Abstract
Context
3-Hydroxy-3-methyl-glutaryl-coenzyme A reductase inhibitors (statins) are widely prescribed. Statins may have important metabolic effects on insulin sensitivity and liver fat, but limited studies have assessed these effects by using euglycemic hyperinsulinemic clamp, stable isotopes, and 1H magnetic resonance spectroscopy (MRS) for liver fat quantification.
Objective
To study the effects of pitavastatin on hepatic fat and insulin sensitivity.
Design
Six-month, double-blind, randomized, placebo-controlled trial.
Setting
Academic clinical research center in Boston, Massachusetts.
Participants
Overweight, insulin-resistant men aged 40 to 65 years who had not received statin therapy for ≥1 year.
Interventions
Pitavastatin 4 mg or placebo daily.
Outcome
The primary endpoints were changes in insulin sensitivity measured by euglycemic hyperinsulinemic clamp and liver fat measured by 1H MRS.
Results
Pitavastatin showed no effect on endogenous glucose production (ΔRa glucose 0.07 ± 0.07 vs 0.04 ± 0.07 mg/kg/min, pitavastatin vs placebo, P = 0.76) or insulin-stimulated glucose uptake during “low dose” (ΔM 0.1 ± 0.1 vs −0.3 ± 0.2 mg/kg/min, P = 0.11) and “high dose” (ΔM −0.5 ± 0.3 vs −0.7 ± 0.4 mg/kg/min, P = 0.70) euglycemic hyperinsulinemic clamps. There was also no effect of pitavastatin on fasting glucose, HbA1c, and 2-hour glucose after 75-g glucose challenge. There was also no change in liver fat fraction (−1 ± 1 vs −0 ± 1%, P = 0.56).
Conclusion
Compared with placebo, pitavastatin did not affect hepatic or whole-body insulin sensitivity, and it did not reduce liver fat.
In a double-blind randomized controlled trial, 6 months of pitavastatin treatment did not change hepatic or whole-body insulin sensitivity or reduce liver fat in abdominally obese men with insulin resistance.
Statins, or 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase inhibitors, have pleiotropic effects beyond inhibition of the cholesterol biosynthetic pathway (1). Statin therapy has the clear benefit of reducing cardiovascular mortality independent of lipid-lowering effects (2, 3). Statins are known to play a role in hepatic response to insulin, and some statins increase the risk of new-onset diabetes (4–6). This diabetogenic effect appears to be dose dependent, with a higher risk of diabetes with more intensive dose therapy (7). This effect does not seem to be sustained among all medications in this class, and it appears that some statins such as pravastatin and lovastatin may be neutral to glucose metabolism (8, 9). A key question on the metabolic effects of statins relates to the effects of statins on hepatic fat. Animal studies and small human studies have suggested that statin therapy may decrease hepatic steatosis and steatohepatitis (10–14). Similarly, limited animal and human data show potential effects of statins on liver enzymes (15, 16) and inflammatory indices (17, 18), as well as hepatic fat scores (15, 16). Taken together, these data suggest potentially important effects of statins on metabolic variables. However, previous randomized, placebo-controlled trials in humans have not simultaneously assessed effects of statins on key indices of muscle and hepatic insulin sensitivity by using state-of-the-art euglycemic clamp and stable isotope methods or assessed liver fat by using established magnetic resonance spectroscopy (MRS) imaging techniques.
Pitavastatin is a moderate-intensity, mildly lipophilic statin that short-term studies suggest may have neutral or beneficial effects on glucose metabolism (19, 20). Based on this evidence, as well as preliminary data suggesting that statins may reduce liver fat, we conducted the current study with two aims: to carefully characterize the effects of pitavastatin on hepatic and whole-body insulin sensitivity among insulin-resistant, abdominally obese men, with the hypothesis that pitavastatin would be neutral to insulin sensitivity even among insulin-resistant patients, and to determine whether pitavastatin treatment over 6 months reduces liver fat in this population, with the hypothesis that pitavastatin would decrease liver fat compared with placebo. In this randomized, placebo-controlled trial, we demonstrated that, after 6 months of treatment with pitavastatin, there was no effect on hepatic or whole-body insulin resistance or on liver fat content in the population studied.
Methods
Participants
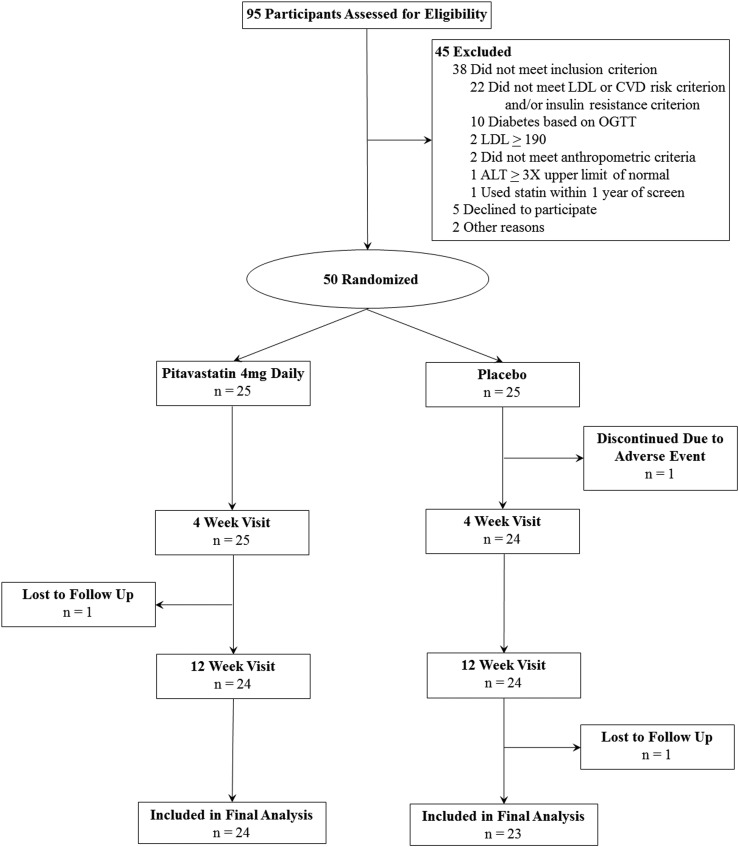
Men between the ages of 40 and 65 years with abdominal obesity and indications of insulin resistance were recruited from the greater Boston area via the clinical research study volunteer program and advertisements in print and electronic media. Ninety-five potential participants were screened at Massachusetts General Hospital (MGH) between March 2015 and October 2017 (Fig. 1). Written informed consent was obtained from each participant before the screening procedures begin. The first participant was enrolled on 9 March 2015, and the final study visit was completed on 27 April 2018. The study was approved by the MGH Institutional Review Board.
Figure 1.
CONSORT flow diagram.
