyroidism.
Acknowledgments
The authors are grateful to Dr. Florin Tuluc in the CHOP Flow Cytometry Core for his assistance with the analysis of EGFP and FRFP expression, to Mr. Brian Hartnett for his excellent technical skills in performing the experiments described here, to Dr. Konstantin A. Lukyanov (Institute of Bioorganic Chemistry, Moscow, Russia) for providing us with the dual reporter minigene, and to Fengxiang Wang and James Snyder, who helped with DNA sample extraction and handling. They are also particularly grateful to the many patients who participated as research subjects in these studies.
Financial Support: This work was supported in part by Grant R01DK079970 from the National Institute of Diabetes and Digestive and Kidney Diseases (to M.A.L.) and by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1TR000003. Additional support for this study was provided by the Institutional Development Fund to the Center for Applied Genomics from The Children's Hospital of Philadelphia (to H.H.).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- EGFP
enhanced green fluorescent protein
- FRFP
far-red mutant of red fluorescent protein
- WES
whole exome sequencing
References
- 1. Clarke BL, Brown EM, Collins MT, Jüppner H, Lakatos P, Levine MA, Mannstadt MM, Bilezikian JP, Romanischen AF, Thakker RV. Epidemiology and diagnosis of hypoparathyroidism. J Clin Endocrinol Metab. 2016;101(6):2284–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grigorieva IV, Thakker RV. Transcription factors in parathyroid development: lessons from hypoparathyroid disorders. Ann N Y Acad Sci. 2011;1237(1):24–38. [DOI] [PubMed] [Google Scholar]
- 3. Nagamine K, Peterson P, Scott HS, Kudoh J, Minoshima S, Heino M, Krohn KJ, Lalioti MD, Mullis PE, Antonarakis SE, Kawasaki K, Asakawa S, Ito F, Shimizu N. Positional cloning of the APECED gene. Nat Genet. 1997;17(4):393–398. [DOI] [PubMed] [Google Scholar]
- 4. Gong W, Gottlieb S, Collins J, Blescia A, Dietz H, Goldmuntz E, McDonald-McGinn DM, Zackai EH, Emanuel BS, Driscoll DA, Budarf ML. Mutation analysis of TBX1 in non-deleted patients with features of DGS/VCFS or isolated cardiovascular defects. J Med Genet. 2001;38(12):e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Unger S, Górna MW, Le Béchec A, Do Vale-Pereira S, Bedeschi MF, Geiberger S, Grigelioniene G, Horemuzova E, Lalatta F, Lausch E, Magnani C, Nampoothiri S, Nishimura G, Petrella D, Rojas-Ringeling F, Utsunomiya A, Zabel B, Pradervand S, Harshman K, Campos-Xavier B, Bonafé L, Superti-Furga G, Stevenson B, Superti-Furga A. FAM111A mutations result in hypoparathyroidism and impaired skeletal development. Am J Hum Genet. 2013;92(6):990–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Parvari R, Hershkovitz E, Grossman N, Gorodischer R, Loeys B, Zecic A, Mortier G, Gregory S, Sharony R, Kambouris M, Sakati N, Meyer BF, Al Aqeel AI, Al Humaidan AK, Al Zanhrani F, Al Swaid A, Al Othman J, Diaz GA, Weiner R, Khan KT, Gordon R, Gelb BD; HRD/Autosomal Recessive Kenny-Caffey Syndrome Consortium . Mutation of TBCE causes hypoparathyroidism-retardation-dysmorphism and autosomal recessive Kenny-Caffey syndrome. Nat Genet. 2002;32(3):448–452. [DOI] [PubMed] [Google Scholar]
