Abstract
Background and Aims:
Maintenance of adequate depth of anesthetic is crucial to prevent awareness and to reduce stress response associated with surgery. Goals of balanced general anesthetic are met by use of adjuvants to facilitate use of lower anesthetic dose, while ensuring adequate anesthetic depth. This study employed BIS monitoring to compare the anesthetic sparing effects of intravenous dexmedetomidine and magnesium sulphate on induction dose of propofol by maintaining a BIS value of 40-50.
Material and Methods:
One hundred and twenty ASA I and II patients undergoing elective surgery under general anesthetic were included in three groups of forty each. Group D received 1 μg/kg of dexmedetomidine, Group M was given 30 mg/kg of magnesium sulphate in 100 ml saline and Group N received 100 ml saline over 15-20 minutes 15 minutes before induction. Data compared were dose of propofol and vecuronium, Ramsay sedation score, BIS values and hemodynamic parameters intraoperatively.
Results:
Propofol required in group D was significantly lower 101.3 ± 16.5 than group M and N with dose of 114 ± 15.5 and 160.50 ± 25.08 respectively (p <0.001). Dose requirement of vecuronium was significantly reduced in group M 5.4 ± 0.8 and group D 6.6 ± 1.2 as compared to N 7.9 ± 1.4 (p <0.001). No significant differences were seen regarding baseline hemodynamics, RSS and BIS values in all groups. After study drug infusion, RSS was 4.59 ± 0.75 in dexmedetomidine group compared to 1.9 ± 0.7 and 1.4 ± 0.5 in group M and N (p <0.001). During maintenance, significantly lower HR, MAP and BIS values were seen in group D and M than N (p <0.001).
Conclusion:
Our study showed that pretreatment with dexmedetomidine and magnesium sulphate significantly reduced the induction dose of propofol by maintaining a constant BIS in value at 40-50. However, both the drugs reduced the time to reach BIS 40-50 but sedation and sparing of propofol was more in dexmedetomidine group.
Keywords: Balanced Anesthetic, BIS targeted induction, dexmedetomidine, magnesium sulphate, propofol sparing
Introduction
The concept of balanced anesthesia is based on the concurrent administration of a combination of several anesthetic drugs to produce the desired effect. Decreased dosages of individual components in the mixture provide safety by preventing the harmful effects produced by large doses of an individual agent.[1]
Because of its pharmacological properties and speedy recovery profile, propofol is a universally used induction agent acting on gamma-aminobutyric acid (GABA) receptors in the central nervous system (CNS). Despite its favorable profile, due to inadequate analgesic properties, higher doses may be required for maintenance of anesthetic depth which can cause adverse cardiorespiratory effects such as myocardial depression, metabolic acidosis, and impaired platelet aggregation.[2] With addition of adjuvants, the requirement of propofol can be reduced.[3] Alpha-2 adrenoceptor agonists, such as dexmedetomidine, are known to possess amnesic, analgesic, sympatholytic, and antinociceptive properties, and therefore, can reduce the requirement of anesthetics and opioids intraoperatively.[4] Administration of MgSO4 has also shown significant reduction in the perioperative requirement of propofol, opioids, and muscle relaxants.[5]
Bi-spectral index (BIS) monitoring provides a simple measure of anesthetic depth through analysis of electrocortical activity.[6] It integrates the frequency-domain, time-domain, and bispectral analysis of raw EEG signals into a numerical value, ranging from 0 (isoelectric EEG) to 100 (fully awake).[7] BIS values of 40–60 are preferred for surgical patients because of deep hypnotic state, unresponsiveness to verbal or surgical stimuli, and low probability of recall in this range; BIS values increase with noxious stimuli.[8]
Role of dexmedetomidine as an adjuvant has been studied widely but adds to the cost being an expensive drug and increases the postanesthesia care unit (PACU) stay due to its prolonged sedative effects. To find a suitable alternative to dexmedetomidine, we employed BIS monitoring to compare the anesthetic sparing effects of intravenous MgSO4 with dexmedetomidine on the induction dose of propofol using BIS 40–50 as a guide to determine the end-points of anesthetic administration and to study intraoperative hemodynamics.
