Abstract
Preoperative assessment is a very crucial step in anesthesia management. Anatomical resection (lobectomy or pneumonectomy) offers best long-term prognosis to a lung cancer patient. At the same time, surgery cannot be offered to a patient who is expected to become ventilator dependent, postoperatively. Hence, it is very important to have an objective preoperative assessment for risk stratification. This review article provides a systematic approach for the prognostication of patients planned for pulmonary resection.
Keywords: Preoperative assessment, spirometry, thoracic surgery
Introduction
Preoperative assessment of patients planned for pulmonary resection is a crucial step in anesthesia management. Pulmonary resection is a high-risk surgery, even in, otherwise healthy patients. Hence, preoperative prognostication and optimization are very important. It is very vital to establish that patients will tolerate one lung ventilation intraoperatively and will have sufficient pulmonary reserve postoperatively. In addition, the impact of comorbidities on the outcome need to be understood. In a patient who is expected to become ventilator dependent in the postoperative period, alternative options in the form of subsegmental resection or radiotherapy can be advised. In a scenario of difficult one lung ventilation, feasibility of surgery with two lung ventilation should be discussed with the surgeon.
There are many guidelines with different criteria for evaluation of a patient planned for lobectomy or pneumonectomy.[1,2,3] Charloux et al. concluded in their study that there is lack of clarity and standardization on the subject.[4] Here, we are presenting a narrative review article based on the clinical practice guidelines by American College of Chest Physician, British Thoracic Society and European Respiratory Society. The aim is to provide systematic approach for preoperative evaluation of lung cancer patients undergoing pulmonary resection.
Level 1
Baseline investigations required before lung cancer surgery include assessment of respiratory mechanics and parenchymal functions. However, any high-risk cardiac patient should be directed for level 3 investigations [Figure 1].
Figure 1.
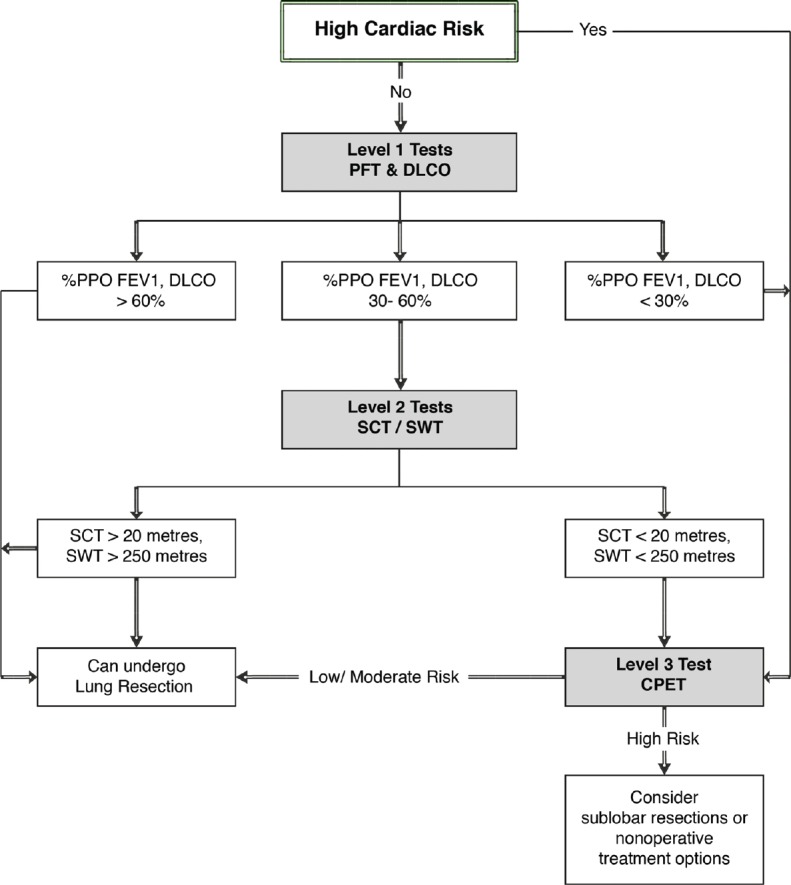
Algorithm for preoperative pulmonary assessment for pulmonary resection
Respiratory mechanics
Spirometry is a commonly used test for assessment of respiratory mechanics. The basic characteristics of spirometry are acceptability and reproducibility. Spirometry should be free from error and artefacts to be acceptable. Error in spirometry can be due to various reasons including coughing, leaks, hesitation, early termination, and variable efforts. Patients should take a deep breath after placing the flow transducer inside the mouth ensuring a tight seal. Afterward, the patient should breathe out as hard, fast, and completely as possible to squeeze out all the air. Spirometry should be done after proper patient training. The expiratory phase during forced vital capacity (FVC) should be more than 6 seconds. Spirometry results are considered reproducible if the variation in the two highest forced expired volume in the first second of expiration (FEV1) or FVC values is less than 5%.