- 7. Vissers LE, van Ravenswaaij CM, Admiraal R, Hurst JA, de Vries BB, Janssen IM, van der Vliet WA, Huys EH, de Jong PJ, Hamel BC, Schoenmakers EF, Brunner HG, Veltman JA, van Kessel AG. Mutations in a new member of the chromodomain gene family cause CHARGE syndrome. Nat Genet. 2004;36(9):955–957. [DOI] [PubMed] [Google Scholar]
- 8. Van Esch H, Groenen P, Nesbit MA, Schuffenhauer S, Lichtner P, Vanderlinden G, Harding B, Beetz R, Bilous RW, Holdaway I, Shaw NJ, Fryns JP, Van de Ven W, Thakker RV, Devriendt K. GATA3 haplo-insufficiency causes human HDR syndrome. Nature. 2000;406(6794):419–422. [DOI] [PubMed] [Google Scholar]
- 9. Pollak MR, Brown EM, Estep HL, McLaine PN, Kifor O, Park J, Hebert SC, Seidman CE, Seidman JG. Autosomal dominant hypocalcaemia caused by a Ca2+-sensing receptor gene mutation. Nat Genet. 1994;8(3):303–307. [DOI] [PubMed] [Google Scholar]
- 10. Li D, Opas EE, Tuluc F, Metzger DL, Hou C, Hakonarson H, Levine MA. Autosomal dominant hypoparathyroidism caused by germline mutation in GNA11: phenotypic and molecular characterization. J Clin Endocrinol Metab. 2014;99(9):E1774–E1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mannstadt M, Harris M, Bravenboer B, Chitturi S, Dreijerink KM, Lambright DG, Lim ET, Daly MJ, Gabriel S, Jüppner H. Germline mutations affecting Gα11 in hypoparathyroidism. N Engl J Med. 2013;368(26):2532–2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nesbit MA, Hannan FM, Howles SA, Babinsky VN, Head RA, Cranston T, Rust N, Hobbs MR, Heath H III, Thakker RV. Mutations affecting G-protein subunit α11 in hypercalcemia and hypocalcemia. N Engl J Med. 2013;368(26):2476–2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ahn TG, Antonarakis SE, Kronenberg HM, Igarashi T, Levine MA. Familial isolated hypoparathyroidism: a molecular genetic analysis of 8 families with 23 affected persons. Medicine (Baltimore). 1986;65(2):73–81. [PubMed] [Google Scholar]
- 14. Arnold A, Horst SA, Gardella TJ, Baba H, Levine MA, Kronenberg HM. Mutation of the signal peptide-encoding region of the preproparathyroid hormone gene in familial isolated hypoparathyroidism. J Clin Invest. 1990;86(4):1084–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee S, Mannstadt M, Guo J, Kim SM, Yi HS, Khatri A, Dean T, Okazaki M, Gardella TJ, Jüppner H. A homozygous [Cys25]PTH(1-84) mutation that impairs PTH/PTHrP receptor activation defines a novel form of hypoparathyroidism. J Bone Miner Res. 2015;30(10):1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ding C, Buckingham B, Levine MA. Familial isolated hypoparathyroidism caused by a mutation in the gene for the transcription factor GCMB. J Clin Invest. 2001;108(8):1215–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li D, Streeten EA, Chan A, Lwin W, Tian L, Pellegrino da Silva R, Kim CE, Anderson MS, Hakonarson H, Levine MA. Exome sequencing reveals mutations in AIRE as a cause of isolated hypoparathyroidism. J Clin Endocrinol Metab. 2017;102(5):1726–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nusynowitz ML, Klein MH. Pseudoidiopathic hypoparathyroidism: hypoparathyroidism with ineffective parathyroid hormone. Am J Med. 1973;55(5):677–686. [DOI] [PubMed] [Google Scholar]