Material and Methods
This prospective randomized double blind study enlisted 120 patients of American Society of Anesthesiologists (ASA) physical status I and II, aged 18–60 years, of either sex, scheduled for elective surgery under general anesthesia after obtaining an ethical committee approval and informed patient consent. The enlisted patients were divided into three groups of 40 each randomly Concealment of randomisation was done by sealed envelope approach. Keeping the precision of estimates of outcome statistics as 95% confidence limits and on the basis of previously published studies, sample size was considered as 40 per group.[9] Patients with hepatic, renal or cardiovascular dysfunction, epilepsy, pregnancy, postural hypotension, anticipated difficult airway, anticipated major blood losses and fluid shifts, patients on sedatives, and drug allergies were excluded from study.
Preanesthetic check-up was done and necessary blood and radiological investigations were ordered. After obtaining an informed written consent, all patients were given tab. alprazolam 0.5 mg a night before the procedure and were kept nil per orally.
On patients’ arrival in the operating room, standard monitors and BIS monitor were attached and an intravenous line was secured. Prior to administering test drug (TPD) ECG; BIS value; SpO2; level of sedation using RAMSAY sedation scale heart rate (HR) and mean arterial pressure (MAP) were noted as a baseline after 5 min of stabilization of the patient. The study drug solution of either dexmedetomidine or MgSO4 or normal saline (control) was prepared in 100 ml normal saline by an independent consultant not involved in this study; the investigator also remained blind regarding the constituents of the solution. Fifteen minutes prior to induction, Group D (n = 40) was infused dexmedetomidine 1 μg/kg, Group M (n = 40) received MgSO4 30 mg/kg and Group N (n = 40) control group was given normal saline infusion over 15–20 min according to the groups allotted.
Five minutes after completion of infusion of test drug (TAD) ECG, BIS value, SpO2, RSS, and vitals were recorded. Preoxygenation was done for 3 min with 100% oxygen via face mask and IV glycopyrrolate 0.01 mg/kg and butorphanol 0.02 mg/kg were administered. Then, induction of anesthesia was facilitated using propofol in titrated doses to achieve BIS range 40–50, which provided adequate depth for laryngoscopy and intubation than 50–60 as per a pilot study conducted by us. Laryngoscopy and intubation were facilitated by 1.5 mg/kg succinylcholine with continuous monitoring of all parameters. BIS values, SpO2, HR, and MAP were recorded after intubation, and then, every 10 min intraoperatively. Muscle relaxation was achieved with inj.vecuronium bromide 0.08 mg/kg bolus dose and 0.02 mg/kg when required. Controlled ventilation was maintained with isoflurane and a mixture of 66% N2O with oxygen. Isoflurane concentration was modified according to the increase or decrease in HR and MAP of 20% from the baseline values. The patients were observed for any adverse effects throughout the procedure and postoperatively. In the event of bradycardia (HR <50 bpm) injection atropine at 0.3 mg IV bolus was given and hypotension (MAP <20% of preinduction value) was managed with injection mephenteramine 6 mg bolus. Arrhythmias were described as supraventricular or ventricular beats >3/min or any rhythm other than sinus. At the end of the surgery, reversal of neuromuscular block was done using IV neostigmine 0.05 mg/kg and glycopyrrolate 0.02 mg/kg and extubation was performed. At 30 min postoperatively, RSS was noted in the recovery room.
Statistical analysis was done with Statistical Package for Social Sciences (SPSS 17 version, SPSS Inc., Chicago, IL, US) 17.0 software. Chi-square test was applied for nonparametric data (age, sex distribution, ASA physical status) and one-way analysis of variance (ANOVA) with post-hoc Tukey HSD tests for parametric numerical data. Results are expressed as mean ± [standard deviation (SD)]. P value of <0.05 was considered significant.