In patients with abnormal values, special attention must be paid to understanding the cause of deranged results. For example, reduction in FEV1 because of muscle weakness will have a different postoperative course compared to chronic obstructive pulmonary disease, even with similar spirometry values.
Lung parenchyma function
Various studies have shown that diffusing capacity of the lung for carbon monoxide (DLCO) is a stronger predictor of postoperative complications in comparison to FEV1.[5,6] It has been established that there is no correlation between DLCO and FEV1.[7] Hence, it is recommended that DLCO in addition to spirometry should be performed as part of baseline investigations.[3] However, it has been reported that most physicians assess DLCO only in patients with compromised lung.[4]
Absolute vs percentage predicted values
British Thoracic Society (BTS) 2001 guidelines suggest absolute preoperative FEV1 of 1.5 L for lobectomy and 2 L for pneumonectomy as selection criteria for surgery and recommend that no further testing is required in the absence of interstitial lung disease or unexpected breathlessness.[8] Whereas ACCP 2013 guideline emphasize the importance of percentage of predicted postoperative values (%PPO) over absolute values.[3] Absolute values when expressed in terms of % PPO may place a low risk patient in the moderate or high risk category. Single cut-off value of FEV1 cannot be applicable to all the ages, built, races, and genders. Values calculated as a percentage of the normal predicted value correlate better with the outcome.[9] Hence, percentage predicted values of lung function values should be used in assessing patients for lung cancer surgery. Percentage predicted parameters are the estimated parameters for late postoperative period at least 3–6 months after the surgical procedure.[9] Percentage predicted values can be calculated by anatomic method or perfusion method. Segment counting is used only for lobectomy. Calculation of values for pneumonectomy should be done by perfusion method only. Either a ventilation or a perfusion scan should be performed in these patients; there is no added advantage of performing both the scans. Consent in lung cancer surgery is for pulmonary resection and does not specify extent of resection. Hence, as per the American College of Chest Physician Clinical Practice guidelines (ACCP CPG) 2013 guideline, any patient planned for pulmonary resection should be assessed for pneumonectomy.[3] Intraoperatively, if cancer is found involving the hilum or crossing the fissure, then there may be need of pneumonectomy in a patient planned for lobectomy.
Formula for calculation of predicted postoperative (PPO) values with anatomic method
PPO FEV1 = preoperative FEV1× (1 – y/z)
PPO DLCO = preoperative DLCO × (1 – y/z)
y = Number of functional or unobstructed lung segments removed.
z = Total number of functional segments.
Assessment of functional or unobstructed segments can be done from preoperative computerized tomography or bronchoscopy [Table 1].
Table 1.
No of segments in various lobes

Formula for PPO with perfusion method
PPO FEV1 = preoperative FEV1× (1 – fraction of total perfusion for the resected lung)
PPO DLCO = preoperative DLCO × (1 – fraction of total perfusion for the resected lung).