- 19. Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25(14):1754–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. DePristo MA, Banks E, Poplin R, Garimella KV, Maguire JR, Hartl C, Philippakis AA, del Angel G, Rivas MA, Hanna M, McKenna A, Fennell TJ, Kernytsky AM, Sivachenko AY, Cibulskis K, Gabriel SB, Altshuler D, Daly MJ. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet. 2011;43(5):491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38(16):e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cingolani P, Platts A, Wang L, Coon M, Nguyen T, Wang L, Land SJ, Lu X, Ruden DM. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly (Austin). 2012;6(2):80–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li D, Yuan H, Ortiz-Gonzalez XR, Marsh ED, Tian L, McCormick EM, Kosobucki GJ, Chen W, Schulien AJ, Chiavacci R, Tankovic A, Naase C, Brueckner F, von Stülpnagel-Steinbeis C, Hu C, Kusumoto H, Hedrich UB, Elsen G, Hörtnagel K, Aizenman E, Lemke JR, Hakonarson H, Traynelis SF, Falk MJ. GRIN2D recurrent de novo dominant mutation causes a severe epileptic encephalopathy treatable with NMDA receptor channel blockers. Am J Hum Genet. 2016;99(4):802–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gordon CT, Petit F, Kroisel PM, Jakobsen L, Zechi-Ceide RM, Oufadem M, Bole-Feysot C, Pruvost S, Masson C, Tores F, Hieu T, Nitschké P, Lindholm P, Pellerin P, Guion-Almeida ML, Kokitsu-Nakata NM, Vendramini-Pittoli S, Munnich A, Lyonnet S, Holder-Espinasse M, Amiel J. Mutations in endothelin 1 cause recessive auriculocondylar syndrome and dominant isolated question-mark ears. Am J Hum Genet. 2013;93(6):1118–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gurskaya NG, Staroverov DB, Zhang L, Fradkov AF, Markina NM, Pereverzev AP, Lukyanov KA. Analysis of alternative splicing of cassette exons at single-cell level using two fluorescent proteins. Nucleic Acids Res. 2012;40(8):e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pan Y, Wang ZG, Liu XY, Zhao H, Zhou N, Zheng GF, Qiu XB, Li RG, Yuan F, Shi HY, Hou XM, Yang YQ. A novel TBX1 loss-of-function mutation associated with congenital heart disease. Pediatr Cardiol. 2015;36(7):1400–1410. [DOI] [PubMed] [Google Scholar]
- 27. McDonald-McGinn DM, Sullivan KE, Marino B, Philip N, Swillen A, Vorstman JA, Zackai EH, Emanuel BS, Vermeesch JR, Morrow BE, Scambler PJ, Bassett AS. 22q11.2 deletion syndrome. Nat Rev Dis Primers. 2015;1:15071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chieffo C, Garvey N, Gong W, Roe B, Zhang G, Silver L, Emanuel BS, Budarf ML. Isolation and characterization of a gene from the DiGeorge chromosomal region homologous to the mouse. Tbx1 gene. Genomics. 1997;43(3):267–277. [DOI] [PubMed] [Google Scholar]
- 29. McDonald-Mcginn DM, Tonnesen MK, Laufer-Cahana A, Finucane B, Driscoll DA, Emanuel BS, Zackai EH. Phenotype of the 22q11.2 deletion in individuals identified through an affected relative: cast a wide FISHing net! Genet Med. 2001;3(1):23–29. [DOI] [PubMed] [Google Scholar]
- 30. de La Rochebrochard C, Joly-Hélas G, Goldenberg A, Durand I, Laquerrière A, Ickowicz V, Saugier-Veber P, Eurin D, Moirot H, Diguet A, de Kergal F, Tiercin C, Mace B, Marpeau L, Frebourg T. The intrafamilial variability of the 22q11.2 microduplication encompasses a spectrum from minor cognitive deficits to severe congenital anomalies. Am J Med Genet A. 2006;140A(14):1608–1613. [DOI] [PubMed] [Google Scholar]
