Abstract
Background and Aims:
Magnesium is a physiological antagonist of NMDA receptor and a calcium channel blocker. This study was designed to test the analgesic effect of magnesium sulfate (MgSO4) when added to epidural anesthesia in mild pre-eclampsia.
Material and Methods:
Sixty parturients with mild pre-eclampsia were allocated randomly to two equal groups. The Placebo group received 20 ml levobupivacaine hydrochloride 0.5% plus 5 ml isotonic saline 0.9% using two separate syringes. The Magnesium group received the same amount of local anesthetic plus 5 ml of 10% MgSO4(500 mg) using two separate syringes. The primary outcome was pain free period. While, the secondary outcomes were the onset of motor block and the time needed to achieve complete motor block. The analgesic profile was evaluated by visual analog scale (VAS) during rest or motion, the time to first request for analgesia, and the total analgesic consumption.
Results:
The pain-free period was significantly longer in the Magnesium group (311.3 ± 21.4) compared to placebo group (153.1 ± 22.18). The total postoperative consumption of fentanyl was significantly lower in the Magnesium group (42.4 ± 5.3) than that in the placebo group (94.4 ± 9.9), with a P value 0. 01. Both the onset time of motor block and the time needed to achieve complete motor block were significantly shorter among the Magnesium group (4.4 ± 1.4 and 8.2 ± 0.4, respectively), with a P value of 0. 01.
Conclusion:
The addition of 500 mg MgSO4 to epidural anesthesia fastens both sensory and motor blockade and improves postoperative analgesic profile.
Keywords: Epidural anesthesia, levobupivacaine, magnesium
Introduction
Neuro-axial anesthesia is the preferable anesthetic technique for pre-eclamptic patients scheduled for cesarean section in the absence of thrombocytopenia.[1,2,3] Epidural anesthesia provides many advantages as it improves utero-placental blood flow and results in a significant reduction in maternal catecholamine levels, which smoothen out the hypertensive surges caused by pain.[4] It can also relieve the stress-induced hypercoagulable status and immunosuppression.[5]
To decrease the onset time of the motor and sensory block and decrease the postoperative analgesic consumption, several agents have been tried such as opioid,[6] neostigmine,[7] clonidine,[8] and n-methyl d-aspartic acid (NMDA) receptor antagonist. The analgesic effect of epidural magnesium sulfate (MgSO4) is because of its noncompetitive antagonism of NMDA receptor and blocking calcium influx.[9]
In obstetric anesthesia, some studies have been tried to test the postoperative analgesic effect of MgSO4 when added to spinal anesthesia,[10,11] combined spinal and epidural anesthesia,[12] or epidural anesthesia.[13,14] These studies have concluded that it increases the postoperative analgesic period by reducing the consumption of postoperative analgesic requirements without additional side effects. However, because all these studies used MgSO4 as an adjuvant to the mixture containing local anesthetics and opioid, the analgesic effect of MgSO4 was primarily attributed to the potentiation of opioid analgesia.
Therefore, this prospective, randomized controlled study was designed to test the analgesic and anesthetic effects of MgSO4 when added to epidural anesthesia in mild pre-eclamptic patients. The first postoperative analgesic request was the primary outcome, and the onset of sensory and motor block were the secondary outcomes. We hypothesized that the addition of MgSO4 alone to levobupivacaine will improve the quality of epidural anesthesia and analgesia.
Material and Methods
After receiving approval from the local Institutional Review Board (IRB) and registering in clinical trial gov. identifier (NCT 02699827), an informed written consent was taken from the parturients prior to enrolment. This prospective, randomized, double-blinded and controlled trial was conducted in the Department of Obstetrics and Gynecology. Sixty pregnant patients suffering from mild pre-eclampsia scheduled for elective cesarean section, aged 20–35 years, and American Society of Anesthesiologists (ASA) Physical Status II were enrolled in this study. Mild pre-eclampsia was defined as the development of hypertension where systolic blood pressure (SPB) was 140–160 mmHg and/or diastolic blood pressure (DPB) was 90–110 mmHg on two occasions at least 6 hours apart after 20 weeks of gestation in a previously normotensive woman.