Subjects were selected to be eligible if they demonstrated both of the following: abdominal obesity, as demonstrated by body mass index (BMI) ≥27 kg/m2 and waist circumference ≥102 cm; and prediabetes, defined by either a fasting blood glucose ≥100 mg/dL and <126 mg/dL or a 2-hour glucose ≥140 mg/dL and <200 mg/dL after standard 75-g oral glucose tolerance test (OGTT), or insulin resistance defined by a homeostasis model assessment of insulin resistance (HOMA-IR) (21) ≥2.0. Subjects were also required to have either 10-year atherosclerotic cardiovascular disease (ASCVD) risk ≥5% by American Heart Association guidelines (22) or low-density lipoprotein cholesterol (LDL-C) ≥100 mg/dL, such that treatment with a statin would be clinically reasonable. Subjects with known ASCVD, LDL-C ≥190 mg/dL, or triglyceride ≥500 mg/dL were excluded, as were subjects who were actively being considered for statin therapy by their physician, because randomization to placebo would not have been acceptable in these cases. Other exclusion criteria were as follows: use of statin in the past year, diagnosis of diabetes or use of antidiabetic medications, use of lipid-modifying therapy, renal disease, alanine aminotransferase (ALT) or aspartate aminotransferase (AST) at least three times the upper limit of normal, creatinine greater than the upper limit of normal, or hemoglobin <10 g/dL.
Study procedures
Screening procedures occurred after a 12-hour overnight fast and included anthropometric measurements, fasting lipid panel, fasting insulin levels, ALT, AST, and a standard 75-g OGTT.
Eligible participants were randomly assigned 1:1 to receive pitavastatin calcium 4 mg vs matching placebo by mouth daily for 6 months. The pitavastatin 4 mg and identical-appearing placebo were supplied by Kowa Pharmaceuticals America, Inc. (Montgomery, AL) and packaged by the MGH Research Pharmacy. Randomization was performed by a biostatistician via block randomization with blocks of varying sizes, and the randomization key was shared only with the pharmacy. All study staff and participants were blinded to treatment assignment until study completion.
Participants underwent a baseline visit that included 1H MRS and stable isotope and clamp assessment, as described here. Participants returned for safety visits at 1 and 3 months and then returned for a final assessment at 6 months that was identical to the baseline assessment and also included repeat OGTT (performed initially at the screening visit). At baseline and at 3 and 6 months, all participants received standardized nutrition and activity counseling by research bionutritionists, who were also blinded to randomization (23, 24).
Euglycemic hyperinsulinemic clamp with stable isotopes at baseline and 6 months
After a 12-hour overnight fast, patients underwent euglycemic hyperinsulinemic clamp procedure under low-dose insulin (20 mU/m2/min) and high-dose insulin (80 mU/m2/min) conditions for 120 minutes each. Patients received an infusion of 20% dextrose at a variable infusion rate to maintain a blood glucose level of 90 mg/dL. Arterialized venous blood was sampled every 5 minutes to measure blood glucose concentration with a point-of-care glucose analyzer (Glucose 201; Hemocue, Cypress, CA). Blood samples were collected for insulin levels at times 0, 80, 100, 120, 200, 220, 240 minutes after the start of the clamp procedure. Hepatic and peripheral insulin-stimulated glucose disposals (M) were calculated during the last 20 minutes of low-dose and high-dose clamp, respectively, with the DeFronzo method (25), as the primary indices of insulin sensitivity. Similarly, M normalized to serum insulin concentration (M/I) was calculated.
A stable isotope infusion of 6,6-2H-glucose (Cambridge Isotope Laboratories, Tewksbury, MA) was infused (3.0 mg/kg priming dose and 0.03 mg/kg/min infusion) starting 2 hours before the start of the clamp. Samples were collected for isotopic analysis (Metabolic Solutions, Nashua, NH) for assessment of fasting endogenous glucose production (Ra glucose) with the modified Steele equation (26).
MRS at baseline and 6 months
1H MRS was performed after 12-hour overnight fast for quantification of hepatic fat (27). Visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT) area were also measured cross-sectionally at L4 with MRI. All imaging was completed on the same scanner (Siemens Trio-3 Tesla; Siemens Medical, Erlangen, Germany), and all scans were reviewed by the same radiologist, who remained blinded to treatment assignment.
Other assessments
Patients underwent laboratory assessment for fasting glucose, insulin, lipid panel, direct LDL-C, HbA1c, ALT, AST, creatine phosphokinase (CPK), and coenzyme Q10 (CoQ10). Glucose, lipid panel, transaminases, and HbA1c were measured by standard clinical assays (Labcorp, Burlington, NC). CoQ10 was measured by liquid chromatography (Labcorp). Serum insulin during the clamp was measured by chemiluminescent assay (Beckman Coulter, Fullerton, CA). Physical activity was evaluated with the Modifiable Activity Questionnaire (28). Food and alcohol intake were quantified with a 3-day food record (Nutrition Data System, Minneapolis, MN). Dual-energy x-ray absorptiometry (Hologic, Discovery A, Marlborough, MA) was performed for total body and regional fat mass (29).
Statistical analysis
The study was powered for detection of clinically significant differences in the coprimary endpoints of changes in insulin-stimulated glucose uptake and hepatic fat fraction. More specifically, the protocol was designed with the plan to enroll 50 participants, with an expected 10% discontinuation rate, which would provide 80% power to detect a change of ≥1.5 mg/kg/min in whole-body insulin sensitivity by using preliminary data, or a change of ≥0.86 SD in hepatic fat fraction at a 2-sided α = 0.05.
The analysis was based on intention to treat, with all available data. Liver fat MRS data from one participant in the placebo group were excluded before unblinding because of inadequate spectral quality. One participant in the placebo group had 6-month insulin values that were outliers by >5 SD; his 6-month insulin values were excluded from analysis. One subject in the pitavastatin group developed a metastatic malignancy in his abdomen before the final visit. Per intention to treat, this subject was retained in the primary analysis, and a sensitivity analysis was also performed excluding him.
Baseline comparisons were expressed as mean ± SEM and compared between groups with the Student two-sample t test. Categorical data comparisons were assessed via the likelihood ratio χ2 test. For comparison of outcomes between the pitavastatin and placebo groups, Student two-sample t test was performed assessing the difference in mean change from baseline to 6 months. Data analysis was performed with JMP Pro, version 13.0.0 (SAS Institute Inc., Cary, NC).
Results
Participant flow
The CONSORT flow diagram is shown in Fig. 1. A total of 95 participants were assessed for eligibility; 50 participants were randomly assigned to receive pitavastatin (n = 25) or placebo (n = 25). One participant in each group was lost to follow-up. One participant was discontinued at the discretion of the study physicians because of concerns about myopathy; however, subsequent testing revealed normal CPK.
Study subjects
Subjects were abdominally obese (mean ± SEM, BMI 36.1 ± 0.8 kg/m2, waist circumference 121 ± 2 cm), with a high VAT area (271 ± 13 cm2). They were insulin resistant (HOMA-IR 3.0 ± 0.2) and had a high liver fat content (16% ± 2%). Mean 10-year ASCVD risk score was 7.1% ± 0.6%, and mean LDL-C was 133 ± 3 mg/dL. No patient reported current alcohol abuse, and mean reported alcohol intake was 9 ± 2 g/day, which is less than one drink per day. Groups were well matched in terms of demographics, smoking status, antihypertensive status, and body composition (Tables 1 and 2). The participants in the treatment group had slightly lower total cholesterol levels at baseline, a difference that was not clinically significant (182 ± 4 vs 196 ± 6; pitavastatin vs placebo, P = 0.05). The groups were also comparable with respect to all metabolic and liver parameters, with no significant differences between the groups (Tables 1 and 2).
Table 1.