Results
The demographic data and the mean duration of surgery was among the three groups [Table 1].
Table 1.
Demographic characteristics of patients in three groups
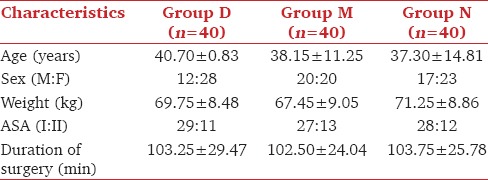
Significantly higher dose of propofol was required in group N compared to groups D and M. The mean induction dose of propofol was significantly less in group D than in group M. Groups D and M both required reduced doses of vecuronium compared to group N and the comparison of group M with D was also significant [Table 2].
Table 2.
Dose requirement of propofol and vecuronium in three groups
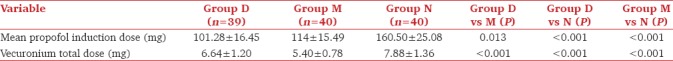
Ramsay sedation scores 5 and 30 minutes after infusion of the study drug were significantly higher in group D compared to those in groups M and N. RSS of group M were also significantly more compared to N at 5 minutes after the drug [Table 3]. Fall in BIS values after study drug infusion was significant for group D with early decline having lowest values after drug and at intubation compared to groups M and N. A significant difference was seen among group D and M till 3 min and the values were comparable thereafter. Groups D and M had significantly lower BIS values compared to group N till 40 minutes and were similar thereafter [Figure 1].
Table 3.
Ramsay sedation scores
Figure 1.
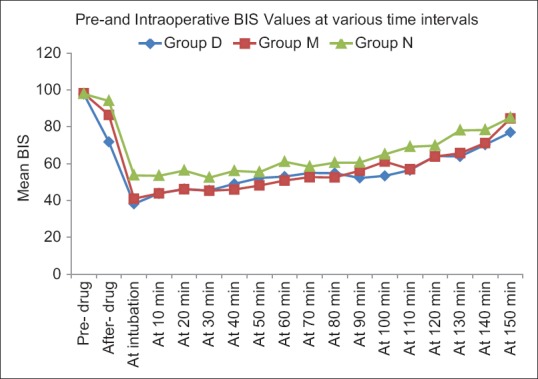
Comparison of BIS values intraoperatively
Mean baseline hemodynamic parameters were similar in three groups. Fall in HR was maximum after study drug in group D as compared to the baseline and other two groups. Post-induction, a statistically significant rise in HR was seen in group N compared to D and M Intraprocedure HR in groups D and M were significantly lower than group N [Figure 2]. MAP values were significantly lower in groups D and M compared to group N after intubation till 10 min and at all times intraoperatively. However, group D had lower MAP values post-infusion and post-intubation compared to groups M and N [Figure 3].
Figure 2.
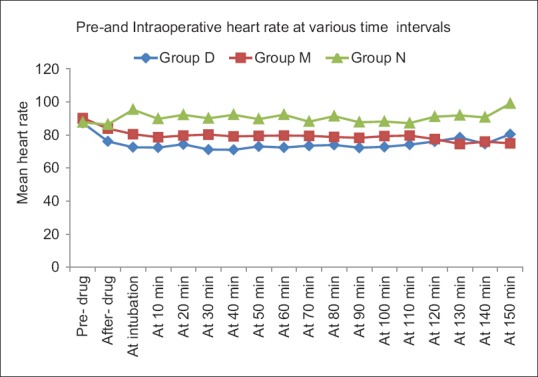
Comparison of heart rate in the intraoperative period
Figure 3.