Patients were excluded if they exhibited any of the following: BMI >40 kg/m2, hepatic or renal impairment, HELLP syndrome, thrombocytopenia, contraindication for epidural block, spine deformity or refusal, history of reaction to any study medication, magnesium therapy, presence of communication difficulties preventing reliable assessment, and fetal distress.
At the preoperative visit, the pain evaluation score using visual analog scale (VAS) 10 cm where (0 cm = no pain and 10 cm = the worst pain) was explained to each parturient. On arrival to the operative theatre, basic monitors were applied and basal vital parameters were monitored in the left wedged supine position. All parturients received intravenous 20 ml/kg Ringer's lactate before and during the epidural block. Oxygen at 8 l/min was administrated via face mask.
The eligible pregnant patients were randomly allocated into two equal groups (n = 30). Concealment of random allocation was done using sealed, unlabeled, and opaque envelops that were sequentially numbered and opened just prior to epidural anesthesia.
Patients in the placebo group received 20 ml levobupivacaine hydrochloride 0.5% followed by 5 ml isotonic saline 0.9% prepared in two separate syringes.
Patients in the Magnesium group had received 20 ml levobupivacaine hydrochloride 0.5% followed by 5 ml of 10% preservative-free MgSO4(500 mg) prepared in two separate syringes.
Blinding was done through the usage of equal amounts of epidural solutions in two identical syringes (20 ml and 5 ml) prepared by an anesthetist not involved in the study or data collection. Epidural injection and pain scores assessment were done by an investigator who was unaware of the type of the drug used.
The epidural anesthesia was performed by an anesthetist who was blinded to the nature of the prepared epidural solution. With the patient in a sitting position and under strict aseptic conditions, an 18-gauge epidural Tuohy needle was introduced at the lumbar L2-3 interspace in the midline. The epidural space was identified by performed the loss of resistance to isotonic saline. Then the multi-orifice catheter was inserted 5 cm cephalic into the epidural space. For confirmation of injection in the epidural space and exclusion of accidental injection, intrathecal or intravenous, injection of 3 ml epidural lidocaine 2% with epinephrine 1:200,000 was given before the epidural solution. The catheter was secured and the patient was turned supine with left lateral tilt to minimize the risk of aortocaval compression. The epidural solution was injected 5 min after the test dose by 5 ml boluses every minute while monitoring patients’ hemodynamics and fetal heart rate. Every time the epidural injection was given after negative aspiration for blood or cerebrospinal fluid.
The sensory block was tested caudal to T6 at 2 min intervals for 30 min after completion of epidural injection using analgesia to pinprick. All dermatomes were tested bilaterally at mid-clavicular line to exclude patchy blocks. If a complete bilateral sensory block did not reach T6 within 30 min, 1.5 ml levobupivacaine at 0.5% increment were injected epidurally over 10 s for each missing segment and assessed after 6 min. The time taken to achieve the sensory block up to T6 level was recorded.
If the sensory block did not reach T6 after 30 min from the block, it was considered as failed epidural block and was excluded from the study. If fetal distress was diagnosed at any time during the epidural anesthesia, urgent cesarean section under general anesthesia or spinal anesthesia was carried out and the patient was excluded from the study.