Baseline Characteristics of the Cohort by Treatment Assignment
| Pitavastatin (n = 25) | Placebo (n = 25) | |
|---|---|---|
| Demographics | ||
| Age, y | 52.8 ± 1.3 | 52.9 ± 1.4 |
| Race, n (%) | ||
| White | 24 (96) | 21 (84) |
| Nonwhite | 1 (4) | 3 (12) |
| Not reported | 0 | 1 (4) |
| Current smoker, n (%) | 4 (16) | 4 (16) |
| Antihypertensive use, n (%) | 9 (36) | 5 (20) |
| Lipid profile | ||
| Total cholesterol, mg/dL | 182 ± 4 | 196 ± 6a |
| LDL-C, mg/dL | 130 ± 4 | 137 ± 5 |
| HDL-C, mg/dL | 41 ± 1 | 42 ± 2 |
| Triglycerides, mg/dL | 132 ± 12 | 152 ± 14 |
| Metabolic parameters | ||
| SBP, mm Hg | 135 ± 3 | 136 ± 3 |
| DBP, mm Hg | 83 ± 2 | 80 ± 2 |
| Fasting glucose, mg/dLb | 91 ± 2 | 91 ± 1 |
| 2-h glucose OGTT, mg/dLb | 139 ± 6 | 131 ± 7 |
| HOMA-IRb | 2.7 ± 0.3 | 3.2 ± 0.3 |
| Creatinine, mg/dL | 0.81 ± 0.03 | 0.88 ± 0.02 |
Continuous data reported as mean ± SEM.
Abbreviations: DBP, diastolic blood pressure; SBP, systolic blood pressure.
P = 0.05 (all other baseline comparisons between treatment groups P > 0.05).
Fasting glucose, 2-h glucose, and HOMA-IR as assessed at screening visit as part of eligibility criteria.
Table 2.
Baseline Values and Outcomes After 6 Mo of Treatment
|
Baseline
|
6 Mo
|
Change Over 6 Mo
|
||||||
|---|---|---|---|---|---|---|---|---|
| Pitavastatin | Placebo | Pitavastatin | Placebo | Pitavastatin (n = 24) | Placebo (n = 23) | Effect Size (95% CI) | P | |
| Body composition | ||||||||
| Weight, kg | 108.6 ± 3.6 | 116.4 ± 4.8 | 107.6 ± 3.6 | 117.4 ± 4.6 | −1.0 ± 0.8 | 1.0 ± 0.8 | −2.0 (−4.4, 0.3) | 0.09 |
| BMI, kg/m2 | 35.6 ± 1.0 | 36.1 ± 1.4 | 35.4 ± 1.0 | 36.4 ± 1.3 | −0.3 ± 0.3 | 0.3 ± 0.3 | −0.6 (−1.4, 0.1) | 0.10 |
| Waist circumference, cm | 118 ± 2 | 122 ± 3 | 117 ± 2 | 123 ± 3 | −0 ± 1 | 1 ± 1 | −1 (−4, 2) | 0.40 |
| VAT area, cm2 | 272 ± 16 | 271 ± 24 | 267 ± 16 | 287 ± 25 | −6 ± 15 | 16 ± 13 | −22 (−62, 19) | 0.29 |
| SAT area, cm2 | 393 ± 25 | 425 ± 36 | 405 ± 35 | 424 ± 33 | 11 ± 14 | −1 ± 12 | 13 (−25, 51) | 0.50 |
| VAT/SAT ratio | 0.75 ± 0.08 | 0.70 ± 0.08 | 0.78 ± 0.10 | 0.73 ± 0.08 | 0.02 ± 0.08 | 0.04 ± 0.05 | −0.01 (−0.20, 0.17) | 0.88 |
| Body fat, % | 35.8 ± 1.0 | 36.7 ± 1.2 | 35.8 ± 0.9 | 37.2 ± 1.1 | 0.1 ± 0.4 | 0.4 ± 0.3 | −0.4 (−1.4, 0.6) | 0.47 |
| Lipid profile | ||||||||
| Total cholesterol, mg/dL | 183 ± 4 | 199 ± 6 | 137 ± 4 | 197 ± 6 | −46 ± 4 | −2 ± 5 | −44 (−57, −32) | <0.0001 |
| LDL-C, mg/dL | 116 ± 4 | 125 ± 5 | 75 ± 3 | 126 ± 4 | −49 ± 3 | −1 ± 4 | −48 (−58, −37) | <0.0001 |
| HDL-C, mg/dL | 41 ± 2 | 43 ± 2 | 39 ± 1 | 43 ± 2 | −2 ± 1 | −0 ± 1 | −1 (−4, 2) | 0.38 |
| Triglycerides, mg/dL | 133 ± 12 | 153 ± 15 | 123 ± 19 | 144 ± 12 | −10 ± 10 | −9 ± 11 | −1 (−31, 29) | 0.95 |
| Metabolic parameters | ||||||||
| HbA1c, % | 5.7 ± 0.1 | 5.7 ± 0.1 | 5.7 ± 0.1 | 5.7 ± 0.1 | 0.0 ± 0.1 | −0.0 ± 0.0 | 0.1 (−0.1, 0.2) | 0.24 |
| Fasting glucose, mg/dL | 98 ± 2 | 97 ± 2 | 96 ± 3 | 99 ± 2 | −2 ± 2 | 2 ± 2 | −4 (−10, 1) | 0.13 |
| 2-hr OGTT glucose, mg/dLa | 139 ± 6 | 132 ± 8 | 141 ± 9 | 138 ± 8 | 3 ± 6 | 7 ± 6 | −3 (−20, 14) | 0.71 |
| HOMA-IR | 2.1 ± 0.4 | 1.6 ± 0.2 | 1.6 ± 0.3 | 2.0 ± 0.3 | −0.5 ± 0.3 | 0.3 ± 0.2 | −0.8 (−1.6, -0.1) | 0.03 |
| Fasting insulin, μU/mL | 8.5 ± 1.5 | 6.7 ± 0.8 | 7.0 ± 1.0 | 7.9 ± 1.2 | −1.9 ± 1.1 | 1.1 ± 1.0 | −3.1 (−6.1, -0.0) | 0.05 |
| Low-dose clamp M, mg/kg/min | 1.4 ± 0.2 | 1.9 ± 0.2 | 1.5 ± 0.2 | 1.6 ± 0.2 | 0.1 ± 0.1 | −0.3 ± 0.2 | 0.4 (−0.1, 0.8) | 0.11 |
| High-dose clamp M, mg/kg/min | 6.4 ± 0.4 | 6.6 ± 0.5 | 5.9 ± 0.4 | 5.9 ± 0.3 | −0.5 ± 0.3 | −0.7 ± 0.4 | 0.2 (−0.9, 1.3) | 0.70 |
| Ra glucose, mg/kg/minb | 1.57 ± 0.06 | 1.55 ± 0.05 | 1.66 ± 0.08 | 1.58 ± 0.07 | 0.07 ± 0.07 | 0.04 ± 0.07 | 0.03 (−0.17, 0.23) | 0.76 |
| CPK, U/L | 135 ± 18 | 197 ± 37 | 137 ± 14 | 194 ± 34 | 2 ± 13 | −3 ± 24 | 5 (−50, 59) | 0.87 |
| CoQ10, μg/mL | 1.0 ± 0.1 | 1.1 ± 0.1 | 0.6 ± 0.0 | 1.0 ± 0.1 | −0.4 ± 0.1 | −0.1 ± 0.1 | −0.4 (−0.7, −0.1) | 0.01 |
| Liver parameters | ||||||||
| ALT, IU/L | 29 ± 3 | 25 ± 3 | 37 ± 8 | 27 ± 3 | 8 ± 8 | 3 ± 2 | 6 (−11, 22) | 0.49 |
| AST, IU/L | 24 ± 2 | 24 ± 2 | 31 ± 5 | 26 ± 3 | 7 ± 5 | 2 ± 2 | 5 (−5, 15) | 0.35 |
| Liver fat content, %c | 17 ± 2 | 14 ± 3 | 17 ± 2 | 14 ± 2 | −1 ± 1 | −0 ± 1 | −1 (−5, 3) | 0.56 |
Data reported as mean ± SEM. P for Student two sample t test (two-tailed) comparing changes between groups. Data shown are for patients who had both baseline and follow-up data available.