PPO FEV1 and PPO DLCO are expressed as a percentage of predicted postoperative(%PPO) values
% PPO FEV1 = computed PPO FEV1 × 100/predicted normal FEV1
% PPO DLCO = computed PPO DLCO × 100/predicted normal DLCO.
Level 2
A patient having % PPO values more than 60% can undergo pulmonary resection (lobectomy or pneumonectomy) with a low risk.[3] If values are 30–60%, then there is a need for second level of tests.[3]
Low technology exercise tests
Six minute walk test
This is a very low technology test with no standardization and hence should not be used for preoperative assessment.[1]
Stair climbing test (SCT)
It is a first-line functional screening test to select patients who can undergo surgery safely. If the height of ascent without any discomfort is more than 22 metre, then the patient is fit to undergo pulmonary resection.[3] There is lack of standardization in this test in terms of duration of stair climbing, speed of ascent, number of steps per flight, height of each step, and the criteria for stopping the test are variable.
Shuttle walk test (SWT)
In this test the patient walks back and forth between two markers set 10 metres apart and the walking speed is increased each minute in a graded fashion, paced by an audio signal. If the patient is too breathless to maintain speed or is unable to maintain the pace or there is any drop in oxygen saturation to less than or equal to 85%, the test is discontinued. A patient unable to complete 25 shuttles (250 metres) on two separate occasions indicates a reduced maximum oxygen consumption (VO2max) of <10 ml/kg/min.[10] If a patient can complete 400 metres on SWT, then the patient is fit to undergo surgery.[3]
Level 3
Positive high-risk cardiac evaluation, % PPO FEV1 or DLCO less than 30%, SCT less than 22 m, or SWT less than 400 metre are indications of cardiopulmonary exercise test (CPET).[3] CPET provides a global and integrated assessment of cardiovascular, respiratory, skeletal muscle, and neurophyschological system to exercise whereas most other tests assess single organ function. It appears illogical to predict the outcome of a major surgical procedure using resting parameters of cardiac and pulmonary functions. Stress echocardiography is commonly used for preoperative evaluation of cardiac functions, but it has its own limitations as the maximum heart rate response to exercise is variable in the general population. Thus, in the current situation, CPET appear to be the gold standard for assessment of patients planned for major surgical procedure. CPET is a reliable and reproducible test as it can differentiate lack of patient motivation to perform the test from other organic causes of cardiorespiratory system. This test also helps in prognostication and exploring therapeutic options.
Few of the important CPET indices are:
-
Maximum aerobic capacity (VO2max) – It is defined as the highest oxygen uptake (VO2) despite work rate increments. VO2max defines the limit of cardiorespiratory response to exercise. Predicted values can be calculated based on gender, age, and height[11]
VO2max men = height (cm)–age (years) × 20
VO2max women = height (cm)–age (years) × 14
Values more than 20ml/kg/min is suggestive of a favorable prognosis,[12,13] and patients with VO2max less than 10 ml/kg/min are very high risk candidates for pulmonary resection.[3] With exercise training it is possible to improve VO2max and hence can be used as a tool to prescribe and follow-up exercise schedule[14]
-
Anerobic threshold (AT) – AT defines the onset of oxygen demand and supply mismatch in muscles leading to anerobic metabolism and lactic acidosis. As per the recommendation of American Heart Association scientific statement, three methods can be used for determination of AT:[15]