Motor block was assessed at 5 min intervals for 30 min using Modified Bromage Scale.[15] The time needed to achieve complete motor block was recorded. In addition, the motor block was reported postoperatively every 2 h till full motor recovery. Abdominal muscle relaxation was assessed by the surgeon who did not know the allocation group in a graded score (1–4) as poor, fair, good, or excellent.[16]
The primary outcome of the study was the time to first analgesic requirement in the postoperative period, which was calculated from the time reaching T6 sensory block till the onset of pain. The VAS was evaluated at 1, 2, 4, 6, 12, 18, and 24 h postoperative either during rest or motion. If the recorded VAS was ≥3, the patient was given diclofenac potassium 75 mg oral tablets as the first rescue analgesia every 12 h. If the VAS was still >3 within 30 min, patients were given incremental dose of 0.5 μg/kg fentanyl. The time of the first rescue analgesic for pain in the postoperative period and the total dose of fentanyl consumption were reported.
Maternal monitoring of hemodynamics was continuously done. Hypotension was defined as systolic blood pressure <100 mmHg or >20% decrease in baseline values and was treated by intravenous ephedrine 5 mg. Bradycardia was defined as HR <50 beat/min or >20% decrease in baseline value was treated by IV 0.5 mg atropine sulfate.
Parturient were monitored for 24 h postpartum period for any complications including nausea, vomiting, bradycardia, hypotension, shivering, sedation, and pruritis. The neonates were assessed clinically by the attending neonatologist with Apgar score at 1 and 5 min after delivery.[17] The umbilical cord blood gas assessment (arterial blood gas/venous blood gas) was done: Apgar score at 1 min was recorded (perinatal asphyxia defined as an Apgar score of <7 at 5 min and evidence of encephalopathy within the first 6 h of life); any requirement for resuscitation such as oxygen supplementation, face-mask application, intubation, naloxone usage, and admission to neonatal intensive care was also noted.
Data analysis
Statistical analysis was done using the IBM® SPSS version 21 (IBM, SPSS Inc, Chicago, IL, USA). The normality of continuous variables was first tested with the Kolmogorov–Smirnov test. Differences between the continuous variables which had normal distribution were done using t-test, while for continuous variables without normal distribution, nonparametric tests were used and differences were computed by the Mann–Whitney U-test. Differences between percentages were compared with the Fisher's exact test. Probability (P value) ≤0.05 was considered statistically significant.
G power program (3.0.10) was used to calculate sample size with priory analysis. Pain-free period was used as the primary outcome. One-tailed Student's t-test for differences between two independent means was performed. Effect size was chosen as 0.8, α error was 0.05, and power (1-ß error) of 0.9 was used. The resulting sample size was 28 patients in each group. A dropout of 5% was expected. Therefore, a total of 60 cases were enrolled in this study.
Results
One hundred pregnant women suffering from mild preeclampsia were evaluated for eligibility to participate in this study. Ten patients refused to sign the consent and 30 patients data did not match with the inclusion criteria. The remaining 60 patients completed the study and none were excluded after allocation into two equal groups, as shown in Figure 1.
Figure 1.
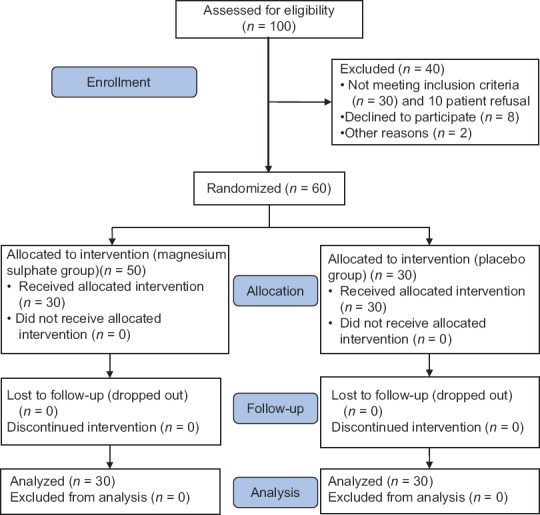
Study flow diagram
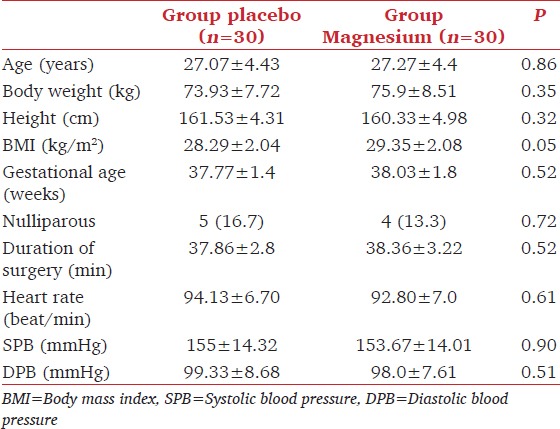
Both groups were comparable regarding the mean age, sex, weight, duration of surgery, and basal hemodynamic readings [Table 1].