OGTT performed at screen visit and not repeated at baseline. Screen values shown for baseline. Change in 2-h glucose between screen visit and 6 mo.
10 participants (5 pitavastatin, 5 placebo) did not have data for stable isotope procedure because of inability to obtain venous access (N = 1) or temporary unavailability of glucose isotope (N = 9).
7 participants (2 pitavastatin, 5 placebo) were unable to complete MRI at baseline because of inability to fit in the scanner or unanticipated claustrophobia.
Effects of pitavastatin on glucose metabolism
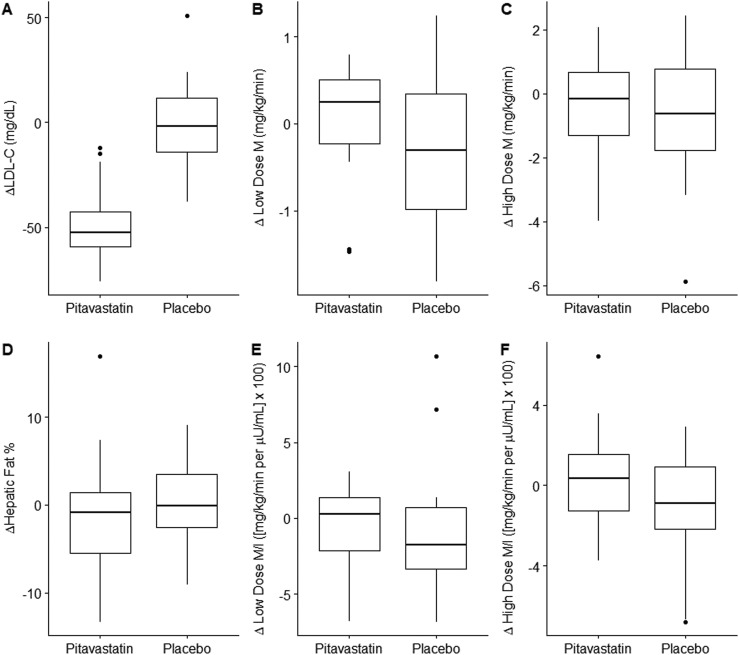
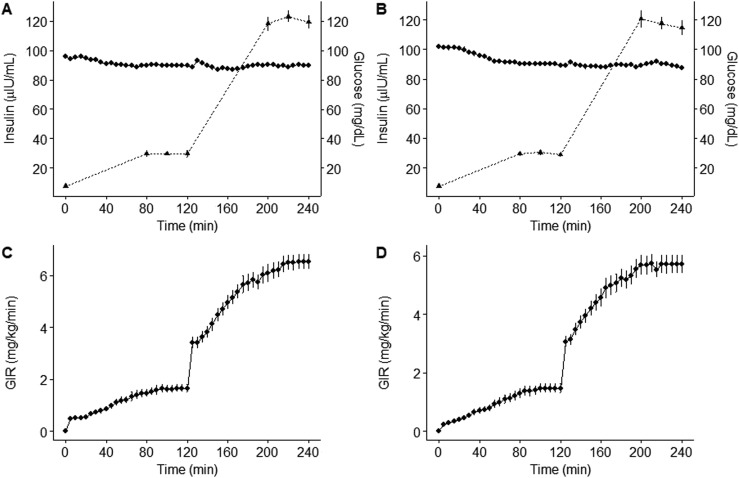
Treatment with pitavastatin had no effect on fasting hepatic gluconeogenesis (Ra glucose) or insulin sensitivity during low-dose or high-dose euglycemic hyperinsulinemic clamp studies (Table 2, Fig. 2). Euglycemic hyperinsulinemic clamp procedures had adequate low-dose (minutes 220 to 240) and high-dose (minutes 340 to 360) steady states achieved at blood glucose levels of 90 mg/dL at baseline and final visits (Fig. 3A–3D). Insulin-stimulated glucose uptake (M) is shown in Table 2. Normalizing glucose utilization to lean body mass instead of total body weight yielded highly similar results. When we adjusted for serum insulin concentrations, results similarly showed no effect of pitavastatin (low-dose clamp ΔM/I −0.8 ± 0.7 vs −1.0 ± 0.9 mg/kg/min per μU/mL × 100, P = 0.87; high dose clamp ΔM/I 0.3 ± 0.5 vs −0.9 ± 0.5 mg/kg/min per μU/mL × 100, P = 0.10, pitavastatin vs placebo, respectively) (Fig. 2). There were also no effects of pitavastatin on HbA1c, fasting glucose, or 2-hour glucose after OGTT. There was a modest, statistically significant reduction in HOMA-IR in the pitavastatin group compared with the placebo group and a trend toward improvement in fasting insulin (Table 2).
Figure 2.
Box and whisker plots showing change from baseline in (A) LDL-C, (B) low-dose clamp M, (C) high-dose clamp M, (D) hepatic fat fraction, (E) low-dose clamp M adjusted for insulin, and (F) high-dose clamp M adjusted for insulin. Data are presented as median (line), 25th and 75th percentiles (box), extremes (whiskers), and outliers (dots). M is insulin-stimulated glucose uptake.
Figure 3.
(A) Baseline and (B) final visit plasma glucose (diamonds) and insulin (triangles, dotted line) concentrations, and (C) baseline and (D) final visit glucose infusion rates during low-dose (min 0 to 120) and high-dose (min 120 to 240) hyperinsulinemic euglycemic clamp. Data are represented as means and SEM (bars). GIR, glucose infusion rate.
Effects of pitavastatin on liver parameters
There were no significant changes in liver fat content (−1% ± 1% vs −0% ± 1%, pitavastatin vs placebo, P = 0.56, Fig. 2) between the pitavastatin and placebo group or in AST or ALT (Table 2).
Effects of pitavastatin on lipids, body composition, and other parameters
As expected, pitavastatin decreased LDL-C substantially compared with placebo (−49 ± 3 vs −1 ± 4 mg/dL, P < 0.0001) and also reduced total cholesterol (Table 2). High-density lipoprotein cholesterol (HDL-C) and triglyceride did not significantly change. Pitavastatin did not change body weight or any measures of body fat distribution (Table 2). Pitavastatin did not change CPK levels. Compared with placebo, there was a reduction in CoQ10 levels with pitavastatin (Table 2). There were no differences in changes in caloric or macronutrient intake over the study between the pitavastatin and placebo groups (Δcaloric intake 6 ± 184 vs −116 ± 187 kcal/day, P = 0.65; Δfat intake 0 ± 10 vs 7 ± 9 g/day, P = 0.63; Δcarbohydrate intake 8 ± 19 vs −18 ± 26 g/day, P = 0.43; Δprotein intake −4 ± 10 vs −8 ± 8 g/day, P = 0.75, pitavastatin vs placebo, respectively). Similarly, changes in activity level over the study were not different between pitavastatin and placebo (Δdaily metabolic equivalent tasks −29 ± 14 vs −9 ± 5, P = 0.17).