- The point of change of gradient of VCO2 – VO2(V slope) [Figure 2]:
- The point at which ventilatory equivalent for oxygen [Minute ventilation (VE)/VO2] increases without a corresponding increase in ventilatory equivalent of carbon dioxide (VE/VCO2)
-
The point where there is a rise of end-tidal oxygen pressure (PETO2) without a concurrent decrease in end-tidal carbon dioxide pressure (PETCO2)
Respiratory exchange ratio – The ratio between VCO2 and VO2 is known as respiratory exchange ratio (RER). This is a very accurate and reliable parameter of patient's efforts. A peak value of ≥1.10 is indicative of excellent patient effort[15]
-
Oxygen pulse – The oxygen pulse gives assessment of stroke volume at peak exercise. It is the ratio of VO2max and maximum heart rate. Calculation of predicted value of oxygen pulse is dependent on the height and gender of the patient[15]
0.28 (height in cm) – 3.3 (sex: male0, female1) – 26.7
Values more than 80% of the predicted is considered normal.[18] Low values of oxygen pulse is suggestive of poor left ventricular function
Ventilatory equivalents of carbon dioxide: The ratio of VE to VCO2 is known as ventilatory equivalent of carbon dioxide. Minute ventilation is regulated by the carbon dioxide produced, and hence, this ratio is quite constant throughout the performance of CPET. High values (≥34) at anerobic threshold is suggestive of gas exchange abnormality[18]
Oxygen desaturation – Fall in oxygen saturation as measured by pulse oximetry is suggestive of diminished ability to increase oxygen transfer through alveolar capillary membrane during exercise. Desaturation of more than 4% is associated with increased rate of postoperative complications[19]
Cardiac indices – Parameters such as peak heart rate achieved, arrhythmias noted, and electrocardiograph changes suggestive of ischemia should be noted. Underlying reason for inability to achieve target heart rate will help identifying cause of reduced exercise tolerance.
Figure 2.
CPET report
Conclusion
Systematic approach to preoperative evaluation of lung cancer is the key to favorable postoperative outcome. Surgical resection offers good long-term survival in case of lung cancer. Sublobar resections or nonsurgical treatment should be considered for a patient who is expected to become ventilator dependent postoperatively. Cardiopulmonary exercise testing provides a fair idea of the expected outcome in the postoperative period.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Brunelli A, Charloux A, Bolliger CT, Rocco G, Sculier JP, Varela G, et al. ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy) Eur Respir J. 2009;34:17–41. doi: 10.1183/09031936.00184308. [DOI] [PubMed] [Google Scholar]
- 2.Lim E, Baldwin D, Beckles M, Duffy J, Entwisle J, Faivre-Finn C, et al. Guidelines on the radical management of patients with lung cancer. Thorax. 2010;65(Suppl 3):iii1–27. doi: 10.1136/thx.2010.145938. [DOI] [PubMed] [Google Scholar]
- 3.Brunelli A, Kim AW, Berger KI, Addrizzo-Harris DJ. Physiologic Evaluation of the Patient With Lung Cancer Being Considered for Resectional Surgery Diagnosis and Management of Lung Cancer, 3rd ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2013;143(Suppl):e166S–90S. doi: 10.1378/chest.12-2395. [DOI] [PubMed] [Google Scholar]
- 4.Charloux A, Brunelli A, Bolliger CT, Rocco G, Sculier JP, Varela G, et al. Lung function evaluation before surgery in lung cancer patients: How are recent advances put into practice? A survey among members of the European Society of Thoracic Surgeons (ESTS) and of the Thoracic Oncology Section of the European Respiratory Society (ERS) Interact Cardiovasc Thorac Surg. 2009;9:925–31. doi: 10.1510/icvts.2009.211219. [DOI] [PubMed] [Google Scholar]