Table 1.
Patient characteristics, duration of surgery and basal hemodynamics in both groups. Data are represented as mean±standard deviation, number or (percentage)
The time needed for the sensory block to reach T6 showed a statistical significant shorter duration in magnesium group than that in the placebo group [Table 2].
Table 2.
The block characteristics and postoperative analgesia. Data are represented as median (range), mean±standard deviation, or numbers (percentage)
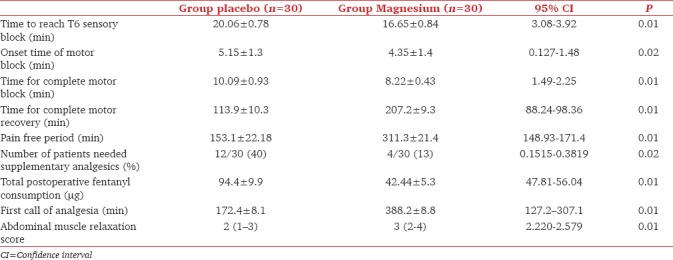
Both the onset time of motor block and time needed to achieve complete motor block were significantly shorter in the magnesium group compared to the placebo group. The full motor recovery was significantly longer in the magnesium group than that in the placebo group as shown in Table 2. The abdominal muscle relaxation was significantly higher in magnesium group than that in the placebo group.
The pain-free period showed statistically longer duration in magnesium group compared to placebo. In addition, the total fentanyl consumption in the postoperative period was significantly lower in magnesium group than that in the placebo group. The time for rescue analgesia for postoperative analgesia was significantly longer in magnesium group than that in the placebo group, as shown in Table 2.
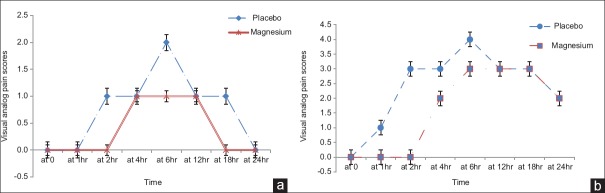
The VAS pain scores during rest were significantly lower in Magnesium group at second, sixth and eighteenth hours [Figure 2a] and those during movement (knee flexion) were significantly lower in the magnesium group in the second and fourth hour postoperatively [Figure 2b].
Figure 2.