Safety and adverse events
Adverse events are shown in Table 3. There were two serious adverse events in the pitavastatin group and one in the placebo group. One participant in the treatment group was hospitalized overnight for acute chest pain with normal cardiac enzymes and was found to have no evidence of an acute myocardial infarction. Another patient in the treatment group received a diagnosis of metastatic cancer as described previously upon completion of the study. A participant in the placebo group was hospitalized for acute perforated appendicitis. There were no confirmed cases of rhabdomyolysis. There were similar rates of nonserious side effects in both groups (Table 3).
Table 3.
Adverse Events
| Pitavastatin (n = 25) | Placebo (n = 25) | |
|---|---|---|
| Serious adverse events | 2 | 1 |
| Nonserious adverse events | 16 | 25 |
| Gastrointestinal symptoms | 5 | 8 |
| Diarrhea or loose stools | 3 | 7 |
| Constipation | 1 | 0 |
| Nausea | 0 | 1 |
| Heartburn | 1 | 1 |
| Excess gas | 1 | 0 |
| Muscle symptoms | 4 | 6 |
| Muscle aches or cramps | 4 | 4 |
| Dark urine | 0 | 1 |
| Rhabdomyolysis | 0 | 0 |
| Elevated CPK level | 0 | 1 |
| Other | 6 | 8 |
| Fatigue | 2 | 5 |
| Headache | 0 | 2 |
| Worsening depression | 1 | 1 |
| Flulike symptoms | 0 | 1 |
| Back pain | 0 | 1 |
| Vivid dreams | 1 | 0 |
Sensitivity analyses
Excluding the patient who developed metastatic cancer, glucose parameters and liver fat remained unchanged between groups, and changes in ALT (1 ± 2 vs 3 ± 2 IU/L, pitavastatin vs placebo, P = 0.45) and AST (2 ± 2 vs 2 ± 2 IU/L, P = 0.85) were also highly similar between groups.
Multivariable linear regression analyses were performed to adjust for the change in weight over the study, which was modestly but nonsignificantly reduced with pitavastatin. Adjusting for changes in weight during the study, there remained no changes in low-dose M (P = 0.20), high-dose M (P = 0.72), HbA1c (P = 0.22), fasting glucose (P = 0.30), 2-hour glucose (P = 0.84), Ra glucose (P = 0.93), and liver fat content (P = 0.68) with pitavastatin compared with placebo, and HOMA-IR showed a similar small decrease with pitavastatin compared with placebo (P = 0.03).
In the subset of patients with ≥5% hepatic fat fraction at baseline (n = 32), changes in liver fat were not meaningfully different than in the entire cohort (Δhepatic fat fraction −1 ± 2 vs −2 ± 1%, pitavastatin vs placebo, P = 0.82).
Adherence to study medication
Adherence as assessed by pill counts at 12-week (97% ± 6% vs 95% ± 6%; pitavastatin vs placebo, P = 0.39) and final visits (96% ± 3% vs 96% ±2%, P = 0.94) was high.
Discussion
In this randomized clinical trial we demonstrated that pitavastatin treatment for 6 months at maximal clinical dosing of 4 mg daily did not affect measures of hepatic or whole-body insulin sensitivity, nor did it reduce liver fat, in men characterized by insulin resistance at baseline. We saw the expected change in LDL-C, and reported compliance was high, demonstrating good adherence to the study regimen.
The effects of statins on glucose appear to differ by intensity and individual formulation. Whereas higher-intensity statins such as atorvastatin and rosuvastatin when used at higher dosages show consistent effects on glucose levels (7, 9), lower-intensity statins such as pravastatin are not consistently shown to worsen glycemia (30–32). Meta-analyses of studies comparing moderate-intensity pitavastatin with either placebo or another statin have suggested no adverse effect on glucose metabolism (20), and a secondary prevention study comparing pitavastatin with atorvastatin showed beneficial effects of pitavastatin on glucose homeostasis (33). In contrast, a retrospective observational study suggested that pitavastatin increased diabetes risk more than other statins (34). Importantly, with increasing clinician awareness of the effect of certain statins to increase glucose, observational studies may increasingly be confounded by indication, as clinicians opt for statins that are thought to have less effect on glycemia in patients at higher risk for diabetes. The current study was designed to rigorously assess the effects of pitavastatin on hepatic and whole-body insulin sensitivity by using stable isotope and euglycemic hyperinsulinemic clamp methods. We elected to use a placebo comparator to assess the effects of pitavastatin compared with untreated natural history rather than a comparator statin with known effects on glucose. This study extends data from a limited number of smaller studies (≤20 participants) of hypercholesterolemic patients investigating one-step clamp data in shorter-term studies (≤2 months) without simultaneous liver fat data (32, 35).
Our results with regard to the effect of pitavastatin on glucose homeostasis are generally neutral, with no changes in hepatic or whole-body insulin sensitivity, HbA1c, or fasting or postprandial glucose and a modest beneficial effect on HOMA-IR with a trend toward reduction in fasting insulin. To assess the possibility that these findings of neutrality may be caused by type 2 error, it is important to examine the 95% CIs around the effect size (Table 2). These 95% CIs exclude clinically meaningful positive or negative effects of pitavastatin on HbA1c, high-dose M, and Ra glucose. For fasting glucose and for low-dose M, the 95% CI of the effect size suggests the possibility of a type 2 error in missing a modest beneficial effect of pitavastatin. Both of these measures are largely reflections of hepatic insulin sensitivity, as is HOMA-IR, and a study with a larger sample size may have shown a small beneficial effect of pitavastatin on these measures. Two-hour glucose has a fair amount of variance, as expected, such that the 95% CI of the effect size does not exclude a modest effect in either direction but does exclude a detrimental effect with clinical importance. Overall, we interpret our data to demonstrate that pitavastatin does not worsen glycemia over 6 months of treatment in an at-risk group of higher-weight, insulin-resistant men. This finding is of clinical relevance because statins are often prescribed for such individuals, and these are detailed data from a randomized controlled trial in humans conducted with state-of-the-art clamp techniques. Thus, this study adds important information to the field.
The underlying mechanism through which statins exert their effect on blood glucose levels is poorly understood (36). One possible mechanism whereby statins may worsen glycemia is reduction in CoQ10, with associated reductions in mitochondrial function, which then cause dysglycemia (and statin-induced myopathy) (37). We did show a reduction in serum CoQ10 levels in the pitavastatin group, but it was not associated with adverse glycemic effects. Importantly, we do not have tissue levels of CoQ10, which may be more relevant with regard to mitochondrial function and tissue-level glucose metabolism.