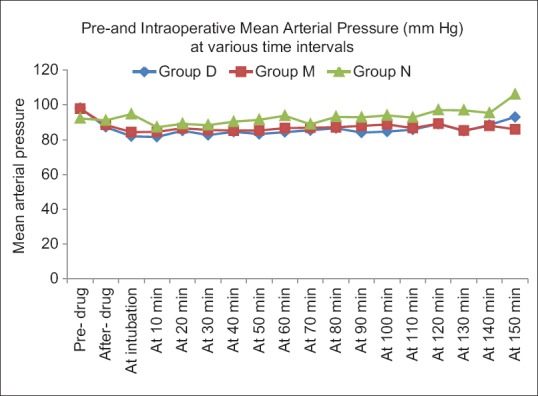
Comparison of mean arterial pressure in the intraoperative period
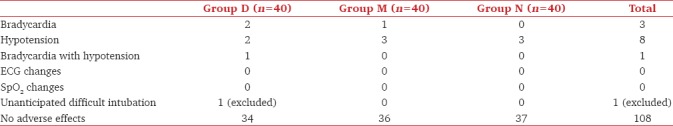
No ECG changes (arrhythmias) or SpO2 fluctuations were seen. No rebound hypertension, vision problems, altered sensorium, chest pain, jaundice, malaise, and fever were noted in the postoperative period. Bradycardia was seen in three patients, hypotension in 8 patients and both problems together were seen in 1 patient. One patient having unanticipated difficult intubation was included in demographic data and complications but was removed from the statistical analysis for dose of propofol, vecuronium and hemodynamics. However, the incidence of complications was similar in the three groups [Table 4].
Table 4.
Intraoperative or postoperative complications in three groups
Discussion
Essential goals of balanced anesthesia are to provide adequate depth of anesthesia, to maintain hemodynamic stability, and to prevent awareness intraoperatively. BIS monitor is a well-established objective and qualitative guide to prevent intraoperative awareness. Our goal was to titrate the dose of propofol to achieve a BIS range 40–50, which was estimated as the feasible range for laryngoscopy and intubation in our pilot study and falls within the recommended range for general anesthesia.[10] By maintaining BIS values between 40–60 during anesthesia, the dose requirement of hypnotic agents was reduced by 11% to 27%.[11] Many studies have been done using adjuvants with continuous infusion of propofol, realizing the need to reduce its dosage. We incorporated the use of adjuvants with BIS monitoring to reduce the bolus dose of propofol in the present study.
Dexmedetomidine 1 μg/kg provides better hemodynamic control over 0.5 μg/kg, and more episodes of hypotension were reported using 2 μg/kg.[12] Similar to a study by Smitha et al., this study was conducted with 1 μg/kg bolus dose of dexmedetomidine to avoid side effects.[13] Elsharnouby noticed episodes of hypotension using MgSO4 40 mg/kg over 15 min prior to induction and intraoperatively 15 mg/kg/h by continuous infusion.[14] However, smooth and gradual decrease of HR and MAP occurred with 30mg/kg MgSO4 in our study.[5]
Reduction in propofol consumption by 29% was seen in our study similar to a 20–30% decrease observed by Choi et al. using MgSO4.[5,15] MgSO4 produces analgesia and sedation by its interference with calcium channels, antagonist action on N-methyl-D-aspartate (NMDA) receptors in CNS, blockade of NMDA-induced currents in a voltage dependant manner, and reducing the release of catecholamines.[16] However, dexmedetomidine caused 37% reduction in the induction dose of propofol by resulting in a decreased neuronal activity and enhancing the vagal activity by activation of α2 receptors located in the postsynaptic terminals in CNS, similar to the findings of Dutta et al. who noticed 30–50% reduction in the dose of propofol.[17,18]
In addition, the vecuronium requirement was reduced by 30% in the MgSO4 group due to prolongation of action of nondepolarizing neuromuscular blocking agents by magnesium ions as a result of inhibition of acetylcholine release at the motor endplate caused by competition of magnesium for calcium channels in the presynaptic nerve terminal. A 30 mg/kg dose of MgSO4 demonstrated a similar reduction in the dose of vecuronium as 40 mg/kg infusion of MgSO4 used by various authors.[19,20] In addition, in the dexmedetomidine group, total dose of vecuronium was reduced by 15%.