(a) Visual analog score during rest and (b) during movement (knee flexion) in the studied groups
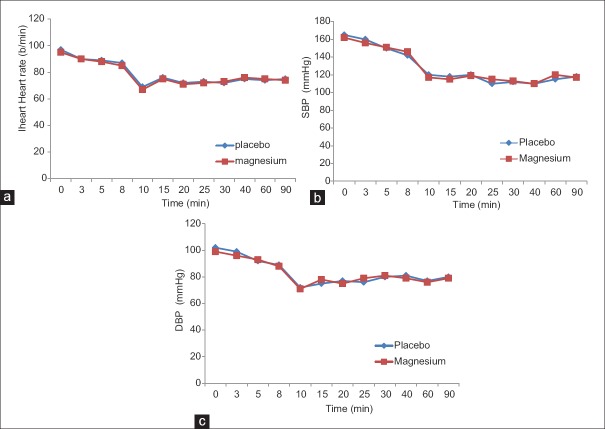
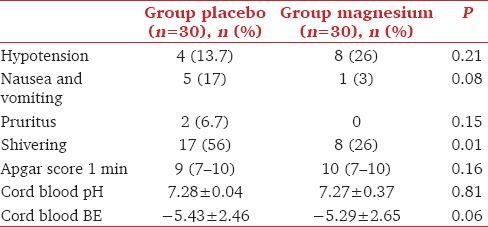
The perioperative hemodynamic variables were comparable in the studied groups, as shown in Figure 3a-c. The incidence of postoperative shivering was significantly lower in the magnesium group than that in the placebo group [Table 3]. The incidence of other complications was similar in the two groups. Neither the neonatal Apgar score nor cord pH or BE had any differences between the studied groups, as shown in Table 3.
Figure 3.
(a) The heart rate, (b) Systolic blood pressure and (c) Diastolic blood pressure in the studied groups
Table 3.
Incidence of maternal complications and neonatal outcomes in the studied groups. Data are represented as mean±standard deviation, median, (range), or number (percentage)
Discussion
This clinical study demonstrated that the epidural MgSO4 had a potent analgesic effect as it prolongs the postoperative pain-free period and first request of analgesia with a significant reduction in analgesic requirements. Moreover, it is a good adjuvant to levobupivacaine as it fastens the sensory and motor block with better abdominal relaxation.
The predominant analgesic effect of epidural magnesium is attributed to its noncompetitive antagonist to NMDA receptor which is ligand-gated ion channels that generate slow excitatory postsynaptic currents at glutamatergic synapses. The sustained activation of NMDA receptor promotes intracellular signaling that culminates in long-term synaptic plasticity, wind up phenomenon, and central sensitization.[18,19] In addition, the antagonist of NMDA receptor prevents the hyperalgesia, allodynia, and the induction of central sensitization.[20,21,22]
The analgesic effect of magnesium was evident by significant reduction of postoperative analgesic consumption which is in accordance with Bilir et al.;[23] similar analgesic effects of magnesium were reported by Yousef and Amr[12] who obtained approximately 153 min prolongation of the duration of postoperative analgesia by addition of 500 mg MgSO4 to epidural bupivacaine and fentanyl. Malleeswaran et al.[10] also obtained approximately 42 min prolongation of the duration of spinal anesthesia by addition of 50 mg MgSO4 to the intrathecal combination of bupivacaine and fentanyl.
The reduction in VAS scores, and lower total analgesic requirements. Are similar to the study by Sun et al.[13] who found comparable effects of both epidural 500 mg and 3 mg morphine regarding analgesia in the first postoperative 6 h.
Most studies depend on the co-administration of magnesium with opioids because they explained the analgesic effect of magnesium by potentiating the antinociceptive effects of opioids because the combined group had the greatest analgesic effect.[11,12,13,14] However, magnesium has its own analgesic effect of blocking the high-voltage-gated N-type calcium channels, and hence inhibits the release of neuropeptides from the sensory nerves as (substance P or calcitonin gene-related peptides). This analgesic effect could be due to the diffusion of magnesium from the epidural space across the dura (13, 14, and 23). Recently, Bahrenberg et al.[24] in their animal study found that the anti-nociceptive effect of MgSO4 alone is greater in magnitude than when MgSO4 and morphine were combined.