Nonalcoholic fatty liver disease (NAFLD) is a health risk to the growing population of obese patients, specifically those with visceral adiposity, and pharmacologic strategies are needed to augment efforts at lifestyle modification. Recent evidence has demonstrated that statins can be safely used in patients with prediabetes and nonalcoholic steatohepatitis (NASH) (38), and there is some evidence in the literature that statins may improve features of NAFLD (16). A small study in HIV-infected patients treated with atorvastatin vs placebo suggested that atorvastatin may reduce hepatosteatosis as assessed by liver/spleen ratio (39), but a larger study of HIV-infected patients randomly assigned to rosuvastatin or placebo showed no effect on liver fat scores calculated from serum metabolic and liver function tests (40). Post hoc analyses of large statin trials have suggested that statins reduce ALT in patients who have elevated transaminases at baseline, suggesting possible NAFLD (41, 42). An open-label study of atorvastatin in patients with NASH demonstrated improvement in steatosis and inflammation on biopsy, although fibrosis worsened in some patients (43), and a randomized controlled trial of simvastatin (N = 10) vs placebo (N = 6) for 1 year showed no improvement in aminotransferases or histologic features of steatosis, inflammation, or fibrosis (44). A 2013 Cochrane review of available evidence concluded that statins may improve aminotransferase levels and ultrasound findings but that trials with larger samples sizes are needed (16). A more recent expert panel recommended use of statins as potentially beneficial for NAFLD and NASH in those who have comorbid cardiovascular disease (CVD) (17). To our knowledge, there are no prospective, randomized, placebo-controlled trials to date investigating whether statins reduce liver fat content as assessed with MRS. Our data show no sign of a reduction in liver fat content, and the 95% CI for the effect size excludes a highly meaningful change. We also saw no change in AST or ALT. Notably, we did not require NAFLD for participation, although baseline levels of liver fat content in our cohort were high, on average well above the threshold for steatosis of 5%. Subset analysis of those who did meet the 5% threshold showed no changes in liver fat content as well. We could not perform liver biopsy, although quantification of liver fat content by 1H MRS correlates very well with histology assessment of steatosis (45). We do not have information on histologic changes in inflammation or fibrosis, and we cannot rule out an effect of statins on one or more of these parameters.
Our study has limitations that should be noted. Not all of our participants had an absolute clinical indication for statins; rather, our eligibility criteria were focused on finding patients at high risk of worsening insulin resistance for whom statin treatment would be reasonable. We included only men for purposes of avoiding the potential cofounding of sex steroids on metabolic indices in a physiology study; thus, our study cannot be generalized to women. Larger future studies including both sexes are needed to investigate whether sex modifies the effect of statins on glycemic parameters. Additionally, we did not include patients >65 years old; statins are certainly prescribed in this older age group, and our results cannot be generalized to that clinical context. If the dysglycemic effects of statins develop over several years, our 6-month study duration may not have been sufficient to see adverse effects. A longer randomized controlled trial, the Long-Term Effects of High-Dose Pitavastatin on Diabetogenicity in Comparison With Atorvastatin in Patients With Metabolic Syndrome, is under way in Korea and will investigate the effect of pitavastatin vs atorvastatin on HbA1c over 2 years (46). Finally, there were some limitations to our clamp and stable isotope assessment. Measuring suppression of Ra glucose during the low-dose clamp would provide a pure measure of insulin’s ability to suppress gluconeogenesis in the liver, but the dosage of insulin used during the low-dose phase of the clamp was high enough that some subjects had complete suppression of gluconeogenesis and a small degree of glucose utilization, such that our data for percentage suppression of gluconeogenesis were not interpretable in some subjects. Additionally, a longer duration of clamp procedure at each insulin dose may have further increased glucose utilization. Nonetheless, data for fasting Ra and for M during low- and high-dose insulin clamp support the conclusion that pitavastatin did not worsen overall glucose homeostasis.
Our study also has important strengths, including enrollment of a cohort with baseline insulin resistance and use of detailed physiologic methods to assess glucose homeostasis. Data from this prospective randomized controlled trial using these techniques significantly extend findings from previous studies that pitavastatin is largely neutral to glucose homeostasis, perhaps with small beneficial effects on some measures. Although a larger sample size would be needed to definitively state that glycemia is unchanged by pitavastatin, our data strongly suggest that pitavastatin does not worsen glycemia. We used a placebo comparator to fully assess pitavastatin’s effect on insulin sensitivity, as opposed to measuring a marginal effect when compared with another statin with known effects on glucose. We did not show an effect of pitavastatin in reducing liver fat content or improving transaminases, although longer studies with liver biopsy may be needed to fully assess possible effects of statins on steatohepatitis. Overall, our findings suggest that pitavastatin may be a good pharmacological option for patients with insulin resistance or prediabetes, at moderate CVD risk in whom a statin might be considered for primary prevention. Additional studies of those with higher CVD risk and absolute indications for statin therapy may also be useful.
Acknowledgments
Financial Support: Funding for the investigator-initiated study and study drug were provided Kowa Pharmaceuticals (to T.L.S. and S.K.G.). M.T., S.K.G., and T.L.S. also received support from National Institutes of Health Center grant P30 DK040561. L.R.B. received support from National Institutes of Health T32HD052961. This was an investigator-initiated study, and the funding sources had no impact on the study design, data collection, data interpretation, and manuscript preparation.
Clinical Trial Information: ClinicalTrials.gov no. NCT02290106 (registered 13 November 2014).
Disclosure Summary: S.K.G. has received research funding through his institution from Kowa Pharmaceuticals on unrelated research, consulting fees and research funding from Theratechnologies, and research funding from Gilead, all unrelated to the current project. T.L.S. has received support for investigator-initiated research unrelated to this study from Novo Nordisk, Inc. C.A.S. is an employee of Kowa Pharmaceuticals America, Inc.