RSS scores were significantly more in the dexmedetomidine group, which prolonged the stay of the patient in the PACU, but no respiratory depression or fall in SpO2 was seen, and on verbal commands, the patients were arousable.[21] Dexmedetomidine provides sedation without respiratory depression which is characteristically different from that of other sedatives such as GABA agonist, propofol. It produces physiological sleep-like phenomenon in the EEG and a characteristic arousable sedation by acting on the α2-adrenoceptors in the locus coeruleus in brainstem where it decreases sympathetic outflow and increases parasympathetic outflow.[22] Dexmedetomidine has shown to produce an additive interaction with propofol for achieving sedative endpoints whereas a narcotic state has also been demonstrated by the use of MgSO4 infusions but with lower RSS scores, which accounted for early discharge from PACU.[15,23]
Titration of propofol to achieve a BIS value 40–50 was helpful to avoid the overdose of the anesthetic, decreased the incidence of intraoperative awareness, and helped maintain a better hemodynamic stability. Significantly lower BIS values were achieved earlier in dexmedetomidine group compared to the other two groups.[24] Kasuya et al. observed that the cut-off BIS values for detecting LOC were lower with dexmedetomidine monoinfusion than with propofol monoinfusion.[25] Similarly, the preoperative infusion of MgSO4 also reduced the anesthetic demands and the time needed to reach a BIS value of 60.[26,27] BIS-guided general anesthesia reduced the exposure time and doses, causing a reduction in neurotoxicity and expedited recovery from anesthesia because sedation produced by dexmedetomidine and MgSO4 helped to achieve the target BIS range earlier and with reduced dose of propofol. BIS has shown to reduce the risk of developing delirium during initial hospitalization and postoperative cognitive dysfunction at 3 months after surgery.[28]
MgSO4 has demonstrated a good hemodynamic stability throughout the intraoperative period with minimum fluctuations. However, HR and MAP in dexmedetomidine group remained significantly lower than placebo group because of its sympatholytic and vagomimetic effects.[29] Magnesium induces hypotension directly by vasodilatation and indirectly by sympathetic blockade.[30] At no point of time intraoperatively did the mean HR, MAP, SBP and DBP values rise above the baseline values in the study population treated with dexmedetomidine and MgSO4.
The advantage of the present study is evaluating the direct effect of dexmedetomidine and MgSO4 on the bispectral index scale by keeping other intraoperative factors almost constant (such as duration of procedure, induction and maintenance techniques, analgesia, and mode of ventilation) for accurate assessment. There are some limitations of our study that we only studied sedation scores but not recovery characteristics. Furthermore, we did not assess analgesic requirement reduction among three groups.
Conclusion
Our study highlights the clinical application of MgSO4 as a useful anesthetic adjuvant that can be safely used for co-administration with propofol to decrease its dosage to maintain a constant BIS in value at 40–50, with an additional vecuronium sparing effect. Hemodynamic stability was more in the MgSO4 group compared to dexmedetomidine with values closer to the baseline. Sedation was exceptionally more in dexmedetomidine treated study population.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Lundy JS. Balanced anesthesia. Minn Med. 1926;9:394–9. [Google Scholar]