Therefore, this study was tailored to evaluate the usage of magnesium against placebo. Consequently, we injected MgSO4 using separate syringes in a sequential manner after injection of local anesthetic. Some studies suggested that MgSO4 would change the chemical and pH characteristics of amide LAs when premixed in the same syringe.[9] Premixing of adjuvant with LAs in the same syringe seems to delay the onset and reduce the duration of the neuraxial block. This may be due to the change in the concentration and/or the chemistry of local anesthetics.[25]
In this study, the addition of MgSO4 significantly enhanced the onset and prolonged the duration of motor block. This result was correlated with Ghatak et al.[26] who studied the addition of magnesium as an anesthetic adjuvant in lower abdominal and lower limb surgeries. The muscle relaxant effect of MgSO4 is due to the calcium channel blocker; MgSO4 prevents the passive release of calcium by the sarcoplasmic reticulum and induces muscle relaxation. Also, it affects neuromuscular transmission as it reduces the presynaptic release of acetylcholine (ACH); the decreased ACH level will affect the postsynaptic muscle receptors and increase the threshold of axonal excitation.[22,23] This is consistent with the results of Arcioni et al. who observed that whether magnesium is added intrathecally or epidurally, it potentiated and prolonged motor block.[27] Better abdominal relaxation is valuable for the surgeon; however, the delayed recovery from motor block may have its disadvantages and may be inappropriate for early mobilization of the parturient.
Further, the significant earlier onset of sensory block to reach T6 in magnesium group (16.65 ± 0.84), which is compatible with the results of Ghatak et al.[26] (11.8 ± 3.3).
Different doses of epidural MgSO4 had been tried; Bilir et al.[23] injected a bolus small dose of 50 mg followed by infusion of 100 mg, but Yousef and Amr[12] used 500 mg MgSO4 which is the maximum dose. In this study, the used dose was the maximum dose but with continuous monitoring for the mother and fetus. In our study, the lower incidence of shivering in the magnesium group was because of the anti-shivering effects of magnesium which have been documented in previous studies that used magnesium intravenous[28] and neuroaxial.[29] They attributed the antishivering effect to the cutaneous vasodilatation preventing sensation of coldness, thus preventing the shivering reflex.[29] The comparable postoperative and intraoperative complication among both groups is because of the choice of intermediate dose. Reduced incidence of pruritis, nausea, vomiting, and hypotension was noted in the MgSO4 group, but without clinical significance. It seems to be related to the reduction of postoperative fentanyl usage.
Limitations of the study
The study design used maximum dose against the placebo. We did not compare different doses of epidural magnesium. Further studies must be done to evaluate if there is a synergistic effect of intravenous magnesium and epidural magnesium in severe eclamptic patients. Epidural MgSO4 seems to be safe for both the mother and the baby because no serious effects were noticed in the study, but its safety profile should be further evaluated, especially its adverse neurological effects. Finally, we did not measure the blood level of magnesium
Conclusion
In conclusion, the addition of MgSO4 to levobupivacaine in epidural anesthesia has dual effects on the anesthetic and analgesic profiles. It fastens both sensory and motor blockade. Moreover, it significantly improves the postoperative pain score and reduces the total analgesic requirement.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Lambert G, Brichant JF, Hartstein G, Bonhomme V, Dewandre PY. Preeclampsia: An update. Acta Anaesthesiol Belg. 2014;65:137–49. [PubMed] [Google Scholar]