Glossary
Abbreviations:
- ALT
alanine aminotransferase
- ASCVD
atherosclerotic cardiovascular disease
- AST
aspartate aminotransferase
- BMI
body mass index
- CoQ10
coenzyme Q10
- CPK
creatine phosphokinase
- CVD
cardiovascular disease
- HDL-C
High-density lipoprotein cholesterol
- HOMA-IR
homeostasis model assessment of insulin resistance
- I
insulin concentration
- LDL-C
low-density lipoprotein cholesterol
- M
glucose disposal
- MGH
Massachusetts General Hospital
- MRS
magnetic resonance spectroscopy
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- OGTT
oral glucose tolerance test
- SAT
subcutaneous adipose tissue
- VAT
visceral adipose tissue
References
- 1. Bhandari S, Gupta P, Quinn P, Sandhu J, Hakimi A, Jones D, Ng L. Pleiotropic effects of statins in hypercholesterolaemia: a prospective observational study using a lipoproteomic based approach. Lancet. 2015;385(suppl 1):S21. [DOI] [PubMed] [Google Scholar]
- 2. Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, Thomason MJ, Mackness MI, Charlton-Menys V, Fuller JH; CARDS investigators . Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364(9435):685–696. [DOI] [PubMed] [Google Scholar]
- 3. Sever PS, Dahlöf B, Poulter NR, Wedel H, Beevers G, Caulfield M, Collins R, Kjeldsen SE, Kristinsson A, McInnes GT, Mehlsen J, Nieminen M, O’Brien E, Ostergren J; ASCOT Investigators . Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial–Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Drugs. 2004;64(suppl 2):43–60. [DOI] [PubMed] [Google Scholar]
- 4. Sattar N, Preiss D, Murray HM, Welsh P, Buckley BM, de Craen AJ, Seshasai SR, McMurray JJ, Freeman DJ, Jukema JW, Macfarlane PW, Packard CJ, Stott DJ, Westendorp RG, Shepherd J, Davis BR, Pressel SL, Marchioli R, Marfisi RM, Maggioni AP, Tavazzi L, Tognoni G, Kjekshus J, Pedersen TR, Cook TJ, Gotto AM, Clearfield MB, Downs JR, Nakamura H, Ohashi Y, Mizuno K, Ray KK, Ford I. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375(9716):735–742. [DOI] [PubMed] [Google Scholar]
- 5. Rajpathak SN, Kumbhani DJ, Crandall J, Barzilai N, Alderman M, Ridker PM. Statin therapy and risk of developing type 2 diabetes: a meta-analysis. Diabetes Care. 2009;32(10):1924–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ; JUPITER Study Group . Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195–2207. [DOI] [PubMed] [Google Scholar]
- 7. Preiss D, Seshasai SR, Welsh P, Murphy SA, Ho JE, Waters DD, DeMicco DA, Barter P, Cannon CP, Sabatine MS, Braunwald E, Kastelein JJ, de Lemos JA, Blazing MA, Pedersen TR, Tikkanen MJ, Sattar N, Ray KK. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. JAMA. 2011;305(24):2556–2564. [DOI] [PubMed] [Google Scholar]
- 8. Sattar NA, Ginsberg H, Ray K, Chapman MJ, Arca M, Averna M, Betteridge DJ, Bhatnagar D, Bilianou E, Carmena R, Ceška R, Corsini A, Erbel R, Flynn PD, Garcia-Moll X, Gumprecht J, Ishibashi S, Jambart S, Kastelein JJ, Maher V, da Silva PM, Masana L, Odawara M, Pedersen TR, Rotella CM, Salti I, Teramoto T, Tokgozoglu L, Toth PP, Valensi P, Vergès B. The use of statins in people at risk of developing diabetes mellitus: evidence and guidance for clinical practice. Atheroscler Suppl. 2014;15(1):1–15. [DOI] [PubMed] [Google Scholar]
- 9. Carter AA, Gomes T, Camacho X, Juurlink DN, Shah BR, Mamdani MM. Risk of incident diabetes among patients treated with statins: population based study. BMJ. 2013;346(4):f2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. de Keyser CE, Koehler EM, Schouten JN, Visser LE, Hofman A, Janssen HL, Stricker BH. Statin therapy is associated with a reduced risk of non-alcoholic fatty liver in overweight individuals. Dig Liver Dis. 2014;46(8):720–725. [DOI] [PubMed] [Google Scholar]
- 11. Athyros VG, Mikhailidis DP, Didangelos TP, Giouleme OI, Liberopoulos EN, Karagiannis A, Kakafika AI, Tziomalos K, Burroughs AK, Elisaf MS. Effect of multifactorial treatment on non-alcoholic fatty liver disease in metabolic syndrome: a randomised study. Curr Med Res Opin. 2006;22(5):873–883. [DOI] [PubMed] [Google Scholar]
- 12. Egawa T, Toda K, Nemoto Y, Ono M, Akisaw N, Saibara T, Hayashi Y, Hiroi M, Enzan H, Onishi S. Pitavastatin ameliorates severe hepatic steatosis in aromatase-deficient (Ar−/−) mice. Lipids. 2003;38(5):519–523. [DOI] [PubMed] [Google Scholar]
- 13. Kamada Y, Kiso S, Yoshida Y, Chatani N, Kizu T, Hamano M, Egawa M, Takemura T, Ezaki H, Furuta K, Hayashi N, Takehara T. Pitavastatin ameliorated the progression of steatohepatitis in ovariectomized mice fed a high fat and high cholesterol diet. Hepatol Res. 2013;43(4):401–412. [DOI] [PubMed] [Google Scholar]
- 14. Hyogo H, Ikegami T, Tokushige K, Hashimoto E, Inui K, Matsuzaki Y, Tokumo H, Hino F, Tazuma S. Efficacy of pitavastatin for the treatment of non-alcoholic steatohepatitis with dyslipidemia: an open-label, pilot study. Hepatol Res. 2011;41(11):1057–1065. [DOI] [PubMed] [Google Scholar]
- 15. Han KH, Rha SW, Kang HJ, Bae JW, Choi BJ, Choi SY, Gwon HC, Bae JH, Hong BK, Choi DH, Han KR. Evaluation of short-term safety and efficacy of HMG-CoA reductase inhibitors in hypercholesterolemic patients with elevated serum alanine transaminase concentrations: PITCH study (PITavastatin versus atorvastatin to evaluate the effect on patients with hypercholesterolemia and mild to moderate hepatic damage). J Clin Lipidol. 2012;6(4):340–351. [DOI] [PubMed] [Google Scholar]
- 16. Eslami L, Merat S, Malekzadeh R, Nasseri-Moghaddam S, Aramin H. Statins for non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Cochrane Database Syst Rev. 2013;(12):CD008623. [DOI] [PubMed]
- 17. Athyros VG, Alexandrides TK, Bilianou H, Cholongitas E, Doumas M, Ganotakis ES, Goudevenos J, Elisaf MS, Germanidis G, Giouleme O, Karagiannis A, Karvounis C, Katsiki N, Kotsis V, Kountouras J, Liberopoulos E, Pitsavos C, Polyzos S, Rallidis LS, Richter D, Tsapas AG, Tselepis AD, Tsioufis K, Tziomalos K, Tzotzas T, Vasiliadis TG, Vlachopoulos C, Mikhailidis DP, Mantzoros C. The use of statins alone, or in combination with pioglitazone and other drugs, for the treatment of non-alcoholic fatty liver disease/non-alcoholic steatohepatitis and related cardiovascular risk. An Expert Panel Statement. Metabolism. 2017;71:17–32. [DOI] [PubMed] [Google Scholar]
- 18. Park HS, Jang JE, Ko MS, Woo SH, Kim BJ, Kim HS, Park HS, Park IS, Koh EH, Lee KU. Statins increase mitochondrial and peroxisomal fatty acid oxidation in the liver and prevent non-alcoholic steatohepatitis in mice. Diabetes Metab J. 2016;40(5):376–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chapman MJ, Orsoni A, Robillard P, Hounslow N, Sponseller CA, Giral P. Effect of high-dose pitavastatin on glucose homeostasis in patients at elevated risk of new-onset diabetes: insights from the CAPITAIN and PREVAIL-US studies. Curr Med Res Opin. 2014;30(5):775–784. [DOI] [PubMed] [Google Scholar]