- 2.Franks NP. Molecular targets underlying general anesthetic. Br J Pharmacol. 2006;147:72–81. doi: 10.1038/sj.bjp.0706441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fabbri LP, Nucera M, Marsili M, Al Malyan M, Becchi C. Ketamine, propofol and low dose remifentanil for ERCP outside the operating room: Is ketamine only a “rescue drug”? Med Sci Monit. 2012;18:575–80. doi: 10.12659/MSM.883354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kang WS, Kim SY, Son JC, Kim JD, Muhammad HB, Kim SH. The effect of dexmedetomidine on the adjuvant propofol requirement and intraoperative hemodynamics during remifentanil-based anesthesia. Korean J Anesthesiol. 2012;62:113–8. doi: 10.4097/kjae.2012.62.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Telci L, Esen F, Akcora D, Erden T, Canbolat AT, Akpir K. Evaluation of effects of magnesium sulphate in reducing intraoperative anaesthetic requirements. Br J Anaesth. 2002;89:594–8. doi: 10.1093/bja/aef238. [DOI] [PubMed] [Google Scholar]
- 6.Glass PS, Bloom M, Kearse L, Rosow C, Sebel P, Manberg P. Bispectral analysis measures sedation and memory effects of propofol, midazolam, isoflurane, and alfentanil in healthy volunteers. Anesthesiol. 1997;86:836–47. doi: 10.1097/00000542-199704000-00014. [DOI] [PubMed] [Google Scholar]
- 7.Chan MT, Gin T. What does the bispectral EEG index monitor? Eur J Anaesthesiol. 2000;17:146–8. doi: 10.1046/j.1365-2346.2000.00613.x. [DOI] [PubMed] [Google Scholar]
- 8.Ekman A, Lindholm ML, Lennmarken C, Sandin R. Reduction in the incidence of awareness using BIS monitoring. Acta Anaesthesiol Scand. 2004;48:20–6. doi: 10.1111/j.1399-6576.2004.00260.x. [DOI] [PubMed] [Google Scholar]
- 9.Hazarika A, Deori AK, Bora J, Deori J, Tiwari PK. Attenuation of haemodynamic responses to laryngoscopy and intubation: A clinical study of dexmedetomidine. Int J Contemp Med Res. 2016;3:3536–8. [Google Scholar]
- 10.Johansen JW. Update on bispectral index monitoring. Best Pract Clin Res Anesthesiol. 2006;20:81–99. doi: 10.1016/j.bpa.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Liu SS. Effects of bispectral index monitoring on ambulatory anesthesia: A meta-analysis of randomized controlled trials and a cost analysis. Anesthesiology. 2004;101:311–5. doi: 10.1097/00000542-200408000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Lawrence CJ, De Lange S. Effects of a single pre-operative dexmedetomidine dose on isoflurane requirements and perioperative haemodynamic stability. Anesthetic. 1997;52:736–44. doi: 10.1111/j.1365-2044.1997.169-az0303.x. [DOI] [PubMed] [Google Scholar]
- 13.Smitha KS, Shukla D, Sathesha M, Rao R, Nethra S, Sudhhesh K. Comparison of two different doses of dexmedetomidine in attenuating hemodynamic changes during laryngoscopy. J Evol Med Dent Sci. 2014;3:13501–8. [Google Scholar]
- 14.Elsharnouby NM, Elsharnouby MM. Magnesium sulphate as a technique of hypotensive anesthetic. Br J Anaesth. 2006;96:727–31. doi: 10.1093/bja/ael085. [DOI] [PubMed] [Google Scholar]
- 15.Choi JC, Yoon KB, Um DJ, Kim C, Kim JS, Lee SG. Intravenous magnesium sulfate administration reduces propofol infusion requirements during maintenance of propofol-N2O anesthesia: Part i: Comparing propofol requirements according to hemodynamic responses: Part ii: Comparing bispectral index in control and magnesium groups. Anesthesiology. 2002;97:1137–41. doi: 10.1097/00000542-200211000-00017. [DOI] [PubMed] [Google Scholar]