- 2.Stocks G. Preeclampsia: Pathophysiology, old and new strategies for management. Eur J Anaesthesiol. 2014;31:183–9. doi: 10.1097/EJA.0000000000000044. [DOI] [PubMed] [Google Scholar]
- 3.Han B, Xu M. A comprehensive analysis of continuous epidural analgesia's effect on labor and neonates in maternal hypertensive disorder patients. Pregnancy Hypertens. 2017;7:33–8. doi: 10.1016/j.preghy.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Ramos-Santos E, Devoe LD, Wakefield ML, Sherline DM, Metheny WP. The effects of epidural anesthesia on the Doppler velocimetry of umbilical and uterine arteries in normal and hypertensive patients during active term labor. Obstet Gynecol. 1991;77:20–6. [PubMed] [Google Scholar]
- 5.Procopio MA, Rassias AJ, DeLeo JA, Pahl J, Hildebrandt L, Yeager MP, et al. The in vivo effects of general and epidural anesthesia on human immune function. Anesth Analg. 2001;93:460–5. doi: 10.1097/00000539-200108000-00044. [DOI] [PubMed] [Google Scholar]
- 6.Parpaglioni R, Baldassini B, Barbati G, Celleno D. Adding sufentanil to levobupivacaine or ropivacaine intrathecal anaesthesia affects the minimum local anaesthetic dose required. Acta Anaesthesiol Scand. 2009;53:1214–20. doi: 10.1111/j.1399-6576.2009.02033.x. [DOI] [PubMed] [Google Scholar]
- 7.Cossu AP, De Giudici LM, Piras D, Mura P, Scanu M, Cossu M, et al. A systematic review of the effects of adding neostigmine to local anesthetics for neuraxial administration in obstetric anesthesia and analgesia. Int J Obstet Anesth. 2015;24:237–46. doi: 10.1016/j.ijoa.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Parker RK, Connelly NR, Lucas T, Serban S, Pristas R, Berman E, et al. Epidural clonidine added to a bupivacaine infusion increases analgesic duration in labor without adverse maternal or fetal effects. J Anesth. 2007;21:142–7. doi: 10.1007/s00540-006-0476-8. [DOI] [PubMed] [Google Scholar]
- 9.Albrecht E, Kirkham KR, Liu SS, Brull R. The analgesic efficacy and safety of neuraxial magnesium sulphate: A quantitative review. Anaesthesia. 2013;68:190–202. doi: 10.1111/j.1365-2044.2012.07337.x. [DOI] [PubMed] [Google Scholar]
- 10.Malleeswaran S, Panda N, Mathew P, Bagga R. A randomised study of magnesium sulphate as an adjuvant to intrathecal bupivacaine in patients with mild preeclampsia undergoing caesarean section. Int J Obstet Anesth. 2010;19:161–6. doi: 10.1016/j.ijoa.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 11.Sayed JA, Fathy MA. Maternal and neonatal effects of adding two different doses of intrathecal magnesium sulphate to bupivacaine fentanyl spinal anesthesia in mild preeclamptic patients undergoing caesarean section. J Am Sci. 2012;8:435–41. [Google Scholar]
- 12.Yousef AA, Amr YM. The effect of adding magnesium sulphate to epidural bupivacaine and fentanyl in elective caesarean section using combined spinal-epidural anaesthesia: A prospective double blind randomised study. Int J Obstet Anesth. 2010;19:401–4. doi: 10.1016/j.ijoa.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 13.Sun J, Wu X, Xu X, Jin L, Han N, Zhou R. A comparison of epidural magnesium and/or morphine with bupivacaine for postoperative analgesia after cesarean section. Int J Obstet Anesth. 2012;21:310–6. doi: 10.1016/j.ijoa.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Hasanein R, El-Sayed W, Khalil M. The value of epidural magnesium sulfate as an adjuvant to bupivacaine to bupivacaine and fentanyl for labor analgesia. Egyptian J Anaesth. 2013;29:219–24. [Google Scholar]