- 20. Vallejo-Vaz AJ, Kondapally Seshasai SR, Kurogi K, Michishita I, Nozue T, Sugiyama S, Tsimikas S, Yoshida H, Ray KK. Effect of pitavastatin on glucose, HbA1c and incident diabetes: a meta-analysis of randomized controlled clinical trials in individuals without diabetes. Atherosclerosis. 2015;241(2):409–418. [DOI] [PubMed] [Google Scholar]
- 21. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. [DOI] [PubMed] [Google Scholar]
- 22. Goff DC Jr, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC Jr, Sorlie P, Stone NJ, Wilson PW, Jordan HS, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC Jr, Tomaselli GF; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25, suppl 2):S49–S73. [DOI] [PubMed] [Google Scholar]
- 23. U.S. Department of Agriculture U.S. Department of Health and Human Services , ed. Dietary Guidelines for Americans. 7th ed. Washington, DC: U.S. Government Printing Office; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. U.S. Department of Health and Human Services , ed. Physical Activity Guidelines for Americans. Washington, DC: U.S. Government Printing Office; 2008. [Google Scholar]
- 25. DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237(3):E214–E223. [DOI] [PubMed] [Google Scholar]
- 26. Finegood DT, Bergman RN, Vranic M. Estimation of endogenous glucose production during hyperinsulinemic-euglycemic glucose clamps. Comparison of unlabeled and labeled exogenous glucose infusates. Diabetes. 1987;36(8):914–924. [DOI] [PubMed] [Google Scholar]
- 27. Bredella MA, Ghomi RH, Thomas BJ, Ouellette HA, Sahani DV, Miller KK, Torriani M. Breath-hold 1H-magnetic resonance spectroscopy for intrahepatic lipid quantification at 3 Tesla. J Comput Assist Tomogr. 2010;34(3):372–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kriska AM, Knowler WC, LaPorte RE, Drash AL, Wing RR, Blair SN, Bennett PH, Kuller LH. Development of questionnaire to examine relationship of physical activity and diabetes in Pima Indians. Diabetes Care. 1990;13(4):401–411. [DOI] [PubMed] [Google Scholar]
- 29. Mazess RB, Barden HS, Bisek JP, Hanson J. Dual-energy x-ray absorptiometry for total-body and regional bone-mineral and soft-tissue composition. Am J Clin Nutr. 1990;51(6):1106–1112. [DOI] [PubMed] [Google Scholar]
- 30. Thakker D, Nair S, Pagada A, Jamdade V, Malik A. Statin use and the risk of developing diabetes: a network meta-analysis. Pharmacoepidemiol Drug Saf. 2016;25(10):1131–1149. [DOI] [PubMed] [Google Scholar]
- 31. Takagi T, Matsuda M, Abe M, Kobayashi H, Fukuhara A, Komuro R, Kihara S, Caslake MJ, McMahon A, Shepherd J, Funahashi T, Shimomura I. Effect of pravastatin on the development of diabetes and adiponectin production. Atherosclerosis. 2008;196(1):114–121. [DOI] [PubMed] [Google Scholar]
- 32. Galvan AQ, Natali A, Baldi S, Frascerra S, Sampietro T, Galetta F, Seghieri G, Ferrannini E. Effect of a reduced-fat diet with or without pravastatin on glucose tolerance and insulin sensitivity in patients with primary hypercholesterolemia. J Cardiovasc Pharmacol. 1996;28(4):595–602. [DOI] [PubMed] [Google Scholar]
- 33. Wang YB, Fu XH, Gu XS, Fan WZ, Jiang YF, Hao GZ, Miao Q, Cao J, Fu B, Li Y. Effects of intensive pitavastatin therapy on glucose control in patients with non-ST elevation acute coronary syndrome. Am J Cardiovasc Dis. 2017;7(4):89–96. [PMC free article] [PubMed] [Google Scholar]
- 34. Cho Y, Choe E, Lee YH, Seo JW, Choi Y, Yun Y, Wang HJ, Ahn CW, Cha BS, Lee HC, Kang ES. Risk of diabetes in patients treated with HMG-CoA reductase inhibitors. Metabolism. 2015;64(4):482–488. [DOI] [PubMed] [Google Scholar]
- 35. Altunbaş H, Balci MK, Karayalçin U. No effect of simvastatin treatment on insulin sensitivity in patients with primary hypercholesterolemia. Endocr Res. 2003;29(3):265–275. [DOI] [PubMed] [Google Scholar]
- 36. Rochlani Y, Kattoor AJ, Pothineni NV, Palagiri RDR, Romeo F, Mehta JL. Balancing primary prevention and statin-induced diabetes mellitus prevention. Am J Cardiol. 2017;120(7):1122–1128. [DOI] [PubMed] [Google Scholar]
- 37. Chan DC, Pang J, Watts GF. Pathogenesis and management of the diabetogenic effect of statins: a role for adiponectin and coenzyme Q10? Curr Atheroscler Rep. 2015;17(1):472. [DOI] [PubMed] [Google Scholar]
- 38. Bril F, Portillo Sanchez P, Lomonaco R, Orsak B, Hecht J, Tio F, Cusi K. Liver safety of statins in prediabetes or T2DM and nonalcoholic steatohepatitis: post hoc analysis of a randomized trial. J Clin Endocrinol Metab. 2017;102(8):2950–2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lo J, Lu MT, Kim EA, Nou E, Hallett TR, Park J, Hoffmann U, Grinspoon SK. Statin effects to reduce hepatosteatosis as measured by computed tomography in patients with human immunodeficiency virus. Open Forum Infect Dis. 2016;3(2):ofw062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kamari VE, Hileman CO, Gholam PM, Kulkarni M, Funderburg N, McComsey GA. Statin therapy does not reduce liver fat scores in patients receiving antiretroviral therapy for HIV infection [published online ahead of print 13 June 2018] Clin Gastroenterol Hepatol. doi: 10.1016/j.cgh.2018.05.058. [DOI] [PMC free article] [PubMed]
- 41. Athyros VG, Tziomalos K, Gossios TD, Griva T, Anagnostis P, Kargiotis K, Pagourelias ED, Theocharidou E, Karagiannis A, Mikhailidis DP; GREACE Study Collaborative Group . Safety and efficacy of long-term statin treatment for cardiovascular events in patients with coronary heart disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) Study: a post-hoc analysis. Lancet. 2010;376(9756):1916–1922. [DOI] [PubMed] [Google Scholar]
- 42. Tikkanen MJ, Fayyad R, Faergeman O, Olsson AG, Wun CC, Laskey R, Kastelein JJ, Holme I, Pedersen TR; IDEAL Investigators . Effect of intensive lipid lowering with atorvastatin on cardiovascular outcomes in coronary heart disease patients with mild-to-moderate baseline elevations in alanine aminotransferase levels. Int J Cardiol. 2013;168(4):3846–3852. [DOI] [PubMed] [Google Scholar]
- 43. Hyogo H, Tazuma S, Arihiro K, Iwamoto K, Nabeshima Y, Inoue M, Ishitobi T, Nonaka M, Chayama K. Efficacy of atorvastatin for the treatment of nonalcoholic steatohepatitis with dyslipidemia. Metabolism. 2008;57(12):1711–1718. [DOI] [PubMed] [Google Scholar]
- 44. Nelson A, Torres DM, Morgan AE, Fincke C, Harrison SA. A pilot study using simvastatin in the treatment of nonalcoholic steatohepatitis: a randomized placebo-controlled trial. J Clin Gastroenterol. 2009;43(10):990–994. [DOI] [PubMed] [Google Scholar]
- 45. Georgoff P, Thomasson D, Louie A, Fleischman E, Dutcher L, Mani H, Kottilil S, Morse C, Dodd L, Kleiner D, Hadigan C. Hydrogen-1 MR spectroscopy for measurement and diagnosis of hepatic steatosis. AJR Am J Roentgenol. 2012;199(1):2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Park JB, Jung JH, Yoon YE, Kim HL, Lee SP, Kim HK, Kim YJ, Cho GY, Sohn DW. Long-term Effects of high-doSe pitavaStatin on Diabetogenicity in comparison with atorvastatin in patients with Metabolic syndrome (LESS-DM): study protocol for a randomized controlled trial. Trials. 2017;18(1):501. [DOI] [PMC free article] [PubMed] [Google Scholar]