- 16.Tramer MR, Schneider J, Marti RA, Rifat K. Role of magnesium sulphate in post operative analgesia. Anesthesiology. 1996;84:340–7. doi: 10.1097/00000542-199602000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Dutta S, Karol MD, Cohen T, Jones RM, Mant T. Effect of dexmedetomidine on propofol requirements in healthy subjects. J Pharm Sci. 2001;90:172–81. doi: 10.1002/1520-6017(200102)90:2<172::aid-jps8>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 18.Ngwenyama NE, Anderson J, Hoernschemeyer DG, Tobias JD. Effects of dexmedetomidine on propofol and remifentanil infusion rates during total intravenous anesthesia for spine surgery in adolescents. Paediatr Anaesth. 2008;18:1190–5. doi: 10.1111/j.1460-9592.2008.02787.x. [DOI] [PubMed] [Google Scholar]
- 19.Fuchs-Buder T, Wilder-Smith OH, Borgeat A, Tassonyi E. Interaction of magnesium sulphate with vecuronium-induced neuromuscular block. Br J Anaesth. 1995;74:405–9. doi: 10.1093/bja/74.4.405. [DOI] [PubMed] [Google Scholar]
- 20.Lee DH, Kwon IC. Magnesium sulphate has beneficial effects as an adjuvant during general anesthetic for caesarean section. Br J Anaesth. 2009;103:861–6. doi: 10.1093/bja/aep265. [DOI] [PubMed] [Google Scholar]
- 21.Yuen VM, Irwin MG, Hui TW, Yuen MK, Lee LH. A double blind, crossover assessment of the sedative and analgesic effects of intranasal dexmedetomidine. Anesth Analg. 2007;105:374–80. doi: 10.1213/01.ane.0000269488.06546.7c. [DOI] [PubMed] [Google Scholar]
- 22.Nelson LE, Lu J, Guo T, Saper CB, Franks NP, Maze M. The alpha2- adrenoceptor agonist dexmedetomidine converges on an endogenous sleep-promoting pathway to exert its sedative effects. Anesthesiology. 2003;98:428–36. doi: 10.1097/00000542-200302000-00024. [DOI] [PubMed] [Google Scholar]
- 23.Peck CH, Meltzer SJ. Anesthesia in human beings by intravenous administration of magnesium sulphate. JAMA. 1916;67:1131–3. [Google Scholar]
- 24.Shin HW, Yoo HN, Kim DH, Lee H, Shin HJ, Lee HW. Preanesthetic dexmedetomidine 1 μg/kg single infusion is a simple, easy, and economic adjuvant for general anesthesia. Korean J Anesthesiol. 2013;65:114–20. doi: 10.4097/kjae.2013.65.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kasuya Y, Govinda R, Rauch S, Mascha EJ, Sessler DI, Turan A. The correlation between bispectral index and observational sedation scale in volunteers sedated with dexmedetomidine and propofol. Anesth Analg. 2009;109:1811–5. doi: 10.1213/ANE.0b013e3181c04e58. [DOI] [PubMed] [Google Scholar]
- 26.Altan A, Turgut N, Yildiz F, Türkmen A, Ustün H. Effects of magnesium sulphate and clonidine on propofol consumption, haemodynamic and post-operative recovery. Br J Anaesth. 2005;94:438–41. doi: 10.1093/bja/aei070. [DOI] [PubMed] [Google Scholar]
- 27.Ray M, Bhattacharjee DP, Hajra B, Pal R, Chatterjee N. Effect of clonidine and magnesium sulphate on anaesthetic consumption, haemodynamics and postoperative recovery: A comparative study. Indian J Anaesth. 2010;54:137–41. doi: 10.4103/0019-5049.63659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matthew TV, Benny CP, Tatia MC, Tony G CODA Trial Group. BIS-guided anesthesia decreases postoperative delirium and cognitive decline. J Neurosurg Anesthesiol. 2013;25:33–42. doi: 10.1097/ANA.0b013e3182712fba. [DOI] [PubMed] [Google Scholar]
- 29.Talke P, Chen R, Thomas B, Aggarwal A, Gottlieb A, Thorborg P. The hemodynamic and adrenergic effects of perioperative dexmedetomidine infusion after vascular surgery. Anesth Analg. 2000;90:834–9. doi: 10.1097/00000539-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 30.Ryu JH, Kang MH, Park KS, Do SH. Effects of magnesium sulphate on intraoperative anaesthetic requirements and postoperative analgesia in gynaecology patients receiving total intravenous anesthetic. Br J Anaesth. 2008;100:397–403. doi: 10.1093/bja/aem407. [DOI] [PubMed] [Google Scholar]