- 15.Bromage PR. A comparison of the hydrochloride and carbon dioxide salts of lidocaine and prilocaine in epidural analgesia. Acta Anaesthesiol Scand Suppl. 1965;16:55–69. doi: 10.1111/j.1399-6576.1965.tb00523.x. [DOI] [PubMed] [Google Scholar]
- 16.Morrison AP, Hunter JM, Halpern SH, Banerjee A. Effect of intrathecal magnesium in the presence or absence of local anaesthetic with and without lipophilic opioids: A systematic review and meta-analysis. Br J Anaesth. 2013;110:702–12. doi: 10.1093/bja/aet064. [DOI] [PubMed] [Google Scholar]
- 17.Weber T. Continuous fetal pH monitoring and neonatal Apgar score. J Perinat Med. 1980;8:158–63. doi: 10.1515/jpme.1980.8.3.158. [DOI] [PubMed] [Google Scholar]
- 18.Hollmann MW, Liu HT, Hoenemann CW, Liu WH, Durieux ME. Modulation of NMDA receptor function by ketamine and magnesium. Part II: Interactions with volatile anesthetics. Anesth Analg. 2001;92:1182–91. doi: 10.1097/00000539-200105000-00020. [DOI] [PubMed] [Google Scholar]
- 19.Woolf CJ, Chong MS. Preemptive analgesia – Treating postoperative pain by preventing the establishment of central sensitization. Anesth Analg. 1993;77:362–79. doi: 10.1213/00000539-199377020-00026. [DOI] [PubMed] [Google Scholar]
- 20.Fawcett WJ, Haxby EJ, Male DA. Magnesium: Physiology and pharmacology. Br J Anaesth. 1999;83:302–20. doi: 10.1093/bja/83.2.302. [DOI] [PubMed] [Google Scholar]
- 21.Dubé L, Granry JC. The therapeutic use of magnesium in anesthesiology, intensive care and emergency medicine: A review. Can J Anaesth. 2003;50:732–46. doi: 10.1007/BF03018719. [DOI] [PubMed] [Google Scholar]
- 22.Dean C, Douglas J. Magnesium and the obstetric anaesthetist. Int J Obstet Anesth. 2013;22:52–63. doi: 10.1016/j.ijoa.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Bilir A, Gulec S, Erkan A, Ozcelik A. Epidural magnesium reduces postoperative analgesic requirement. Br J Anaesth. 2007;98:519–23. doi: 10.1093/bja/aem029. [DOI] [PubMed] [Google Scholar]
- 24.Bahrenberg A, Dzikiti BT, Fosgate GT, Stegmann FG, Tacke SP, Rioja E, et al. Antinociceptive effects of epidural magnesium sulphate alone and in combination with morphine in dogs. Vet Anaesth Analg. 2015;42:319–28. doi: 10.1111/vaa.12211. [DOI] [PubMed] [Google Scholar]
- 25.Sachan P, Kumar N, Sharma JP. Efficacy of premixed versus sequential administration of clonidine as an adjuvant to hyperbaric bupivacaine intrathecally in cesarean section. Anesth Essays Res. 2014;8:20–5. doi: 10.4103/0259-1162.128898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghatak T, Chandra G, Malik A, Singh D, Bhatia VK. Evaluation of the effect of magnesium sulphate vs. clonidine as adjunct to epidural bupivacaine. Indian J Anaesth. 2010;54:308–13. doi: 10.4103/0019-5049.68373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arcioni R, Palmisani S, Tigano S, Santorsola C, Sauli V, Romanò S, et al. Combined intrathecal and epidural magnesium sulfate supplementation of spinal anesthesia to reduce post-operative analgesic requirements: A prospective, randomized, double-blind, controlled trial in patients undergoing major orthopedic surgery. Acta Anaesthesiol Scand. 2007;51:482–9. doi: 10.1111/j.1399-6576.2007.01263.x. [DOI] [PubMed] [Google Scholar]
- 28.Gozdemir M, Usta B, Demircioglu RI, Muslu B, Sert H, Karatas OF. Magnesium sulfate infusion prevents shivering during transurethral prostatectomy with spinal anesthesia: A randomized, double-blinded, controlled study. J Clin Anesth. 2010;22:184–9. doi: 10.1016/j.jclinane.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 29.Faiz SH, Rahimzadeh P, Iman F, Bakhtiari A. Intrathecal injection of magnesium sulphate: Shivering prevention during cesarean section: A randomized double-blinded controlled study. Korean J Anesthesiol. 2013;65:293–8. doi: 10.4097/kjae.2013.65.4.293. [DOI] [PMC free article] [PubMed] [Google Scholar]