- 5.Ferguson MK, Little L, Rizzo L, Popovich KJ, Glonek GF, Leff A, et al. Diffusing capacity predicts morbidity and mortality after pulmonary resection. J Thorac Cardiovasc Surg. 1988;96:894–900. [PubMed] [Google Scholar]
- 6.Cerfolio RJ, Bryant AS. Different diffusing capacity of the lung for carbon monoxide as predictors of respiratory morbidity. Ann Thorac Surg. 2009;88:405–10. doi: 10.1016/j.athoracsur.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 7.Ferguson MK, Vigneswaran WT. Diffusing capacity predicts morbidity after lung resection in patients without obstructive lung disease. Ann Thorac Surg. 2008;85:1158–64. doi: 10.1016/j.athoracsur.2007.12.071. [DOI] [PubMed] [Google Scholar]
- 8.Guidelines on the selection of patients with lung cancer for surgery. Thorax. 2001;56:89–108. doi: 10.1136/thorax.56.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yuan N, Fraire JA, Margetis MM, Skaggs DL, Tolo VT, Keens TG. The effect of scoliosis surgery on lung function in the immediate postoperative period. Spine. 2005;30:2182–5. doi: 10.1097/01.brs.0000181060.49993.4a. [DOI] [PubMed] [Google Scholar]
- 10.Wang J, Olak J, Ultmann RE, Ferguson MK. Assessment of pulmonary complications after lung resection. Ann Thorac Surg. 1999;67:1444–7. doi: 10.1016/s0003-4975(99)00255-6. [DOI] [PubMed] [Google Scholar]
- 11.Agnew N. Preoperative cardiopulmonary exercise testing. Continuing Educ Anaesth Crit Care Pain. 2010;10:33–7. [Google Scholar]
- 12.Loewen GM, Watson D, Kohman L, Herndon JE, 2nd, Shennib H, Kernstine K, et al. Cancer and Leukemia Group B. Preoperative exercise Vo2 measurement for lung resection candidates: Results of Cancer and Leukemia Group B Protocol 9238. J Thorac Oncol. 2007;2:619–25. doi: 10.1097/JTO.0b013e318074bba7. [DOI] [PubMed] [Google Scholar]
- 13.Bayram AS, Candan T, Gebitekin C. Preoperative maximal exercise oxygen consumption test predicts postoperative pulmonary morbidity following major lung resection. Respirology. 2007;12:505–10. doi: 10.1111/j.1440-1843.2007.01097.x. [DOI] [PubMed] [Google Scholar]
- 14.Jones LW, Peddle CJ, Eves ND, Haykowsky MJ, Courneya KS, Mackey JR, et al. Effects of presurgical exercise training on cardiorespiratory fitness among patients undergoing thoracic surgery for malignant lung lesions. Cancer. 2007;110:590–8. doi: 10.1002/cncr.22830. [DOI] [PubMed] [Google Scholar]
- 15.Balady GJ, Arena R, Sietsema K, Myers J, Coke L, Fletcher GF, et al. Clinician's guide to cardiopulmonary exercise testing in adults: A scientific statement from the American Heart Association. Circulation. 2010;122:191–225. doi: 10.1161/CIR.0b013e3181e52e69. [DOI] [PubMed] [Google Scholar]
- 16.Snowden CP, Prentis JM, Anderson HL, Roberts DR, Randles D, Renton M, et al. Submaximal cardiopulmonary exercise testing predicts complications and hospital length of stay in patients undergoing major elective surgery. Ann Surg. 2010;251:535–41. doi: 10.1097/SLA.0b013e3181cf811d. [DOI] [PubMed] [Google Scholar]
- 17.Wilson RJ, Davies S, Yates D, Redman J, Stone M. Impaired functional capacity is associated with all-cause mortality after major elective intra-abdominal surgery. Br J Anaesth. 2010;105:297–303. doi: 10.1093/bja/aeq128. [DOI] [PubMed] [Google Scholar]
- 18.Joint Statement of the American Thoracic Society (ATS) and the American College of Chest Physicians (ACCP). ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167:211–77. doi: 10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- 19.Ninan M, Sommers KE, Landreneau RJ, Weyant RJ, Tobias J, Luketich JD, et al. Standardized exercise oximetry predicts postpneumonectomy outcome. Ann Thorac Surg. 1997;64:328–32. doi: 10.1016/S0003-4975(97)00474-8. [DOI] [PubMed] [Google Scholar]