- 31. Choe CP, Crump JG. Tbx1 controls the morphogenesis of pharyngeal pouch epithelia through mesodermal Wnt11r and Fgf8a. Development. 2014;141(18):3583–3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kong P, Racedo SE, Macchiarulo S, Hu Z, Carpenter C, Guo T, Wang T, Zheng D, Morrow BE. Tbx1 is required autonomously for cell survival and fate in the pharyngeal core mesoderm to form the muscles of mastication. Hum Mol Genet. 2014;23(16):4215–4231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Piotrowski T, Ahn DG, Schilling TF, Nair S, Ruvinsky I, Geisler R, Rauch GJ, Haffter P, Zon LI, Zhou Y, Foott H, Dawid IB, Ho RK. The zebrafish van gogh mutation disrupts tbx1, which is involved in the DiGeorge deletion syndrome in humans. Development. 2003;130(20):5043–5052. [DOI] [PubMed] [Google Scholar]
- 34. Yagi H, Furutani Y, Hamada H, Sasaki T, Asakawa S, Minoshima S, Ichida F, Joo K, Kimura M, Imamura S, Kamatani N, Momma K, Takao A, Nakazawa M, Shimizu N, Matsuoka R. Role of TBX1 in human del22q11.2 syndrome. Lancet. 2003;362(9393):1366–1373. [DOI] [PubMed] [Google Scholar]
- 35. Zweier C, Sticht H, Aydin-Yaylagül I, Campbell CE, Rauch A. Human TBX1 missense mutations cause gain of function resulting in the same phenotype as 22q11.2 deletions. Am J Hum Genet. 2007;80(3):510–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ogata T, Niihori T, Tanaka N, Kawai M, Nagashima T, Funayama R, Nakayama K, Nakashima S, Kato F, Fukami M, Aoki Y, Matsubara Y. TBX1 mutation identified by exome sequencing in a Japanese family with 22q11.2 deletion syndrome-like craniofacial features and hypocalcemia. PLoS One. 2014;9(3):e91598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gordon J, Bennett AR, Blackburn CC, Manley NR. Gcm2 and Foxn1 mark early parathyroid- and thymus-specific domains in the developing third pharyngeal pouch. Mech Dev. 2001;103(1-2):141–143. [DOI] [PubMed] [Google Scholar]
- 38. Liu Z, Yu S, Manley NR. Gcm2 is required for the differentiation and survival of parathyroid precursor cells in the parathyroid/thymus primordia. Dev Biol. 2007;305(1):333–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liao J, Kochilas L, Nowotschin S, Arnold JS, Aggarwal VS, Epstein JA, Brown MC, Adams J, Morrow BE. Full spectrum of malformations in velo-cardio-facial syndrome/DiGeorge syndrome mouse models by altering Tbx1 dosage. Hum Mol Genet. 2004;13(15):1577–1585. [DOI] [PubMed] [Google Scholar]
- 40. Lindsay EA, Vitelli F, Su H, Morishima M, Huynh T, Pramparo T, Jurecic V, Ogunrinu G, Sutherland HF, Scambler PJ, Bradley A, Baldini A. Tbx1 haploinsufficieny in the DiGeorge syndrome region causes aortic arch defects in mice. Nature. 2001;410(6824):97–101. [DOI] [PubMed] [Google Scholar]
- 41. Jerome LA, Papaioannou VE. DiGeorge syndrome phenotype in mice mutant for the T-box gene, Tbx1. Nat Genet. 2001;27(3):286–291. [DOI] [PubMed] [Google Scholar]
- 42. Funke B, Epstein JA, Kochilas LK, Lu MM, Pandita RK, Liao J, Bauerndistel R, Schüler T, Schorle H, Brown MC, Adams J, Morrow BE. Mice overexpressing genes from the 22q11 region deleted in velo-cardio-facial syndrome/DiGeorge syndrome have middle and inner ear defects. Hum Mol Genet. 2001;10(22):2549–2556. [DOI] [PubMed] [Google Scholar]
- 43. Arnold JS, Werling U, Braunstein EM, Liao J, Nowotschin S, Edelmann W, Hebert JM, Morrow BE. Inactivation of Tbx1 in the pharyngeal endoderm results in 22q11DS malformations. Development. 2006;133(5):977–987. [DOI] [PubMed] [Google Scholar]
- 44. Xu YJ, Chen S, Zhang J, Fang SH, Guo QQ, Wang J, Fu QH, Li F, Xu R, Sun K. Novel TBX1 loss-of-function mutation causes isolated conotruncal heart defects in Chinese patients without 22q11.2 deletion. BMC Med Genet. 2014;15(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Torres-Juan L, Rosell J, Morla M, Vidal-Pou C, García-Algas F, de la Fuente MA, Juan M, Tubau A, Bachiller D, Bernues M, Perez-Granero A, Govea N, Busquets X, Heine-Suñer D. Mutations in TBX1 genocopy the 22q11.2 deletion and duplication syndromes: a new susceptibility factor for mental retardation. Eur J Hum Genet. 2007;15(6):658–663. [DOI] [PubMed] [Google Scholar]
- 46. Lopez-Rivera E, Liu YP, Verbitsky M, Anderson BR, Capone VP, Otto EA, Yan Z, Mitrotti A, Martino J, Steers NJ, Fasel DA, Vukojevic K, Deng R, Racedo SE, Liu Q, Werth M, Westland R, Vivante A, Makar GS, Bodria M, Sampson MG, Gillies CE, Vega-Warner V, Maiorana M, Petrey DS, Honig B, Lozanovski VJ, Salomon R, Heidet L, Carpentier W, Gaillard D, Carrea A, Gesualdo L, Cusi D, Izzi C, Scolari F, van Wijk JA, Arapovic A, Saraga-Babic M, Saraga M, Kunac N, Samii A, McDonald-McGinn DM, Crowley TB, Zackai EH, Drozdz D, Miklaszewska M, Tkaczyk M, Sikora P, Szczepanska M, Mizerska-Wasiak M, Krzemien G, Szmigielska A, Zaniew M, Darlow JM, Puri P, Barton D, Casolari E, Furth SL, Warady BA, Gucev Z, Hakonarson H, Flogelova H, Tasic V, Latos-Bielenska A, Materna-Kiryluk A, Allegri L, Wong CS, Drummond IA, D’Agati V, Imamoto A, Barasch JM, Hildebrandt F, Kiryluk K, Lifton RP, Morrow BE, Jeanpierre C, Papaioannou VE, Ghiggeri GM, Gharavi AG, Katsanis N, Sanna-Cherchi S. Genetic drivers of kidney defects in the DiGeorge syndrome. N Engl J Med. 2017;376(8):742–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jensen M, Kooy FR, Simon T, Reyniers E, Girirajan S, Tassone F. A higher rare CNV burden in the genetic background potentially contributes to intellectual disability phenotypes in 22q11.2 deletion syndrome. Eur J Med Genet. 2018;61(4):209–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Guo T, Repetto GM, McDonald McGinn DM, Chung JH, Nomaru H, Campbell CL, Blonska A, Bassett AS, Chow EWC, Mlynarski EE, Swillen A, Vermeesch J, Devriendt K, Gothelf D, Carmel M, Michaelovsky E, Schneider M, Eliez S, Antonarakis SE, Coleman K, Tomita-Mitchell A, Mitchell ME, Digilio MC, Dallapiccola B, Marino B, Philip N, Busa T, Kushan-Wells L, Bearden CE, Piotrowicz M, Hawula W, Roberts AE, Tassone F, Simon TJ, van Duin EDA, van Amelsvoort TA, Kates WR, Zackai E, Johnston HR, Cutler DJ, Agopian AJ, Goldmuntz E, Mitchell LE, Wang T, Emanuel BS, Morrow BE. ; International 22q11.2 Consortium/Brain and behavior Consortium. Genome-wide association study to find modifiers for tetralogy of Fallot in the 22q11.2 deletion syndrome identifies variants in the GPR98 locus on 5q14.3. Circ Cardiovasc Genet. 2017;10(5):pii:e001690. [DOI] [PMC free article] [PubMed] [Google Scholar]